Abstract
Objective: To induce acinar-differentiation from human dental pulp cells for potential application in aiding treatment of dry-eye syndromes. Method: Human dental pulp cells were co-cultured with human submandibular gland acinar cells using a transwell construction for 2 weeks. The two populations of cells were physically separated while chemical and biochemical components can be exchanged. Fibroblasts were included as a negative control. Expression of amylase, cytokeratin 8 and vimentin were examined by immune-staining. Amylase activity was measured using an AMS Assay Kit. Result: Cobblestone-like islands, a feature of acinar cells, appeared in the dental pulp cells which were co-cultured with salivary gland cells for one week and increased in number and size after two weeks. Antibody detected amylase in 30 and 50% of the pulp cells 1 and 2 weeks in the co-culture, respectively. Cytokeratin 8 increased while vimentin decreased. All these changes indicate an acinar-like differentiation of the dental pulp cells. None of these changes were observed in fibroblasts which were also co-cultured with salivary gland cells, indicating that the acinar-like differentiation is specific for the dental pulp cells. Neither of the changes were observed in dental pulp cells when not co-cultured with the salivary gland cells, indicating that induction is specific and essential. Conclusions: Human dental pulp cells have the potential to differentiate into acinar-like cells which may provide an autologous source for cellular therapy for dry-eye syndromes.
Keywords: Dental pulp cells, differentiation, salivary glands, regenerative, fibroblasts, dry-eye syndromes
Introduction
Sjogren syndrome is a chronic autoimmune disease of the exocrine glands associated with dryness of the eyes and mouth that severely impairs the quality of life [1,2]. The prevalence is estimated as 0.1~4.8% in Europa and 0.77% in China [3,4].
The lacrimal gland is the most common site involved. Impairment of secretion from the acinar cells can lead to symptoms including burning sensation, serious ocular conjunctiva, susceptibility to corneal infection and ulcers, and in server cares, even blindness [5].
The saliva gland is the second most common site involved. Patients with salivary gland hypofunction often suffer from conditions including dry mouth, dysphagia, rampant caries, susceptibility to fungus infection and malnutrition, all leading to poor quality of life [6].
So far, there is no established curative treatment. The current measures mainly aim to ameliorate the symptoms which does not solve the problems [7]. For some severe cases of dysfunctioning acinar gland, transplantation of the mandibular gland is an option to prevent loss of vision and to enable the patients a normal life to certain extent [8]. However, this major surgical procedure demands extensive experience and skill from the surgeon. More importantly, such transplantation leads to damage of the donor site, and is therefore controversial. In addition, since the saliva gland is also affected in the Sjogren syndrome, the transplanted gland is expected to function only for limited time period [9].
Advance in regenerative medicine raises the hope of using autologous cells and tissues for compensating and supporting dysfunctional organs and tissues. In the case of the Sjogren syndrome, injecting autologous acinar-cell-like cells may provide a strategy for treating dry eyes or at least for ameliorating the symptoms. Recently, bioengineered lacrimal glands from embryo-stem/progenitor cells have been successfully transplanted into mice which produced acini and ducts [10]. Other studies injected stem/progenitor cells from the lacrimal gland to the defect ones with improvement in structure and function of the latter in mice. However, using such embryonic or lacrimal gland stem/progenitor cells is not realistic in humans. An alternative practicable strategy would be inducing other adult human pluripotent cells into acinar-cell-like cells.
Human dental pulp cells are cells from roots of teeth which exhibit features of pluripotent stem/progenitor cells and therefore provide a promising cell source for tissue engineering and regeneration medicine [11]. Many people will likely have some of their teeth, for example and especially, their wisdom teeth, extracted for clinical reasons or prevention purpose sometime in their life. Cells cultured from the teeth of a patient are autologous to this patient which salvages the problem of host rejection. Dental pulp cells can be induced into osteogenic, adipogenic and chondrogenic cells. We therefore hypothesized that dental pulp cells can also be induced into the lineage of acinar cells [12]. However, there is no established cultural condition for such induction in vitro.
The present study was designed to test the feasibility of inducing acinar-differentiation from dental pulp cells by co-culturing them with cells derived from mandibular gland. We used a transwell construction which prevents mix of the two types of cells while enables chemical exchange and interaction. As acomparison, we included a human fibroblast culture.
Materials and methods
Culturing human salivary gland cells
At submandibular sialadenectomy surgeries carried out in our Hospital for patients with blocked saliva glands, the removed glands were saved from biowaste for the study. The study protocol was approved by the local authority and all patients gave written consent for using their removed gland specimen for the study. All specimens were anonymized for privacy protection. The salivary glands were dissected into small fragments in the laboratory and used to culture the gland cells by the explant method. The gland cells were identified by labeling with an antibody for the enzyme amylase (Amylase-G10 1:150, cat NO. SC-46657, SANTA Inc, USA) and were used for inducing the pulp cells.
Culture and purification of human dental pulp cells
Human dental pulp tissues were obtained from teeth extracted at our hospital for medical indications. The primary culturing was carried out using the explant methods. Expression of CD29 and CD44 was examined using flow-cytometry. Pluripotent of the pulp cells were checked by their differentiation into adipogenic and ostepgenic cells which were stained with Oil Red-O and Alzerin red [12].
Inducing acinar differentiation using a transwell construction
The dental pulp cells and the gland cells were co-cultured in a transwell plate (4-μm pore size, Corning, USA) where 4×104 acinar cells were seeded in the upper inserts and 1×104 pulp cells in the lower wells (Figure 1A). Transwell membrane inserts have 0.4 μm pores which prevent migration of cells while enable exchange of the medium with component secreted by the cells. Media in both the upper and the lower chambers were changed every two days. The co-culture was continued for 2 weeks. Morphology of the cells were observed daily. After one and two weeks, expression of amylase, cytokeratin 8 (CK8; cat. no. ab9023; dilution, 1:800; Abcam, USA) and vimentin (anti-vimentin 1:1000, cat# ab8069, Abcam, USA) were examined using antibodies and counter-staining with DAPI or the hematoxylin.
Figure 1.
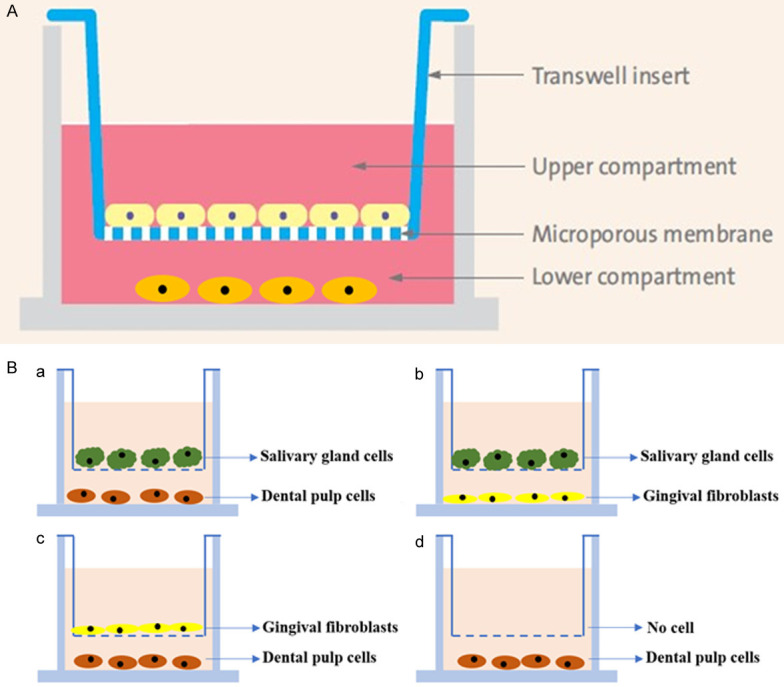
Study design. A: The transwell-construction. Two populations of cells were cultured in the upper and lower compartment, separated by a microporous membrane which allows passage of chemical and biochemical molecules. B: 4 combinations of the inducing and differentiating cells.
Amylase activity
To measure amylase activity, the upper inserts with inducing cells were removed and the medium in the lower differentiating cells changed to medium without color pH indicator. After 3 days, the supernatants were used for measuring amylase activity using the AMS assay kit (Nanjing jiancheng Corp., Nanjing, China). The activity was calculated as: Amylase activity (U/dl) = (blank OD value-test OD value)/blank OD×80. For each sample, the measurement was carried out in 4 replicates. The values were given in means and standard deviation. Comparing the amylase activities in two samples was carried out using the t-test with two-tailed hypothesis. The significant level was set at 0.05.
Results
Human salivary gland cells and dental pulp cells
Salivary gland cells migrating from the explants in primary culture were observed in phase contrast microscope. After two days of plating the dissected fragments of the gland tissue, cells migrating from the explants were observed. These cells appeared in polygonal shape which is typical for epithelial gland cells. After 6 to 8 days, these cells reached 80-90% confluence.
Production of amylase is one of the most characteristic function of salivary gland acinar cells. Immuno-staining revealed that cells in culture were positive for amylase in their cytoplasm.
5 to 7 days after plating the explants of dental pulp tissue, cells started migrating from the explants. In the primary dental pulp cells, the morphology of the cells was from ellipse to a spindle, strip or irregular shape.
Acinar-like differentiation of pulp cells
The primary dental pulp cells and the gland cells obtained as above were cultured together in a transwell construction which enables exchange of chemical and biochemical components of the two physically separated cell populations. The gland cells were in the upper insert and the pulp cells in the lower wells (Figure 1A). After one week, morphology change began in the pulp cells (Figure 2A). Acinar cell-like polygonal shaped cells appeared among the elongated ovate pulp cells. These acinar cell-like cells packed together compactly, forming an island-structure (Figure 2A, yellow arrows). On top of the islands, bubbles were visible which were possibly secretion from the cells. Proportion of these acinar cell-like cells, size and number of the cell-islands, and bubbles over them further increased with time. However, 3 weeks after the co-culture, the cell-islands started to detach the surface.
Figure 2.
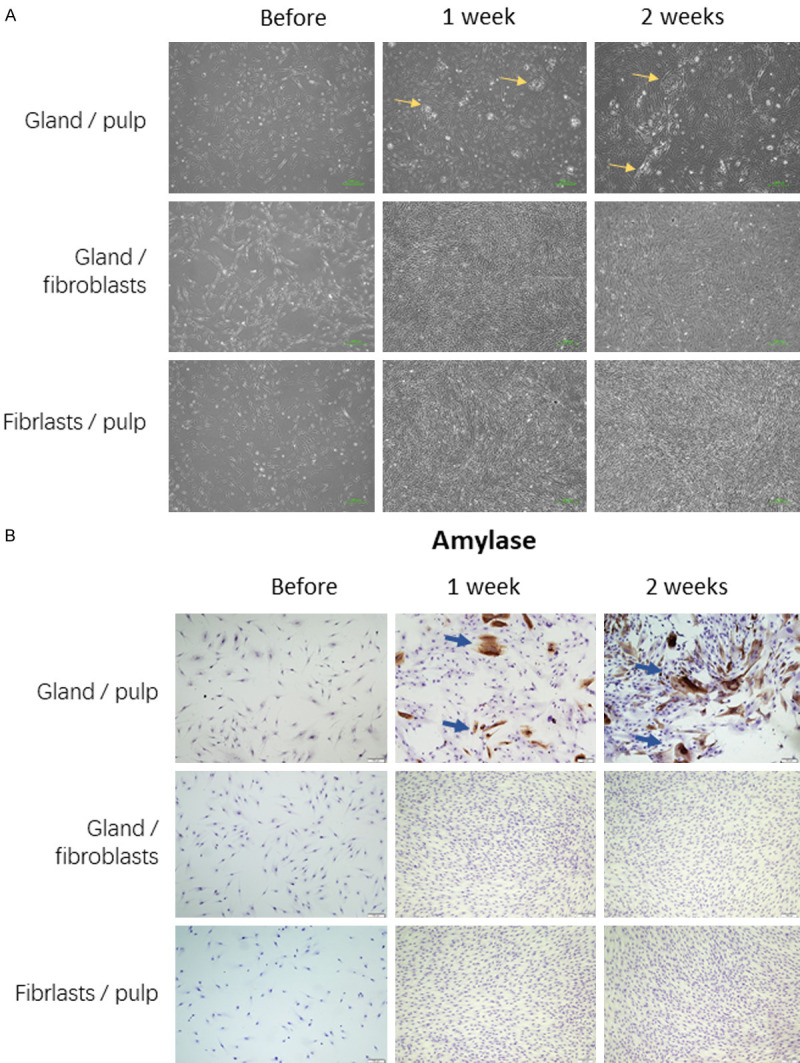
Acinar-like differentiation of the dental pulp cells co-cultured with salivary gland cells (gland/pulp): island-like structures were visible (A) and amylase-positive cells were evident (B). These chances were not visible in the human gingival fibroblasts co-cultured with salivary gland cells (gland/fibroblasts) and not in the dental pulp cells without salivary gland cells (fibroblasts/pulp).
Amylase is a characteristic feature of acinar cells. Immunostaining indeed revealed expression of amylase in approximately 30% of the pulp cells with induction of gland-cells in the upper insert for one week (Figure 2B, blue arrows). After two-weeks, the amylase positive cell population increased to approximately 50%. In contrast, none of the fibroblasts was positive for amylase. Similarly, no pulp cells without acinar-induction by gland-cells in the upper chambers were positive for amylase expression (Figure 2B).
In concordance, increased amylase activity was measured in the supernatant of pulp cells co-cultured with the gland cells (Figure 3, green bar). This increase did not reach the significant level (P=0.06) for one week co-cultured, but became highly significant (P<0.001) after two weeks co-culture. In contrast, no such increase in amylase activity was measured in the two negative controls (Figure 3). For example, the amylase activity of the gland-induced fibroblasts was significantly lower than that of the gland-induced pulp cells (P=0.02 and <0.001 for one and two weeks co-culture).
Figure 3.
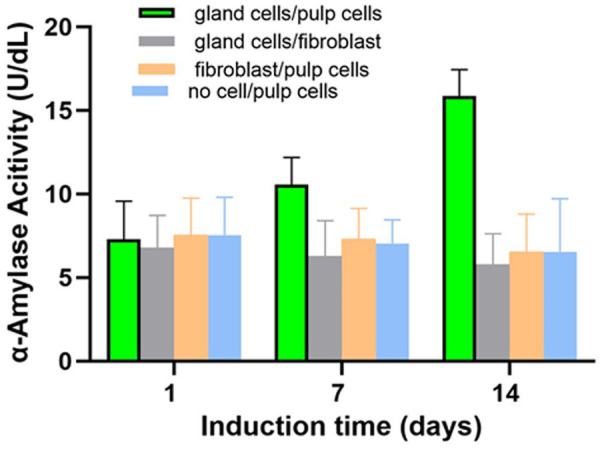
Amylase activity increased in the supernatant of the dental pulp cells which have been co-cultured with salivary gland cells (green bars) for 1 (P=0.06) and 2 weeks (P<0.001). To exclude the amylase activity from the salivary gland cells, the inserts with inducing cells were they were removed and the cells in the lower compartments were cultured for another 3 days before the measure. No such increase was measured in the supernatant of the human gingival fibroblast which has been co-cultured with salivary gland cells (gray bars). Consequently, the amylase activity of the gland-co-cultured pulp cells was significantly higher (P=0.02 on day 7 and P<0.001 on day 14). Without co-culturing with the salivary gland cells, the amylase activity did not increase in the dental pulp cells neither (yellow bars).
As further supporting evidence for the acinar-like differentiation, cytokeratin, an epithelial marker, was also increased in the dental pulp cells which were co-cultured with the gland cells. In concordance, vimentin, a mesenchymal marker, control, decreased indicating that the dental pulp cells were losing the stem/progenitor features in the acinar-differentiating process.
This acinar-like differentiation potential seems to be a specific feature of dental pulp cells, since no morphological and expression changes were observed in the fibroblasts induced under the identical condition (Figures 2, 3 and 4). Instead, the fibroblasts continued to grow. In another negative control, the pulp cells were co-cultured with fibroblasts in the upper inserts, no acinar-like differentiation was observed (Figures 2, 3 and 4).
Figure 4.
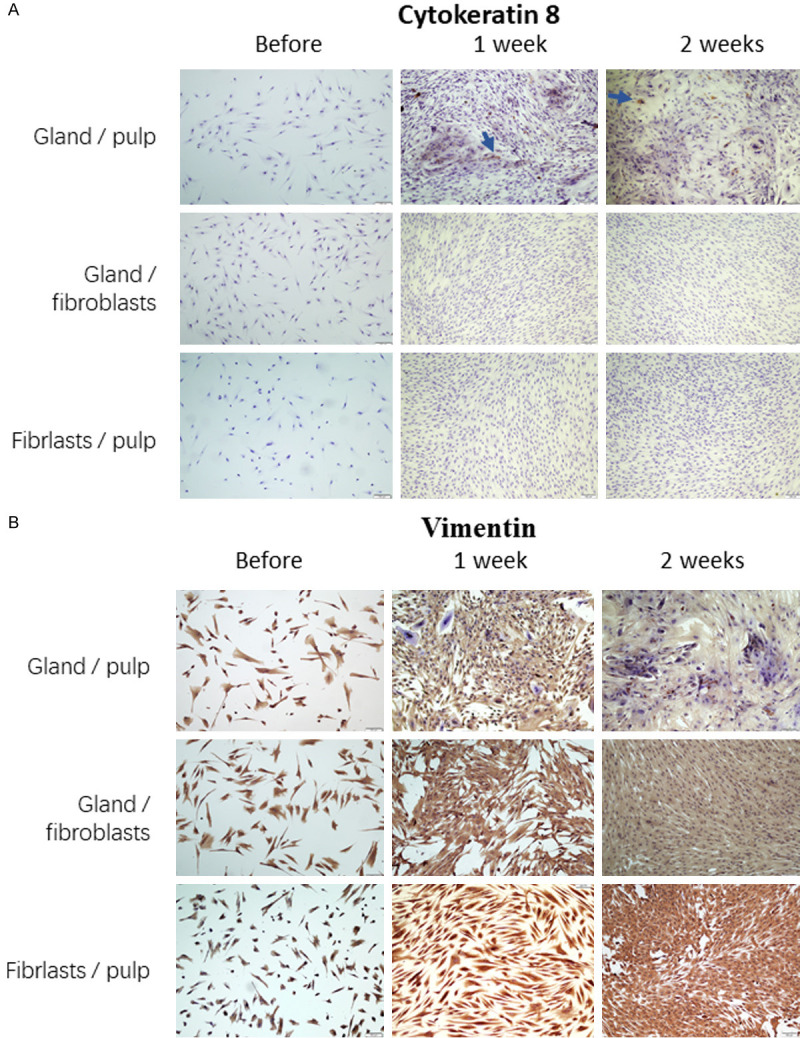
A: Increasing expression of cytokeratin 8, a marker for epithelial cells, in the acinar-like differentiating pulp cells acinar-like differentiation of the dental pulp cells co-cultured with salivary gland cells (gland/pulp). B: Decreasing expression of vimentin, a mesenchymal marker, in the acinar-like differentiating pulp cells acinar-like differentiation of the dental pulp cells co-cultured with salivary gland cells (gland/pulp). These chances were not visible in the human gingival fibroblasts co-cultured with salivary gland cells (gland/fibroblasts) and not in the dental pulp cells without salivary gland cells (fibroblasts/pulp).
Discussion
We succeeded in inducing acinar-like differentiation from human dental pulp cells. The induction is realized by co-culturing salivary gland cells in a transwell construction. Since the two populations of cells share the same medium, the acinar-differentiation of the pulp cells in the lower wells was likely induced by factors and components secreted from the salivary gland cells in the upper inserts.
For induction of differentiation in various lineages such as the adipogenic and osteogenic ones, conditions have been established which are based on a special combination of small molecules. However, for acinar-differentiation, no such simple conditions are known. A recent study succeeded in enriching lacrimal gland cells from human embryonic stem cells by transfecting synthetic messenger RNA for several transcription factors [13]. However, transfection is not a practicable approach for medicine application.
Co-culturing is an alternative strategy for inducing differentiation. Indeed, differentiation into the acinar-lineage has been achieved from adipose stromal cells when co-cultured with salivary gland cells [14]. However, no detail of the co-culturing construction was given in that study and no transwell is mentioned. It is possible that the two kinds of cells were cultured in mixture.
In the present study, we used a transwell construction to carry out the “co-culturing”. This construction enables exchange of chemicals and biochemical molecules. However, the membrane of the trans-inserts does not allow passage of the cells. Therefore, the two types of cells remain physically separated. An important advantage of this semi-co-culture is that the two types of cell can have different origins. While the pluripotent cells in the lower wells can be autologous for application without host rejection, the inducing cells in the upper inserts can have a different origin. In the present study, human salivary gland cells were used as the inducing cells. However, other sources of inducing cells and tissues are also theoretically possible, for example, cell lines derived from human or non-human gland tissues. Alternatively, conditioned medium of gland cells may also have effect in inducing acinar-differentiation of pluripotent cells.
We used a ratio of 4 to 1 of the inducing cells to the differentiating cells. However, it is possible, that other ratios may also work. Furthermore, since the acinar-like differentiation is reached in approximately 30% of the pulp cells, it is possible that these cells will secrete factors and therefore enable the condition for further differentiation of other pulp cells. In addition, future studies also need to explore the potential of other types of pluripotent cells including bone marrow cells and adipose stromal cells in this acinar-differentiation.
Human stem/progenitor cells are postulated to have some immune-suppressing activity [15,16]. Therefore, in addition to their potential acinar-function after the induction, dental pulp cells may also contribute to suppress some dysregulated immunity which damages the original lacrimal cells in the dry-eye syndrome.
In summary, results of the present study demonstrated the feasibility of inducing acinar-differentiation from human dental pulp cells by co-culturing them with salivary gland cells using a transwell construction without mixing the two cell populations. The acinar-like cells may have application potential in cellular therapy for dry-eye syndromes as autologous cell source.
Acknowledgements
We thank Mrs. Susan Eick for correcting the errors in spelling and grammar. This paper was funded by the key research and development project of Hebei Province Department of Science and Technology, No. 17277745D; The Scientific Research Project of the Health Committee of Hebei Province Hebei. No. 20191080 and the Scientific Research Project of the Education Department of Hebei Province, No. ZH2012033.
Disclosure of conflict of interest
None.
References
- 1.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjogren’s syndrome. A clinical, pathological, and serological study of sixty-two cases. 1965. Medicine (Baltimore) 1992;71:386–401. discussion 401-383. [PubMed] [Google Scholar]
- 2.Meijer JM, Meiners PM, Huddleston Slater JJ, Spijkervet FK, Kallenberg CG, Vissink A, Bootsma H. Health-related quality of life, employment and disability in patients with Sjogren’s syndrome. Rheumatology (Oxford) 2009;48:1077–1082. doi: 10.1093/rheumatology/kep141. [DOI] [PubMed] [Google Scholar]
- 3.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westhoff G, Zink A. Epidemiology of primary Sjorgren’s syndrome. Z Rheumatol. 2010;69:41–49. doi: 10.1007/s00393-009-0518-3. [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC. Differential diagnosis of dry eye conditions. Adv Dent Res. 1996;10:9–12. doi: 10.1177/08959374960100011801. [DOI] [PubMed] [Google Scholar]
- 6.Both T, Dalm VA, van Hagen PM, van Daele PL. Reviewing primary Sjogren’s syndrome: beyond the dryness-From pathophysiology to diagnosis and treatment. Int J Med Sci. 2017;14:191–200. doi: 10.7150/ijms.17718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, Them NC, Berg T, Elena C, Casetti IC, Milanesi C, Sant’antonio E, Bellini M, Fugazza E, Renna MC, Boveri E, Astori C, Pascutto C, Kralovics R, Cazzola M Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–1551. doi: 10.1182/blood-2013-11-539098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murube-del-Castillo J. Transplantation of salivary gland to the lacrimal basin. Scand J Rheumatol Suppl. 1986;61:264–267. [PubMed] [Google Scholar]
- 9.Schroder C, Hakim SG, Collin JR, Sieg P, Geerling G. Long-term follow-up after autologous submandibular gland transplantation in scarring keratoconjunctivitis with absolute dry eyes. Ophthalmologe. 2003;100:1079–1084. doi: 10.1007/s00347-003-0861-8. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, Ikeda K, Shimmura S, Kawakita T, Tsubota K, Tsuji T. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497. doi: 10.1038/ncomms3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Zheng Y, Eichhorn W, Klatt J, Henningsen A, Kluwe L, Friedrich RE, Gosau M, Smeets R. Enriching stem/progenitor cells from dental pulp cells by low-density culturing. In Vivo. 2019;33:23–29. doi: 10.21873/invivo.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirayama M, Kawakita T, Tsubota K, Shimmura S. Challenges and strategies for regenerating the lacrimal gland. Ocul Surf. 2016;14:135–143. doi: 10.1016/j.jtos.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama M. Advances in functional restoration of the lacrimal glands. Invest Ophthalmol Vis Sci. 2018;59:DES174–DES182. doi: 10.1167/iovs.17-23528. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 16.Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114–126. doi: 10.1111/nyas.12815. [DOI] [PubMed] [Google Scholar]


