Abstract
The purpose of this study is to explore the expression and clinical significance of KIF23 in ovarian cancer (OV) and identify potential targets for clinical treatment. Oncomine, GEO, and TCGA databases were used to analysis the expression of KIF23 in OV. The prognostic value of KIF23 gene was analyzed by the Kaplan-Meier plotter database. The molecular mechanism of KIF23 activity was analyzed from the perspective of immunology, gene mutation, copy number variation (CNV). Finally, immunohistochemistry was conducted to validate the expression of KIF23, univariable and multivariate cox analysis were used to determine its relationship with clinical characteristics and OV prognosis. It showed that highly expressed KIF23 is an adverse independent prognostic biomarker for OV patients. Genomics analysis showed that KIF23 expression was associated with mutations such as FLG2 and TTN, and was significantly enriched in DNA replication and the cell cycle tumor-related signaling pathways. Immunology analysis showed that KIF23 is closely related to the immune infiltration. KIF23 can not only performed as a prognosis signature in OV but also as a target of immune molecular therapeutics.
Keywords: KIF23, ovarian cancer, prognosis, biomarker, immune infiltration, immunohistochemistry
Introduction
OV has the highest fatality rate among gynecological malignant tumors [1]. Due to its still unknown pathogenesis and lack of sensitive screening methods, 70 to 80% of patients are diagnosed with advanced-stage disease and have a 5-year survival rate of less than 45%. Therefore, it is critical to explore the mechanisms underlying the development and progression of OV and identify useful biomarkers for this disease [2].
The kinesin protein superfamily (kinesin family, KIF) belongs to the class of molecular motors. Its globular head has ATPase activity, which can obtain energy by hydrolyzing ATP and changing its configuration [3]. Kinesins are involved in the transport of vesicles, organelles, chromosomes, and RNA-binding proteins in cells, the formation of spindles and intermediates, and the separation of chromosomes. Abnormal expression of KIF family members plays an important role in tumor development [4,5]. Kinesin family member 23 (KIF23), of the kinesin 6 family, is localized to the mitotic spindle region. It plays an important role in mitotic cytoplasmic separation [6,7]. High KIF23 expression levels affect normal cytokinesis and centrosome formation, which leads to cell division arrest or abnormalities, resulting in aneuploid cells that cause tumorigenesis. High KIF23 expression can also regulate AKT activity, levels of phosphorylation, and the proteasome degradation pathway, leading to tumor invasion [8,9].
KIF23 is highly expressed in a variety of tumors, such as breast cancer, gastric cancer, and lung cancer. KIF23 overexpression is significantly associated with tumor grade, invasion, and prognosis in breast cancer [5]. The high expression of KIF23 in glioma cells may be related to transcriptional activation, and KIF23 knockdown can significantly inhibit glioma cell proliferation in vitro and in vivo [10]. Zhao C et al. found that KIF23 expression is significantly elevated in glioma samples. MiR-424 acts as a tumor suppressor and inhibits cell migration and EMT by targeting KIF23 in gliomas [11]. Murakami et al. [12] found that KIF23 expression levels are significantly elevated in gastric cancer and associated with poor prognosis. Kato et al. [13] found that KIF23 expression is significantly increased in NSCLC tissues, especially in adenocarcinoma tissues, and patients with high expression typically have a poor prognosis.
Previously, we found that KIF23 was a poor prognostic indicator for endometrial cancer [14]. In this study, we analyzed KIF23 expression in multiple databases and studied the correlation between its expression and clinical stage, tumor grade, and patient prognosis in OV. Samples (n=167) representing a range of pathologies were collected from surgical patients and analyzed by immunohistochemistry to verify the role of KIF23. Finally, we explored the molecular pathways and functions of KIF23 involved in the development of OV. Our results improve the understanding of the roles and mechanisms of KIF23 in the development of OV.
Materials and methods
Data extraction from Oncomine, GEO and TCGA databases
The Oncomine database has an integrated data mining platform. In this database, the conditions for filtering and mining data can be set to accommodate specific needs. The screening conditions for the current study were as follows: ① “Cancer Type: Ovary cancer”, ② “Gene: KIF23”, ③ “Analysis Type: Cancer vs Normal Analysis”, and ④ threshold conditions of P < 0.01, fold-change > 2, and gene rank of top 10%.
Three OV datasets were analyzed from the GEO database (GSE14407 [15], GSE18520 [16], and GSE54388 [17]). All three datasets were based on the GPL570 platform ([HG-U133_Plus_2] Affymetrix Human Genome U133 + 2.0 Array). GSE14407 contained 12 samples each of serous papillary OV and normal ovarian epithelium. GSE18520 contained 53 high-grade serous papillary carcinoma samples and ten para-cancerous samples. GSE54388 contained 16 well or moderately differentiated OV samples and six normal ovarian epithelial samples. The datasets were processed, calibrated, standardized, and log2-converted using the R package. The KIF23 expression was extracted from the three datasets, and differential expression box plots were drawn using “ggpubr” in the R package.
An OV dataset consisting of 374 tumor tissue samples was downloaded and pre-processed from the TCGA database (https://tcga-data.nci.nih.gov/tcga/). KIF23 expression was ranked from low to high, and the samples were divided into four equal parts. The first and last 25% of the samples were selected as the low expression and high expression groups, respectively.
Kaplan-Meier plotter for survival analysis
The Kaplan-Meier Plotter database (http://kmplot.com) [18] contains 10188 cancer samples, including 4142 breast cancer, 1648 OV, 2437 lung cancer, and 1065 gastric cancer patient samples, that allow the assessment of the impact of 54675 genes on patient survival. Patients with OV were divided into two groups based on KIF23 expression levels. Survival analysis was performed using the Kaplan-Meier Plotter (http://kmplot.com/analysis/). The screening conditions were as follows: ① “Cancer: Ovary Cancer”, ② “Gene: KIF23”, ③ “Survival: OS/PFS”, and ④ “Follow up threshold: 120 months”. Subgroup conditions, including clinical stage, tissue classification, and mutation status, were defined in the database. The prognosis of OV patients with different stages, tumor grades, and mutations was analyzed based on KIF23 expression levels. The hazard ratios (HRs) with 95% confidence intervals (CIs) and log-rank p-values were generated.
Sample sources and clinical data
Ovarian tissues (n=167) were collected from surgical patients hospitalized at Shengjing Hospital of China Medical University from 2008 to 2015. All patients provided written informed consent, and complete clinical data were obtained. This study was approved by the Ethics Committee of China Medical University. None of the enrolled patients received radiotherapy, chemotherapy, or hormone therapy before surgery. Pathologists diagnosed all pathological ovarian sections, consisting of 115 epithelial OV tumors (79 serous carcinomas, nine mucinous carcinomas, 18 endometrial carcinomas, and nine clear cell carcinomas), 20 borderline epithelial ovarian tumors, 20 benign epithelial ovarian tumors, and 12 normal ovarian tissues. The average age of all patients (16-84 yo) was 53.52 years. The median ages of patients with malignant, borderline, and benign tumors, and normal ovaries were 55 (16-79 yo), 52 (19-84 yo), 45 (28-79 yo), and 50.5 (35-67 yo), respectively. No statistically significant differences were noted between the groups. Of the 115 epithelial OV samples, 62 were poorly differentiated, and 53 were moderately differentiated. Based on the International Federation of Obstetrics and Gynecology (FIGO, 2009), 47 cases were grades I-II and 68 cases were grades III-IV. In addition, there were 28 cases of pelvic or para-aortic lymph node metastasis, while 87 had no metastasis.
Immunohistochemistry
Ovarian tissues were fixed in 10% formalin and processed into 5-mm thick paraffin sections. The samples were dewaxed with discontinuous concentrations of ethanol and blocked to inhibit endogenous peroxidase. The sections were heated in a microwave for antigen retrieval, cooled to room temperature, and blocked by incubation in goat serum for 30 min at 37°C. Samples were incubated in rabbit anti-KIF23 (Abcam, 1:200 dilution) overnight at 4°C, followed by incubation with horseradish peroxidase-coupled goat anti-rabbit secondary antibody at 37°C for 30 min. Nuclei were stained blue by hematoxylin. Sections were then dehydrated, cleared by xylene, and mounted. The SP kit was used according to the manufacturer’s instructions. Samples were deemed KIF23-positive when intense granular staining in the cell nucleus and cytoplasm was present.
Stained cells were classified based on their color intensity using the following scoring system: non-staining, light yellow, brownish-yellow, and dark brown, which were recorded as 0, 1, 2, and 3, respectively. Five fields were randomly evaluated for each slice using 400× magnification. The percentage of stained cells was also scored as follows: < 5% (0), 5 to 25% (1), 26 to 50% (2), 51 to 75% (3), and > 75% (4). The final score was calculated by multiplying the staining and percentage scores: 0 to 2 (-), 3 to 4 (+), 5 to 8 (++), and 9 to 12 scores (+++). Final scores of 3 to 12 were considered to represent positive expression, and 5 to 12 were considered high positive expression.
GSEA analysis, function and pathway enrichment analysis
GSEA analysis was performed using GSEA version 3.0 software. The c2.cp.kegg.v6.1.symbols.gmt dataset was downloaded from the MsigDB database on the GSEA website. The high-low grouping expression spectrum data and the attribute file were subjected to enrichment analysis by default weighted enrichment statistics, and the random combination number was set to 1000.
Proteins that interact with KIF23 were found in the cBioportal (http://www.cbioportal.org/) [19] database. Based on the p-values, the top 50 significantly related molecules were screened using DAVID Bioinformatics Resources (http://david.abcc.Ncifrrf.gov/) [20]. Gene Ontology (GO) functional analysis was performed on the genes corresponding to the above proteins, and the significance of the enrichment to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was calculated using a hypergeometric test according to the following formula:
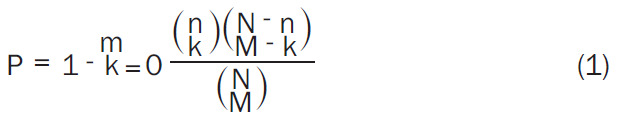 |
in which N is the number of genome-wide genes, M is the number of genes annotated to a given pathway in the whole genome, n is the number of genes in the network, and m is the number of genes annotated to a given pathway.
Copy number alterations (CNAs)
Somatic CNAs (SCNAs) were defined by GISTIC 2.0 (Version 2.0.23, ftp://ftp.broadinstitute.org/pub/GISTIC2.0/GISTIC_2_0_23.tar.gz), which includes deep deletion (-2), arm-level deletion (-1), diploid/normal (0), arm-level gain (1), and high amplification (2). The distributions of immune subsets at each copy number status in OV are provided in box plots. The infiltration level for each SCNA category was compared with the normal level using a two-sided Wilcoxon rank-sum test.
Statistical analysis
Data were analyzed using SPSS 22.0 soft- ware (IBM Corporation, Armonk, NY, USA). Chi-squared and Fisher’s exact tests were used to analyze counting data, and the t-test was used to analyze measurement data. Survival curves were analyzed by KM and log-rank tests. The Cox regression model was used to analyze the relationships between KIF23 expression and clinical data. P < 0.05 indicated statistically significant differences.
Results
KIF23 expression in Oncomine and GEO databases
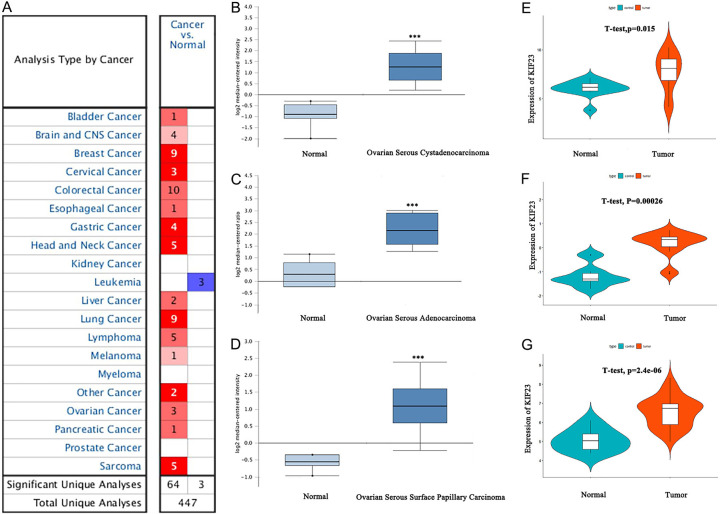
A total of 447 KIF23 studies of different types of cancer were collected from the Oncomine database (Figure 1A). Of these studies, 67 showed statistically significant differences in the expression of KIF23 (64 studies showed significant increases; three studies showed significant decreases). Furthermore, analysis of KIF23 expression in independent OV datasets [21-23], which contained 657 OV samples and 22 normal samples, showed that KIF23 expression levels in all OV groups were significantly higher than in the normal group (P < 0.01) (Figure 1B-D). These results were verified using three independent OV microarrays (GSE14407, GSE18520, and GSE54388) from the GEO database. Together, these results indicate that KIF23 expression in OV tissues was significantly higher than that of adjacent non-cancerous tissues (P < 0.001) (Figure 1E-G).
Figure 1.
KIF23 expression in Oncomine and GEO databases. (A) KIF23 expression in all tumor studies in the Oncomine database (B-D). Differential expression of KIF23 in the OV dataset of the Oncomine database (***P < 0.01) (E-G). KIF23 expression in GSE14407, GSE54388, and GSE18520 from the GEO database.
Correlation between KIF23 mRNA expression and clinical pathological parameters in OV using the TCGA database
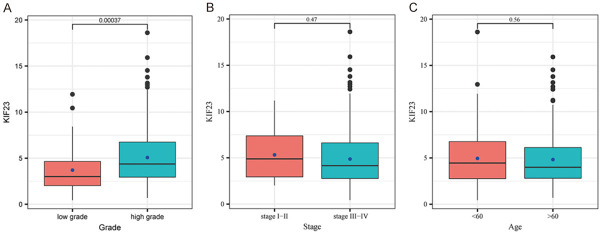
To investigate the relationship between KIF23 expression and clinical-pathological parameters in OV, we used the pathological data for OV from the TCGA database, which contained the complete clinical data for 360 cases, including clinical stage, tumor grade, and patient age. Statistical analysis showed that high KIF23 expression was significantly correlated with poor differentiation (P < 0.05) (Table 1). However, KIF23 expression did not significantly correlate with the FIGO stage or age. Box plots of KIF23 expression and the clinical-pathological parameters are presented in Figure 2A-C.
Table 1.
Relationship between KIF23 and clinical pathological parameters of OV
| Item | KIF23 expression | Total | P-value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| FIGO stage | P > 0.05 | |||
| I-II | 11 | 10 | 21 | |
| III-IV | 202 | 137 | 339 | |
| Grade | ||||
| Low Grade (1/2) | 37 | 9 | 46 | P < 0.05 |
| High Grade (3) | 186 | 128 | 314 | |
| Age | P > 0.05 | |||
| > 60 | 117 | 54 | 271 | |
| < 60 | 115 | 74 | 189 | |
Figure 2.
Box plots for the correlations between KIF23 expression and clinical pathological parameters. A. Expression of KIF23 in patients with high or low-grade OV. B. Expression of KIF23 in patients with stage I-II or stage III-IV OV. C. Expression of KIF23 in OV patients > 60 or < 60 years old.
Relationship between KIF23 mRNA expression and OV prognosis
For overall survival (OS), the qualifying data consisted of 1,656 OV cases was analyzed. The overall survival of patients with high KIF23 expression was significantly worse than that of patients with low KIF23 expression (HR 1.31; CI 1.13 to 1.53; log-rank P=0.00032) (Figure 3A). Analysis of qualifying data from 587 OV cases for the relationship between progression-free survival (PFS) and KIF23 expression yielded similar results (HR 1.27; CI 1.12 to 1.45; log-rank P=0.00026) (Figure 3B). Thus, the disease-free survival time of patients with high KIF23 expression was significantly shorter than that of patients with low KIF23 expression. The analysis also demonstrated that patients with low KIF23 expression had a better prognosis for mutant TP53 OV (Figure 3C), while low KIF23 expression did not show significant difference in the prognosis of wild-type TP53 OV patients (Figure 3D).
Figure 3.
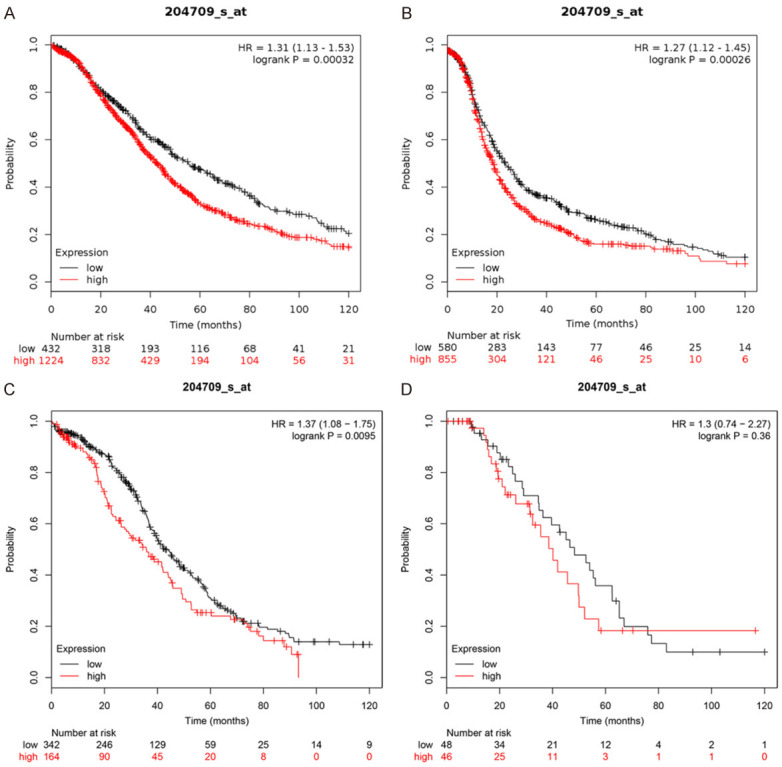
Relationship between prognosis and KIF23 gene expression and TP53 mutations in OV patients. A. Relationship between KIF23 expression and OS in patients with OV. B. Relationship between KIF23 expression and PFS in patients with OV. C. Relationship between KIF23 expression and prognosis in patients with TP53 mutant-associated OV. D. Relationship between KIF23 expression and prognosis in patients with TP53 wild type OV. Red represents high expression, and the black line represents low expression, the abscissa represents month and ordinate represents survival rate.
Relationship between KIF23 mRNA levels and prognosis of patients at different stages of OV
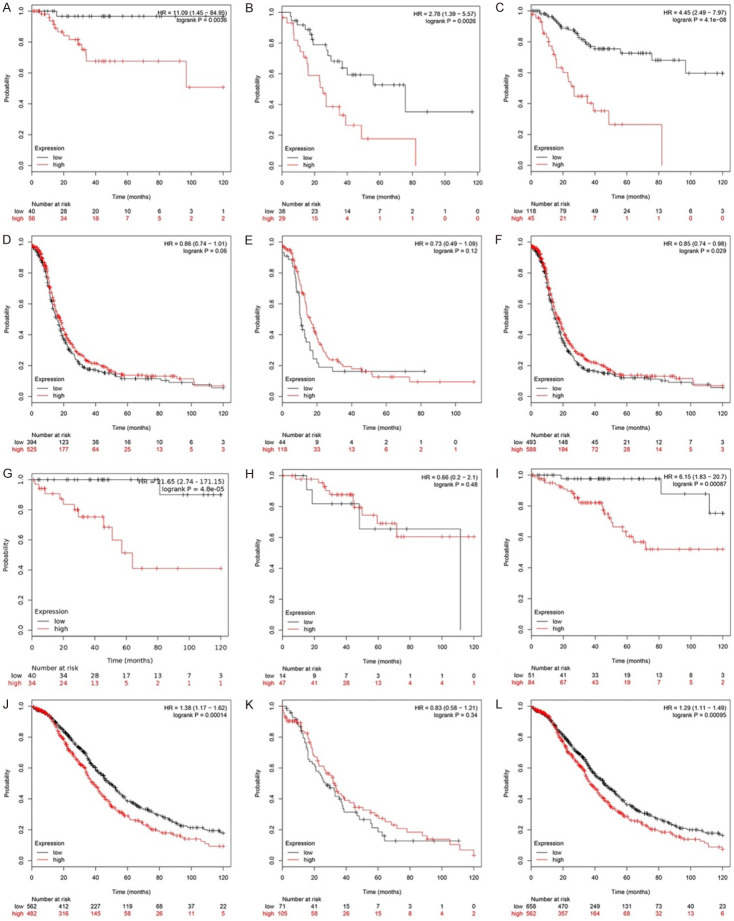
For PFS, higher KIF23 expression levels in early-stage OV (stages I/II/I + II) were associated with a worse prognosis than low expression levels (Table 2; Figure 4A-C). The HR for Stage I OV was 11.09 (CI 1.45 to 84.95; log-rank P=0.004), indicating that KIF23 may be a negative prognostic indicator for lower stage OV patients. The HR was less than 1 for stage III and IV OV (Table 2; Figure 4D-F). Similar results were obtained for OS. The HRs were 21.65 (CI 2.74 to 171.15; log-rank P=0.000) for stage I, 6.15 (CI 1.83 to 20.7; log-rank P=0.001) for stage I + II, and 1.29 (CI 1.11 to 1.49; log-rank P=0.001) for stage III + IV (Table 2; Figure 4G-L). Based on these results, KIF23 could be used as an indicator of poor overall prognosis for all stages of OV.
Table 2.
Relationship between prognosis and KIF23 mRNA expression in patients with different stages of OV
| PFS (n=2000) | OS (n=3241) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Stage | Hazard Ratio | P-value | Stage | Hazard Ratio | P-value |
| I (n=107) | 11.09 (1.45-84.95) | 0.004 | I (n=107) | 21.65 (2.74-171.15) | 4.8e-05 |
| I + II (n=179) | 4.45 (2.49-7.97) | 4.1e-08 | I + II (n=179) | 6.15 (1.83-20.7) | 0.001 |
| II (n=72) | 2.78 (1.39-5.57) | 0.003 | II (n=72) | 0.66 (0.2-2.1) | 0.480 |
| III (n=1079) | 0.86 (0.74-1.01) | 0.060 | III (n=1079) | 1.38 (1.17-1.62) | 0.000 |
| III + IV (n=1268) | 0.85 (0.74-0.98) | 0.029 | III + IV (n=1268) | 1.29 (1.11-1.49) | 0.001 |
Figure 4.
Relationship between prognosis and KIF23 mRNA expression in patients with different stages of OV. A-C. Relationship between KIF23 expression and PFS in patients with early-stage OV (stage I/II/I + II). D-F. Relationship between KIF23 expression and PFS in patients with advanced OV (stage III/IV). G-I. Relationship between KIF23 expression and OS in patients with early-stage OV (stage I/II/I + II). J-L. Relationship between KIF23 expression and OS in patients with advanced OV (stage III/IV) (Red represents high expression, the black line represents low expression. The abscissa represents the month, the ordinate represents survival rate).
Relationship between KIF23 mRNA levels and prognosis of patients with different grades of OV
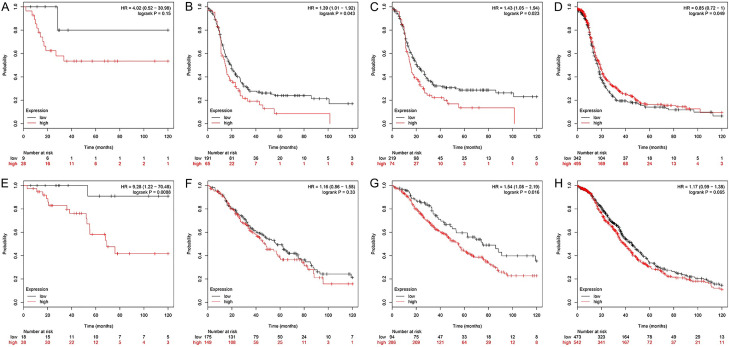
For PFS, higher KIF23 expression levels in grade 1 + 2 were associated with a worse prognosis (Table 3; Figure 5A-C). The HR was 1.43 (CI 1.05 to 1.94; log-rank P=0.023) for grade 1 + 2 OV and 0.85 (CI 0.72 to 1.00; log-rank P=0.050) for grade 3 (Figure 5D). For OS, the HR for high KIF23 expression levels was 9.28 (CI 1.22 to 70.48; log-rank P=0.009) for grade 1 OV and 1.54 (CI 1.08 to 2.19; log-rank P=0.016) for grade 1 + 2 (Table 3; Figure 5E, 5F). There were no significant differences between grades 2 and 3 (Table 3; Figure 5G, 5H), and the HR was 5.27 (CI 1.69 to 16.38; log-rank P=0.002) for grade 4.
Table 3.
Relationship between prognosis and KIF23 mRNA expression in patients with different grades of OV
| PFS (n=1822) | OS (n=3386) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Grade | Hazard Ratio | P-value | Grade | Hazard Ratio | P-value |
| 1 (n=56) | 4.02 (0.52-30.98) | 0.150 | 1 (n=56) | 9.28 (1.22-70.48) | 0.009 |
| 1 + 2 (n=381) | 1.43 (1.05-1.94) | 0.023 | 1 + 2 (n=381) | 1.54 (1.08-2.19) | 0.016 |
| 2 (n=325) | 1.39 (1.01-1.92) | 0.043 | 2 (n=325) | 1.16 (0.86-1.58) | 0.330 |
| 3 (n=1024) | 0.85 (0.72-1) | 0.050 | 3 (n=1024) | 1.17 (0.99-1.38) | 0.065 |
| 4 (n=20) | 5.27 (1.69-16.38) | 0.002 | |||
Figure 5.
Relationship between prognosis and KIF23 mRNA levels in patients with different grades of OV. A-D. Relationship between KIF23 expression and PFS in patients with Grade 1, Grade 1 + 2, Grade 2, Grade 3 OV. E-H. Relationship between KIF23 expression and OS in patients with Grade 1, Grade 1 + 2, Grade 2, Grade 3 OV.
Relationship between KIF23 mRNA expression and prognosis of platinum/paclitaxel-treated OV patients
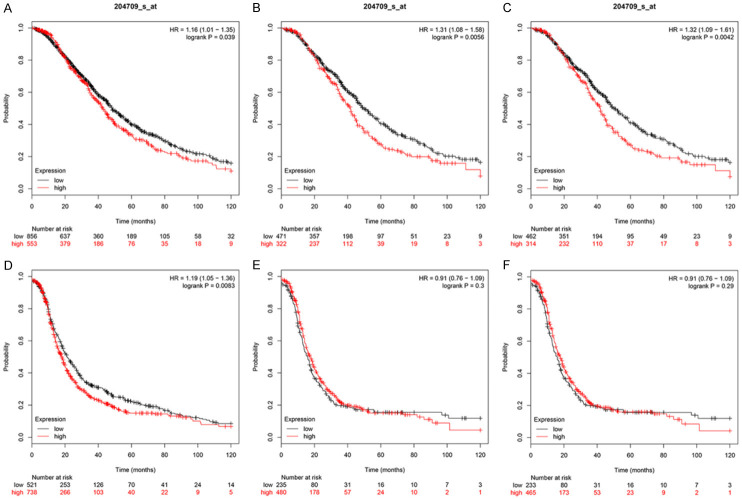
For the analysis of OS, the total number of eligible OV cases treated with platinum-based chemotherapy was 1409. High KIF23 expression resulted in an HR of 1.16 (CI 1.01 to 1.35; log-rank P=0.039) (Figure 6A). The total number of OV patients that received paclitaxel chemotherapy was 793. The HR for high KIF23 expression was 1.31 (CI 1.08 to 1.58; log-rank P=0.0056) (Figure 6B). A total of 776 patients received platinum-paclitaxel combination chemotherapy. The HR for high KIF23 expression in these patients was 1.32 (CI 1.09 to 1.61; log-rank P=0.0042) (Figure 6C), suggesting that KIF23 overexpression could lead to chemotherapy resistance in OV, which affects the overall survival of patients. KIF23 may represent a therapeutic target for the treatment of this disease.
Figure 6.
Relationship between KIF23 mRNA expression and chemotherapy in patients with OV. A-C. Relationship between KIF23 expression and OS in patients with OV treated with platinum, paclitaxel, and platinum/paclitaxel combined chemotherapy. D-F. Relationship between KIF23 expression and PFS in patients with OV treated with platinum, paclitaxel, and platinum/paclitaxel combined chemotherapy (Red represents high expression, the black line represents low expression, the abscissa represents the month, the ordinate represents survival rate).
For PFS, the analysis of the relationship of KIF23 expression and prognosis for 1259 OV patients that received platinum-based chemotherapy yielded an HR of 1.19 (CI 1.05 to 1.36; log-rank P=0.0083) (Figure 6D), suggesting that overexpression of KIF23 may cause resistance to platinum-based chemotherapy. However, KIF23 had no significant effect on the PFS of OV patients treated with paclitaxel chemotherapy alone or platinum-paclitaxel combination therapy (Figure 6E, 6F).
KIF23 expression in different ovarian tissues using IHC
To verify the results of the database analyses, we performed KIF23 IHC on 167 ovarian samples. KIF23 staining primarily occurred in both the nucleus and cytoplasm. The results showed that KIF23 expression was significantly upregulated in epithelial ovarian cancer (Figure 7). The positive expression and high positive expression rates in the epithelial ovarian cancer group were 94.78% and 87.83%, respectively, which was significantly higher than that of the borderline (40% and 10%, respectively), benign (30% and 5%, respectively), and normal (25% and 0%) groups (P < 0.05 for all comparisons) (Table 4).
Figure 7.

KIF23 immunohistochemistry in ovarian tissues. A. Ovarian epithelial malignant tumor. B. Ovarian epithelial borderline tumor. C. Ovarian epithelial benign tumor. D. Normal ovarian tissues (The lower image is a partial enlargement of the image in the box above).
Table 4.
KIF23 immunohistochemistry in ovarian tissues
| Group | n | Low | High | Positive rate (%) | High positive rate (%) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| (-) | (+) | (++) | (+++) | ||||
| Malignant | 115 | 6 | 8 | 37 | 64 | 94.78* | 87.83# |
| Borderline | 20 | 12 | 6 | 2 | 0 | 40 | 10 |
| Benign | 20 | 14 | 5 | 1 | 0 | 30 | 5 |
| Normal | 12 | 9 | 3 | 0 | 0 | 25 | 0 |
Compared with the positive rate of borderline, benign, and normal groups, KIF23 in the malignant group was higher (all P < 0.05).
Compared with the high positive rate of borderline, benign, and normal groups, KIF23 in the malignant group was higher (all P < 0.05).
Relationship between KIF23 mRNA levels and clinical pathological parameters of OV patients using IHC
Based on the IHC analysis, 115 malignant ovarian epithelial tumor samples were divided into low KIF23 expression (-/+) and high KIF23 expression (++/+++) groups. High KIF23 expression levels were significantly correlated with increased FIGO stage and poor differentiation (P < 0.05). KIF23 expression levels did not significantly correlate with lymph node metastasis or pathological type (Table 5).
Table 5.
Relationships between expression of KIF23 and clinical pathological parameters of 115 OV patients
| Items | n | Low | High | High positive rate (%) | P-value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| (-) | (+) | (++) | (+++) | ||||
| FIGO stage | < 0.05 | ||||||
| I-II | 47 | 4 | 7 | 17 | 19 | 76.6 | |
| III-IV | 68 | 2 | 1 | 20 | 45 | 95.59 | |
| Differentiation | < 0.05 | ||||||
| Well-Moderate | 54 | 5 | 5 | 18 | 25 | 79.63 | |
| Poorly | 61 | 1 | 3 | 19 | 39 | 95.08 | |
| Lymph node metastasis | < 0.05 | ||||||
| Yes | 28 | 0 | 0 | 10 | 18 | 100 .00 | |
| No | 87 | 6 | 8 | 27 | 46 | 83.91 | |
| Pathological subtype | > 0.05 | ||||||
| Serous | 79 | 2 | 4 | 27 | 46 | 92.41 | |
| Mucinous | 9 | 0 | 1 | 3 | 5 | 88.89 | |
| Endometrioid | 18 | 2 | 2 | 4 | 10 | 77.78 | |
| Clear cell carcinoma | 9 | 2 | 1 | 3 | 3 | 66.67 | |
Relationship between KIF23 mRNA levels and prognosis of OV patients using IHC
Follow-up evaluations with 115 OV patients occurred through June 2019. Univariate analysis demonstrated that KIF23 expression levels (HR 0.098; CI 0.013 to 0.711; log-rank P=0.022), FIGO stage (HR 0.192; CI 0.092 to 0.401; log-rank P=0.000), degree of differentiation (HR 1.885; CI 1.020 to 3.484; log-rank P=0.043), and lymph node metastasis (HR 0.268; CI 0.141 to 0.510; log-rank P=0.000) significantly correlated with overall survival (Table 6). The 5-year survival probability of the KIF23 high expression group was significantly lower than that of the KIF23 low expression group (Figure 8A). Patients with stage III-IV disease had a shorter survival time than those with stage I-II (Figure 8B). Patients with poorly differentiated tumors had shorter survival times than those with well or moderately differentiated tumors (Figure 8C). Patients with lymph node metastasis had shorter survival times than patients with no metastasis (Figure 8D). Multivariate analysis demonstrated that high KIF23 expression (HR 0.129; CI 0.018 to 0.955; log-rank P=0.045), advanced clinical stage (HR 0.239; CI 0.105 to 0.545; log-rank P=0.001), and poor differentiation (HR 0.532; CI 0.267 to 1.0579; log-rank P=0.035) were independent risk factors for poor prognosis (Table 6).
Table 6.
Cox regression analysis of overall survival of ovarian epithelial serous tumors
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI of HR | P-value | HR | 95% CI of HR | P-value | |
| Age (< 60 vs ≥ 60 years) | 0.595 | 0.333~1.063 | 0.079 | 0.653 | 0.358~1.192 | 0.165 |
| KIF23 expression (low vs high) | 0.098 | 0.013~0.711 | 0.022 | 0.129 | 0.018~0.955 | 0.045 |
| FIGO stage (I-II vs III-IV) | 0.192 | 0.092~0.401 | 0.000 | 0.239 | 0.105~0.545 | 0.001 |
| Lymph node metastasis (yes vs no) | 0.268 | 0.141~0.510 | 0.000 | 0.532 | 0.267~1.057 | 0.072 |
| Differentiation (poor vs well-moderate) | 1.885 | 1.020~3.484 | 0.043 | 1.982 | 1.051~3.739 | 0.035 |
| Pathological subtype (serous vs nonserous) | 0.508 | 0.245~1.051 | 0.068 | 1.036 | 0.489~2.194 | 0.926 |
Figure 8.
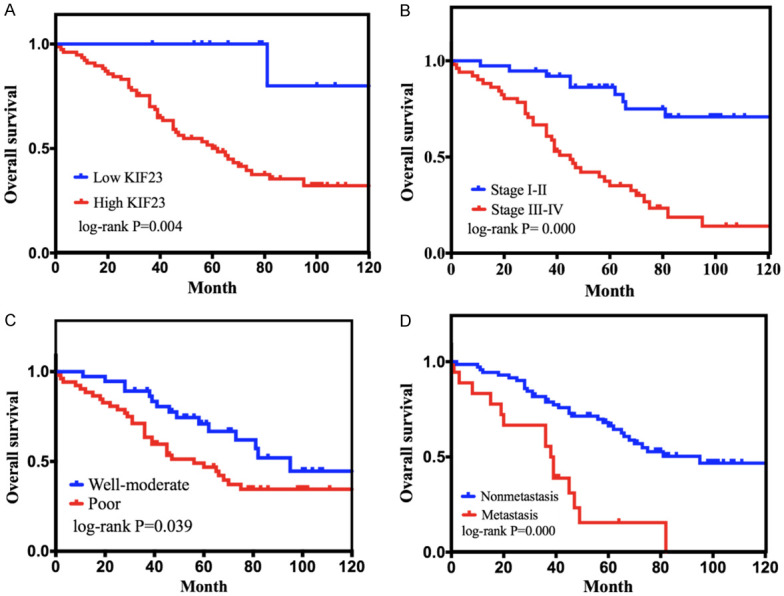
Kaplan-Meier curves for overall survival of patients with epithelial OV. A. Correlation of KIF23 expression with overall survival. B. Correlation of clinical stage with overall survival. C. Correlation of the degree of differentiation with overall survival. D. Correlation of lymph node metastasis with overall survival.
Molecular mechanism of KIF23 involved in the development of OV
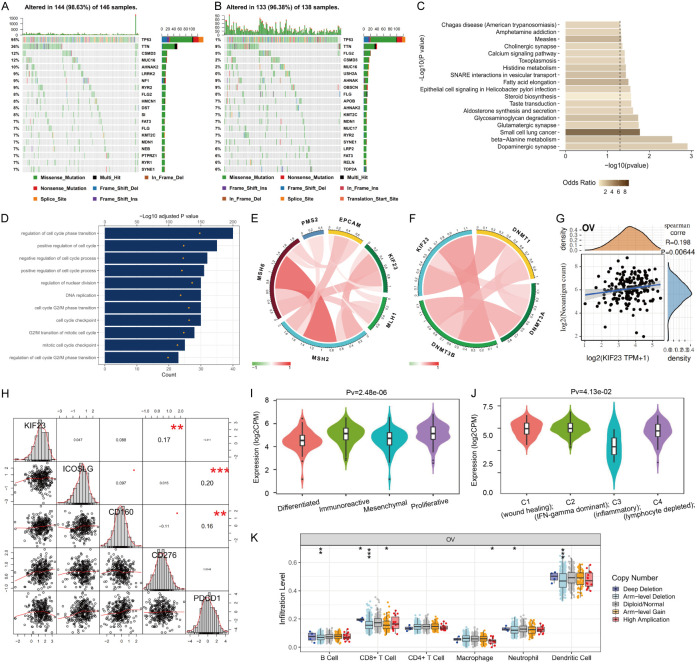
DNA methylation is one of the most important pathways of epigenetic modification. DNA methyltransferases, including DNMT1, DNMT3A, and DNMT3B, are responsible for the methylation pattern of genomic DNA. Recent studies have shown that there is a correlation between abnormal DNA methylation and the occurrence, development, recurrence and drug resistance of OV [24,25]. We further analyzed the correlation between KIF23 and methyltransferase (DNMT1, DNMT3A, DNMT3B) [26]. The results showed that KIF23 was positively correlated with DNMT1 and DNMT3B (Figure 9F), indicating that KIF23 participates in paclitaxel/platinum chemotherapy resistance through abnormal DNA methylation by regulating methyltransferase in OV.
Figure 9.
Molecular mechanism of KIF23 in the development of OV. A. Distribution of common single nucleotide mutations in OV with 98.63% samples altered in KIF23 low expressed group (The bar graph on the right represents the number of mutations of each gene in the total number of samples). B. Distribution of common single nucleotide mutations in OV with 96.38% samples altered in the KIF23 high expressed group (The bar graph on the right represents the number of mutations of each gene in the total number of samples). C. KEGG analysis of enrichment pathway of KIF23 high expression mutant group. D. GO functional enrichment analysis of biological behavior of genes related to KIF23 expression. E. Correlation between KIF23 and MMR key genes (Green represents a negative correlation, red represents positive correlation; the darker the color, the greater the correlation). F. Correlation between KIF23 expression and methyltransferase (Green represents negative correlation, red represents positive correlation; the darker the color, the greater the correlation). G. Correlation between KIF23 expression and Neoantigen count (The abscissa represents KIF23 expression, the ordinate represents Neoantigen count). H. Correlation between KIF23 expression and immune checkpoint protein. I. Correlation between KIF23 expression and molecular subtypes of OV. J. Correlation between KIF23 expression and immune subtypes of OV. K. Correlation between KIF23 expression and infiltration level of different somatic copy number changes in OV (The abscissa represents copy number change, including deep deletion (-2), arm-level deletion (-1), diploid/normal (0), arm-level gain (1), and high amplification (2). Ordinate represents infiltration level).
Any gene mutation of the DNA mismatch repair (MMR) gene family will cause DNA replication errors that cannot be repaired, resulting in genetic instability and higher somatic mutations, which promote cancer malignancy [27]. In exploring the relationship between KIF23 and the expression of key MMR genes, we found a positive correlation between KIF23 and MSH2 and MSH6 (Figure 9E). Somatic mutations were further analyzed using the TCGA database. Gene expression data were analyzed for 433 samples, and a total of 284 samples had overlapping mutations. The median KIF23 expression level was calculated. Low expression samples had KIF23 levels below the median, and high expression samples had KIF23 levels above the median. The FLG2 mutation was significantly enriched in patients with low KIF23 expression, whereas TP53 mutations were significantly enriched in both KIF23 populations. In the high KIF23 expression group, the lower TTN mutant expression group was significantly enriched. Mutations in USH2A, OBSCN, NF1, MUC17, and DST were significantly different under different expression conditions (Figure 9A, 9B). However, the role of these mutations in OV has not been thoroughly investigated. Using KEGG pathway analysis, we found that the high KIF23 expression mutation group was significantly enriched for dopaminergic synapse, beta-alanine metabolism, and glycosaminoglycan degradation (Figure 9C). Using the TCGA OV gene expression profile data (Pearson |R| > 0.5), 160 genes closely related to KIF23 expression were identified. GO functional enrichment analysis was performed to explore the biological behavior and molecular function of these genes. We found that gene expression associated with KIF23 expression was mainly involved in the regulation of cell cycle phase transition, cell cycle checkpoint, G2/M transition of the cell cycle, and DNA replication (Figure 9D).
Neoantigen is a new antigen encoded by the mutant gene of tumor cells. Using the immune activity of the new tumor antigen, the new antigen vaccine can be designed and synthesized according to the mutation of tumor cells, and personalized immunotherapy can be provided to patients [28]. The number of new antigens in each tumor sample was counted, and there was a positive correlation between the expression of the KIF23 gene and the number of antigens in OV (Figure 9F). In addition, there was a positive correlation between KIF23 and immune checkpoint protein CD276 (Figure 9H). Based on the expression of mRNA, TCGA-OV samples have been classified into four subtypes: Differentiated, Immunoreactive, Mesenchymal, and Proliferative [29]. The analysis of the relationships between the expression of KIF23 and the four subtypes showed that the expression of KIF23 was lowest in the Differentiated subgroup and the highest in the Immunoreactive subgroup (Figure 9I). Further analysis of the relationship between the expression of KIF23 and immune subtypes of OV [30] showed that the expression of KIF23 was the highest in subgroup C1 (Figure 9J). As infiltrating lymphocytes in the tumor site are the main participants in the anti-tumor immune response, we quantified the number of different types of lymphocytes in each OV sample, including B cells, CD8 + T cells, CD4 + T cells, macrophages, neutrophils, and dendritic cells. Of all lymphocytes, CD8 + T cells showed the most significant difference in OV samples with different KIF23 copy numbers (Figure 9K) demonstrating the correlation between immune cell infiltration and somatic copy number. The above results confirm that the expression level of KIF23 is closely related to the immune infiltration of OV, and KIF23 is expected to become a new target for immunotherapy.
Discussion
The fatality rate of OV ranks first among gynecological malignant tumors, and 4/5 of the patients are diagnosed with advanced OV [31]. At present, the pathogenesis of OV is not clear, and there are few studies on KIF23 in OV. Therefore, it is vital to explore the relationship between KIF23 and OV.
The kinesin superfamily (KIF) is involved in a variety of normal cellular biological activities, such as cell mitosis and intracellular transport of vesicles and organelles [32]. Overexpression of certain kinesins, such as Eg5, can induce excessive spindle separation, causing uneven distribution of genetic material, thereby forming aneuploid progeny cells, which are involved in cancer invasion and metastasis [33,34]. Downregulation of certain kinesins, such as KIF20B, can cause mitotic arrest or cytokinesis defects, triggering apoptosis through p53 or other signaling pathways [33,35]. Studies have shown that KIF23, as a member of the kinesin superfamily 6, is highly expressed in gastric cancer and is positively correlated with the pTNM stage and poor prognosis. Knocking down KIF23 can inhibit the proliferation of gastric cancer cells [36]. Sun [37] et al. confirmed that TCF-4 regulates KIF23 expression at the transcriptional level, knocking down KIF23 by CHIP and dual luciferase gene reports, and glioma cells show dual and multinucleated cell bodies, thereby inhibiting tumor cell proliferation [38]. Liu [39] demonstrated that KIF23 could promote the proliferation, migration, and invasion of human hepatocellular carcinoma cells by activating the Akt signal transduction pathway. It is shown that KIF23 plays an essential role in the occurrence and development of tumors.
Through the joint analysis of Oncomine, GEO, and TCGA databases, the results showed that KIF23 was highly expressed in OV, and the high expression of KIF23 was significantly related to tissue differentiation. The overall survival time of patients with high expression of KIF23 was significantly less than that of patients with low expression of KIF23. The immunohistochemistry results showed that the high expression of KIF23 was significantly correlated with the FIGO stage and tissue differentiation, but the results of the bioinformatics analysis did not indicate a relationship between high expression of KIF23 and the FIGO stage. We speculate that this inconsistency may be related to sample size and ethnic differences. The results of survival analysis showed that the overall survival time of patients with high expression of KIF23 was significantly worse than that of patients with low expression, consistent with the conclusion of bioinformatics analysis. Therefore, we propose that KIF23 can be used as a biomarker to guide the early clinical diagnosis and efficacy detection of OV. TP53 mutations have been reported to exist in more than 50% of the advanced epithelial serous ovarian cancers, and the frequency of TP53 mutations can be as high as 80% when purified tumor samples are used for sequence analysis [40-43]. A TP53 mutation is involved in the occurrence and development of epithelial serous ovarian cancer [44]. Therefore, we analyzed the survival curve of OV patients according to the status of TP53 to determine which patients can benefit from testing of TP53 gene targeting. The results suggest that patients with low expression of KIF23 gene have a better prognosis in TP53 mutant OV. Zhang [45] found that the presence of TP53 mutations in OV aggravates genomic instability and promotes the expression of MDR1, which in turn activates chemotherapy resistance. Murakami et al. [46] isolated nine upregulated genes, including KIF23 from paclitaxel-resistant cell lines, by microarray analysis, indicating that KIF23 may be involved in the process of paclitaxel resistance in patients with gastric cancer peritoneal metastasis. Therefore, we contend that the expression of KIF23 may be related to chemotherapy resistance in OV. Our results show that overexpression of KIF23 can lead to platinum/paclitaxel or combined drug resistance, affecting the overall survival. For PFS, overexpression of KIF23 only leads to platinum chemotherapy resistance but has no significance in paclitaxel chemotherapy resistance or platinum combined paclitaxel chemotherapy resistance of OV patients. Therefore, targeted KIF23 therapy can be regarded as a promising OV treatment strategy.
We further explored the molecular mechanism of KIF23 involvement in the occurrence, development, and chemotherapy resistance of OV. As one of the critical DNA repair systems in vivo, the MMR system plays a vital role in maintaining genome stability [47]. Some studies have shown that early diagnosis of OV is easier in patients with an MMR gene deficiency, the pathological type is usually endometrioid, and the number of tumor-infiltrating lymphocytes is higher [48]. Compared with MMR proficient patients, the PFS is longer and the prognosis is better. Our results showed that there was a positive correlation between the expression of KIF23 and MSH2 and MSH6. OV has traditionally been considered as scarcely immunogenic. However, evidence of mechanisms of immune evasion and occasional durable responses to immune checkpoints-inhibitors (ICIs) contradict this statement [49]. Clinical trials of the anti-PD1 drugs such as nivolumab and anti-CTLA drugs such as ipilimumab are ongoing, indicating the prospect of immunotherapy in OV. Wei [50] constructed an immune-related endogenous competitive regulatory network in lung adenocarcinoma and discovered LINC00337 may up-regulate the expression of PBK and KIF23 through competitive binding of has-mir-373 and has-mir-519d. The relationship between KIF23 and immune infiltration in OV is still unknown. We further explored the correlation between the expression of KIF23 and the infiltration level of different somatic copy number changes and found that of all lymphocytes, the number of CD8 + T cells showed the most significant difference in OV samples with different KIF23 copy number. OV patients with high expression of KIF23 may have a poor prognosis because of their low number of infiltrating lymphocytes. In exploring the immune mechanism of KIF23, the results showed that the expression of KIF23 was positively correlated with the expression of immune checkpoint protein CD276. Some studies have shown that CD276 upregulates the expression of BCL-2 protein by activating the PI3K/AKT signal pathway, which leads to chemotherapy resistance of OV [51]. As a potential immune checkpoint molecule, CD276 has broad potential in tumor immunotherapy [52]. It is speculated that inhibiting the expression of KIF23 may change the anti-tumor immune response and improve the efficacy of immunotherapy based on immune checkpoint therapy. FLG2 mutations were significantly enriched in patients with low KIF23 expression, and TP53 mutations were significantly enriched in both high and low expression groups of KIF23. In the KIF23 high expression group, TTN mutations were significantly richer than that in the low expression group, and the mutations of USH2A, OBSCN, and NF1, MUC17, and DST were significantly different under different expression conditions. Kunstman [53] discovered a USH2A mutation in anaplastic thyroid carcinoma by whole exon sequencing. The mutant peptides presented by the MHC II (major histocompatibility complex) in cancer are important targets for cancer immunotherapy. Some studies have shown that TNN and USH2A are the most common mutant genes of neoantigen that can predict prognosis [54]. The OBSCN mutation is closely related to breast cancer and colorectal cancer [55,56]. However, the role of these mutations in OV has rarely been discussed. Speculating on the role of the KEGG pathway of gene mutation, we found that the high expression mutant group of the KIF23 gene was significantly enriched in the dopaminergic synapse, beta-alanine metabolism, glycosaminoglycan degradation, and other pathways. Beta-alanine plays an anti-tumor role by inhibiting the migration of cervical cancer and kidney tumor cells [57]. Glycosaminoglycan is involved in multiple signal cascades required for angiogenesis, invasion, and metastasis [58]. Therefore, we suggest that KIF23 can affect the occurrence and development of OV by regulating these pathways.
Copy number variation (CNV) is closely related to the genetic and phenotypic diversity of cancer [59]. By identifying the copy number variation of the whole genome of OV, the regions experiencing frequent increase and decrease in copy number have been identified. In addition, high-level amplification of CCNE1, RB1, MYC, ERBB2, PIK3CA, EVI1, AKT2, NOTCH3, and FGFR1 genes can be used as a predictive marker of OV [60-62]. However, the copy number of KIF23 in OV has not been studied. In this study, we showed that the KIF23 gene expression increased significantly with the amplification of sample copy number. Our results demonstrated that the high expression of KIF23 in OV is partly caused by copy number amplification. GSEA gene enrichment analysis showed that highly expressed KIF23 genes were significantly enriched in the concentration of DNA replication and cell cycle genes. The expression of genes related to KIF23 expression is mainly involved in biological behaviors such as regulation of cell cycle phase transition, cell cycle checkpoint, cell cycle G2/M phase transition, G2/M transition of the mitotic cell cycle, and DNA replication, among others.
In conclusion, KIF23 is highly expressed in epithelial OV, its high expression indicates a poor prognosis, and it can be used as an independent marker to predict the prognosis of OV patients. The large bioinformatics sample analysis can bypass the error caused by the small sample size of a single study, and the immunohistochemical results are consistent with bioinformatics results. One novel finding of our study is that patients with low expression of KIF23 have a better prognosis in TP53 mutant OV. KIF23 can be used as a target of molecular therapeutics for the treatment of OV by affecting OS and PFS. Through multi-omic analysis, we also determined for the first time that the expression of KIF23 in OV is related to FLG2, TTN, and other mutations, and it is significantly enriched in tumor signal pathways such as DNA replication and the cell cycle. Our research provides direction for clinical treatment and molecular mechanism research of OV, increasing the possibility of the development of a cure. Our research in KIF23 will continue via cell function experiments.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81672590, No. 81472437), Shengjing Freedom researchers’ plan (201804). This study was approved by the Ethics Committee of China Medical University. We greatly thank the China Scholarship Council (CSC) for supporting the research and work of Mingjun Zheng (No. 201908210291).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chou JL, Su HY, Chen LY, Liao YP, Hartman-Frey C, Lai YH, Yang HW, Deatherage DE, Kuo CT, Huang YW, Yan PS, Hsiao SH, Tai CK, Lin HJ, Davuluri RV, Chao TK, Nephew KP, Huang TH, Lai HC, Chan MW. Promoter hypermethylation of FBX032, a novel TGF-p/SMAIM target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab Invest. 2010;90:414–25. doi: 10.1038/labinvest.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 4.Hirokawa N, Tanaka Y. Kinesin superfamily proteins (KIFs): various functions and their relevance for important phenomena in life and diseases. Exp Cell Res. 2015;334:16–25. doi: 10.1016/j.yexcr.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Zou JX, Duan Z, Wang J, Sokolov A, Xu J, Chen CZ, Li JJ, Chen HW. Kinesin family deregulation coordinated by bromodomain protein ANCCA and histone methyltransferase MLL for breast cancer cell growth, survival, and tamoxifen resistance. Mol Cancer Res. 2014;12:539–49. doi: 10.1158/1541-7786.MCR-13-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12:527–39. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C, Bossy-Wetzel E, Jiang W. Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem J. 2005;389:373–81. doi: 10.1042/BJ20050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Erikson RL. The nuclear localization signal of mitotic kinesin-like protein Mklp-1: effect on Mklp-1 function during cytokinesis. Biochem Biophys Res Commun. 2007;353:960–4. doi: 10.1016/j.bbrc.2006.12.142. [DOI] [PubMed] [Google Scholar]
- 9.Sablin EP. Kinesins and microtubules: their structures and motor mechanisms. Curr Opin Cell Biol. 2000;12:35–41. doi: 10.1016/s0955-0674(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Fusaki N, Ohta S, Iwahori Y, Iizuka Y, Inagawa K, Kawakami Y, Yoshida K, Toda M. Downregulation of kif23 suppresses glioma proliferation. J Neurooncol. 2012;106:519–29. doi: 10.1007/s11060-011-0706-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Wang XB, Zhang YH, Zhou YM, Yin Q, Yao WC. MicroRNA-424 inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting KIF23 and functions as a novel prognostic predictor. Eur Rev Med Pharmacol Sci. 2018;22:6369–6378. doi: 10.26355/eurrev_201810_16049. [DOI] [PubMed] [Google Scholar]
- 12.Murakami H, Ito S, Tanaka H, Kondo E, Kodera Y, Nakanishi H. Establishment of new intraperitoneal paclitaxel-resistant gastric cancer cell lines and comprehensive gene expression analysis. Anticancer Res. 2013;33:4299–307. [PubMed] [Google Scholar]
- 13.Kato T, Wada H, Patel P, Hu HP, Lee D, Ujiie H, Hirohashi K, Nakajima T, Sato M, Kaji M, Kaga K, Matsui Y, Tsao MS, Yasufuku K. Overexpression of KIF23 predicts clinical outcome in primary lung cancer patients. Lung Cancer. 2016;92:53–61. doi: 10.1016/j.lungcan.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Zheng MJ, Gou R, Zhang WC, Nie X, Wang J, Gao LL, Liu JJ, Li X, Lin B. Screening of prognostic biomarkers for endometrial carcinoma based on a ceRNA network. PeerJ. 2018;6:e6091. doi: 10.7717/peerj.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen NJ, Walker LD, Matyunina LV, Logani S, Totten KA, Benigno BB, McDonald JF. Gene expression profiling supports the hypothesis that human ovarian surface epithelia are multipotent and capable of serving as ovarian cancer initiating cells. BMC Med Genomics. 2009;2:71. doi: 10.1186/1755-8794-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, Park DC, Hao K, Yip DK, Donninger H, Ozbun L, Samimi G, Brady J, Randonovich M, Pise-Masison CA, Barrett JC, Wong WH, Welch WR, Berkowitz RS, Birrer MJ. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–32. doi: 10.1016/j.ccr.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung TL, Leung CS, Wong KK, Gutierrez-Hartmann A, Kwong J, Gershenson DM, Mok SC. ELF3 is a negative regulator of epithelial-mesenchymal transition in ovarian cancer cells. Oncotarget. 2017;8:16951–16963. doi: 10.18632/oncotarget.15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanczky A, Nagy A, Bottai G, Munkacsy G, Paladini L, Szabo A, Santarpia L, Gyorffy B. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2,178 breast cancer patients. Breast Cancer Res Treat. 2016;160:439–446. doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 21.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2001;98:1176–81. doi: 10.1073/pnas.98.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshihara K, Tajima A, Komata D, Yamamoto T, Kodama S, Fujiwara H, Suzuki M, Onishi Y, Hatae M, Sueyoshi K, Fujiwara H, Kudo Y, Inoue I, Tanaka K. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates zeb2 in tumor progression and prognosis. Cancer Sci. 2009;100:1421–8. doi: 10.1111/j.1349-7006.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TCGA (The Cancer Genome Atlas) Ovarian Serous Cystadenocarcinoma Gene Expression Data [Google Scholar]
- 24.Barton CA, Hacker NF, Clark SJ, O’Brien PM. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol Oncol. 2008;109:129–39. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Balch C, Fang F, Matei DE, Huang TH, Nephew KP. Minireview: epigenetic changes in ovarian cancer. Endocrinology. 2009;150:4003–4011. doi: 10.1210/en.2009-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem. 2010;17:2141–51. doi: 10.2174/092986710791299966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada C, Shionoya S, Fujino Y, Tokuhiro H, Akahoshi T, Uchida T, Ohtani H. Genomic instability of microsatellite repeats and its association with the evolution of chronic myelogenous leukemia. Blood. 1994;83:3449–56. [PubMed] [Google Scholar]
- 28.Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28:22–7. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang TX, Fu L. The immune Landscape of cancer. Cancer Commun (Lond) 2019;39:79. doi: 10.1186/s40880-019-0427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein LS, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol. 1999;15:141–83. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- 33.Castillo A, Morse HC, Godfrey VL, Naeem R, Justice MJ. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res. 2007;67:10138–47. doi: 10.1158/0008-5472.CAN-07-0326. [DOI] [PubMed] [Google Scholar]
- 34.Wordeman L. How kinesin motor proteins drive mitotic spindle function: lessons from molecular assays. Semin Cell Dev Biol. 2010;21:260–8. doi: 10.1016/j.semcdb.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu XR, Cai Y, Cao X, Wei RC, Li HL, Zhou XM, Zhang KJ, Wu S, Qian QJ, Cheng B, Huang K, Liu XY. A new oncolytic adenoviral vector carrying dual tumour suppressor genes shows potent anti-tumour effect. J Cell Mol Med. 2012;16:1298–309. doi: 10.1111/j.1582-4934.2011.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XL, Ji YM, Song R, Li XN, Guo LS. KIF23 promotes gastric cancer by stimulating cell proliferation. Dis Markers. 2019;2019:9751923. doi: 10.1155/2019/9751923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Zhang C, Yang Z, Wu Y, Wang H, Bao Z, Jiang T. KIF23 is an independent prognostic biomarker in glioma, transcriptionally regulated by TCF-4. Oncotarget. 2016;7:24646–55. doi: 10.18632/oncotarget.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi S, Fusaki N, Ohta S, Iwahori Y, Iizuka Y, Inagawa K, Kawakami Y, Yoshida K, Toda M. Downregulation of KIF23 suppresses glioma proliferation. J Neurooncol. 2012;106:519–29. doi: 10.1007/s11060-011-0706-2. [DOI] [PubMed] [Google Scholar]
- 39.Liljeholm M, Irvine AF, Vikberg AL, Norberg A, Month S, Sandström H, Wahlin A, Mishima M, Golovleva I. Congenital dyserythropoietic anemia type III (CDA III) is caused by a mutation in kinesin family member, KIF23. Blood. 2013;121:4791–9. doi: 10.1182/blood-2012-10-461392. [DOI] [PubMed] [Google Scholar]
- 40.Kupryjanczyk J, Thor AD, Beauchamp R, Merritt V, Edgerton SM, Bell DA, Yandell DW. p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci U S A. 1993;90:4961–5. doi: 10.1073/pnas.90.11.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen WH, Reles A, Runnebaum IB, Sullivan-Halley J, Bernstein L, Jones LA, Felix JC, Kreienberg R, el-Naggar A, Press MF. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18:29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Chan WY, Cheung KK, Schorge JO, Huang LW, Welch WR, Bell DA, Berkowitz RS, Mok SC. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156:409–417. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salani R, Kurman RJ, Giuntoli R, Gardner G, Bristow R, Wang TL, Shih IM. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2008;18:487–91. doi: 10.1111/j.1525-1438.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 44.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Zhuang G, Sun X, Shen Y, Wang W, Li Q, Di W. TP53 mutation-mediated genomic instability induces the evolution of chemoresistance and recurrence in epithelial ovarian cancer. Diagn Pathol. 2017;12:16. doi: 10.1186/s13000-017-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vikberg AL, Vooder T, Lokk K, Annilo T, Golovleva I. KIF23Mutation analysis and copy number alterations of in non-small-cell lung cancer exhibiting over-expression. Onco Targets Ther. 2017;10:4969–4979. doi: 10.2147/OTT.S138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994;266:1959–60. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- 48.Xiao X, Dong D, He W, Song L, Wang Q, Yue J, Xie L. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149:146–154. doi: 10.1016/j.ygyno.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol. 2018;151:381–389. doi: 10.1016/j.ygyno.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei B, Kong W, Mou X, Wang S. Comprehensive analysis of tumor immune infiltration associated with endogenous competitive RNA networks in lung adenocarcinoma. Pathol Res Pract. 2019;215:159–170. doi: 10.1016/j.prp.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Zhao Y. B7-H3 induces ovarian cancer drugs resistance through an PI3K/AKT/BCL-2 signaling pathway. Cancer Manag Res. 2019;11:10205–10214. doi: 10.2147/CMAR.S222224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22:3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, Rubinstein JC, Choi M, Kiss N, Nelson-Williams C, Mane S, Rimm DL, Prasad ML, Höög A, Zedenius J, Larsson C, Korah R, Lifton RP, Carling T. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24:2318–29. doi: 10.1093/hmg/ddu749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai W, Zhou D, Wu W, Tan WL, Wang J, Zhou C, Lou Y. MHC class II restricted neoantigen peptides predicted by clonal mutation analysis in lung adenocarcinoma patients: implications on prognostic immunological biomarker and vaccine design. BMC Genomics. 2018;19:582. doi: 10.1186/s12864-018-4958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Hu Z, DeLisi C. Mutated pathways as a guide to adjuvant therapy treatments for breast cancer. Mol Cancer Ther. 2016;15:184–9. doi: 10.1158/1535-7163.MCT-15-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandurangan M, Enkhtaivan G, Mistry B, Patel RV, Moon S, Kim DH. β-Alanine intercede metabolic recovery for amelioration of human cervical and renal tumors. Amino Acids. 2017;49:1373–1380. doi: 10.1007/s00726-017-2437-y. [DOI] [PubMed] [Google Scholar]
- 58.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–97. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 59.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, Aerts J, Andrews TD, Barnes C, Campbell P, Fitzgerald T, Hu M, Ihm CH, Kristiansson K, Macarthur DG, Macdonald JR, Onyiah I, Pang AW, Robson S, Stirrups K, Valsesia A, Walter K, Wei J Wellcome Trust Case Control Consortium. Tyler-Smith C, Carter NP, Lee C, Scherer SW, Hurles ME. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–12. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etemadmoghadam D, deFazio A, Beroukhim R, Mermel C, George J, Getz G, Tothill R, Okamoto A, Raeder MB, Harnett P, Lade S, Akslen LA, Tinker AV, Locandro B, Alsop K, Chiew YE, Traficante N, Fereday S, Johnson D, Fox S, Sellers W, Urashima M, Salvesen HB, Meyerson M, Bowtell D AOCS Study Group. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15:1417–27. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SW, Kim JW, Kim YT, Kim JH, Kim S, Yoon BS, Nam EJ, Kim HY. Analysis of chromosomal changes in serous ovarian carcinoma using high-resolution array comparative genomic hybridization: potential predictive markers of chemoresistant disease. Genes Chromosomes Cancer. 2007;46:1–9. doi: 10.1002/gcc.20384. [DOI] [PubMed] [Google Scholar]
- 62.Gorringe KL, Jacobs S, Thompson ER, Sridhar A, Qiu W, Choong DY, Campbell IG. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin Cancer Res. 2007;13:4731–9. doi: 10.1158/1078-0432.CCR-07-0502. [DOI] [PubMed] [Google Scholar]