Abstract
In this study, we investigated whether CD47 antibody could attenuate isoproterenol (ISO)-induced cardiac hypertrophy in mice and H9C2 cells. Cardiac hypertrophy was induced by intraperitoneal (i.p.) injection of ISO (60 mg.kg-1.d-1 in 100 µl of sterile normal saline) daily for 14 days, and cell hypertrophy was induced by ISO (10-5 mol/l) for 48 h. The injury was confirmed by increased levels of lactate dehydrogenase (LDH) and creatine kinase MB (CK-MB), increased heart-to-body weight (HW/BW) ratio and visible cardiac fibrosis. Apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. Autophagic flux in H9c2 cells was monitored by TEM and mRFP-GFP-LC3 virus transfection. The expression levels of Cleaved caspase-3, Cleaved caspase-9 and autophagy-related proteins were detected by Western blotting. CD47 antibody significantly limited ISO-induced increases in LDH, CK-MB, HW/BW ratio and attenuated cardiac fibrosis, oxidative stress, and apoptosis in the heart; CD47 antibody promoted autophagy flow and decreased cell apoptosis in cardiac tissues. Moreover, autophagy inhibitor chloroquine (CQ) reversed the effect of CD47 antibody treatment. In conclusion, CD47 antibody reduced ISO-induced cardiac hypertrophy by improving autophagy flux and rescuing autophagic clearance.
Keywords: Cardiac hypertrophy, CD47 antibody, oxidative stress, apoptosis, autophagy
Introduction
Cardiac hypertrophy, occurring during the clinical course of stress-induced heart failure [1-3], is characterized by an abnormal enlargement of the heart muscle resulting from increased myocyte cell size and abnormal proliferation of non-muscle cells [4]. Cardiac hypertrophy is controlled by a complex signal transduction and gene regulatory network [5,6]. Recently, autophagy, a dynamic process involving the bulk degradation of cytoplasmic organelles and proteins, has been proven to participate in the pathogenesis of cardiac hypertrophy [7-12].
Isoproterenol (ISO), a nonselective b-adrenergic receptor (b-AR) agonist, has been widely used as a stimulus for cardiac hypertrophy [13,14] due to its convenience and rapidity in yielding reproducible results [15]. The pathophysiological and morphological aberrations in the heart of myocardial necrotic rat model are comparable with those in human myocardial infarction [16,17]. These b-adrenergic effects can result in cardiac “infarct-like” lesions in experimental animals [18], similar to those in patients with myocardial infarction [19]. ISO-induced cardiac hypertrophy is accompanied by a significant decrease in autophagy activity [20-22].
CD47 is a widely expressed cell receptor [23] and activator of NADPH-oxidase-mediated reactive oxygen species (ROS) production in vascular cells [24]. Previous studies have identified the role of CD47 in limiting blood flow [23] and metabolism [25], and suggested the additional benefits by therapeutic targeting of CD47 in myocardial infarction [26]. CD47 transcript has been reported to increase in ventricular biopsies from patients of left ventricular heart failure (LVHF) [27]. CD47-knockout mice displayed protection from transverse aortic constriction (TAC)-driven LVHF with enhanced cardiac functions, decreased cellular hypertrophy and fibrosis [26], and CD47 deficiency conferred cell survival through the activation of autophagic flux against radiation injury [28-30]. Moreover, CD47-blocking antibody has been used in research of various diseases including tumor and atherosclerosis [31-36]. So far, the specific effect of CD47 antibody on ISO-induced cardiac hypertrophy remains unclear. In this study, we investigated the effect of CD47 antibody on cardiac hypertrophy, fibrosis and myocyte apoptosis in mouse and cell models with ISO-induced cardiomyocyte hypertrophy.
Materials and methods
Animals
Eighty C57/BL6 male mice, 8-10 weeks old, weighing 22-28 g, were obtained from Nanjing University. The mice were housed in a Specific Pathogen Free (SPF) facility in the Animal Core Facility of Nanjing Medical University under standard temperature conditions with a 12 h light/dark cycle and fed ad libitum. All experimental protocols and animal handling procedures were performed according to the “Guide for the Care and Use of Laboratory Animals” (National Academic Press, USA, 1996). The animal study was approved by the Institutional Animal Care and Use Committee of the Nanjing Medical University.
Animal model of cardiac hypertrophy
Animal model of cardiac remodeling was established by intraperitoneally injection of ISO (I5627, Sigma-Aldrich, USA; 60 mg.kg-1.d-1, dissolved in sterile normal saline) once daily for 14 consecutive days [37]. The animals were then allowed to recover with free access to food and water. At 24 h after the last administration, the mice were euthanized using intraperitoneal injection of sodium pentobarbital (50 mg/kg) under general anesthesia, and the heart tissues were dissected and weighed. The ratio of heart weight to body weight (relative weight of heart) was calculated for each group as index of cardiac hypertrophy. The blood and left ventricles were harvested for subsequent examination.
Grouping and experimental protocol
Two studies were performed. In study 1, a total of 80 mice were randomly allocated into 4 groups.
Group 1 (IgG): Animals received IgG antibody (0.4 μg/g body weight in 150 μl sterile normal saline, i.p., sc-2026, Santa Cruz Biotechnology, USA).
Group 2 (IgG+ISO): Animals received IgG antibody (0.4 μg/g body weight in 150 μl sterile normal saline, i.p.) treatment twice weekly for 4 weeks after injection of ISO (60 mg.kg-1.d-1 in sterile normal saline 100 μl, i.p.) daily for 14 days.
Group 3 (CD47): Animals received CD47 antibody (0.4 μg/g body weight in 150 μl sterile normal saline, i.p., sc-12731, Santa Cruz Biotechnology, USA) [26,38] treatment twice weekly for 4 weeks without injection of ISO.
Group 4 (CD47+ISO): Animals received CD47 antibody (0.4 μg/g body weight in 150 μl sterile normal saline, i.p.) treatment twice weekly for 4 weeks after injection of ISO (60 mg.kg-1.d-1 in sterile normal saline 100 μl, i.p.) daily for 14 days [26]. All mice were sacrificed under general anesthesia using intraperitoneal injection of sodium pentobarbital (50 mg/kg). The blood was collected from the punctured heart and the right kidney was stored at -80°C for mRNA and protein extraction.
In study 2, H9c2 cells were assigned to 4 groups.
Group 1 (IgG): Cells received IgG antibody (7 μg/ml, sc-2025, Santa Cruz Biotechnology, USA).
Group 2 (IgG+ISO): Cells received IgG antibody (7 ug/ml) and cultured for 12 h [39]. Subsequently, after adding ISO (10-5 mol/l), cells were cultured for another 48 h [40].
Group 3 (CD47): Cells received CD47 antibody (7 μg/ml, sc-53050, Santa Cruz Biotechnology, USA).
Group 4 (CD47+ISO): Cells received CD47 antibody (7 ug/ml) and cultured for 12 h [39]. Subsequently, after adding ISO (10-5 mol/l), the cells were cultured for another 48 h [40].
Group 5 (CD47+ISO+CQ): Cells received CD47 antibody (7 ug/ml) for 2 h and CQ (10 mmol/L C6628, Sigma, St. Louis, MO, USA) for 1 h [39]. Subsequently, after adding ISO (10-5 mol/l), the cells were cultured for another 48 h [40]. CQ was used to inhibit lysosomal acidification and autophagosome-lysosome fusion [41]. All experiments were repeated for three times.
Echocardiography
The systolic heart function of the mice was evaluated by echocardiography. Briefly, mice were anesthetized with inhalation of 2% isoflurane in the overhead position on a heating pad (40°C). The body temperature of each mouse was monitored and maintained at 37°C throughout the experiments and spontaneous breath was allowed. Echocardiography was performed using a 35-MHz phased-array ultrasound system (Vevo 2100, Visual Sonics Inc., Toronto, Ontario, Canada). To minimize data variation, cardiac function was assessed when the heart rate was within the range of 550-650/minute. Data of 3 consecutive heart cycles in each mouse were digitally recorded and analyzed. The following parameters were measured from M-mode images taken from the parasternal short-axis view at papillary muscle level: interventricular septum (IVS), left ventricular internal dimension (LVID), left ventricular volume (LV vol.), left ventricle mass (LV mass), left ventricular fractional shortening (FS) and left ventricular ejection fraction (EF).
Biochemical assays
All blood samples were allowed to clot at room temperature and centrifuged at 2,000 g for 10 min to harvest serum. The biochemical indicators including lactate dehydrogenase (LDH), creatine kinase MB (CK-MB), superoxide dismutase (SOD), malonaldehyde (MDA) were measured spectrophotometrically using commercially available kits for LDH (A020-2), CK-MB (H197), SOD (A001-1), MDA (A003-1) (Jiancheng Bioengineering Institute, Nanjing, China). The heart weight to body weight ratio was calculated by dividing heart weight (mg) by body weight (g).
Histological and morphometric analysis
The heart was placed in a 10% potassium chloride solution immediately at end-diastole after removal from the euthanized mice, washed with saline solution, and then placed in 4% paraformaldehyde at 4°C overnight. The heart was transversely cut close to the apex to expose the left and right ventricles. The samples were then dehydrated in an ethanol gradient, rinsed in xylene, and embedded in paraffin. Finally, paraffin blocks were cut into 4 μm sections, stained in H&E for histopathology and Masson for collagen deposition and then visualized by light microscopy. Photos were analyzed using Image-Pro Plus.
ROS staining
Total ROS was stained with dihydroethidium (DHE, D-23107; Invitrogen, USA) on fresh frozen sections. The hearts taken out of the euthanized mice were mounted in OCT embedding compound (3801480; Leica, Germany), frozen at -80°C and cut into 5 um thick sections using a cryostat and thawed. The sections were mounted onto gelatin-coated histological slides. 5 uM DHE dissolved in DMSO was added to fresh frozen mouse heart sections after dilution in PBS, incubated in darkness at 37°C for exactly 30 min, and then rinsed twice with cold PBS. The photos were taken immediately.
Quantitative real time polymerase chain reaction (Q-PCR)
Total mRNA was extracted from heart samples using TRIzol reagent (B5704-1, Takara, Dalian, China) and then treated with DNase I (2212, Takara, Dalian, China) according to the manufacturer’s protocol. The quality and quantity of RNA were determined using a spectrophotometer (NanoDrop 2000c, Thermo Scientific, USA). cDNA was immediately synthesized using a PrimeScriptTM RT Reagent Kit (RR037A, Takara, Dalian, China) according to the manufacturer’s instructions. Q-PCR was performed using a Light Cycler PCR QC Kit (Roche, Switzerland) and a 7300 Real-Time PCR System (LC96, Roche, Switzerland). The primer sequences used were as follows: natriuretic peptide precursor type A (ANP) (NM_008725.3) forward: AAGAGGGCAGATCTATCGGA, reverse: TTGGCTTCCAGGCCATAATTG; brain natriuretic peptide (BNP) (NM_001287348.1) forward: TCTTGTGCCCAAAGCAGCTT, reverse: ATGGATCTCCTGAAGGTGCT; and β-actin (NM_007393.3) forward: CACGGTTGGCCTTAGGGTTCAG, reverse: GCTGTATTCCCCTCCATCGTG. The housekeeping gene β-actin was used as an internal reference. Data analysis was performed and graphs were produced using GraphPad Prism 5 software.
In situ TUNEL staining assay
A terminal deoxynucleotidyltransferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay was performed according to the manufacturer’s instructions (11684817910, Roche, Switzerland). Heart tissues were fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, sectioned into 4-μm-thick sections and placed on numbered polylysine-coated glass slides. Deparaffinized tissue sections were incubated with proteinase K (20 mg/ml, Sigma, USA) in a humidified chamber for 15 min, and endogenous peroxidase activity was blocked by a 10-min 3% H2O2 treatment. The sections were incubated with TdT labeling buffer at 37°C for 1 h in a moist chamber and then counterstained with DAPI. TUNEL-positive cells showed brown and the nuclei blue. Five random fields per slide (five slides per animal, seven animals per group) were examined. The nuclei number of TUNEL-positive cells (Numerator) and the total number of cellular nuclei (denominator) were counted as previously mentioned [42]. The rate of TUNEL-positive cells in each field was analyzed using Image Pro Plus 6.0 software.
LC3 puncta quantification
The mRFP-GFP-LC3 virus was purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China), and titers were determined (1×108). H9c2 cells were first transfected with the mRFP-GFP-LC3 virus following the manufacturer’s instructions. After treatment, the cells were fixed with 4% paraformaldehyde. The GFP-LC3 punctate dot structures in individual live H9c2 cells were imaged using a fluorescence microscope. Autophagy was quantified by calculating the percentage of GFP-positive (green) puncta, and upon fusion with lysosomes, the puncta became mRFP-positive (red). The nuclei were counterstained with 4’, 6-Diamidino-2-Phenylindole (DAPI) (Sigma-Aldrich). The images were taken using the Confocal Imaging System (Zeiss LSM710, ZEISS, Germany).
Transmission electron microscopy (TEM)
For TEM examination, H9C2 cells were digested and centrifuged (800× g for 5 min at 25°C) to form cell pellets, fixed in 2.5% glutaraldehyde for 48 h at 4°C, post fixed in 0.5% osmium tetroxide, dehydrated and embedded in epoxy resin. Ultrathin sections (90 nm thick) were made and examined using TEM (Tecnai G2 Spirit Bio TWIN; FEI Ltd.) at accelerating voltage of 80 kV and ×10,000 magnification. Electron micrographs (five fields of view per cell) were randomly examined (five cells at four corners and in the middle of the field were selected) for each experiment.
Western blotting
Both cell and tissue proteins were extracted by homogenizing samples in 1× RIPA buffer with 1 mmol/L phenyl methyl sulfonyl fluoride (PMSF) and protease inhibitor cocktail. Protein concentrations were determined using the Coomassie PlusTM Protein Assay reagent. The total protein (30 mg) was separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane. Each membrane was blocked with 5% nonfat milk in TBST buffer (100 mM NaCl, 10 mM Tris-HCl, pH 7.4, 0.1% Tween-20) for 1 h prior to incubation with primary antibodies against LC3 (4108, Cell Signaling Technology, USA), LAMP-2 (sc-71492, Santa Cruz Biotechnology, USA), ATG5 (12994, Cell Signaling Technology, USA), ATG5 (8558, Cell Signaling Technology, USA), Cleaved caspase-3 (9664, Cell Signaling Technology, USA), Cleaved caspase-9 (7237, Cell Signaling Technology, USA) and GAPDH (sc-166574, Santa Cruz Biotechnology, USA) at 4°C overnight followed by incubation with a goat anti-rabbit IgG HRP-conjugated secondary antibody (sc-2004, Santa Cruz Biotechnology, USA) or a goat anti-mouse IgG HRP-conjugated secondary antibody (sc-2005, Santa Cruz Biotechnology, USA). Then, the membranes were washed 3 times in TBST. The blots were imaged using a ChemiDoc XRS+ Molecular Imager (Bio-Rad) with Pierce ECL Western Blotting Substrate (32209, Thermo Scientific) and analyzed using image analysis software (ImageJ 1.42). The housekeeping protein GAPDH was used as an internal reference. Western blotting quantification was corrected for GAPDH expression prior to normalization.
Statistical analysis
All assays were independently performed for 3 times. All data were presented as the means ± standard error of the means and statistically analyzed using SPSS software (version 13.0). One-way ANOVAs were used to determine statistical significance, and Bonferroni post hoc tests were used for further evaluation of the data. A value of P < 0.05 was considered statistically significant.
Results
CD47 antibody inhibited ISO-induced cardiac hypertrophy
An increase in the heart to body weight ratio in ISO-induced myocardial infarcted mice indicated cardiac hypertrophy [43]. Hematoxylin-eosin (H&E) staining of the hypertrophic heart paraffin sections showed that the mice injected with ISO had eccentric hypertrophy (Figure 1A). Heart weight to body weight ratio (HW/BW) was significantly increased in IgG+ISO group compared with the IgG group (P < 0.01, Figure 1B). Importantly, the HW/BW ratio of mice from the CD47+ISO group was significantly lower than those in the IgG+ISO group (P < 0.05). The observed increase in the heart weight in ISO-treated mice might be attributed to the elevated water content, oedematous intramuscular space and extensive necrosis of cardiac muscle fibers followed by the invasion of damaged tissues caused by the inflammatory cells [44].
Figure 1.
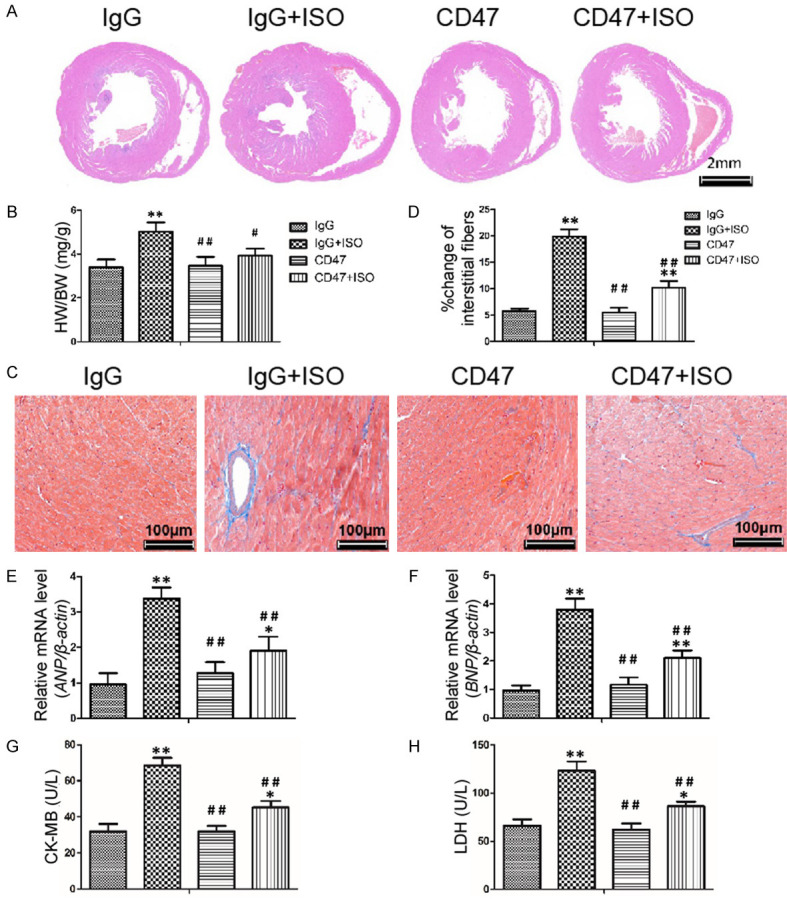
CD47 antibody inhibited ISO-induced cardiac hypertrophy. A. Representative H&E staining of mouse hearts from the four groups. B. Heart weight to body weight ratio (HW/BW) was significantly increased after ISO treatment, n=7 per group. C. The levels of fibrosis in the four groups were detected by Masson’s trichrome staining. D. Quantification of the volume of interstitial fibrosis was performed using Image-Pro Plus (n=6). CD47 antibody abolished the effects of ISO in increasing cardiac fibrosis. E, F. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were increased in the ISO-induced mice which were reversed by CD47 antibody treatment. G, H. CD47 antibody treatment decreased CK-MB and LDH levels induced by ISO, n=7 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SD. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group; #P < 0.05 and ##P < 0.01 versus the IgG+ISO group.
ISO-induced cardiac hypertrophy is associated with increased fibrosis in the myocardium [45]. Cardiac fibrosis is characterized by excessive extracellular matrix accumulation and fibroblast deposition, which can eventually destroy organ architecture and abolish normal cardiac functions [45-47]. In this study, fibrosis detected by Masson’s trichrome staining for collagen was negligible in hearts of mice from the IgG and CD47 groups, whereas fibrosis significantly increased in mice of the IgG+ISO group (P < 0.01, Figure 1C, 1D). Compared with the ISO group, fibrosis was significantly lower in mice from the CD47+ISO group (P < 0.01).
Pathological hypertrophy and heart failure are accompanied by altered expression of a large number of genes that are correlated with loss of cardiac functions [48]. Hypertrophic genes, natriuretic peptide precursor type A (ANP) and brain natriuretic peptide (BNP) are molecular markers of cardiac hypertrophy [48]. Cardiac expression of hypertrophy-related genes ANP and BNP were detected by Q-PCR (Figure 1E, 1F). The mRNA levels of ANP and BNP significantly increased in the ISO group compared with the IgG group (P < 0.01). The expression levels of ANP and BNP were significantly lower than those in the IgG+ISO group (P < 0.01), suggesting an inhibitory effect of CD47 antibody on the expression of hypertrophy-related genes.
CD47 antibody attenuated ISO-induced cardiac dysfunction
Cytosolic enzymes such as CK-MB and LDH, which serve as the diagnostic markers, leak out from the damaged tissues to blood stream when cell membrane ruptures or becomes permeable [49]. The effects of CD47 antibody on cardiac function index are shown in Figure 1G, 1H. There was no significant difference in LDH and CK-MB levels between the IgG and CD47 groups. ISO treatment in the IgG+ISO group increased LDH and CK-MB levels compared with the control group (P < 0.01). CD47 antibody treatment in the CD47+ISO group decreased LDH and CK-MB levels compared with the ISO group (P < 0.01).
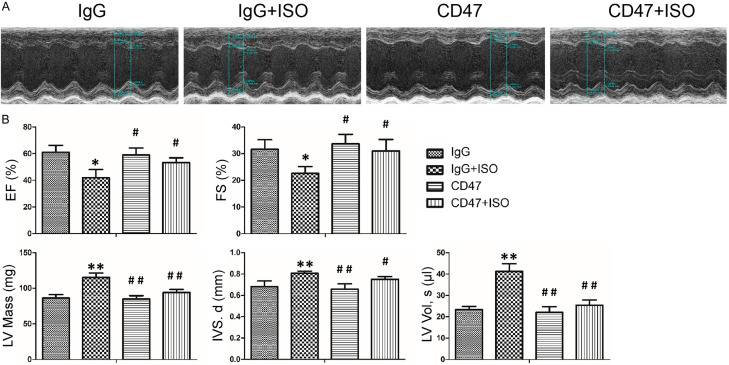
The echocardiography revealed significant impairment in LV function after ISO infusion, including EF and FS (Figure 2A, 2B). Also, consistent with the H&E results (Figure 1A, 1B), the two-week application of ISO in mice from the IgG+ISO group produced a marked increase in heart size, including interventricular septum (IVS, d), left ventricular volume (LV Vol.), left ventricle mass (LV Mass), compared with the control group (P < 0.05 or P < 0.01, respectively, Figure 2B). These physiological changes were inhibited by CD47 antibody treatment (P < 0.05 or P < 0.01).
Figure 2.
CD47 antibody attenuated ISO-induced cardiac dysfunction. A. Left ventricular (LV) function was measured by echocardiography. B. Echocardiography revealed significant impairment in LV function after ISO infusion, as evidenced by changes in EF; FS; LV mass; IVS, d; and LV vol. CD47 antibody treatment improved cardiac function. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SD, n=7 per group. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group; #P < 0.05 and ##P < 0.01 versus the IgG+ISO group.
CD47 antibody protected against ISO-induced oxidative stress in cardiac tissues
The effects of CD47 antibody on oxidative stress level in cardiac tissues are shown in Figure 3A, 3B. There was no significant difference in MDA and SOD levels between the IgG and the CD47 groups. ISO treatment in the IgG+ISO group increased MDA level, attenuated SOD activity in cardiac tissues compared with the IgG group (P < 0.01). CD47 antibody treatment in the CD47+ISO group decreased MDA levels and promoted SOD activity compared with the ISO group (P < 0.05 or P < 0.01).
Figure 3.
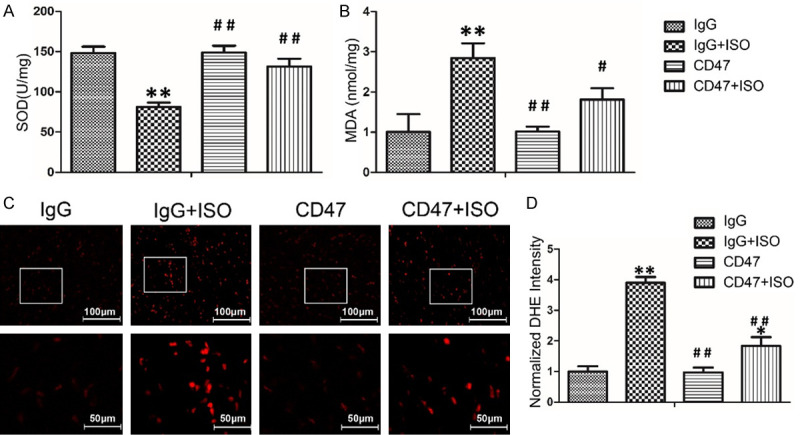
CD47 antibody protected against ISO-induced oxidative stress in cardiac tissues. A, B. CD47 antibody treatment increased the SOD activity and decreased the MDA level in serum induced by ISO, n=7 per group. C. The ROS levels in the hearts of mice from all groups were revealed by DHE staining of frozen sections. D. CD47 antibody treatment decreased ROS fluorescence intensity. The fluorescence intensity of DHE staining was analyzed using Image-Pro Plus, n=7 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SD. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group; #P < 0.05 and ##P < 0.01 versus the IgG+ISO group.
To assess the effects of ISO on ROS production, we visualized intracellular generation of the ROS moiety O2- with the fluoroprobe DHE staining (Figure 3C). Confocal microscopy showed cardiac tissue sections from the ISO group had a widespread and marked increase in DHE fluorescence compared with those from the IgG group (P < 0.01, Figure 3D). CD47 antibody treatment decreased ROS fluorescence intensity in the CD47+ISO group compared with that in the IgG+ISO group (P < 0.01).
CD47 antibody protected against ISO-induced cardiomyocyte apoptosis
In the TUNEL assay, the nuclei of the TUNEL-positive (apoptotic) cells were stained green, indicating apoptosis (Figure 4A). There was no significant difference in apoptosis rates between the IgG and the CD47 groups (Figure 4B). The levels of apoptosis were indicated as the percentage of TUNEL-positive cells among total cells (Figure 4B). Few apoptotic cells were observed in the IgG and the CD47 groups, whereas the IgG+ISO group displayed more TUNEL-positive cells than the IgG and the CD47 groups (P < 0.01). As expected, CD47 antibody treatment decreased the number of TUNEL-positive cells significantly, and fewer apoptotic cells were observed in the CD47+ISO group compared with the IgG+ISO group (P < 0.01).
Figure 4.
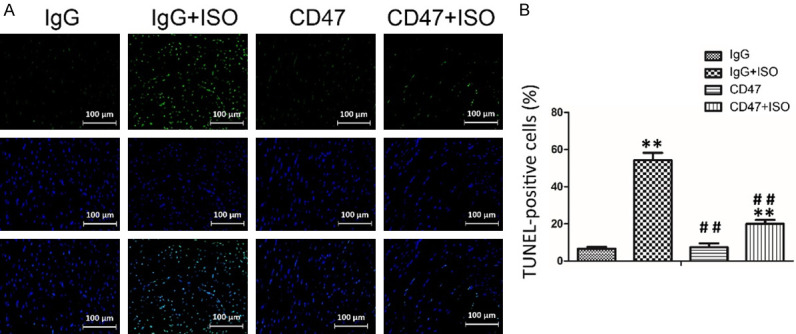
CD47 antibody protected against ISO-induced myocardial cells apoptosis. A. Apoptosis was analyzed using in situ TUNEL fluorescence staining. The nuclei of TUNEL-positive (apoptotic) cells were stained green. Five random fields per section (five sections per tissue from each mouse) were examined in each experiment. B. CD47 antibody treatment decreased the number of TUNEL-positive cells, n=7 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and expressed as the mean ± SD. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group; #P < 0.05 and ##P < 0.01 versus the IgG+ISO group.
CD47 antibody protected against ISO-induced accumulation of autophagosomes in H9c2 cells
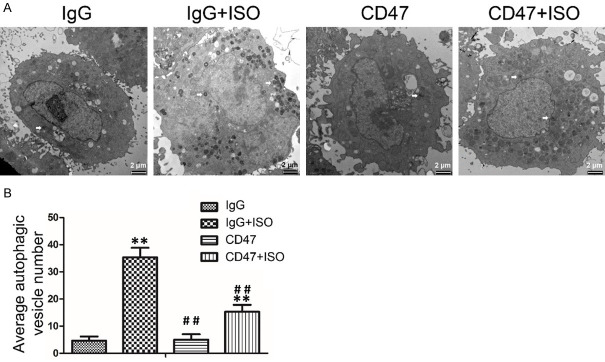
TEM was performed to detect the accumulation of autophagosomes in H9c2 cells. The results showed no significant difference in apoptotic rates between the IgG and the CD47 groups (Figure 5A, 5B). Compared with the IgG group, ISO treatment increased autophagic vesicle number in the IgG+ISO group (P < 0.01). Compared with the IgG+ISO group, CD47 treatment decreased autophagic vesicle number in the CD47+ISO group (P < 0.01).
Figure 5.
CD47 antibody reduced the accumulation of autophagosome in ISO-treated H9c2 cells. A. Autophagic vesicles (AV) were detected by TEM in H9c2 cells subjected to ISO treatment. AVs by TEM were measured. The figures were representative images of three different experiments. B. CD47 treatment decreased AV number. AVs in different groups were quantified and presented as bar graphs, n=7 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and shown as the mean ± SD. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group, #P < 0.05 and ##P < 0.01 versus the IgG+ISO group.
CD47 antibody protected against ISO-induced cardiac hypertrophy by autophagy activation
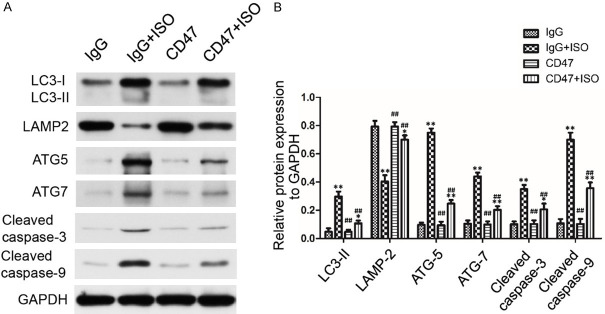
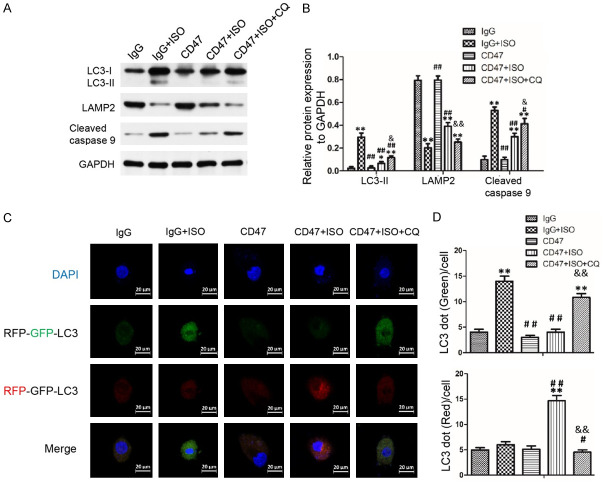
The present study investigated the expression of autophagosomal marker LC3 II, autophagy-associated protein ATG5 and ATG7, autophagosome clearance related protein LAMP2, Cleaved caspase-3 and Cleaved caspase-9 in heart tissues and H9c2 cells using Western blot analysis. As shown in Figures 6A, 6B and S1A, there was no significant difference in protein expression levels of LC3 II, ATG5, ATG7, LAMP2, Cleaved caspase-3 and Cleaved caspase-9 between the IgG and the CD47 groups. ISO treatment in the IgG+ISO group increased LC3 II, ATG5, ATG7 protein levels and decreased LAMP2 protein level compared with the IgG group (P < 0.01). CD47 antibody treatment in the CD47+ISO group decreased LC3 II, ATG5, ATG7 protein levels and increased LAMP2 protein level compared with the IgG+ISO group (P < 0.01). The expression levels of Cleaved caspase-3 and Cleaved caspase-9 were significantly higher in the IgG+ISO group than the control group (P < 0.01). Down-regulation of Cleaved caspase-3 and Cleaved caspase-9 was observed in the CD47+ISO group compared with the IgG+ISO group (P < 0.01). However, results shown in Figures 7A, 7B and S1B indicated that chloroquine treatment in the CD47+ISO+CQ group reversed the effect of CD47 treatment in H9c2 cells. Compared with the CD47+ISO group, chloroquine treatment decreased LAMP2 protein level, increased LC3-II and Cleaved caspase-9 protein levels in the CD47+ISO group (P < 0.05 or P < 0.01, respectively).
Figure 6.
CD47 antibody protected against ISO-induced injury through rescuing impaired autophagy flux. A. Representative Western blots photographs depicting protein levels of LC3 II, LAMP2, ATG5, ATG7, Cleaved caspase-3 and Cleaved caspase 9 in cardiac tissues. The figures were representative images of three different experiments. B. CD47 antibody promoted autophagy flow and inhibited apoptosis. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and shown as the mean ± SD, n=7 per group. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group, #P < 0.05 and ##P < 0.01 versus the IgG+ISO group, respectively.
Figure 7.
Blockage of autophagy reversed the protective effect of CD47 antibody on ISO induced H9C2 cells injury. A. Representative Western blots photographs depicting protein levels of LC3 II, LAMP2 and Cleaved caspase 9 in H9c2 cells. The figures were representative images of three different experiments. B. Autophagy inhibitor chloroquine (CQ) treatment reversed the autophagy flow promotion of CD47 antibody in H9c2 cells. The protein expression levels were quantitatively analyzed, n=7 per group. C. Representative images showing the LC3 fluorescent signals in H9C2 cells transfected with mRFP-GFP-LC3. D. LC3 dots in different groups were quantified and presented as bar graphs, n=7 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test and shown as the mean ± SD. Statistical significance: *P < 0.05 and **P < 0.01 versus the IgG group, #P < 0.05 and ##P < 0.01 versus the IgG+ISO group, &P < 0.05 and &&P < 0.01 versus the CD47+ISO group, respectively.
CD47 antibody protected against ISO-induced cardiac hypertrophy by activating autophagic flux in H9c2 cells
H9c2 cells were transfected with the mRFP-GFP-LC3 virus for effective and convenient monitoring of autophagic flux (Figure 7C, 7D). Autophagosome induction can result in an increase of green puncta, and the combination of autophagosome and lysosome can result in an increase of red puncta [50]. The cells in the IgG and the CD47 groups presented diffused and weak green and red dots in cytoplasm compared with the IgG+ISO group. Compared with the IgG group, ISO treatment increased green puncta number in the IgG+ISO group (P < 0.01). Compared with the IgG+ISO group, CD47 treatment decreased autophagosome clearance in the CD47+ISO group (P < 0.01). Compared with the CD47+ISO group, chloroquine treatment in the CD47+ISO+CQ group reversed the effect of CD47 treatment (P < 0.01).
Discussion
Cardiac hypertrophy is featured by increased cell size, enhanced protein synthesis, and heightened organization of the sarcomere, and associated with the aggregation of misfolded proteins and damage of sub-cellular organelles including mitochondria, which can be cleared by a bulk degradation autophagic process [4,7]. Ventricular hypertrophy usually involves decreased autophagy, which facilitates the accumulation of unfolded proteins and worsens cardiac functions, whereas autophagy is enhanced during the regression of hypertrophy [51]. In the present study, ISO-injected mice showed significant elevated serum levels of the described marker enzymes, LDH and CK-MB. This study also showed that HW/BW ratios, cardiac fibrosis and the expressions of hypertrophic genes, ANP and BNP, were significantly increased by ISO treatment; while CD47 antibody reversed these changes. We have thus confirmed that CD47 antibody can exert cardioprotective effects against ISO-induced cardiac hypertrophy by inhibiting heart failure, cardiac fibrosis and cardiac hypertrophy.
ROS is also important for the development of cardiac hypertrophy [52-54]. Various experimental and clinical studies have shown that an enormous number of ROS, such as superoxide, hydrogen peroxide and hydrogen radicals, are generated in failing myocardium [55]. Although a low level of ROS is essential for normal physiological function, excessive ROS produced in dysfunctional mitochondria may be compromised and eventually overwhelmed, which can result in inflammation, apoptosis, heart dysfunction and hypertrophy [56-59]. In the present study, ISO administration resulted in marked decrease in SOD and elevation in MDA and ROS levels, which is in line with the previous reports [55,60,61]. CD47 antibody treatment increased serum SOD, while at the same time, decreased serum MDA, a lipid peroxidation index, and ROS level. Thus, the anti-oxidant mechanism should be one pathway through which CD47 antibody protects cardiac functions against ISO-induced myocardial injury.
Autophagy is beneficial to the heart in pressure overload, b-adrenergic stress and other forms of stress. Although the pathogenesis of cardiac hypertrophy is complex and remains to be clarified, emerging evidence has indicated that autophagy, an evolutionarily conserved catabolic pathway, participates in pathological cardiac hypertrophy [8,62-64]. Several molecular mechanisms, including LC3 II, ATG5, ATG7 and LAMP2, respond to autophagy regulation [65-68]. Therefore, the present study examined the changes in the levels of these autophagy-associated proteins in mice with ISO-induced cardiac hypertrophy. In mammalian cells, LC3 II is produced from LC3 I and modified into a membrane-bound form to prompt its localization to autophagosomes [69]. Thus, LC3 II is considered to be an autophagosomal marker [70]. ATG5 and ATG7, which are required for autophagosome formation and degrade upon autophagosome processing, increased in ISO-treated hearts [22,26]. In this study, the expression of LC3 II, ATG5 and ATG7 increased significantly and was associated with the reduction of cell apoptosis in ISO-treated hearts, suggesting that excessive activation of autophagy induced by ISO may be detrimental for myocardial cell survival. Clinical studies have also demonstrated that adrenergic blockers significantly improve cardiac functions and reduce cardiac apoptosis in patients with heart failure [71,72]. Notably, CD47 antibody treatment significantly downregulated the expression of LC3 II, ATG5 and ATG7 and improved the survival rate of the myocardial cells. These results indicated that CD47 antibody promoted cell survival through inhibiting the excessive activation of autophagy induced by ISO.
To further explore the role of CD47 in autophagic flux, we assessed the expression of LAMP2 and the number of autophagosomes in cardiomyocytes. LAMP2 is an important lysosome membrane protein in autophagosome-lysosome fusion [67,68]. LAMP2 knockdown impairs autophagy in ventricular myocytes of adult rats and causes cell death at levels comparable to autophagy inhibition with 3MA [73]. LAMP2 deficiency [74,75] caused by mutations in individuals with Danon disease [76], a disease characterized by autophagosome accumulation in multiple tissues including the myocardium [75], can lead to extensive myocardial fibrosis [77]. In this study, we observed a rapid decline in LAMP2 abundance and an increase in the number of autophagosomes in the IgG+ISO group. CD47 antibody treatment was found to improve autophagosome processing by upregulating LAMP2 and decreasing the number of autophagosomes. However, CQ can neutralize the effect of CD47 antibody treatment by inhibiting autophagosome processing. The results suggested that the inhibition of autophagy flux induced autophagosome accumulation, which is an important pathogenic mechanism for cardiac hypertrophy. The activation of autophagic flux by rapamycin has been reported to prevent cardiac hypertrophy induced by thyroid hormone treatment [78,79]. Pressure overload due to transverse aortic constriction also revealed that autophagic flux decreased during hypertrophic responses [8]. The activation of cardiomyocyte autophagic flux may be a novel therapeutic strategy against cardiac hypertrophy and dysfunction.
In conclusion, these findings demonstrated the protective effect of CD47 antibody against cardiac hypertrophy through improving autophagy flux, rescuing autophagic clearance and improving cardiac performance. CD47 antibody, if further confirmed in vivo, may offer a potential therapeutic approach to prevent cardiac hypertrophy.
Acknowledgements
This study was supported by the Natural Science Foundation of China (No. 81627802).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Koshman YE, Patel N, Chu M, Iyengar R, Kim T, Ersahin C, Lewis W, Heroux A, Samarel AM. Regulation of connective tissue growth factor gene expression and fibrosis in human heart failure. J Card Fail. 2013;19:283–294. doi: 10.1016/j.cardfail.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirish P, Li N, Liu JY, Lee KS, Hwang SH, Qiu H, Zhao C, Ma SM, López JE, Hammock BD, Chiamvimonvat N. Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci U S A. 2013;110:5618–5623. doi: 10.1073/pnas.1221972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 5.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–2039. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Oparil S. Pathogenesis of ventricular hypertrophy. J Am Coll Cardiol. 1985;5:57–65. doi: 10.1016/s0735-1097(85)80528-3. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. J Biol Chem. 2010;285:8509–8514. doi: 10.1074/jbc.R109.025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Meyer GR, Martinet W. Autophagy in the cardiovascular system. Biochim Biophys Acta. 2009;1793:1485–1495. doi: 10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Mellor KM, Reichelt ME, Delbridge LM. Autophagy anomalies in the diabetic myocardium. Autophagy. 2011;7:1263–1267. doi: 10.4161/auto.7.10.17148. [DOI] [PubMed] [Google Scholar]
- 13.Molojavyi A, Lindecke A, Raupach A, Moellendorf S, Köhrer K, Gödecke A. Myoglobin-deficient mice activate a distinct cardiac gene expression program in response to isoproterenol-induced hypertrophy. Physiol Genomics. 2010;41:137–145. doi: 10.1152/physiolgenomics.90297.2008. [DOI] [PubMed] [Google Scholar]
- 14.Feng XJ, Gao H, Gao S, Li Z, Li H, Lu J, Wang JJ, Huang XY, Liu M, Zou J, Ye JT, Liu PQ. The orphan receptor NOR1 participates in isoprenaline-induced cardiac hypertrophy by regulating PARP-1. Br J Pharmacol. 2015;172:2852–2863. doi: 10.1111/bph.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balakumar P, Singh AP, Singh M. Rodent models of heart failure. J Pharmacol Toxicol Methods. 2007;5:61–10. doi: 10.1016/j.vascn.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- 17.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- 18.York M, Scudamore C, Brady S, Chen C, Wilson S, Curtis M, Evans G, Griffiths W, Whayman M, Williams T, Turton J. Characterization of troponin responses in isoproterenol-induced cardiac injury in the Hanover Wistar rat. Toxicol Pathol. 2007;35:606–617. doi: 10.1080/01926230701389316. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Knapton A, Lipshultz SE, Weaver JL, Herman EH. Isoproterenolinduced cardiotoxicity in Sprague-Dawley rats: correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 2008;36:277–278. doi: 10.1177/0192623307313010. [DOI] [PubMed] [Google Scholar]
- 20.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 21.Balakumar P, Jagadeesh G. Multifarious molecular signaling cascades of cardiac hypertrophy: can the muddy waters be cleared? Pharm Res. 2010;62:365–383. doi: 10.1016/j.phrs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Sun D, Liu Z, Li M, Hong H, Liu C, Gao S, Li H, Cai Y, Chen S, Li Z, Ye J, Liu P. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res. 2016;172:96–112. doi: 10.1016/j.trsl.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:615–621. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csányi G, Yao M, Rodríguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011;30:154–161. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc. 2014;3:e000670. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, Abu-Asab M, Wink DA, Tsokos M, Roberts DD. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing C, Bian L, Wang M, Keep RF, Xi G, Hua Y. Enhancement of hematoma clearance with CD47 blocking antibody in experimental intracerebral hemorrhage. Stroke. 2019;50:1539–1547. doi: 10.1161/STROKEAHA.118.024578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brierley CK, Staves J, Roberts C, Johnson H, Vyas P, Goodnough LT, Murphy MF. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59:2248–2254. doi: 10.1111/trf.15397. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Zhang D, Yang C, Duan X, Li X, Zhong D. Two validated liquid chromatography-mass spectrometry methods with different pretreatments for the quantification of an anti-CD47 monoclonal antibody in rat and cynomolgus monkey serum compared with an electrochemiluminescence method. J Pharm Biomed Anal. 2019;175:112792. doi: 10.1016/j.jpba.2019.112792. [DOI] [PubMed] [Google Scholar]
- 34.Andrechak JC, Dooling LJ, Discher DE. The macrophage checkpoint CD47: SIRPα for recognition of ‘self’ cells: from clinical trials of blocking antibodies to mechanobiological fundamentals. Philos Trans R Soc Lond B Biol Sci. 2019;37420:180217. doi: 10.1098/rstb.2018.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, Mohanakumar T, Frazier WA, Lin Y, Chapman WC. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–309. doi: 10.1016/j.canlet.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Huang M, Shen S, Wu X, Wu X, Lv P, Zhang H, Wang H, Li X. Qiliqiangxin attenuates isoproterenol-induced cardiac remodeling in mice. Am J Transl Res. 2017;9:5585–5593. [PMC free article] [PubMed] [Google Scholar]
- 38.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Yin C, Feng L, Wang C, Sheng G. Ara-C and anti-CD47 antibody combination therapy eliminates acute monocytic leukemia THP-1 cells in vivo and in vitro. Genet Mol Res. 2015;14:5630–5641. doi: 10.4238/2015.May.25.15. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Peng J, Wang C, Deng H, Li Y. Calcitonin gene-related peptide suppresses isoprenaline-induced cardiomyocyte apoptosis through regulation of microRNA-1 and microRNA-133a expression. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:964–971. doi: 10.3969/j.issn.1672-7347.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Godar RJ, Liu H, Diwan A. Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death. Autophagy. 2012;8:297–309. doi: 10.4161/auto.18658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang CY, Lai CC, Huang PH, Yang AH, Chiang SC, Huang PC, Tseng KW, Huang CH. Magnolol reduces renal ischemia and reperfusion injury via inhibition of apoptosis. Am J Chin Med. 2017;45:1–19. doi: 10.1142/S0192415X1750077X. [DOI] [PubMed] [Google Scholar]
- 43.Roy SJ, Mainzen Prince PS. Protective effects of sinapic acid on cardiac hypertrophy, dyslipidemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. Eur J Pharmacol. 2012;699:213–218. doi: 10.1016/j.ejphar.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Xie YH, Yang Q, Wang SW, Zhang BL, Wang JB, Cao W, Bi LL, Sun JY, Miao S, Hu J, Zhou XX, Qiu PC. Cardioprotective effect of Paeonol and Danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7:e48872. doi: 10.1371/journal.pone.0048872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minnaard-Huiban M, Emmen JM, Roumen L, Beugels IP, Cohuet GM, van Essen H, Ruijters E, Pieterse K, Hilbers PA, Ottenheijm HC, Plate R, de Gooyer ME, Smits JF, Hermans JJ. Fadrozole reverses cardiac fibrosis in spontaneously hypertensive heart failure rats: discordant enantioselectivity versus reduction of plasma aldosterone. Endocrinology. 2008;149:28–31. doi: 10.1210/en.2007-0584. [DOI] [PubMed] [Google Scholar]
- 46.Tao H, Shi KH, Yang JJ, Huang C, Liu LP, Li J. Epigenetic regulation of cardiac fibrosis. Cell Signal. 2013;25:1932–1938. doi: 10.1016/j.cellsig.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. 2012;7:e35144. doi: 10.1371/journal.pone.0035144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 49.Sabeena Farvin KH, Anandan R, Kumar SH, Shiny KS, Sankar TV, Thankappan TK. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol Res. 2004;50:231–236. doi: 10.1016/j.phrs.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Palfi A, Toth A, Hanto K, Deres P, Szabados E, Szereday Z, Kulcsar G, Kalai T, Hideg K, Gallyas F Jr, Sumegi B, Toth K, Halmosi R. PARP inhibition prevents postinfarction myocardial remodeling and heart failure via the protein kinase C/glycogen synthase kinase-3β pathway. J Mol Cell Cardiol. 2006;41:149–159. doi: 10.1016/j.yjmcc.2006.03.427. [DOI] [PubMed] [Google Scholar]
- 51.Goswami SK, Das DK. Autophagy in the myocardium: dying for survival? Exp Clin Cardiol. 2006;11:183–188. [PMC free article] [PubMed] [Google Scholar]
- 52.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Stanely Mainzen Prince P, Rajakumar S, Dhanasekar K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol. 2011;668:233–240. doi: 10.1016/j.ejphar.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 54.Radhiga T, Rajamanickam C, Sundaresan A, Ezhumalai M, Pugalendi KV. Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie. 2012;94:1135–1142. doi: 10.1016/j.biochi.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicology. 2006;228:259–268. doi: 10.1016/j.tox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 58.Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 59.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 60.Priscilla DH, Prince PS. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Upaganlawar A, Gandhi C, Balaraman R. Effect of green tea and vitamin E combination in isoproterenol induced myocardial infarction in rats. Plant Foods Hum Nutr. 2009;64:75–80. doi: 10.1007/s11130-008-0105-9. [DOI] [PubMed] [Google Scholar]
- 62.Yin X, Peng C, Ning W, Li C, Ren Z, Zhang J, Gao H, Zhao K. miR-30a downregulation aggravates pressure overload-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2013;379:1–6. doi: 10.1007/s11010-012-1552-z. [DOI] [PubMed] [Google Scholar]
- 63.Guo R, Hu N, Kandadi MR, Ren J. Facilitated ethanol metabolism promotes cardiomyocyte contractile dysfunction through autophagy in murine hearts. Autophagy. 2012;8:593–608. doi: 10.4161/auto.18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–149. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levine B, Ranganathan R. Autophagy: snapshot of the network. Nature. 2010;466:38–40. doi: 10.1038/466038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, Von Figura K, Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–3368. doi: 10.1091/mbc.E02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Effect of metoprolol CR XL in chronic heart failure: metoprolol CR XL randomised Intervention trial in congestive heart failure (MERITHF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 72.Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36:2072–2080. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 73.Maruyama R, Goto K, Takemura G, Ono K, Nagao K, Horie T, Tsujimoto A, Kanamori H, Miyata S, Ushikoshi H, Nagashima K, Minatoguchi S, Fujiwara T, Fujiwara H. Morphological and biochemical characterization of basal and starvation-induced autophagy in isolated adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:1599–1607. doi: 10.1152/ajpheart.91449.2007. [DOI] [PubMed] [Google Scholar]
- 74.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lüllmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 76.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 77.Maron BJ, Roberts WC, Arad M, Haas TS, Spirito P, Wright GB, Almquist AK, Baffa JM, Saul JP, Ho CY, Seidman J, Seidman CE. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301:1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakaoka M, Iwai-Kanai E, Katamura M, Okawa Y, Mita Y, Matoba S. An alpha-adrenergic agonist protects hearts by inducing Akt1-mediated autophagy. Biochem Biophys Res Commun. 2015;456:250–256. doi: 10.1016/j.bbrc.2014.11.067. [DOI] [PubMed] [Google Scholar]
- 79.Kuzman JA, O’Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148:3477–3484. doi: 10.1210/en.2007-0099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.