Abstract
Atherosclerosis is a chronic pathological process characterized by the accumulation of inflammation. Overactivation of the sympathetic nervous system accelerates the progression of atherosclerosis. Renal denervation (RDN) reduces the activity of the sympathetic nerve system (SNS) by disrupting sympathetic nerves surrounding renal arteries. We sought to determine whether RDN could mitigate atherosclerosis through the suppression of inflammation. First, we investigated the correlation between plasma norepinephrine concentrations and circulatory inflammation in the progression of atherosclerosis. Then, forty ApoE-/- mice underwent renal denervation or a sham operation after 6 weeks or 12 weeks of feeding with a high-fat diet. The effects of RDN on atherosclerosis in mice were explored. In the development of atherosclerosis, positive correlations were found between SNS activation and the accumulation of circulatory myeloid cells and inflammatory cytokines. In the second part of the study, inhibition of the increase in plaque size was found in both RDN groups compared with that in the sham operation (SO) groups (P<0.05), and RDN also ameliorated inflammation in plaques. Furthermore, RDN attenuated the accumulation of circulating neutrophils and monocytes (P<0.05), which is associated with a significant reduction in levels of several circulating inflammatory cytokines related to hemopoiesis (P<0.05). Flow cytometry analysis revealed comparable levels of neutrophils and monocytes in the bone marrow between all four groups. However, RDN decreased the production and proportions of neutrophils and monocytes in the spleen and reduced splenic sympathetic activity (P<0.05). In summary, our study reveals a novel link between SNS activity and inflammation in atherosclerosis and identifies RDN as a potential anti-inflammatory therapeutic strategy for the treatment of atherosclerosis by restricting the production of splenic immune cells.
Keywords: Renal denervation, atherosclerosis, inflammation, sympathetic nerve system
Introduction
Atherosclerosis is a chronic pathological process characterized by the accumulation of lipids and increases in inflammation in both the circulation and arterial vessel walls [1-3]. To date, lipid-lowering and anti-inflammatory therapies are the two major strategies that have been used to combat atherosclerosis. In addition, as a concomitant condition and an independent risk factor for stroke, hypertension, diabetes and cardiovascular disease, sympathetic overactivation caused by these diseases aggravates the progression of atherosclerosis, suggesting that sympathetic nerve system (SNS) is also a factor that promotes the progression of atherosclerosis [4-9].
Renal denervation (RDN) reduces SNS activity by disrupting the sympathetic nerves surrounding renal arteries [10-13]. It was initially used as an interventional treatment for resistant hypertension, and its use has gradually expanded to alleviate the pathological state of overall sympathetic overactivation and improve metabolism caused by conditions such as myocardial infarction and metabolic syndrome, which are the promoting factors of atherosclerosis [14-17].
Although an earlier study reported that RDN can inhibit the development of atherosclerosis in normotensive mice independently of lowering blood pressure, later studies have come to the opposite conclusion, which is that RDN accelerates the development of atherosclerosis in hypertensive mice [18,19]. To our knowledge, there are very few articles about RDN in atherosclerosis, and most of them are limited to exploring the related phenomena. Little is known about the underlying mechanism.
Therefore, based on the contradictory conclusions of previous studies and the pathogenesis of atherosclerosis, we wanted to determine the effect of SNS on atherosclerosis and whether RDN can relieve atherosclerosis independently of its progression. For this purpose, we designed this experiment to explore the relationship between SNS or RDN and atherosclerosis. We hypothesized that RDN can relieve atherosclerosis either by inhibiting inflammation or improving lipid metabolism. The results may have broader clinical applications for RDN and provide explanations for potential hyperresponsiveness to RDN in hypertensive patients complicated by atherosclerosis.
Materials and methods
Animals and experimental protocol
All animal-related protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Zhongshan Hospital, Fudan University.
Male ApoE-/- mice (5 weeks old, n=56) were purchased from Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China. After one week of acclimation, all mice were given a high-fat diet (HFD; D12109C, Research Diets, NJ, USA), which was defined as the baseline.
Eight ApoE-/- mice (6 W group) were sacrificed after 6 weeks on a HFD, and another 8 were sacrificed after 12 weeks (12 W group). For the main experiment, the remaining 40 mice were evenly divided into two subgroups: the early and late groups. After 6 weeks on a HFD, 20 mice from the early group were subjected to renal denervation (6 W-RDN group) or a sham operation (6 W-SO group). The remaining 20 mice from the late group were subjected to RDN (12 W-RDN group) or a SO (12 W-SO group) after 12 weeks on a HFD. Ten mice were included in each group. After surgery, mice were maintained on a HFD for an additional 6 weeks until they were sacrificed, and samples were harvested (Figure 1A).
Figure 1.
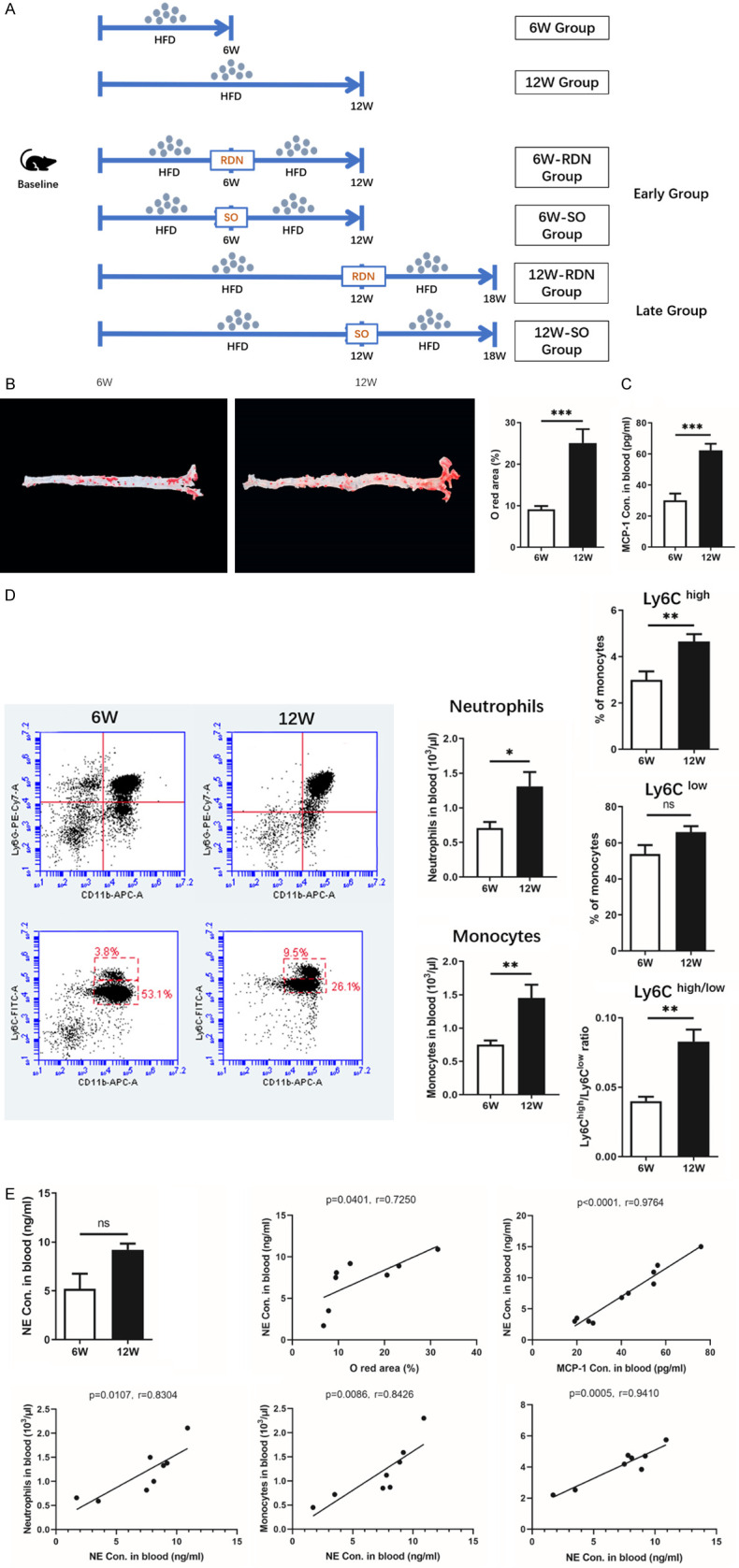
SNS sympathetic activity is associated with an increased level of inflammation in atherosclerosis. A. A diagram of the experimental design. B. Representative images and quantification analysis of aortic oil red O staining after 6 and 12 weeks of a high-fat diet (HFD). With the extension of the HFD feeding time, atherosclerotic plaques in the aorta developed gradually. C and D. Circulatory levels of MCP-1 and inflammatory cells, as assessed by flow cytometry after 6 weeks or 12 weeks of a HFD, were increased with prolonged feeding of the HFD. E. A positive correlation was found between the norepinephrine (NE) concentration and the plaque size and circulating inflammatory cells. Data were analyzed by an unpaired t test or 1-way ANOVA and expressed as the mean ± SEM, where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with the 6 W groups. n=4-8 per group.
All mice were euthanized by cervical dislocation under 4% isoflurane inhalation with 0.5 L/min of 100% O2. Blood and tissues were collected accordingly for further processing and analysis.
Renal denervation
Bilateral RDN was performed as previously described. In brief, mice were first anaesthetized with 4% isoflurane (0.5 L/min of 100% O2) in an induction chamber, and 1.5% isoflurane (0.5 L/min of 100% O2) was used for anesthesia maintenance. The abdomen was opened through a ventral midline laparotomy. After gently moving the small intestine and other abdominal organs aside, the visible intrarenal nerves, fat, and connective tissue wrapped around renal vessels were severed. Then, the renal vessels were covered with 10% phenol in 95% ethanol solution to further destroy the remaining nerves. The abdominal cavity was then closed with a stratified suture.
For SO mice, all procedures were identical to those used for the RDN mice, except that the renal nerves were kept intact.
Biological parameters
The blood pressure and heart rate were measured without anesthesia by a noninvasive tail-cuff BP-2000 Blood Pressure Analysis System (Visitech System, Apex, NC) every 6 weeks from baseline until the mice were sacrificed. Body weight was monitored every other week from baseline.
Plasma renin activity was detected by a renin assay kit (Sigma, MO, USA), and angiotensin II and aldosterone were measured by ELISA (R&D, MO, USA) by following the manufacturers’ instructions. Cholesterol, triglycerides, LDL, and HDL in plasma and the liver were determined by the corresponding kits (Nanjing Jiancheng, Nanjing, China).
RDN verification and norepinephrine measurement
To verify the success of RDN, the renal arteries distal from the excision site in all mice were stained with hematoxylin and eosin (HE) and tyrosine hydroxylase (TH; Abcam, MA, USA) immunohistochemical stains. NE concentrations in the renal cortex and blood were measured by phase isocratic high-performance liquid chromatography (HPLC). Splenic sympathetic activity was measured using the same methods.
Histological analysis
Formalin-fixed aortas free of the surrounding adventitia were opened longitudinally, rinsed with 60% isopropanol, and then stained with 0.5% oil red O (Sigma Aldrich, MO, USA) in isopropanol. Hearts embedded in OCT were sectioned at the point at which the three leaflets of the aortic root emerged. A total of 15 slides spanning all portions of the aortic root were stained with HE, Masson’s trichrome, 0.5% oil red O, and antibodies against αSMA and CD68 (Abcam, MA, USA), with 3 slides for each stain. The distal renal arteries were embedded in paraffin for HE and TH (Abcam, Ma, USA) immunohistochemical staining. Samples of spleen were embedded for immunohistochemistry and immunofluorescent staining.
Quantification of gene expression by real-time RT-PCR
Total RNA was extracted from the aorta with a RNeasy Mini Kit (Qiagen, Hombrechtikon, Switzerland). Reverse transcription was performed using Prime Script RT Master Mix (Takara, Osaka, Japan). Quantitative PCR was performed using SYBR Premix Ex Taq II (Takara, Osaka, Japan) on a CFX96 Real-Time PCR detection system (Bio-Rad, Cressier, Switzerland) in 96-well PCR plates. Gene expression analysis was performed by determining the relative gene expression compared with the expression of the control gene GAPDH. Data were analyzed using the 2-ΔΔCt method. The pairs of mouse primers are listed in Supplementary Table 1.
Inflammation measurements
Plasma MCP-1 was detected using an ELISA kit (Sigma Aldrich, MO, USA). The cytokine profile was analyzed using a fluorescence-based multiplex ELISA array chip (RayBiotech, GA, USA). The screened cytokines included G-CSF, GM-CSF, IL-1a, IL-1b, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, IL-21, IL-23, IFNγ and TNFα.
Flow cytometry analysis
Flow cytometry of inflammatory cells in peripheral blood, femurs and spleens was conducted with a BD FACS Aria II (BD Biosciences, NJ, USA) and analyed by FlowJo 7 software (Treestar, USA). The antibodies and fluorophores were purchased from BioLegend (CA, USA) and BD Biosciences and are listed in Supplementary Table 2.
Statistical analysis
All data are presented as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, CA, USA). The results were analyzed using an unpaired t test or 1-way ANOVA for comparisons. P values <0.05 were defined as statistically significant.
Results
Sympathetic activity is associated with an increased level of circulatory inflammation in atherosclerosis
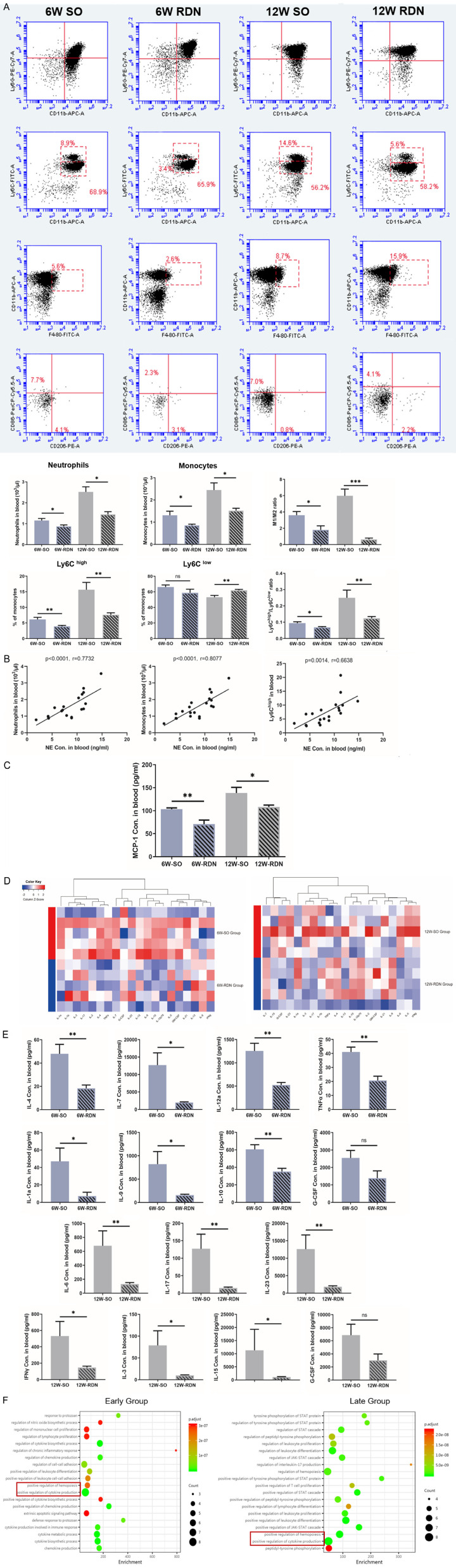
We first simulated the different stages of atherosclerosis. Sixteen ApoE-/- mice were fed a HFD for 6 weeks or 12 weeks. Aortas in mice from the 12 W group exhibited larger plaque areas and higher levels of inflammation in the circulation than those in mice from the 6 W group (Figure 1B). Specifically, the MCP-1 content in the peripheral blood of 12 W mice was significantly higher than that of 6 W mice (Figure 1C). Flow cytometry analysis showed that the proportions of circulatory neutrophils, monocytes and proinflammatory Ly6Chigh monocytes also increased over time (Figure 1D).
In addition, with the progression of atherosclerosis, circulatory sympathetic activity increased, which was manifested by the tendency of plasma NE toward elevation. Through further analysis, we found that the circulatory NE concentration was positively correlated with plaque size and plasma MCP-1. Correlations between the plasma NE concentration and the proportions of circulatory neutrophils, monocytes and inflammatory monocytes were also detected (Figure 1E).
RDN reduces sympathetic activity
The above results indicate that chronic SNS activation may be involved in the progression of atherosclerosis. Next, we designed an experiment to investigate the effect of RDN on atherosclerosis by reducing SNS activation. Forty ApoE-/- mice fed a HFD for 6 or 12 weeks were divided into four groups prior to RDN or the sham operation. As shown in the representative photomicrographs, the TH staining intensity in both RDN groups was significantly reduced compared with that in the corresponding sham groups (Figure 2A). Renal NE levels were decreased significantly by approximately 70% in both the early and late groups after RDN, whereas the decrease in plasma NE was relatively mild, indicating successful denervation (Figure 2B and 2C).
Figure 2.
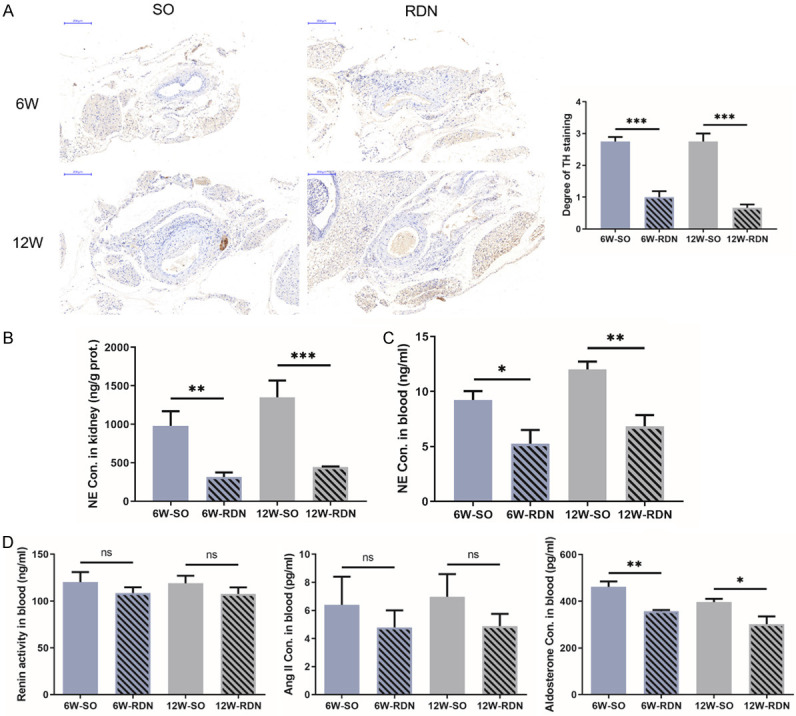
RDN reduces sympathetic activity. A. Representative photomicrographs of hematoxylin and eosin (HE) and tyrosine hydroxylase (TH) staining of intrarenal arteries of ApoE-/- mice from the early and late groups. The intact neural structure in the adventitia was destroyed, and TH intensity, a marker of the activity of the sympathetic nerve system (SNS) in both renal denervation (RDN) groups, was significantly reduced compared with that in the corresponding sham groups. The TH staining intensity was scored on a scale of 0 to 3, where 0 indicated no reaction, 1 indicated a patchy/very weak reaction, 2 indicated a weak to moderate reaction, and 3 indicated a strong reaction. B and C. NE concentrations in the kidneys and peripheral blood of ApoE-/- mice were increased with the extension of HFD feeding, which was alleviated by RDN. D. The plasma renin activity and angiotensin II (Ang II) concentration were comparable in all 4 groups. Aldosterone levels decreased significantly after RDN. Data were analyzed by an unpaired t test or 1-way ANOVA and expressed as the mean ± SEM, where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with the corresponding SO group. n=5-10 per group.
Since the SNS also has a certain regulatory effect on the RAAS system, we measured the concentration of relevant indicators in peripheral blood. The plasma renin activity and angiotensin II concentration were comparable in all 4 groups. However, aldosterone levels decreased significantly after RDN (Figure 2D).
In addition, the blood pressure and heart rate remained relatively consistent among the four groups, and there was no difference in body weight between the RDN and SO groups (Supplementary Table 3).
RDN mitigates plaque progression
Six weeks after the operation, atherosclerotic plaques in the aortas of ApoE-/- mice were quantified by oil red O-staining, and the positive area was decreased in the aortas from the RDN groups (Figure 3A). In the early group, the plaque area in the 6 W-RDN group was smaller than that in the 6 W-SO group. Although the plaque area in the 12 W-RDN group was increased compared with that in the 6 W-RDN group, the progression rate was reduced significantly in the 12 W-RDN group compared with that in the corresponding 12 W-SO group.
Figure 3.
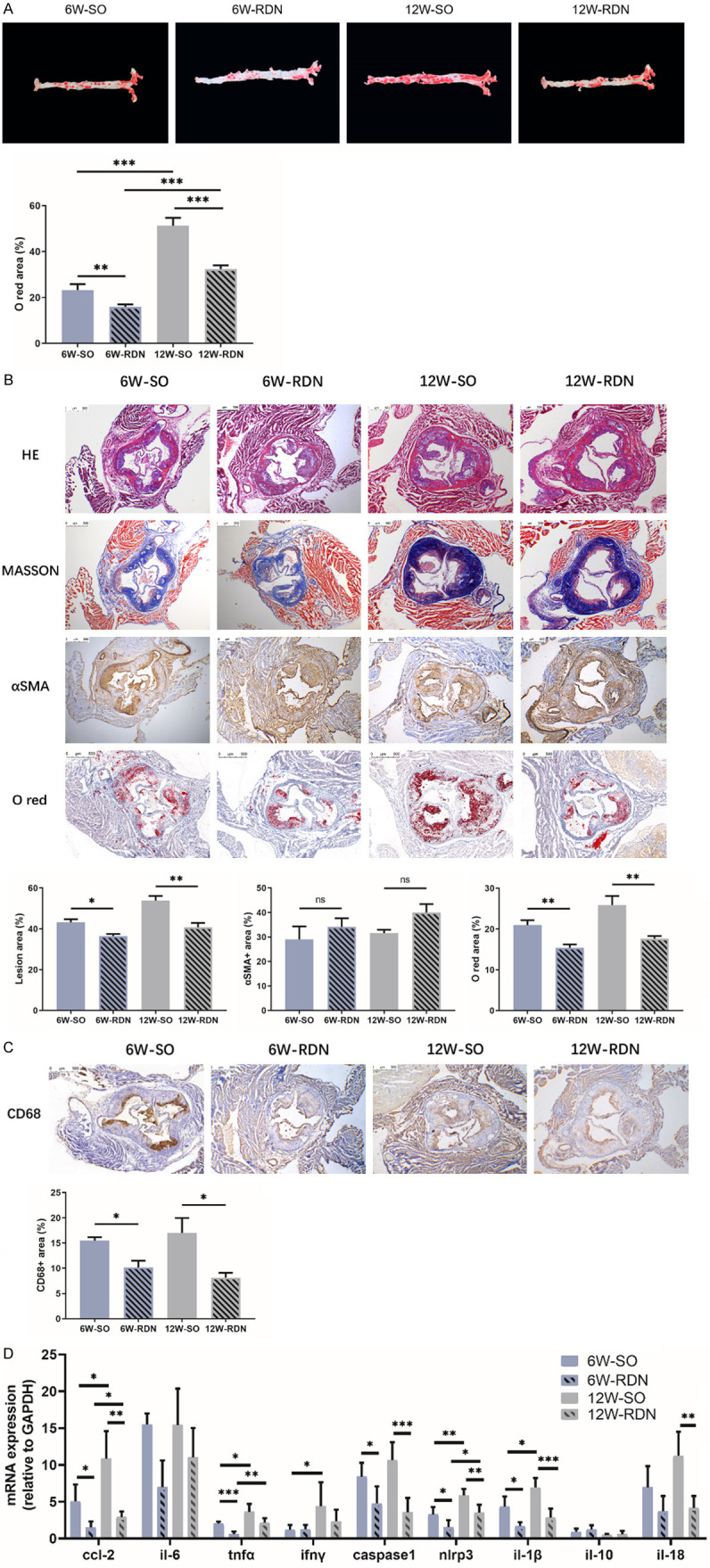
RDN attenuates the progression and inflammation of plaques. A. Representative images and quantification of aortic oil red O-staining in ApoE-/- mice from the early and late groups. Six weeks after the operation, atherosclerotic plaques in the aortas of ApoE-/- mice were quantified by oil red O staining. The positive area was decreased in the aortas from both RDN groups. B and C. Representative photomicrographs and quantification of HE, Masson’s trichrome, a-SMA, oil red O and CD68 staining of aortic root cross-sectional lesions of ApoE-/- mice from the early and late groups. RDN delayed the progression and inflammation of atherosclerotic plaques in both the early and late groups as measured by HE, Masson’s trichrome, oil red O and CD68 staining, whereas the a-SMA-positive area remained unchanged by RDN. D. The effect of RDN on the mRNA expression of Ccl-2, Il-6, Tnfα, Ifnγ, Caspase1, Nlrp3, Il-1β and Il-18 was significantly decreased by RDN in ApoE-/- mice from the early and late groups. Data were analyzed by an unpaired t test or 1-way ANOVA and expressed as the mean ± SEM, where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with the corresponding SO group. n=5-10 per group.
The aortic root cross-sectional lesion area also showed a similar significant difference. Based on the HE and Masson staining, RDN delayed the progression of atherosclerotic plaques in both the early and late groups. When comparing the 12 W-RDN and 6 W-RDN groups, the progression of the aortic root plaques was inhibited but significantly different between the 12 W-SO and 6 W-SO groups. Although the smooth muscle cell content remained relatively constant in the 4 groups, as determined by α-smooth muscle cell actin immunostaining, with the prolongation of the administration of the HFD, the oil red O-stained positive area increased gradually. When comparing the early and late groups, we found that the rate of increase of the oil red O-stained positive area was reduced after RDN (Figure 3B).
RDN does not improve lipid metabolism
As dyslipidemia plays a certain role in the initiation and development of atherosclerosis, we therefore explored whether RDN improves atherosclerotic plaques by regulating lipid metabolism. No differences were found in lipid metabolism-related mRNA expression or the total cholesterol and triglyceride concentrations (Supplementary Figure 1A and 1B). The circulatory concentrations of total cholesterol, triglycerides, LDL and HDL were equivalent after RDN or SO (Supplementary Figure 1C).
RDN reduces inflammation in plaques
Inflammation is another important contributor to plaque development. The effect of RDN on inflammation in atheroma was assessed in all 4 groups. As the plaque progressed, more inflammatory cells infiltrated into the lesion. There were fewer CD68 immunohistochemical-stained areas in the RDN groups than in the SO groups, suggesting that the infiltration of circulating inflammatory cells into the plaque was reduced after RDN (Figure 3C). In accordance with this finding, the production of inflammatory cytokines in the aorta was reduced. Briefly, in the 6 W-RDN group, the mRNA expression of Ccl-2, Il-6, Tnfα, Caspase1, Nlrp3, and Il-1β were significantly lower than that in the 6 W-SO group. The mRNA expression of Ccl-2, Tnfα, Caspase1, Nlrp3, Il-1β and Il-18 were also significantly reduced at the mRNA level in the 12 W-SO group (Figure 3D).
RDN relieves inflammation in the circulation
Inflammatory cells infiltrating the plaque originate from the circulation. Since the first part of our study revealed that the levels of circulating inflammatory cells and chemokines were correlated with the NE concentration, we sought to determine whether blocking the renal sympathetic nerve had an impact on inflammation in the circulation. Flow cytometry analysis of peripheral blood was performed. As atherosclerosis progressed, circulatory neutrophils and proinflammatory monocytes increased, but RDN diminished these increases. Although the proportion of Ly6Clow monocytes decreased over time, this trend was neutralized by RDN. The increases in the monocyte Ly6Chigh/low ratio and macrophage M1/M2 ratio were also diminished in both the 6 W-and 12 W-RDN groups compared with those in the corresponding SO groups (Figure 4A). Consistently, correlations were also detected between the plasma NE concentration and the levels of circulatory neutrophils, monocytes and proinflammatory monocytes (Figure 4B). Then, we determined whether the reduction in the number of myeloid cells was associated with the decrease in chemokines. Therefore, we detected the level of circulating MCP-1 and found that the accumulation of MCP-1 was mitigated by RDN (Figure 4C).
Figure 4.
RDN relieves inflammation in the circulation. A. As assessed by flow cytometry, the levels of circulating neutrophils, monocytes and inflammatory monocytes in ApoE-/- mice from both RDN groups were lower than those in the corresponding SHAM groups, and there were decreased proportions of Ly6C high/low monocytes and M1/M2 macrophages. B. A positive correlation was found between the plasma NE concentration and levels of circulatory neutrophils, monocytes and proinflammatory monocytes in all four groups. C. The increase in the concentration of circulatory MCP-1 in ApoE-/- mice was mitigated by RDN in both the early and late groups. D. Heatmap of 20 inflammatory cytokines within the early and late groups showing an overall decreased level of inflammation in both RDN groups. E. Concentration of inflammatory cytokines with significance measured by cytokine microarrays. F. GO analysis of biological processes identified the regulation of hemopoiesis and cytokine production as major changes within the early and late groups. Data were analyzed by an unpaired t test or 1-way ANOVA and expressed as the mean ± SEM, where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with the corresponding SO group. n=5-10 per group.
Along with inflammatory cells, inflammatory cytokines in the circulation were systematically decreased by RDN, and as shown in the heatmap, this tendency was more obvious in the early group (Figure 4D). In the early groups, the plasma levels of IL-1a, IL-4, IL-7, IL-9, IL-10, IL-12a and TNFα were significantly lower in the 6 W-RDN group. In the late groups, the plasma levels of IL-3, IL-6, IL-15, IL-17, IL-23 and IFNγ were significantly lower in the 12 W-RDN group. G-CSF decreased in both the early and late groups, but the decreases were not significant (Figure 4E). GO analysis revealed that in both the early and late groups, the main physiological pathways inhibited by RDN involved the positive regulation of cytokine production and the hematopoietic system (Figure 4F).
RDN suppresses the production of inflammatory cells in the spleen
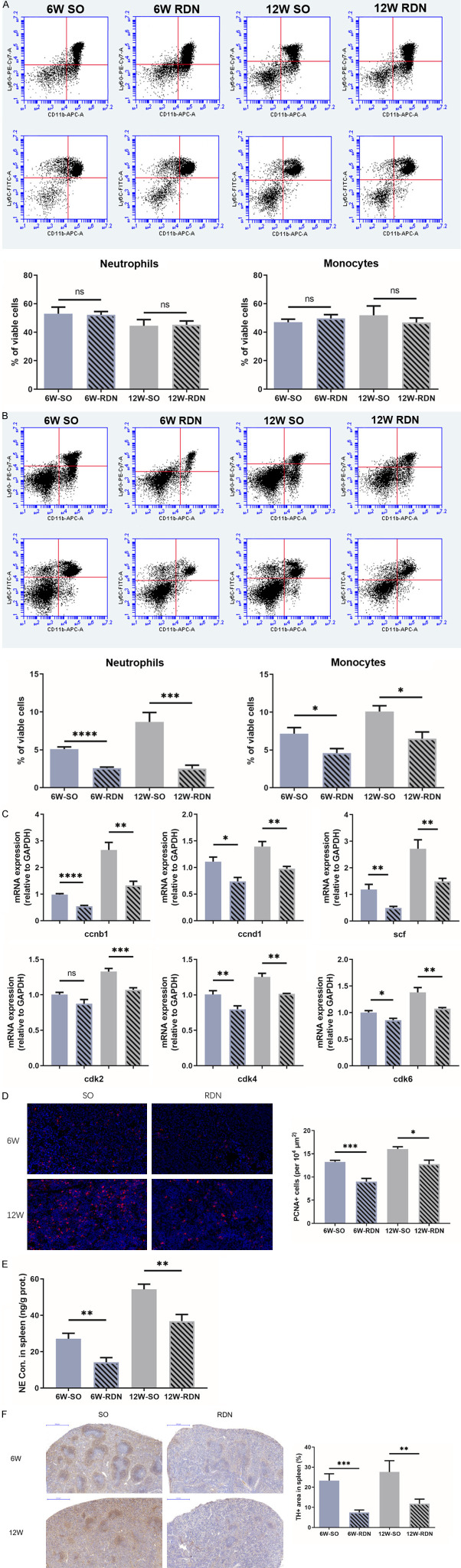
Circulating inflammatory cells are mainly derived from the hematopoietic system. Based on the GO analysis indicating that RDN can inhibit the positive regulation of hematopoiesis in atherosclerosis, we determined whether the reduction in circulating inflammatory cells was echoed by changes in the BM and spleen. In the stable state, bone marrow is responsible for the production and mobilization of immune cells. Therefore, we first measured the proportion of immune cells in bone marrow. However, flow cytometry showed that the proportions of neutrophils and monocytes remained at relatively constant levels in all 4 groups (Figure 5A).
Figure 5.
RDN suppresses the production of inflammatory cells in the spleen. A and B. As assessed by flow cytometry, RDN had no effect on the proportions of neutrophils and monocytes in bone marrow but reduced their proportions in spleen. C. The mRNA expression of genes related to the cell cycle (Ccnb1, Ccnd1, Cdk2, Cdk4 and Cdk6) as well as Scf increased over time and were increased in the sham groups. D. Quantification of proliferating cell nuclear antigen (PCNA)-positive cells in spleen showed the relatively decreased proliferation rate in the RDN groups. E. The increase in the splenic NE concentration in ApoE-/- mice was reduced by RDN. F. Representative photomicrographs of TH staining in the spleen of ApoE-/- mice from the early and late groups. As a marker of SNS activity, the splenic TH intensity in both renal denervation (RDN) groups was significantly reduced compared with that in the corresponding sham groups. Data were analyzed by an unpaired t test or 1-way ANOVA and expressed as the mean ± SEM, where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 compared with the corresponding SO group. n=5-10 per group.
Then, we explored whether the spleen is responsible for the regulation of circulating inflammatory cells by RDN. As expected, with the development of atherosclerosis, the proportions of neutrophils and monocytes in the spleen increased, and these increases were mitigated by RDN (Figure 5B). Additionally, the expression of genes related to the cell cycle (Ccnb1, Ccnd1, Cdk2, Cdk4 and Cdk6) as well as Scf increased over time and were higher in the sham groups than the RDN groups (Figure 5C). These trends were further reinforced by the detection of the expression of PCNA protein in the spleen tissue, which was suppressed by RDN (Figure 5D). These data indicate that RDN reduced the overactivation of the production of myeloid cells during the development of atherosclerosis.
Since leukocytosis in the spleen is regulated by the SNS, we measured the markers of sympathetic neural activity. We found that the splenic NE concentration in both SO groups was significantly higher than that in the RDN groups, and similar results were found in the TH-positive area of the spleen, suggesting an overall reduction of SNS activity in the spleen (Figure 5E and 5F). Taken together, these results indicated that RDN decreased sympathetic neural activity in the spleen and prevented the production of monocytes and neutrophils.
Discussion
In the first part of the study, we first revealed that the atherosclerotic plaque area and the levels of circulating inflammatory cells were correlated with SNS activity during the progression of atherosclerosis. Subsequent experiments confirmed that RDN in ApoE-/- mice had an inhibitory effect on plaque progression at different time points, specifically in terms of plaque size and the oil red O-stained positive area, that resulted from an overall decrease in circulatory and plaque inflammation. Further analysis suggests that RDN may have an inhibitory effect on atherosclerosis by regulating hematopoiesis and that both sympathetic activity and the proportions of neutrophils and monocytes in the spleen decreased. Taken together, the results of our study revealed the relationship between SNS activation and chronic inflammation in atherosclerosis and identified that RDN plays an anti-inflammatory role in atherosclerosis by inhibiting splenic inflammatory cell production. In addition, the anti-inflammatory effect was independent of the timing of the RDN intervention in atherosclerosis, but early RDN was better than late RDN.
Abnormal lipid metabolism initiates inflammation and atherogenesis but targeting LDL levels alone may not be sufficient to reduce immune cell production to normal levels [2,20-24]. This can be partially explained by the activation of central neural circuits by the continuous release of inflammatory cytokines and chemokines in atherosclerosis. Activated sympathetic efferent nerves carry impulses to peripheral organs [8,25-27]. Increased activity of sympathetic neurons in the spleen induces granulocyte-monocyte progenitor (GMP) proliferation and differentiation, stimulates inflammatory cells to further release inflammatory factors and chemokines and increases the plaque load [28-30]. In this study, we observed a correlation between the plasma concentration of NE and the levels of circulating inflammatory cells in atherosclerosis, reinforcing the important role of SNS activation in the production of leukocytes in atherosclerosis.
From the perspective of histology, we confirmed that RDN can improve the plaque size and composition. The protective effects occurred in the absence of changes in liver and plasma lipids, suggesting that instead of improving lipid metabolism, the beneficial effects of RDN occur through other mechanisms. In addition, although Wang et al. speculated that RDN exerted an anti-atherosclerosis effect via aldosterone blockade, RDN performed on hypertensive ApoE mice infused with Ang II accelerated plaque growth [18,19]. Angiotensin-aldosterone system (RAAS) inhibition by RDN may be weaker than RAAS stimulation by exogenous Ang Il. SNS and RAAS may influence atherosclerosis through two relatively independent pathways, suggesting that in addition to RAAS inhibition, there should be other mechanisms involved in the inhibitory effect of RDN on atherosclerosis. The anti-inflammatory effects of RDN have been demonstrated in response to in situ renal inflammation and various acute inflammatory responses [31-34]. We hypothesized that the sympathetic regulation of chronic inflammation is affected by RDN.
Previous studies have confirmed that circulating neutrophils promote the recruitment and infiltration of monocytes into plaques [35,36]. Consistent with this evidence, in our study, the local plaque showed a decrease in CD68-positive cells, which may explain the decrease in local inflammatory factors and the oil red O-stained area of the plaque, as Ly6Chigh cells are the main cells in lesions that secrete inflammatory cytokines and engulf liposomes. We showed that after RDN, the decrease in circulating proinflammatory Ly6Chigh monocytes was accompanied by a change in the ratio of proinflammatory/anti-inflammatory cells. In addition, since the presence of inflammatory factors suggest that RDN inhibits the positive regulation of hematopoiesis in atherosclerosis, we explored the sources of inflammatory cells. The spleen is a reservoir for inflammatory cells. All of the splenic monocytes that infiltrated into the plaque were pro-inflammatory Ly6Chigh [28]. Previous studies have found that the spleen, unlike the bone marrow, maintains inflammation as well as chronicity [37]. Our data also showed that RDN had a considerable effect on the spleen, as it reduced the production and proportions of mature monocytes and neutrophils, which remained unchanged in the bone marrow. Splenic leukocytosis is regulated by the central sympathetic nervous system [38]. Splenic sympathectomy or β2 blockers have been reported to mitigate inflammation-induced extramedullary hematopoiesis [4,5,28]. Consistently, increases in splenic myeloid cells, NE concentration and TH-positive areas were observed in both SO groups, and RDN reduced sympathetic neural activity in the spleen. However, unlike the inhibitory effect of RDN on the release of splenic inflammatory cells observed under acute sympathetic activation, our study found that in atherosclerosis, the proportions of mature monocytes and neutrophils in the spleen decreased, suggested by decreased cell proliferation markers [34]. These differences suggest that RDN, which is partially mediated by β2 signals, reduces the production of monocytes and neutrophils in the spleen by inhibiting splenic sympathetic activity. However, more studies are needed in the future to explore the specific mechanism by which RDN reduces leukocyte production.
Currently, as statin intervention alone cannot restore circulating inflammatory cells to normal levels, many anti-inflammatory drugs for atherosclerosis are being studied, such as canakinumab, tocilizumab, and colchicine [39-41]. Most of them are in the preclinical stage, and their clinical effect remains to be further determined. RDN was originally regarded as an interventional treatment for hypertension. In this study, we explored its chronic anti-inflammatory effect on atherosclerosis via the regulation of the production of splenic immune cells and related inflammatory cytokines. These benefits of RDN are not affected by the timing of processing. On the one hand, it has been suggested that the antihypertensive mechanism of RDN involves not only the inhibition of SNS and RAAS and the antagonism of the release of vasoconstrictor substances but also, in part, anti-inflammation. On the other hand, although the anti-hypertensive effect of RDN has been widely recognized, optimal selection of the patient population has not been determined. However, it is certain that the level of sympathetic activation is a relevant measure. Since hypertension and atherosclerosis are common comorbidities that are both associated with activation of the SNS, individuals with combined hypertension and atherosclerosis may be hyperresponsive and benefit more from RDN.
In conclusion, based on the immunomodulatory effect of the SNS on atherosclerosis, we found that RDN played an anti-inflammatory role in atherosclerosis by reducing the production and number of inflammatory cells released into the blood that infiltrated into the plaque (Figure 6). The underlying mechanism remains to be explored, but our study is helpful in identifying the wider clinical application prospects of RDN and provides a potential therapeutic strategy for hypertensive patients complicated by atherosclerosis.
Figure 6.
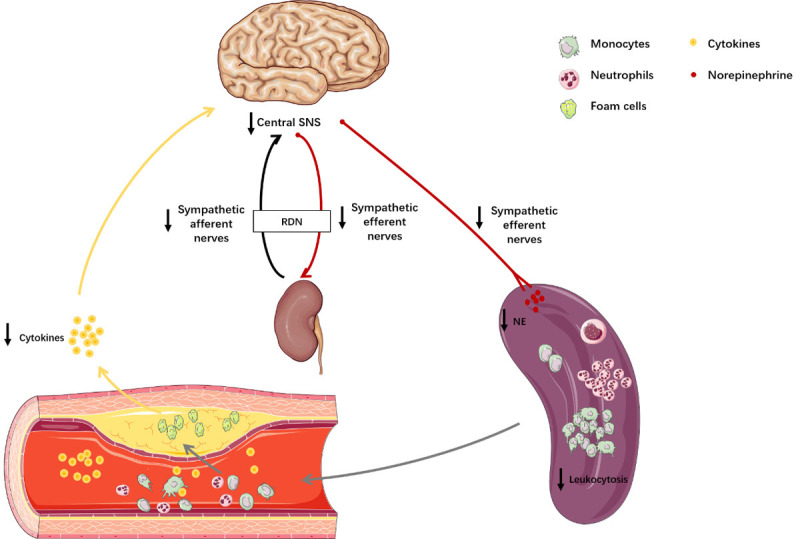
Schematic diagram illustrating how RDN plays an anti-inflammatory role in atherosclerosis. The inflammatory cytokines released in atherosclerosis pass through the blood-brain barrier, stimulating the sympathetic nerve center. Excitatory sympathetic nerve signals are then transmitted to the kidney and spleen. RDN reduces the excitability of the SNS. Decreased sympathetic signaling in the spleen attenuates the production of monocytes and neutrophils. As a result, there is a decrease in the number of inflammatory cells released into the blood and infiltrating into the plaque.
Acknowledgements
The authors acknowledge Mr. Jianguo Jia for his advice and help with the histological analysis. This work was supported by the National Natural Science Fund of China (grant numbers 81670319 and 81521001) and the Shanghai Municipal Science and Technology Commission (grant number 15441900100).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumer Y, McCurdy S, Weatherby TM, Mehta NN, Halbherr S, Halbherr P, Yamazaki N, Boisvert WA. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun. 2017;8:1129. doi: 10.1038/s41467-017-01186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Vasamsetti SB, Florentin J, Coppin E, Stiekema LCA, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ESG, Kim K, Dutta P. Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity. 2018;49:93–106. e107. doi: 10.1016/j.immuni.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res. 2015;116:407–417. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Sharea A, Lee MKS, Whillas A, Michell DL, Shihata WA, Nicholls AJ, Cooney OD, Kraakman MJ, Veiga CB, Jefferis AM, Jackson K, Nagareddy PR, Lambert G, Wong CHY, Andrews KL, Head GA, Chin-Dusting J, Murphy AJ. Chronic sympathetic driven hypertension promotes atherosclerosis by enhancing hematopoiesis. Haematologica. 2019;104:456–467. doi: 10.3324/haematol.2018.192898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvillo L, Gironacci MM, Crotti L, Meroni PL, Parati G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat Rev Cardiol. 2019;16:476–490. doi: 10.1038/s41569-019-0178-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Su E, Wang H, Guo C, Lawrence DA, Eitzman DT. Traumatic brain injury leads to accelerated atherosclerosis in apolipoprotein E deficient mice. Sci Rep. 2018;8:5639. doi: 10.1038/s41598-018-23959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 11.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 12.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D’Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7. [DOI] [PubMed] [Google Scholar]
- 13.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M SPYRAL HTN-OFF MED trial investigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 14.Iyer MS, Bergman RN, Korman JE, Woolcott OO, Kabir M, Victor RG, Clegg DJ, Kolka C. Renal denervation reverses hepatic insulin resistance induced by high-fat diet. Diabetes. 2016;65:3453–3463. doi: 10.2337/db16-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selejan SR, Linz D, Tatu AM, Hohl M, Speer T, Ewen S, Mahfoud F, Kindermann I, Zamyatkin O, Kazakov A, Laufs U, Bohm M. Sympathoadrenergic suppression improves heart function by upregulating the ratio of sRAGE/RAGE in hypertension with metabolic syndrome. J Mol Cell Cardiol. 2018;122:34–46. doi: 10.1016/j.yjmcc.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Bohm M, Ewen S, Wolf M. Renal denervation halts left ventricular remodeling and dysfunction in heart failure: new shores ahead. J Am Coll Cardiol. 2018;72:2622–2624. doi: 10.1016/j.jacc.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Yamada S, Fong MC, Hsiao YW, Chang SL, Tsai YN, Lo LW, Chao TF, Lin YJ, Hu YF, Chung FP, Liao JN, Chang YT, Li HY, Higa S, Chen SA. Impact of renal denervation on atrial arrhythmogenic substrate in ischemic model of heart failure. J Am Heart Assoc. 2018;7:e007312. doi: 10.1161/JAHA.117.007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang J, Guo C, Luo W, Kleiman K, Eitzman DT. Renal denervation attenuates progression of atherosclerosis in apolipoprotein E-deficient mice independent of blood pressure lowering. Hypertension. 2015;65:758–765. doi: 10.1161/HYPERTENSIONAHA.114.04648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Dinh TN, Nield A, Krishna SM, Denton K, Golledge J. Renal denervation promotes atherosclerosis in hypertensive apolipoprotein E-deficient mice infused with angiotensin II. Front Physiol. 2017;8:215. doi: 10.3389/fphys.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Diepen JA, Berbee JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228:306–315. doi: 10.1016/j.atherosclerosis.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Hara T, Phuong PT, Fukuda D, Yamaguchi K, Murata C, Nishimoto S, Yagi S, Kusunose K, Yamada H, Soeki T, Wakatsuki T, Imoto I, Shimabukuro M, Sata M. Protease-activated receptor-2 plays a critical role in vascular inflammation and atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2018;138:1706–1719. doi: 10.1161/CIRCULATIONAHA.118.033544. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 2020;17:137–144. doi: 10.1038/s41569-019-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart RA, White HD, Kirby AC, Heritier SR, Simes RJ, Nestel PJ, West MJ, Colquhoun DM, Tonkin AM Long-Term Intervention With Pravastatin in Ischemic Disease (LIPID) Study Investigators. White blood cell count predicts reduction in coronary heart disease mortality with pravastatin. Circulation. 2005;111:1756–1762. doi: 10.1161/01.CIR.0000160924.73417.26. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 26.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–373. doi: 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C (high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beis D, von Kanel R, Heimgartner N, Zuccarella-Hackl C, Burkle A, Ehlert U, Wirtz PH. The role of norepinephrine and alpha-adrenergic receptors in acute stress-induced changes in granulocytes and monocytes. Psychosom Med. 2018;80:649–658. doi: 10.1097/PSY.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 30.Yin X, Zhou L, Han F, Han J, Zhang Y, Sun Z, Zhao W, Wang Z, Zheng L. Beta-adrenoceptor activation by norepinephrine enhances lipopolysaccharide-induced matrix metalloproteinase-9 expression through the ERK/JNK-c-Fos pathway in human THP-1 Cells. J Atheroscler Thromb. 2017;24:55–67. doi: 10.5551/jat.35204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD, Htun NM, Duval J, Hammond L, Eisenhardt SU, Flierl U, Schlaich MP, Peter K. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension. 2017;69:323–331. doi: 10.1161/HYPERTENSIONAHA.116.08373. [DOI] [PubMed] [Google Scholar]
- 33.Hilderman M, Qureshi AR, Abtahi F, Witt N, Jagren C, Olbers J, Delle M, Lindecrantz K, Bruchfeld A. The cholinergic anti-inflammatory pathway in resistant hypertension treated with renal denervation. Mol Med. 2019;25:39. doi: 10.1186/s10020-019-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Wei Z, Li Y, Wang J, Hu J, Yin Y, Xie J, Xu B. Renal denervation restrains the inflammatory response in myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2020;115:15. doi: 10.1007/s00395-020-0776-4. [DOI] [PubMed] [Google Scholar]
- 35.Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17:327–340. doi: 10.1038/s41569-019-0326-7. [DOI] [PubMed] [Google Scholar]
- 36.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 37.McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF. Social stress mobilizes hematopoietic stem cells to establish persistent splenic myelopoiesis. Cell Rep. 2018;25:2552–2562. e2553. doi: 10.1016/j.celrep.2018.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez SD, Kozic B, Molinaro CA, Thyagarajan S, Ghamsary M, Lubahn CL, Lorton D, Bellinger DL. Chronically lowering sympathetic activity protects sympathetic nerves in spleens from aging F344 rats. J Neuroimmunol. 2012;247:38–51. doi: 10.1016/j.jneuroim.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 40.Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, Aakhus S, Damas JK, Aukrust P, Wiseth R, Gullestad L. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 41.Khan R, Spagnoli V, Tardif JC, L’Allier PL. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis. 2015;240:497–509. doi: 10.1016/j.atherosclerosis.2015.04.783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.