Abstract
Tumor-infiltrating immune cells have been recognized to be associated with prognosis and response to immunotherapy; however, genes related to immune microenvironment of clear cell renal cell carcinoma (ccRCC) remains unclear. To better understand the effects of genes involved in immune and stromal cells on prognosis, we used Cancer Genome Atlas Kidney Renal Clear Cell Carcinoma (TCGA-KIRC), DAVID database and ESTMATE algorithm, and divided the patients into low and high groups according to immune (median: 1038.45) and stromal scores (median: 667.945), respectively. We found the immune scores were significantly correlated with clinicopathological parameters and overall survival (OS). Based on immune scores, 890 DEGs were significantly associated with OS among the 1433 up-regulated genes. Based on top 10 DEGs (IL10RA, FCER1G, SASH3, TIGIT, RHOH, IL12RB1, AIF1, LPXN, LAPTM5 and SP140), cases with number of up-regulated genes ≥ 5 were associated poor OS (P = 0.002). In addition, the mean differences of percentages of CD8 T cells (11.32%), CD4 memory resting T cells (-4.52%) and mast resting cells (-3.55%) between low and high immune scores were the most significant. Thus, combination of these genes might use to predict the efficacy of immunotherapy. Further analyses of these genes were warrant to explore their potential association with the prognosis of ccRCC.
Keywords: Clear cell renal cell carcinoma, immune microenvironment, differentially expressed genes, prognosis
Introduction
Renal cell carcinoma (RCC) is one of the most common malignancies in urological oncology, and clear cell RCC (ccRCC), the most predominant histological type, accounts for more than 80% of RCCs [1]. Generally, ccRCC has been proved to be with highly infiltrated immune cells [2]. Meanwhile, approximate 1% spontaneous regression RCC, were attributed to immune-mediated [3]. Therefore, ccRCC was one of the earliest malignancies in history to be treated with immunotherapy, and heretofore remains one of the most responsive tumors [4-6]. In recent years, the progress of molecular immunology has promoted the discovery of immune checkpoint inhibitors (ICIs), which were successfully applied into clinical practice. Although complete response dramatically occurred up to 9% in patients with immunotherapy [7], still only part of population could benefit from either immunotherapy alone or immunotherapy combined with TKIs (ORR: 25-59%) [7-12]. Commonly speaking, response of ccRCC to immunotherapy was not satisfied as we expected [13]. And none of current biomarkers could determine immune infiltration and predict immunotherapy response [14]. This has prompted researchers around the world to keep exploring new potential biomarkers which can predict efficacy of immunotherapy and help clinical decision making.
In the context of immuno-oncology, growing evidences suggested that tumor microenvironment (TME) plays an important role in tumor progression, metastasis and therapeutic resistance [20,21]. Immune and stromal cells are the two main types of non-tumor cells in TME [22]. It has been reported that regulatory T cells (Tregs), tumor associated macrophages (TAM) and CD8+ T cells have different effects on tumor cells [23-27]. Meanwhile, multiple researches have demonstrated that immune microenvironment is closely related to ccRCC [28-30]. Whereas, the mechanisms of TME highly infiltrated with immune cells, spontaneous tumor remission in the course of treatment of primary tumor and the treatment response to immunotherapy in ccRCC still remain obscure. Since the immune microenvironment is a complex space consisted of various kinds of immune cells, identification of these cells alone is insufficient to assess the complex microenvironment. Considering the limitations of technique, such as the difficulty to simultaneously assess the number of miscellaneous cell types and the high demanded amount of tissue, Yoshihara et al. designed a calculation to quantitatively predict immune and stromal scores based on ESTIMATE algorithm (Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data), which utilizes specific genes in immune and stromal cells to predict scores of tumor purity and infiltration extent [31,32].
The cancer genome atlas (TCGA) is an open database aims to apply high-throughput genomic sequencing techniques to assist better understanding of cancer and thus improve the ability to prevent, diagnose and treatment of cancer [33,34]. In order to analyze the influences of immune and stromal related genes on the prognosis of ccRCC, we aimed to classify ccRCC cases of TCGA into low and high score groups based on immune and stromal scores. Subsequently, we planned to identify differentially expressed genes between low and high groups and to screen the most significant genes to predict outcomes of ccRCC.
Materials and methods
Acquisition of gene expression profile data
We obtained level 3 gene expression profile of ccRCC patients (KIRC) from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). In addition, clinical and pathological data were also downloaded. The presence of infiltrating stromal/immune cells in tumor tissues of ccRCC was predicted by a tool of ESTIMATE algorithm created by Yoshihara et al.
Relationship between immune/stromal score and clinicopathological parameters and overall survival (OS)
Based on the immune/stromal scores mentioned before, we classified cases of ccRCC into low and high groups by medians according to immune and stromal scores, respectively. Subsequently, we analyzed correlations between immune or stromal scores and T stage, histologic type, tumor stage and top 4 mutated genes in ccRCC (VHL, PBRM1, SETD2 and BAP1). We used non-parametric test to analyze differences between low and high group. Additionally, survival analysis using log rank test was conducted to assess difference between low and high groups of immune/stromal scores, and Kaplan-Meier curves were plotted by R software.
Identification of differentially expressed genes (DEGs) between low and high groups stratified by immune or stromal scores
DEGs between low and high groups were identified by performing data analysis according to limma package from R software [35]. The following cut-off points were adopted to screen DEGs: > 1.5 of fold change with adjusted P value < 0.05. Heatmaps, volcano plot and venn diagram were plotted by R software using pheatmap package, ggplot2 and gplots.
Functional enrichment analysis of DEGs
DAVID (The Database for Annotation, Visualization and Integrated Discovery) is a classic database (https://david.ncifcrf.gov/) mainly used for gene functional enrichment analysis [36]. DAVID provides a comprehensive annotation of biological function, and we used the database to conduct gene ontology (GO) functional annotation and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis. GO annotation included three parts: biological process (BP), molecular function (MF) and cell composition (CC). Visual results of the above analysis were presented by bubble plot conducted by using R software with ggplot2 package. P value < 0.05 was considered as cut-off point of statistically significant.
Construction of protein-protein interaction (PPI) network and module analysis
We used a biological database named STRING (Search Tool for the Retrieval of Interacting Genes) for PPI analysis. The role of the database (http://string.embl.de/) was to conduct a network of PPI for identified DEGs on the basis of the proven and predicted PPIs, and then analyze the potential interactions among proteins [37]. After online analysis, TSV format files were downloaded for further analysis in Cytoscape software (version 3.5.1) [38]. We used Cytoscape software to visualize PPI network and explore top 3 significant modules of network with 10 or more nodes.
Survival analysis
Analysis of overall outcome was performed by R software with survival package. Additionally, Kaplan-Meier plots were conducted by R software with survminer package. We calculated hazard ratio (HR) and log-rank P value for presentation. P value < 0.05 was considered as cut-off point of statistically significant.
The composition differences of 22 immune cell subtypes between low and high immune scores
In order to quantify the abundance of 22 immune subtypes in ccRCC specimens, the CIBERSORT deconvolution algorithm was applied [39]. Specifically, gene expression data was uploaded to the CIBERSORT web portal (http://cibersort.stanford.edu/) to calculate proportions of immune cells using the LM22 signature at 1,000 permutations. After acquisition of the infiltration data of each case, we planned to find the difference of abundance of immune infiltration cells between low and high groups of immune scores.
Oncomine and clinical proteomic tumor analysis consortium (CPTAC) database analysis
In this study, we tried to validate the mRNA and protein expression of top 10 DEGs based on two existing cancer database: Oncomine (https://www.oncomine.org/) and CPTAC (http://ualcan.path.uab.edu/analysis-prot.html). Filters of Oncomine database were set: primary filters were set as differential analysis (cancer versus normal tissue) and cancer type (clear cell renal cell carcinoma). Data type was set as mRNA, then differential expression of mRNA between ccRCC and normal tissue was displayed. For CPTAC, Z-values represent standard deviations from the median across samples for the given cancer type. Log2 Spectral count ratio values from CPTAC were first normalized within each sample profile, then normalized across samples.
Results
Immune scores were significantly correlated with clinicopathological parameters and OS
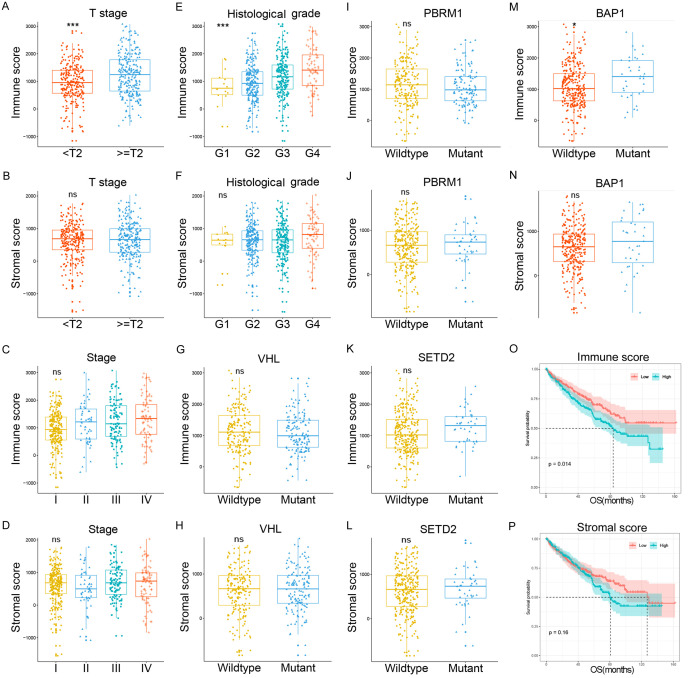
Among all 537 cases downloaded from TCGA portal, 191 (35.6%) cases were female, 346 (64.4%) cases were male. The median age was 61 y (ranged from 26 to 90 y). According to ESTIMATE algorithm mentioned before, the distributions of immune and stromal scores were ranged from -11.57.91 to 2030.4 and from 1158.94 to 3076.4, respectively. As presented in Figure 1, cases with T stage ≥ 2 were associated with higher immune scores rather than stromal scores (Figure 1A, 1B). Tumor stage was not statistically different in both immune and stromal scores (Figure 1C, 1D). For histological grade, immune scores increased with escalation of nuclear grade (Figure 1E), whereas no such trend was observed in stromal scores (Figure 1F).
Figure 1.
Immune scores were significantly correlated with clinicopathological parameters and OS. (A and B) Represented the associations of T stage with immune and stromal scores, cases with T stage ≥ 2 were associated with higher immune score rather than stromal score. (C and D) Showed there were no relationship between tumor stage and immune or stromal scores. (E and F) Indicated immune scores were increased with escalation of nuclear grade, but no such trend was observed in stromal scores. From (G-N), correlations of top 4 mutated genes of TCGA with immune and stromal scores were presented. Except for BAP1, immune scores were not correlated with mutations of VHL, PBRM1 and SETD2, while stromal scores were not statistically associated with all four genes. We further analyzed the correlations of immune and stromal scores with OS. (O and P) Showed Kaplan-Meier survival curves of immune and stromal scores for OS.
Four top mutated genes, including VHL, PBRM1, SETD2 and BAP1, were observe from the TCGA.I Immune scores were correlated with mutations of BAP1 while stromal scores were not statistically associated with all four genes (Figure 1G-N). We further analyzed the correlations of immune and stromal scores with OS, and Kaplan-Meier survival curves showed that cases with high immune scores were associated poorer OS (Figure 1O), whereas stromal scores were not correlated with OS (Figure 1P).
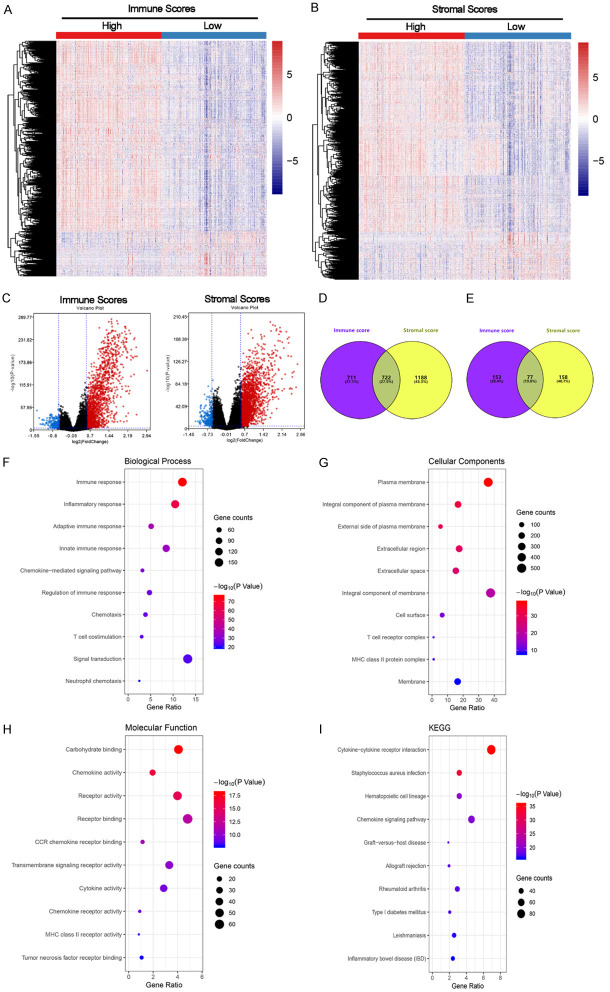
Gene expression profiles were distinct between the low and high groups stratified by immune and stromal scores. As shown in Figure 2A-C, the gene expression profiles presented by RNAseq data were significantly different between low and high groups in both immune and stromal scores. Based on immune scores, a total of 1433 genes were up-regulated and 1910 genes were down-regulated. For stromal scores, the numbers of up-regulated and down-regulated genes were 230 and 235, respectively. The numbers of up-regulated and down-regulated genes in both immune and stromal scores were 722 and 77, respectively (Figure 2D, 2E).
Figure 2.
Gene expression profiles were distinct between low and high groups stratified by immune and stromal scores. A-C. Showed that the gene expression profiles were significantly different between low and high groups in both immune and stromal scores. Based on immune scores, a total of 1433 genes were up-regulated and 1910 genes were down-regulated. For stromal scores, the numbers of up-regulated and down-regulated genes were 230 and 235, respectively. D and E. Represented numbers of genes up-regulated or down-regulated genes in both immune and stromal scores. F-I. Indicated functional enrichment analysis of the 1433 up-regulated genes. These genes were mainly involved in the biological process of immune and inflammatory response, the cellular components of plasma membrane, the molecular function of carbohydrate binding and the KEEG pathway of cytokine-cytokine receptor interaction.
It is worth noting that DEGs identified from low and high groups are relatively independent between immune scores and stromal scores. And based on the prognosis results of immune and stromal scores (Figure 1O, 1P), stromal scores had no value in predicting prognosis. Therefore, we conducted all subsequent analysis based on DEGs identified from immune scores in the present study. Functional enrichment analysis of the 1433 up-regulated genes revealed that these genes were mainly involved in the biological process of immune and inflammatory response (Figure 2F), the cellular components of plasma membrane (Figure 2G), the molecular function of carbohydrate binding (Figure 2H) and the KEEG pathway of cytokine-cytokine receptor interaction (Figure 2I).
Individual DEGs and OS
We explored the relationships of the 1433 up-regulated genes to OS and a total of 890 DEGs were statistically significant (Table S1). Among them, 855 genes were shown to predict poor OS and only 35 genes were shown to predict favorable OS. Kaplan-Meir survival curves of selected genes (Top 10 DEGs) were shown in Figure 3 and high expression of each gene was associated with poor OS.
Figure 3.
Associations of top 10 individual DEGs with OS. (A-J) Showed the relationship of top 10 DEGs and OS. Patients were divided into two groups (low and high) according to median mRNA expression of each gene. As shown in the figure, all genes were statistically associated with OS, and all genes were associated with unfavorable survival. All patients with high expression stratified by top 10 DEGs reached median OS, and only patients with high expression of AIF1, IL12RB1 and IL10RA reached median OS (F, H and I).
PPI network among prognostic DEGs
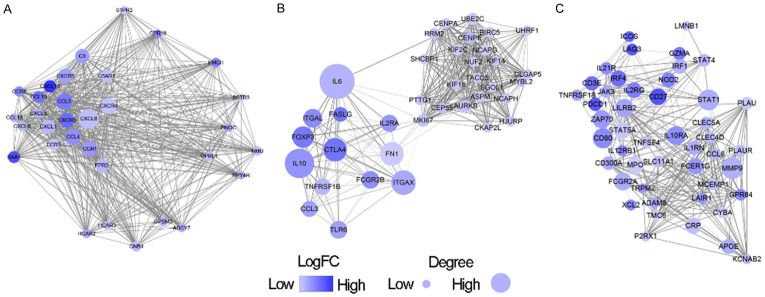
We obtained the protein-protein interaction among DEGs by using the STRING tool. After downloading the data, we plotted PPI network through Cytoscape software for subsequent analysis. The whole network was composed of 733 nodes and 7001 edges, and a total of 7 modules were screened through MCODE. For further analysis, we selected the three most important modules (Figure 4). In the module 1, a total of 32 nodes and 495 edges form the network, and CXCL8, CXCR3/4/5 and CCL4/5 were the most important nodes (Figure 4A). In the module 2 (Figure 4B), 55 nodes involving 639 edges were formed in the network. The main nodes included IL6, IL10, CTLA4, FOXP3, and ITGAX. The module 3 was consisted of 47 nodes and 297 edges, including MMP9, STAT1, IL10RA and CD80 as the most important nodes (Figure 4C). To describe the types of proteins in the PPI network in terms of immune microenvironment, we additionally presented each protein’s extracellular/subcellular locations (secreted, extracellular and cell surface proteins), and its function (Table S2). Specifically, module 1 and module 3 were characterized as secreted or membrane proteins, as these proteins accounted for 93.8% (30/32) and 70.2% (33/47) of the whole network, respectively. For module 2, most proteins were classified into intracellular (58.2%, 32/55).
Figure 4.
PPI network among prognostic DEGs. A. Represented module 1, consisted of 32 nodes and 495 edges. B. Represented module 2, formed by 55 nodes and 639 edges. C. Represented module 3, involving 47 nodes and 297 edges.
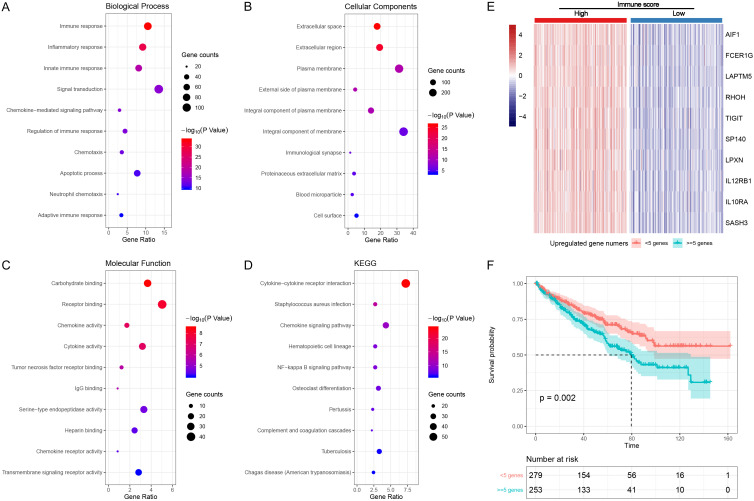
Functional enrichment analysis of DEGs with prognostic value and the role of top 10 hub genes in predicting OS. Functional enrichment analysis of the 890 DEGs revealed that these genes were mainly involved in immune and inflammatory response (Figure 5A), plasma membrane (Figure 5B), carbohydrate binding (Figure 5C) and cytokine-cytokine receptor interaction (Figure 5D), which were identical to the results of the 1433 up-regulated genes and PPI network analysis.
Figure 5.
Functional enrichment analysis of DEGs with prognostic value and the role of top 10 hub genes in predicting OS. (A-D) Represented functional enrichment analysis, these genes were mainly involved in immune and inflammatory response, plasma membrane, carbohydrate binding and cytokine-cytokine receptor interaction. (E) Represented the heatmap of these genes between low and high immune score groups and (F) represented Kaplan-Meir survival curve of OS in cases classified by number of up-regulated genes ≥ 5 or not.
We further classified cases into two groups based on number of up-regulated genes in Top 10 DEGs (IL10RA, FCER1G, SASH3, TIGIT, RHOH, IL12RB1, AIF1, LPXN, LAPTM5 and SP140). Figure 5E showed the heatmap of these genes between low and high immune score groups. Kaplan-Meir survival curve was plotted to verify the prognostic value of the classification. Cases with number of up-regulated genes ≥ 5 were associated poor OS (P = 0.002, Figure 5F).
The compositions of CD8 T cells, CD4 memory resting T cells and mast resting cells were significant different between low and high immune scores. Among 22 subtypes of immune infiltration cells, up to 72.7% (16/22) of cells were statistically different between low and high immune scores (Figure 6). The mean differences of percentages of CD8 T cells (11.32%), CD4 memory resting T cells (-4.52%) and mast resting cells (-3.55%) between low and high immune scores were the most significant, with CD8 T cells the greatest (increased from 7.64% to 18.96%, P < 0.001). It is noteworthy that the trend of change in cell composition of CD8 T cells were opposite to CD4 memory resting T cells and mast resting cells (Figure 6).
Figure 6.
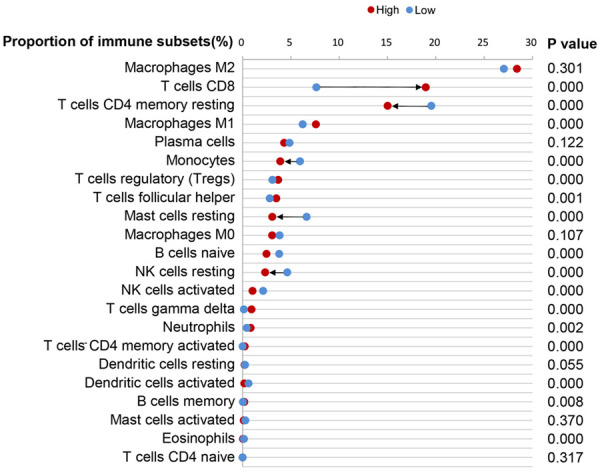
The compositions of cells in the immune microenvironment between low and high immune scores. The compositions of CD8 T cells, CD4 memory resting T cells and mast resting cells were significant different between low and high immune scores (arrow).
Validation of DEGs
We further validated top 10 DEGs in patients from Oncomine and CPTAC database. As shown in Table S3, only LPXN was not differentially expressed between clear cell renal cell carcinoma and normal tissue, with other 9 genes observed with higher mRNA expression in carcinoma than normal tissue. For protein expression, data of 6 DEGs were found in the CPTAC database, and all were over-expressed in the carcinoma compared to normal tissue, except for RHOH (Figure S1).
Discussion
As the in-depth research on molecular mechanism of incidence and evolution of solid tumors including renal cell carcinoma, ICIs were used to block co-inhibitory signals, prevent inactivation of T cells and then facilitate T cells to kill tumor cells [40,41]. The unsatisfied treatment response keeps impelling researchers concentrate on developing and optimizing biomarkers. Current biomarkers focusing on immune status and prognostic models were not sufficient in screening out appropriate patients to receive ICIs treatment. Thus, it is important to focus on the comprehensive immune status in TME. Based on this, we aimed to predict the prognosis of ccRCC by calculating immune and stromal scores in the aspect of comprehensive level, and explore potential immune related genes to predict prognosis of ccRCC.
In the present study, we found that immune score was a poor prognostic factor of OS. Since stromal score was not associated with prognosis, all subsequent analysis was based on immune score. After comparing gene expression profiles of cases with low and high scores, a total of 1433 genes related to immune response were identified. Functional enrichment analysis showed that most of these genes were involved in tumor microenvironment. Further survival analysis revealed that 890 genes were found to be associated with prognosis, and PPI network analysis also found that these genes were associated with immune or inflammatory responses, such as IL6, IL10, CTLA4, FOXP3, and ITGAX in module 1. We further presented proteins location for these differentially expressed genes were analyzed by using immune microenvironment score. The results showed that module 1 and model 3 were characterized by secreted and membrane proteins, and module 2 was mainly consisted by intracellular proteins. This might provide potential hints for future research on mechanism of immune microenvironment and provide insights into improvement of the prediction and efficacy of immunotherapy in oncology. Eventually, we identified top 10 prognosis-related DEGs to be integrated as one comprehensive parameter to predict prognosis of ccRCC, which were IL10RA, FCER1G, SASH3, TIGIT, RHOH, IL12RB1, AIF1, LPXN, LAPTM5 and SP140. Kaplan-Meier survival analysis suggested that patients with number of up-regulated genes ≥ 5 have shorter OS compared to patients with number of up-regulated genes < 5.
The top 10 prognosis-related DEGs were found to be mainly correlated with immune and inflammatory process. IL10RA (Interleukin 10 Receptor Subunit Alpha) was mainly expressed on most hematopoietic cells and functioned as anti-inflammatory factor [42,43]. FCER1G (Fc Fragment Of IgE Receptor Ig) was associated with allergic reactions and played a role in tumor development and squamous carcinogenesis [44,45]. In addition, FCER1G was associated with progression and prognosis of ccRCC [46]. Mutations or abnormal expressions of SASH3 (SAM And SH3 Domain Containing 3), RHOH (Ras Homolog Family Member H), LPXN (leupaxin) and LAPTM5 (lysosomal protein transmembrane 5) have been demonstrated to be associated with the development and progression multiple malignancies [47-56]. TIGIT (T cell immunoglobulin and ITIM domain), IL12RB1 (Interleukin 12 Receptor Subunit Beta 1), AIF1 (allograft inflammatory factor 1) and SP140 (SP140 nuclear body protein) mainly involved in the process of immune and inflammation. As a novel immune checkpoint receptor similar to PD-1, TIGIT is upregulated on CD8+ TILs and Tregs in multiple tumors, exhibiting therapeutic benefits in animal models [57,58]. IL12RB1 promotes delayed type hypersensitivity and autoimmunity [59]. AIF1 is mainly associated with allograft rejection, autoimmune diseases and vasculopathy, etc. [60]. SP140 was demonstrated to be associated with multiple sclerosis [61].
We also compared subtypes of immune cells between low and high immune scores to find potential factors attributed to the situation of high immune score. It seemed that increased compositions of CD8 T cells and decreased proportions of CD4 memory resting T cells and mast resting cells were the most important in contributing to higher immune score. Therefore, the higher immune score, the worse prognosis may be mainly related to CD8 T cells, which is consistent with previous research evidence, suggesting that the reactions of immune cells were more pronounced as the progression of tumor grade/biological malignancy, possibly due to the increased antigenicity of tumor cells [62,63].
Immunohistochemistry (IHC), flow cytometry (FCM) and next-gene sequencing (NGS) were the three major tools to assess immune status. Previous reports about immune status and prognosis were mainly based on IHC and FCM, which focused on single or partial subtypes of immune cells [64-66]. It has been found that immune scores (scored by densities of CD3+ and CD8+ by IHC) can accurately estimate the risk of recurrence in colon cancer, and these results support the inclusion of immune scores as a new component of the TNM immune cancer classification [67]. In terms of ccRCC, Kawashima et al. classified patients’ immune status into 3 groups using 5 markers (PD-1, TIM-3, ICOS, CD45RA, and CD25). The classification of the above 3 groups was significantly associated with tumor grade [68]. Theoretically, the prediction of immunotherapy efficacy based on molecular markers of tumor and/or immune cells alone is not comprehensive and the clinical effect is mostly not satisfactory. ICIs are different from antiangiogenic drugs in that they act directly on tumor microenvironment rather than tumor cells. Therefore, a growing number of researchers are focusing on the predictive value of comprehensive evaluation. In the field of ccRCC, efforts have been made in characterizing immune status in a more comprehensive way. Xu et al. has developed novel signatures of immune infiltration based on calculating immune and stromal scores [69]. In the present study, we identified top 10 immune microenvironment-related genes and these genes were integrated into one parameter to represent immune status. Survival analysis demonstrated that this integrated parameter can distinguish patients with good or poor prognosis. However, further researches are warrant to explore the potential of predictive value of these genes in the immunotherapy of ccRCC.
Acknowledgements
This work was supported by Natural Science Foundation of China (NSFC 81974398, 81902577 and 81672547) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No.0040205301E21).
Disclosure of conflict of interest
None.
Supporting Information
Abbreviations
- ccRCC
clear cell renal cell carcinoma
- DEGs
differentially expressed genes
- ICIs
immune checkpoint inhibitors
- TME
tumor microenvironment
- Tregs
regulatory T cells
- TAM
tumor associated macrophages
- TCGA
the cancer genome atlas
- IHC
Immunohistochemistry
- FCM
flow cytometry
- NGS
next-gene sequencing
- GO
gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- BP
biological process
- MF
molecular function
- CC
cell composition
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 3.Janiszewska AD, Poletajew S, Wasiutynski A. Spontaneous regression of renal cell carcinoma. Contemp Oncol (Pozn) 2013;17:123–127. doi: 10.5114/wo.2013.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10:6342s–6346s. doi: 10.1158/1078-0432.CCR-040029. [DOI] [PubMed] [Google Scholar]
- 5.Inman BA, Harrison MR, George DJ. Novel immunotherapeutic strategies in development for renal cell carcinoma. Eur Urol. 2013;63:881–889. doi: 10.1016/j.eururo.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 11.David FM, Lee JL, Szczylik C, Donskov F, Malik J, Alekseev BY, James MG, Larkin , Matveev VB, Gafanov RA, Tomczak P, Scott ST, Poul FG, Pawel JW, Shin SJ, Pouliot F, Gordoa TA, Li W, Rodolfo FP, Schloss C, Michael BA, Deaconess BI. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort A of KEYNOTE-427. J. Clin. Oncol. 2018;36 abstr 4500. [Google Scholar]
- 12.Robert JM, Powles T, Michael BA, Escudier B, David FM, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, Robert EH, Ravaud A, Boris YA, Michael DS, Uemura M, Donaldson F, Li S, Mahrukh AH, Schiff C, Brian IR. IMmotion151: arandomized phase III study of atezolizumab plus Bevacizumab vs Sunitinib in untreated metastatic renal cell carcinoma (mRCC) J. Clin. Oncol. 2018;36 abstr 578. [Google Scholar]
- 13.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M, Bronte V, Scarpa A, Bria E, Tortora G. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa AF, Jegede O, Haas NB, Flaherty KT, Pins MR, Messing EM, Manola J, Wood CG, Kane CJ, Jewett MAS, Dutcher JP, DiPaola RS, Carducci MA, Uzzo RG. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J. Clin. Oncol. 2019;37:2062–2071. doi: 10.1200/JCO.19.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, Chambers T, Salgado R, Savas P, Loi S, Birkbak NJ, Sansregret L, Gore M, Larkin J, Quezada SA, Swanton C. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 18.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, Sznol M, Hainsworth J, Rathmell WK, Stadler WM, Hutson T, Gore ME, Ravaud A, Bracarda S, Suarez C, Danielli R, Gruenwald V, Choueiri TK, Nickles D, Jhunjhunwala S, Piault-Louis E, Thobhani A, Qiu J, Chen DS, Hegde PS, Schiff C, Fine GD, Powles T. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 21.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 27.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, Kluger H, Stadler WM, Perez-Gracia JL, McNeel DG, Curti B, Harrison MR, Plimack ER, Appleman L, Fong L, Albiges L, Cohen L, Young TC, Chasalow SD, Ross-Macdonald P, Srivastava S, Jure-Kunkel M, Kurland JF, Simon JS, Sznol M. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:5461–5471. doi: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senbabaoglu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A, Weinhold N, Lee W, Manley BJ, Khalil DN, Kaffenberger SD, Chen Y, Danilova L, Voss MH, Coleman JA, Russo P, Reuter VE, Chan TA, Cheng EH, Scheinberg DA, Li MO, Choueiri TK, Hsieh JJ, Sander C, Hakimi AA. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17:231. doi: 10.1186/s13059-016-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YP, Zhang Y, Lv JW, Li YQ, Wang YQ, He QM, Yang XJ, Sun Y, Mao YP, Yun JP, Liu N, Ma J. Genomic analysis of tumor microenvironment immune types across 14 solid cancer types: immunotherapeutic implications. Theranostics. 2017;7:3585–3594. doi: 10.7150/thno.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA, Beroukhim R, Pellman D, Levine DA, Lander ES, Meyerson M, Getz G. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Trevino V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song E, Song W, Ren M, Xing L, Ni W, Li Y, Gong M, Zhao M, Ma X, Zhang X, An R. Identification of potential crucial genes associated with carcinogenesis of clear cell renal cell carcinoma. J Cell Biochem. 2018;119:5163–5174. doi: 10.1002/jcb.26543. [DOI] [PubMed] [Google Scholar]
- 34.Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019;16:539–552. doi: 10.1038/s41585-019-0211-5. [DOI] [PubMed] [Google Scholar]
- 35.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 37.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman AM, Liu CL, Green MR. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 41.Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol. 2017;28:1484–1494. doi: 10.1093/annonc/mdx151. [DOI] [PubMed] [Google Scholar]
- 42.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, Snapper SB. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 45.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Yuan L, Wang Y, Wang G, Zhu Y, Cao R, Qian G, Xie C, Liu X, Xiao Y, Wang X. Co-expression network analysis identified FCER1G in association with progression and prognosis in human clear cell renal cell carcinoma. Int J Biol Sci. 2017;13:1361–1372. doi: 10.7150/ijbs.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Peng M, Xue W, Fan Z, Wang T, Lian J, Zhai Y, Lian W, Qin D, Zhao J. Integrated analysis of dysregulated long non-coding RNAs/microRNAs/mRNAs in metastasis of lung adenocarcinoma. J Transl Med. 2018;16:372. doi: 10.1186/s12967-018-1732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schieffer KM, Choi CS, Emrich S, Harris L, Deiling S, Karamchandani DM, Salzberg A, Kawasawa YI, Yochum GS, Koltun WA. RNA-seq implicates deregulation of the immune system in the pathogenesis of diverticulitis. Am J Physiol Gastrointest Liver Physiol. 2017;313:G277–G284. doi: 10.1152/ajpgi.00136.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanati N, Iancu OD, Wu G, Jacobs JE, McWeeney SK. Network-based predictors of progression in head and neck squamous cell carcinoma. Front Genet. 2018;9:183. doi: 10.3389/fgene.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdem-Eraslan L, Heijsman D, de Wit M, Kremer A, Sacchetti A, van der Spek PJ, Sillevis Smitt PA, French PJ. Tumor-specific mutations in low-frequency genes affect their functional properties. J Neurooncol. 2015;122:461–470. doi: 10.1007/s11060-015-1741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troeger A, Williams DA. Hematopoietic-specific Rho GTPases Rac2 and RhoH and human blood disorders. Exp Cell Res. 2013;319:2375–2383. doi: 10.1016/j.yexcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voena C, Chiarle R. RHO family GTPases in the biology of lymphoma. Cells. 2019;8:646. doi: 10.3390/cells8070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woldu SL, Hutchinson RC, Krabbe LM, Sanli O, Margulis V. The Rho GTPase signalling pathway in urothelial carcinoma. Nat Rev Urol. 2018;15:83–91. doi: 10.1038/nrurol.2017.184. [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Wang G, Luo Y, Wang Y, Xie C, Jiang W, Xiao Y, Qian G, Wang X. Downregulation of LAPTM5 suppresses cell proliferation and viability inducing cell cycle arrest at G0/G1 phase of bladder cancer cells. Int J Oncol. 2017;50:263–271. doi: 10.3892/ijo.2016.3788. [DOI] [PubMed] [Google Scholar]
- 55.Dierks S, von Hardenberg S, Schmidt T, Bremmer F, Burfeind P, Kaulfuss S. Leupaxin stimulates adhesion and migration of prostate cancer cells through modulation of the phosphorylation status of the actin-binding protein caldesmon. Oncotarget. 2015;6:13591–13606. doi: 10.18632/oncotarget.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou T, Zhou L, Wang L, Kazobinka G, Chen Y, Zhang X, Chen Z. Leupaxin promotes bladder cancer proliferation, metastasis, and angiogenesis through the PI3K/AKT pathway. Cell Physiol Biochem. 2018;47:2250–2260. doi: 10.1159/000491536. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Zhang H, Chen L, Feng Z, Gao L, Li Q. TIGIT expression is upregulated in T cells and causes T cell dysfunction independent of PD-1 and Tim-3 in adult B lineage acute lymphoblastic leukemia. Cell Immunol. 2019;344:103958. doi: 10.1016/j.cellimm.2019.103958. [DOI] [PubMed] [Google Scholar]
- 58.Liu XG, Hou M, Liu Y. TIGIT, a novel therapeutic target for tumor immunotherapy. Immunol Invest. 2017;46:172–182. doi: 10.1080/08820139.2016.1237524. [DOI] [PubMed] [Google Scholar]
- 59.Robinson RT. IL12Rbeta1: the cytokine receptor that we used to know. Cytokine. 2015;71:348–359. doi: 10.1016/j.cyto.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao YY, Yan DJ, Chen ZW. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell Immunol. 2013;284:75–83. doi: 10.1016/j.cellimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Karaky M, Fedetz M, Potenciano V, Andres-Leon E, Codina AE, Barrionuevo C, Alcina A, Matesanz F. SP140 regulates the expression of immune-related genes associated with multiple sclerosis and other autoimmune diseases by NF-kappaB inhibition. Hum Mol Genet. 2018;27:4012–4023. doi: 10.1093/hmg/ddy284. [DOI] [PubMed] [Google Scholar]
- 62.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 63.Mella M, Kauppila JH, Karihtala P, Lehenkari P, Jukkola-Vuorinen A, Soini Y, Auvinen P, Vaarala MH, Ronkainen H, Kauppila S, Haapasaari KM, Vuopala KS, Selander KS. Tumor infiltrating CD8(+) T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology. 2015;4:e1002726. doi: 10.1080/2162402X.2014.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–1196. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, van den Broek M, Beisel C, Stadler MB, Gedye C, Reis B, Pe’er D, Bodenmiller B. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169:736–749. e718. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Borger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grutzmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Leonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Musina AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 68.Kawakami F, Sircar K, Rodriguez-Canales J, Fellman BM, Urbauer DL, Tamboli P, Tannir NM, Jonasch E, Wistuba II, Wood CG, Karam JA. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer. 2017;123:4823–4831. doi: 10.1002/cncr.30937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu WH, Xu Y, Wang J, Wan FN, Wang HK, Cao DL, Shi GH, Qu YY, Zhang HL, Ye DW. Prognostic value and immune infiltration of novel signatures in clear cell renal cell carcinoma microenvironment. Aging (Albany NY) 2019;11:6999–7020. doi: 10.18632/aging.102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.