Abstract
Spinal cord injury (SCI) can lead to different levels of paraplegia. Studies have shown that exercise exerts wide protective effects against various diseases, and microRNAs (miRNAs) are involved in its beneficial effects. However, the specific role of miRNAs in the protective effects of exercise against SCI remains unclear. Here, we showed that exercise exerted protective effects against SCI as evidenced by increased locomotor activity and spinal cord cell survival in rats with SCI. Exercise upregulated circulating miR-21, detected by miRNA microarray, in rats with SCI. Treating SCI rats with agomiR-21 upregulated circulating miR-21 and exerted protective effects against SCI. Additionally, downregulating miR-21 using antagomir-21 abolished the protective effects of exercise on SCI. Programmed cell death protein 4 (PDCD4) was found to be the target of miR-21. These results suggested that exercise protects against SCI, at least partly, through miR-21-mediated suppression of PDCD4.
Keywords: Exercise, miR-21, PDCD4, spinal cord injury
Introduction
Spinal cord injury (SCI) is a traumatic event to the spinal cord that could result in permanent damage to sensory and motor function. More than 25000 patients worldwide suffer from SCI yearly [1]. SCI can be caused by tumors, traffic accidents, trauma, and other sporting injuries [2]. Secondary acute spinal cord injury includes free radical production, inflammation, and apoptosis, which is of great importance. However, human and animal subjects can spontaneously recover from these injuries through adaptive changes. These changes manifest in neuronal properties [3,4], cortical maps [5,6], and rostral and/or caudal collateral sprouting in the lesion zone [7,8]. This recovery can happen without therapy such as pharmacological agents or surgery. As a non-invasive therapeutic intervention, exercise (Ex) can effectively protect spinal cord tissue by alleviating hindlimb muscle mass [9], maintaining stable lumbar motoneurons patterns [10] and increasing neurotrophic factor expression levels in injury sites [11]. However, the specific molecular and cellular mechanism of Ex against SCI remains unknown.
Complex pathophysiologic changes occur in the spinal cord, following SCI, including diverse molecular dysfunction and signaling pathways. MiRNAs are a cluster of endogenous noncoding small RNAs that down-regulate gene expression by binding to the 3’-UTR of target mRNAs at a post-transcriptional level, leading to translational inhibition or degradation. About 20-30% of genes, which are translated to proteins, are modulated by miRNAs [12-14]. miRNAs are of great importance in cellular processes [15-18]. SCI can induce widespread molecular and signal alterations, including specific protein and miRNA expression [19,20]. Microarrays were used to identify the regulation of 60 miRNAs in the SCI group, in comparison to the sham one [21]. Cluster analysis suggested lots of miRNAs relating to pathology post-SCI such as apoptosis, oxidation, and inflammation. Several previous researches suggest that miRNAs-21 is over-expressed after SCI [22,23] and may contribute to recovery and regeneration. For example, Chen’s group [24] found increasing PDCD4 expression levels and apoptotic cells by inhibiting miR-21 in glioblastoma cells. Lei’s group showed that many miRNA expressions changed over time, coupled with up-modulated miR-21, after brain damage [25]. SCI can also result in neuromuscular damage, however, some studies have indicated that daily exercise of the affected hind limbs could lead to a great decrease in the formation of dendritic branches [26].
Here, we tried to find evidence on changes in miR expression after Ex, using miRNA arrays and observed miR-21 upregulation. After miR-21 overexpression by agomir-21, spinal cord tissue protection after SCI increased with less apoptotic cells. miR-21 knockdown by antagomir-21 also confirmed that Ex can alleviate functional deficit. To further explore the molecular mechanism, we observed that PDCD4 was closely associated with miR-21 expression levels. Therefore, this paper showed that exercise can protect against spinal cord injury through miR-21-mediated PDCD4 suppression, which could provide a mechanism for exercise treatment following SCI.
Materials and methods
Spinal cord injury
All surgical interventions and postoperative care were conducted under the Guidelines of the Institutional Animal Care and Use Committee and approved by the Ethics Committee. Adult male Sprague-Dawley rats (200-230 g), after being injected with 10% chloral hydrate (3 mg/kg), underwent the left lateral hemisection model of SCI as described previously [27]. Briefly, a longitudinal incision was made in the midline to perform laminectomy in the thoracic region T8/T10. After the spinal cord was exposed, a lateral hemisection was made with a surgical blade in the left of the spinal cord. The injury led to the creation of a gap at the length of 2-4 mm alongside the rostralcaudal axis that was extended to the midline. Post-injury, we put rats in recovery cages with ampicillin (100 mg/kg) lasting 7 days to prevent postoperative infection, including bladder voiding, and for daily postoperative care [28].
Exercise
Swim training was used as the exercise model in this study based on Magnuson’s research, which demonstrated the significant effect of swimming on severe thoracic spinal cord contusion injury in rats [29]. The water temperature was maintained at 25-28°C [30]. Rats received swim training 7 days post-injury, twice daily 5 days per week lasting for 4 weeks. The first training day involved two 10 min sessions with a minimum of 4 hours off, then gradually increased to 90 min/day over one week.
Animal groups
Rats were randomly divided into four groups in the first set of experiments, including Sed+sham, Exe+sham, Sed+SCI, Exe+SCI (n=6 rats for each group). Rats were exercised at 7 days after surgery. Rats were randomly divided into the other four groups in the second set of experiments, including Sham+agomiR-NC, Sham+agomiR-21, SCI+agomiR-NC, SCI+agomiR-21 (n=6 rats for each group). AgomiR-NC or agomiR-21 (10 nmol) was intramyocardially injected into rats after the surgery at an interval of 7 days. Rats were randomly divided into another four groups in the third set of experiments, including Sed+antigomiR-NC, Sed+antigomiR-21, Exe+antigomiR-NC, Exe+antigomiR-21 (n=6 rats for each group). SCI was induced in all rats. AntigomiR-NC or antigomiR-21 (10 nmol) was intramyocardially injected into rats after the surgery at an interval of 7 days. The sequences of agomiR, antigomiR and NC were shown in Table S1.
miRNA library construction and sequencing
miRNA library preparation and sequencing were conducted by a commercial service (Ribobio, Guangzhou, China). Briefly, total RNA was extracted from plasma. Both 3’ and 5’ adaptors were ligated, followed by reverse transcription and polymerase chain reaction amplification. The polymerase chain reaction products derived from the 18 to 30 nucleotide long RNA molecules were purified by electrophoresis and sequenced using the Illumina HiSeq 2500 platform (San Diego, CA, USA).
Behavioral assessment
We checked locomotor activity for 60 days after the injury using a Basso, Beattie, and Bresnahan (BBB) score as described previously [27]. The rating scales of BBB range from 0, meaning no functional improvement in hindlimb junctions, to 21, denoting normal functional behavior similar to a healthy rat. Two blinded investigators independently observed the movements of animals for 5 min in an open field, with the result being an average of both investigators’ scores.
Intrathecal injection of antagomir-21 and negative control antagomir
The SCI and sham rats were randomly separated into 2 respective groups (10 rats per group), a negative control antagomir group (antagomir-NC), treated with antagomir-NC (1 μL/h, 20 nmol/mL). The other group was treated with antagomir-21 (1 μL/h, 20 nmol/mL). The entire experiment was conducted in a warm environment. Catheter and minipump implantation surgeries were performed right after the injuries. Either negative control antagomir (antagomir-NC) or antagomir-21 (Shanghai GenePharma Ltd., Shanghai, China) was dissolved in PBS to 20 nmol/mL and loaded into osmotic minipumps (ALZET, Cupertino, CA, USA). The mixture was then continuously infused into the rats, post-injury or laminectomy, at a rate of 1 uL/h, according to instructions [31]. A fractional laminectomy at T12/T13 with a small needlepoint incision was necessary for the intrathecal catheter placement. The catheter was inserted into the incision and secured to muscle and bone by 4/0 silk threads, followed by attachment to the osmotic minipump. Rats were then injected with penicillin G during surgery.
TUNEL
A DeadEndTM Fluorometric TUNEL Detection System (Promega, Beijing, China) was used, conforming to the manufacturer’s protocol, to determine apoptosis. Briefly, spinal cord neurons were washed with PBS containing 4% formaldehyde for 15 min, then permeabilized with PBS containing 0.2% Triton X-100 for another 10 min under the same conditions. After washing twice with PBS, the cells were cultured with a terminal deoxytransferase (TdT) mixture at 37°C for 1 h, then washed twice. The nuclei were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) and fluorescent images captured using a Nikon Eclipse Ti microscope.
Quantitative real-time RT-PCR
We extracted RNA from the spinal cord segments, including the injury site (about 1 cm-long segment around the injury site) with TRIzol (Invitrogen, Carlsbad, CA, USA), per the manufacturer’s protocol. Roche Lightcycler 480 Detection System (Shanghai, China) was used to amplify and detect the RNAs. We used U6 as an internal control for template normalization and the primers were from Ambion, Inc. (Austin, Texas, USA). Fluorescent signals were calibrated against an internal reference, and the threshold cycle (Ct) was set within the exponential phase of the PCR. Before each target amplification, the relative gene expression was calculated by comparing cycle times. The target PCR Ct values were normalized by subtracting the U6 Ct value, which then provided a ΔCt value. The relative expression level between treatments was then calculated using the following equation: relative gene expression =2 - (ΔCt, sample - Ct, control).
Western blot analysis
Rats were euthanized with 10% chloral hydrate overdose. The SCI samples, containing the injury site (about 1 cm-long segment around the injury site) were harvested, separated using 7.5% or 10% SDS-polyacrylamide gel electrophoresis, then transferred onto Hybond-C Extra nitrocellulose membranes (GE Amersham). The membranes were blocked with 10% dry milk and 0.1% bovine serum albumin (BSA; Fraction V) in PBS for 1 h at room temperature, incubated overnight at 4°C with anti-PDCD4 polyclonal antibody (diluted in PBS containing 0.1% BSA) and β-actin as negative control, and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG for 1 h at 37°C, after washing. Relative intensities were analyzed using ImageJ with β-actin as the internal control. Each experiment was conducted in triplicate.
Statistical analyses
All values are presented as mean ± SEM. Statistical analyses were performed by SPSS software. Data were compared with one-way ANOVA or two-way ANOVA, with all ANOVA tests followed by an unpaired t-test, as appropriate. Bonferroni’s correction for multiple comparisons was used. Differences were considered significant when P<0.05.
Results
The effect of exercise after SCI
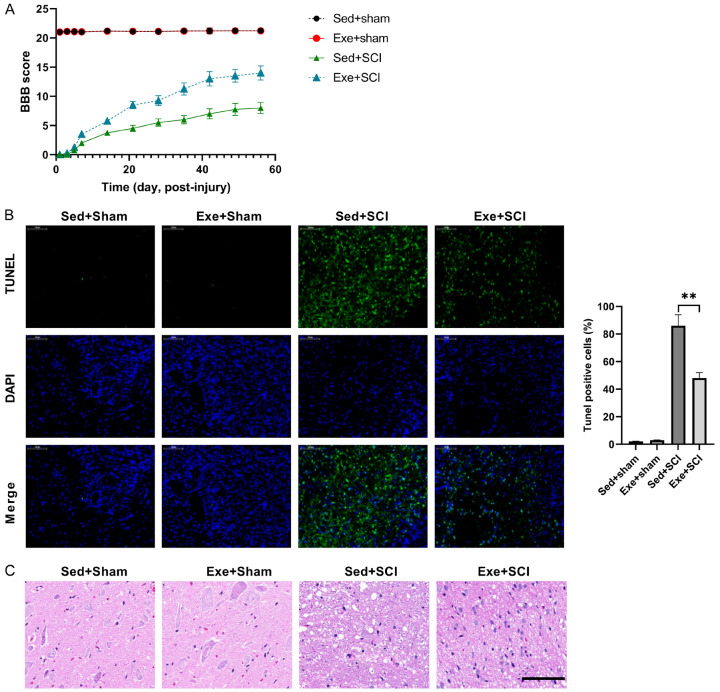
SCI model was established in rats to test the effects of exercise. SCI rats exhibited bilateral hind limb paralysis. The BBB score of the sham group rats, with/without exercise, remained unchanged (Figure 1A). Hindlimb activity of the SCI group improved gradually over the experiment period, as suggested by the increase in BBB score, with/without exercise. Compared with the sedentary group, the degree of recovery in the exercise rats was better at 60 days post-injury, with BBB scores of 14±1.22 and 8±0.94, respectively (Figure 1A). This indicates that exercise can protect against SCI.
Figure 1.
Exercise protects against SCI. (A) Rat recoveries were assessed from days 1 to 60, following SCI by BBB score. (B) Exercise protects cells from apoptosis after SCI-injury, confirmed by hematoxylin and eosin staining (C). n=8.
TUNEL staining at the lesion site post-surgery was used to determine the potential cellular mechanism behind exercise protecting against SCI. SCI increased cell apoptosis and this was alleviated by exercise at the lesion stie post-surgery in rats (Figure 1B). In addition, H/E staining showed that there were significant inflammatory cell infiltrate and rimmed vacuoles at the lesion site post-surgery in SCI rats, and exercise alleviated these changes (Figure 1C). These results suggested that exercise has a protective effect against SCI.
MiR-21 overexpression alleviates cell apoptosis following SCI
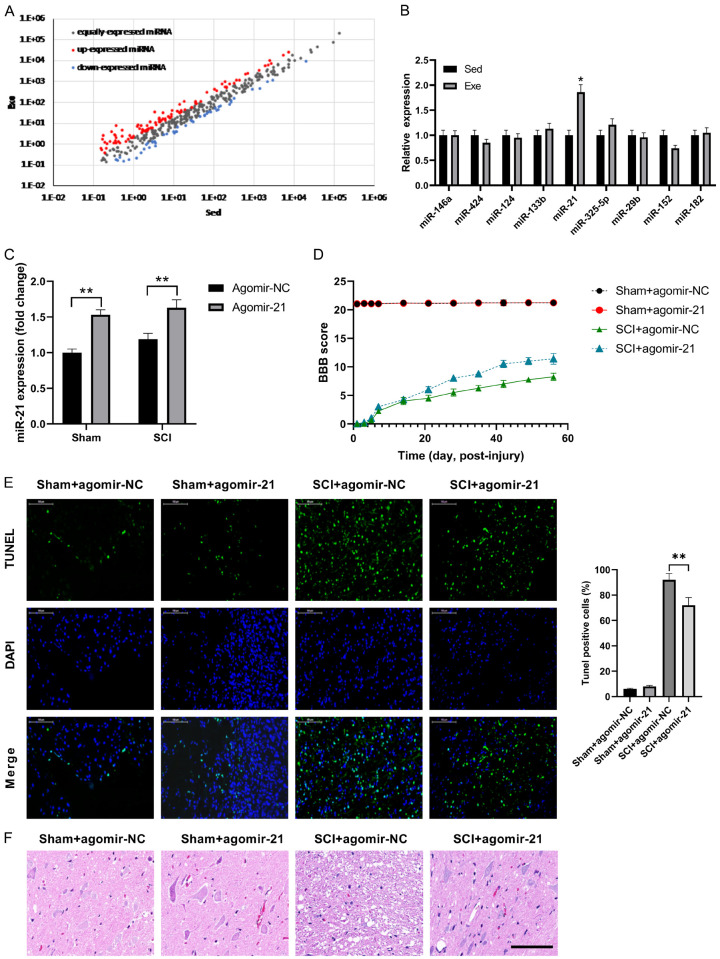
To explain the potential molecular function of exercise after SCI, we detected alterations in circulating miRNA expression by miRNA sequencing in SCI rats with and without exercise. A total of 9 differentially expressed miRNAs (fold change >2.0; P<0.05) were detected (Figure 2A). These results were further detected using qRT-PCR and found that miR-21 was upregulated by exercise (Figure 2B). To further understand the importance of miR-21 and exercise, agomir-21 and agomir-NC were used to increase miR-21 expression and as a negative control, respectively. Compared to the negative control, agomir-21 greatly up-regulated miR-21 expression in both groups (Figure 2C and 2D), resulting in an improved BBB score in the SCI group. TUNEL staining at the lesion site post-surgery was used to analyze the potential role of agomir-21 on cell apoptosis. Fewer TUNEL-positive cells were observed in the agomir-21 group following SCI, suggesting that miR-21 exerted anti-apoptosis effect against SCI (Figure 2E). These results were confirmed by H&E staining at the lesion site post-surgery, which showed that agomir-21 alleviated the inflammatory cell infiltrate and rimmed vacuoles at the lesion site post-surgery in SCI rats (Figure 2F).
Figure 2.
Exercise-upregulated miR-21 protects against SCI. (A) miRNA profiling assays were performed in plasma of SCI rats. (B) qRT-PCR analysis confirmed that miR-21 was up-regulated by exercise. (C) Agomir-21 increased miR-21 expression in both sham and SCI groups. Agomir-21 has little effect on the sham group, while significantly increasing the BBB score (D) and cell viability (E) of the SCI group, compared with agomir-NC. (F) The result of hematoxylin and eosin staining of tissue sections from mice. n=8. *P<0.05; **P<0.01.
Exercise promotes against SCI via upregulating miR-21
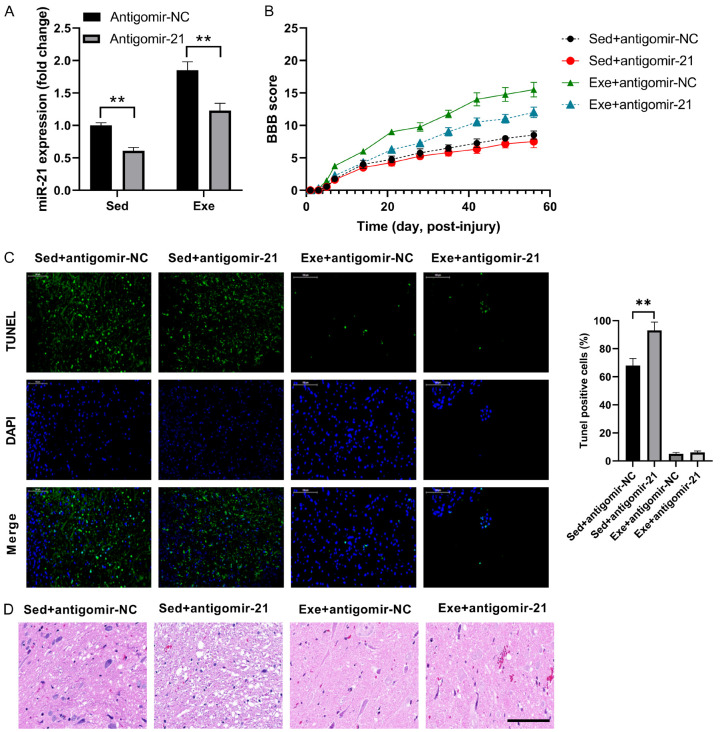
After determining the protective effect of exercise and miR-21 overexpression in cell recovery, following SCI, we intended to figure out the potential connection between them by miR-21 knockdown using antagomir-21. Even though antagomir-21 decreased miR-21 expression, miR-21 expression was up-regulated in exercise group, compared to the sedentary group (Figure 3A), demonstrating that miR-21 is an important exercise-associated factor. The BBB scores of all four groups were directly proportional to the miR-21 expression (Figure 3B). The effect of miR-21 on apoptosis following SCI was further analyzed by TUNEL staining. A higher number of TUNEL-positive cells were found in the antagomir-21 and sedentary groups (Figure 3C). The alleviated inflammatory cell infiltrate and rimmed vacuoles by exercise were abolished by antagomir-21 in SCI rats (Figure 3D). Based on that, we concluded that exercise can protect against SCI by upregulating miR-21 expression.
Figure 3.
Antagomir-21 abolishes the protective effects of exercise on SCI. A. miR-21 expression levels in SCI rats. B. BBB score of SCI rats. C. TUNEL results in SCI rats. D. Cell morphology with hematoxylin and eosin staining of tissue sections. n=8. *P<0.05; **P<0.01.
PDCD4 is a miR-21 target gene
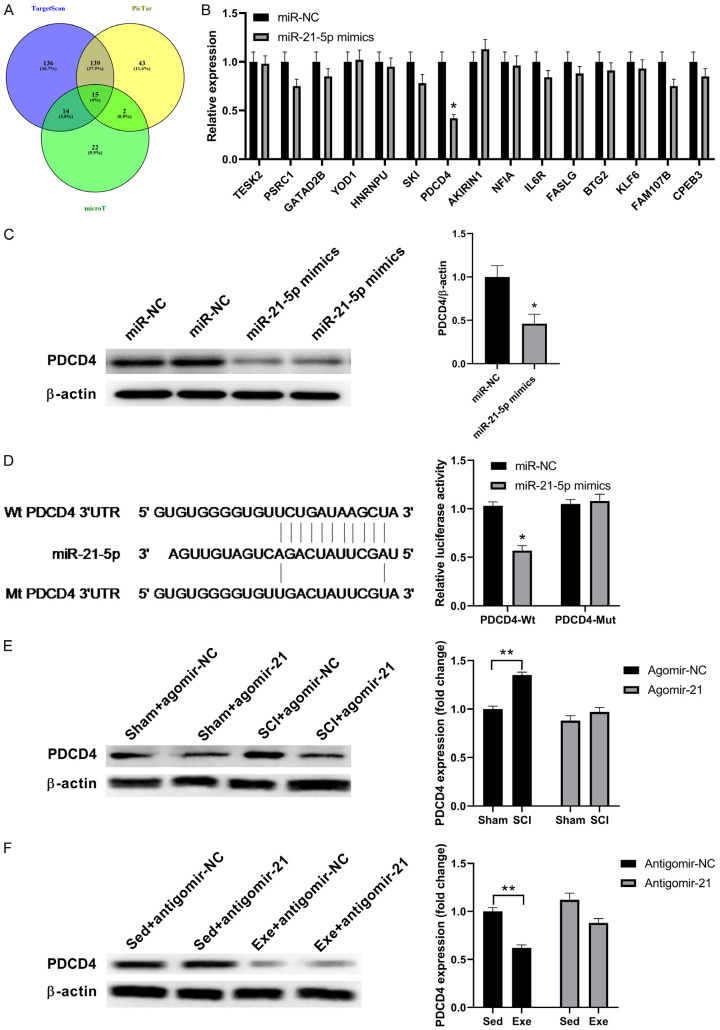
By regulating target genes, miRNA can activate their biological functions. Each miRNA can target thousands of genes as determined in a Venn diagram aggregation analysis using three target gene prediction programs (PicTar, microT, and TargetScan). All four programs showed the same central target genes (15 genes) (Figure 4A), which suggested that miR-21 more likely bound 15 target genes. To further determine the specific genes, we included miR-21-5p analogs as miR-21 genes. The real-time RT-PCR showed that PDCD4 expression was significantly inhibited, compared to others, suggesting that PDCD4 is the target, which was confirmed by western blot data (Figure 4B and 4C). The miRbase showed that human miRNA-2 and rat PDCD4 3’-UTRs had a high-energy binding site at 281-288 bp (Figure 4D). To determine whether miR-21 directly targets PDCD4, we cloned the 3’-UTR downstream of rat PDCD4. By co-transfection of wt-PDCD4 and mt-PDCD4 with the miR-21-5p analogs, we demonstrated that miR-21 greatly reduced luciferase activity in wt-PDCD4-transfected cells by 40±4.32%, whereas no significant difference was observed in mt-PDCD4.
Figure 4.
miR-21 targets the PDCD4 gene. A. miR-21 molecular targets predicted by bioinformatics. B. The expression levels of different miRNAs with miR-21-5p analogs. C. The quantification of PDCD4 protein expression levels, following transfection with miR-NC and miR-21-5p. D. PDCD4 3’-UTR sequence alignment with miR-21-5p and relative luciferase expression in rat PDCD4 luciferase reporters co-transfected with miR-21-5p-expressing constructs in cells. E. Respective western blot images and the PDCD4 protein expression levels in sham and SCI groups with/without agomir-21. F. Respective western blot images and PDCD4 protein expression levels in the SCI group with/without antagomir-21. β-actin served as an internal control. miR-21 significantly down-regulated PDCD4 protein expression. n=5. *P<0.05; **P<0.01.
As shown in Figure 4E, upregulation of miR-21 using agomiR-21 abolished the effects of SCI in upregulating PDCD4. In addition, downregulation of miR-21 using antigomiR-21 attenuated the effects of exercise in downregulating PDCD4 (Figure 4F). These results reinforced the notion that PDCD4 is the target of miR-21.
miR-21 is shuttled by exosomes
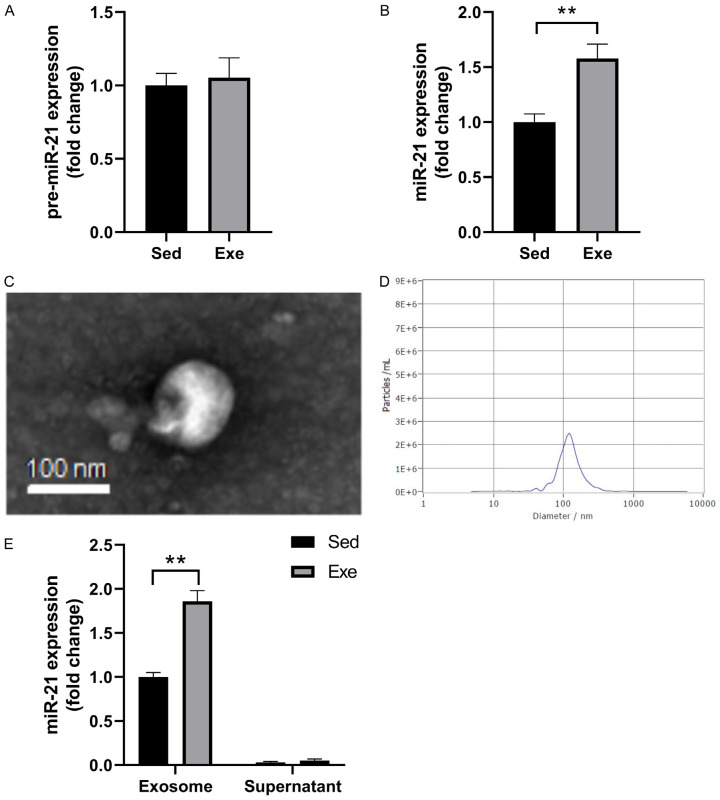
To determine the source of miR-21 in cells, we extracted spinal cord samples in the thoracic region T8/T10. from sedentary and exercise rats and detected pre-miR-21 and miR-21 expressions. There was no difference in the pre-miR-21 expression level in the exercise group, compared to the sedentary group (Figure 5A and 5B), however, there was a 40% increase in miR-21 expression levels (P<0.01). To determine whether the miR-21 was shuttled by circulating exosomes, exosomes were isolated from the plasma of SCI rats. Additionally, we observed the exosome with SEM and determined its diameter of about 100 nm (Figure 5D). MiR-21 expression level was analyzed in both the exosome and cell supernatant; miR-21 was shuttled by exosomes (Figure 5E).
Figure 5.
miR-21 is exosome-shuttled. The quantification of pre-miR-21 (A) and miR-21 (B) expression in both exercise and sedentary groups. The SEM image (C) and size (D) of the exosome. (E) The amount of miR-21 expressed in the exosome and supernatant. n=4. **P<0.01.
Discussion
In this study, we found that exercise increases circulating miR-21 expression following SCI. Down-regulating miR-21 expression by antagomir-21 exacerbated functional deficiency, tissue damage, and apoptosis. These results showed that exercise protects against the damage caused by spinal cord injury by up-regulating miR-21 expression levels, mainly shuttled in the exosome.
Recently, studies have shown that miRNAs are involved in the development and prognosis of SCI through multiple mechanisms which are associated with apoptosis, regeneration, inflammation, and demyelination [16]. Apoptosis, a crucial pathway of cell death in many neurological diseases, is critical in SCI. Recent studies have shown that miR-21 exerts anti-apoptotic effects in cancer cells [19], and its expression is upregulated in some central nervous system disease [7,20]. There is also evidence that miR-21 was upregulated 3 days following SCI in rats [21]. Moreover, it has been shown that miR-21 has protective effects in the injury of different organs. For example, miR-21 expression was increased in the injured region of myocardial infarction, which exerts a protective role [22]; miR-21 attenuated the apoptosis of cardiac cells induced by oxidative stress [23], and it was involved in ischemic preconditioning-mediated cardiac protection [24]. Moreover, miR-21 expression was upregulated after traumatic brain injury [20,25]. After stroke, the overexpression of miR-21 protected neurons from ischemia by targeting Fasl [26]. In a rat SCI model, inhibition of miR-21 by antagomir-21 increased apoptosis [9]. Here, we found that exercise upregulated circulating miR-21 expression which contributes to exercise’s protective effects against SCI.
PDCD4, a pro-apoptotic gene, is one of the most widely researched miR-21 target genes. Previous studies have suggested that PDCD4 can be modulated and targeted by miR-21 in breast cancer [32]. PDCD4 induces the apoptosis of pancreatic β cells, and this process is regulated by miR-21 [28]. PDCD4 is also involved in cardiovascular function by regulating the apoptosis of cardiomyocytes and vascular smooth muscle cells [24]. Here, we found that exercise exerted protective effect against SCI through upregulating miR-21 and suppression of PDCD4. PDCD4 is the target of miR-21 in the exercise’s protection against SCI. These results are consistent with previous studies, indicating that miR-21 expression levels negatively regulate PDCD4 gene expression, proving PDCD4 a target of miR-21. Therefore, exercise-modulated miR-21 protects against SCI by retraining the pro-apoptotic PDCD4 gene expression.
Conclusion
In conclusion, exercise significantly up-regulates miR-21 expression in SCI rats. Additionally, miR-21 most likely achieves the anti-apoptotic effect by targeting PDCD4. Exercise protects against the damage caused by SCI through miR-21-mediated suppression of PDCD4.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (no. 81701896), Natural Science Basic Research Program of Shaanxi (no. 2020JM-682), and The Fundamental Research Funds for the Central Universities (no. xjj2018jchz03).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2016;39:493–4. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Johnson CS, Wrathall JR. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol. 2007;203:502–511. doi: 10.1016/j.expneurol.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Kastin AJ, Pan W. Neural plasticity after spinal cord injury. Curr Pharm Des. 2005;11:1441–1450. doi: 10.2174/1381612053507855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–70. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 7.Murray M, Goldberger ME. Restitution of function and collateral sprouting in cat spinal-cord - partially hemisected animal. J Comp Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Fazekas D, Koltai M, Türei D, Módos D, Pálfy M, Dúl Z, Zsákai L, Szalay-Bekő M, Lenti K, Farkas IJ, Vellai T, Csermely P, Korcsmáros T. SignaLink 2 - a signaling pathway resource with multi-layered regulatory networks. BMC Syst Biol. 2013;7:7. doi: 10.1186/1752-0509-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Abe M, Bonini NM. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol. 2013;23:30–36. doi: 10.1016/j.tcb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths-Jones S. The microRNA registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 19.Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle neuronal death. Ann Neurol. 2003;53:454–468. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- 20.De Biase A, Knoblach SM, Di Giovanni S, Fan CG, Molon A, Hoffman EP, Faden AI. Gene expression profiling of experimental traumatic spinal cord injury as a function of distance from impact site and injury severity. Physiol Genomics. 2005;22:368–381. doi: 10.1152/physiolgenomics.00081.2005. [DOI] [PubMed] [Google Scholar]
- 21.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–9. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30:1349–1360. doi: 10.1089/neu.2012.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei P, Li Y, Chen X, Yang S, Zhang J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009;1284:191–201. doi: 10.1016/j.brainres.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 26.Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–197. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- 27.Nazemi Z, Nourbakhsh MS, Kiani S, Heydari Y, Ashtiani MK, Daemi H, Baharvand H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145–158. doi: 10.1016/j.jconrel.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.