Abstract
Background: Osteoarthritis (OA) is an aging-related chronic degenerative joint disease. A number of miRNAs have been found to be involved in the development of OA, but the role of miR-613 in OA remains unclear. Thus, this study aimed to investigate the role of miR-613 during the progression of OA. Methods: CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. Cell viability, cell proliferation and cell apoptosis in CHON-001 cells were assessed by CCK-8, immunofluorescence, and flow cytometry assays, respectively. In addition, the dual luciferase reporter system assay was used to determine the interaction of miR-613 and fibronectin 1 in CHON-001 cells. Results: The level of miR-613 was significantly decreased in IL-1β-treated CHON-001 cells. Overexpression of miR-613 markedly inhibited IL-1β-induced apoptosis in CHON-001 cells. In addition, upregulation of miR-613 obviously alleviated IL-1β-induced inflammatory response and cartilage matrix degradation in CHON-001 cells. Meanwhile, fibronectin 1 was identified as a direct binding target of miR-613 in CHON-001 cells. Overexpression of miR-613 alleviated IL-1β-induced injury in CHON-001 cells via downregulating the expression of fibronectin 1. Furthermore, overexpression of miR-613 alleviated cartilage degradation, and reduced OARSI scores and subchondral bone thickness in a mouse model of OA. Conclusion: Our data indicated that overexpression of miR-613 could inhibit IL-1β-induced injury in CHON-001 cells via decreasing the level fibronectin 1 in vitro, and alleviate the symptoms of OA in vivo. Therefore, miR-613 might be a potential therapeutic option for the treatment of OA.
Keywords: Osteoarthritis, microRNA-613, fibronectin 1, apoptosis
Introduction
Osteoarthritis (OA) is the most common form of degenerative joint disease, which accompanied by cartilage degeneration [1]. Gender, age, obesity, joint injury and genetic factors are most common risk factors for OA [2,3]. OA can lead to chronic pain, swelling, stiffness and disability, which affects the quality of life in patients with OA [4,5]. Previous reports indicated that degradation of cartilage, chondrocytes senescence and degradation of the extracellular matrix (ECM) are main features of OA [6,7]. Interleukin-1β (IL-1β) is a kind of proinflammatory cytokines, which could lead to the loss of cartilage [8]. In addition, chondrocytes under inflammatory conditions lead to the production of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), and then result in matrix degradation [9]. However, the pathogenesis of OA has not been fully elucidated. Thus, it is necessary to explore new biomarkers for the diagnosis and treatment of OA.
MicroRNAs (miRNAs) are a class of noncoding RNAs, which are 21-25 nucleotides in size [10]. Previous study indicated that miRNAs could regulate gene expression at the transcriptional and post-transcriptional levels by direct binding to 3’ untranslated regions (3’UTRs) of target mRNAs [11]. In addition, miRNAs play vital roles in several cellular processes, including survival, proliferation, differentiation, apoptosis and metabolism [12]. Moreover, miRNAs have emerged as promising mediators in skeletal pathophysiology [13]. Zhu et al indicated that overexpression of miR-21-5p could promote hyaline cartilage production [14]. Zhong et al indicated that miR-335-5p could inhibit the inflammatory response in human OA chondrocytes [15].
Liang et al found that miR-613 could inhibit the proliferation of rheumatoid arthritis synovial fibroblasts [16]. However, the biological role of miR-613 in OA remains unclear. In this study, an in vitro OA-like chondrocyte model was established using IL-1β-stimulated human CHON-001 cells, and a surgical model of murine OA in mice was established to mimic human OA in vivo. Herein, we identified that overexpression of miR-613 may alleviate the progression of human OA in vitro and in vivo, which may inform novel therapeutic strategies for OA.
Materials and methods
Cell culture and treatment
Human chondrocyte cell line CHON-001 was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific) and 100 U/mL penicillin under 5% CO2 atmosphere with 95% humidity at 37°C. CHON-001 cells were cultured with 10 ng/mL IL-1β (Sigma-Aldrich, St. Louis, USA) for 24 h, in order to mimic an in vitro model of OA.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA samples from cultured CHON-001 cells were extracted using the TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. For reverse transcription of miRNA, the TaqMan microRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA. For reverse transcription of mRNA, RNAs were reversely transcribed into cDNAs using a PrimeScript RT reagent kit (Takara Bio Inc. Shiga, Japan). After that, qPCR amplification was performed using the SYBR Premix Ex Taq II kit (Takara) on a Real-Time quantitative thermal block (Bioneer, Daejeon, South Korea). U6 and actin were used as the internal control for normalizing miR-613 and fibronectin 1 expressions respectively. Data were analyzed using the 2-ΔΔCT method. MiR-613, forward: 5’-GACCTCAGCCTCAACTGAATTG-3’; reverse: 5’-CTCAACTGGTGTCGTGGAGTC-3’. U6, forward: 5’-CTCGCTTCGGCAGCACAT-3’; reverse: 5’-AACGCTTCACGAATTTGCGT-3’. Fibronectin 1, forward: 5’-GTGGCAGAAGGAATATCTCGG-3’; reverse: 5’-TCTAAAGGCATGAAGCACTCAA-3’. Actin, forward: 5’-GTCCACCGCAAATGCTTCTA-3’; reverse: 5’-TGCTGTCACCTTCACCGTTC-3’.
Cell transfection
MiR-613 agonist (50 nM; 5’-AGGAAUGUUCCUUCUUUGCC-3’), miR-613 antagonist (50 nM; 5’-GAAUUACAAGGAAGAAACGG-3’) and negative control (NC) were purchased from RiboBio (Guangzhou, China). CHON-001 cells were transfected with miR-613 agonist, miR-613 antagonist and NC using Lipofectamine 2000 reagent (Thermo Fisher Scientific). The culture medium was changed at 6 h after transfection, and then cells were cultured for another 48 h in DMEM contained 10% FBS.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using the Cell Counting Kit-8 (CCK-8, Dojindo, Gaithersburg, USA). CHON-001 cells (5000 cells per well) were seeded onto a 96-well plate and incubated overnight at 37°C. After that, cells were transfected with miR-613 agonist or miR-613 antagonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. Later on, 10 μL CCK-8 reagent was added into each well, and the cultures were incubated for another 2 h at 37°C. Subsequently, a Microplate Reader (Bio-Rad, Hercules, USA) was applied to measure the absorbance of each well at 450 nM.
Ki67 immunofluorescence assay
The CHON-001 cells (5 × 104 cells per well) were seeded onto a 6-well plate and incubated overnight at 37°C. Later on, cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. After that, cells were fixed in 4% paraformaldehyde, and then washed twice with PBS at room temperature. Next, cells were incubated with primary antibody against Ki67 (1:200, Abcam Cambridge, MA, USA) overnight at 4°C, and then incubated with fluorescein-conjugated goat anti-rabbit IgG antibody (1:200, Abcam) at room temperature for 1 h. Cells were counterstained with DAPI for 5 min. Subsequently, cells were imaged using a fluorescence microscope (DMi8, Leica, Buffalo Grove, IL, USA).
Flow cytometry
Cell apoptosis was determined using the Annexin V-FITC/PI apoptosis detection kit (Sigma-Aldrich, St. Louis, USA). CHON-001 cells were washed three times with PBS, and then stained with 5 μL of propidium iodide (PI) and 5 μL of Annexin V-FITC at room temperature for 15 min in the presence of 50 μg/ml RNase A (Sigma). Subsequently, a flow cytometer (FACScan™, BD Biosciences, Franklin Lakes, NJ, USA) was used to measure the percentage of apoptotic cells.
Western blot assay
Total proteins were quantified using BCA protein assay kit (Beyotime Institute of Biotechnology), and then separated by 10% SDS-PAGE. After that, proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific). Later on, the membrane was blocked in 5% skimmed milk at room temperature for 1 h. After washing three times with TBST, the membrane was probed with the primary antibodies against Bax (1:1000, Abcam), Bcl-2 (1:1000, Abcam), Active caspase 3 (1:1000, Abcam), TLR4 (1:1000, Abcam), p-p65 (1:1000, Abcam), p65 (1:1000, Abcam), COL2A1 (1:1000, Abcam), Aggrecan (1:1000, Abcam), ADAMTS5 (1:1000, Abcam), MMP13 (1:1000, Abcam), Runx2 (1:1000, Abcam), Fibronectin 1 (1:1000, Abcam), E-cadherin (1:1000, Abcam), N-cadherin (1:1000, Abcam), and β-actin (1:1000, Abcam) at 4°C overnight. Subsequently, secondary antibodies (Abcam) were incubated with the membranes at 1:5000 dilution at room temperature for 1 h. The membrane was then visualized using an electrochemiluminescence (Thermo Fisher Scientific). β-actin was acted as the internal control.
ELISA
CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. After that, samples of the supernatant were collected from CHON-001 cells. Then, ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to assess the production of IL-6, TNF-α and IL-1β in CHON-001 cells.
Dual-luciferase reporter assay
Fibronectin 1 segment was synthesized with either wild-type (WT) or mutant (MT) seed region and then cloned into the psiCHECK-2 vector. After that, WT-fibronectin 1 or MT-fibronectin 1 plasmid was co-transfected with miR-613 agonist and NC respectively using lipofectamine 2000 in CHON-001 cells for 48 h. Subsequently, the dual luciferase reporter assay system (Promega, Madison, USA) was used to detect luciferase activity with renilla luciferase activity as endogenous control.
Mouse OA model
4-6-weeks C57BL/6 mice were purchased from the Hubei Provincial Center for Laboratory Animal, and mice were randomized into 3 groups: control, OA, and OA + miR-613 agonist group. The model of OA was established by anterior cruciate ligament transection (ACLT) in the right knees, as described previously [17,18]. Mice in OA + miR-613 agonist group were received an intraperitoneal injection of miR-613 agonist once-daily after surgery for 8 weeks. After that, all mice were euthanized, and the knee joint tissues were collected. The animal experiments were approved by the Animal Care Committee of the First Affiliated Hospital of Zhengzhou University.
Immunohistochemistry (IHC)
Joint samples were fixed in 4% paraformaldehyde, and then embedded in wax blocks. Each paraffin-embedded joint sample was cut into 5 μm, and then stained with Safranin-O/Fast Green (Sigma-Aldrich) to determine cartilage destruction. A fluorescence microscope was used to observed stained histological sections. Subchondral bone thickness was determined by an AxioVision software according to Safranin-O-stained sections. In addition, the synovitis scores and osteoarthritis research society international (OARSI) system was assessed histologically using scoring system as previously described [19].
Statistical analysis
All data were repeated in triplicate. Data are presented as the mean ± standard deviation (S.D.). All statistical analyses were performed using GraphPad Prism software (version 7.0, La Jolla, CA, USA). The results of RT-qPCR, western blot assay and the CCK-8, immunofluorescence, apoptosis and ELISA assays were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s tests. The results were considered significant at *P < 0.05.
Results
MiR-613 was downregulated in IL-1β-stimulated CHON-001 cells
To investigate the role of miR-613 on IL-1β-stimulated CHON-001 cells, RT-qPCR was applied. As shown in Figure 1A, the level of miR-613 was dramatically decreased in CHON-001 cells after exposure to IL-1β for 24 h. In addition, miR-613 agonist markedly upregulated the level of miR-613 in CHON-001 cells, compared with the NC group (Figure 1B). Moreover, IL-1β-induced miR-613 downregulation in CHON-001 cells were notably reversed following transfection with miR-613 agonist (Figure 1C). These data indicated that miR-613 was downregulated in IL-1β-stimulated CHON-001 cells.
Figure 1.
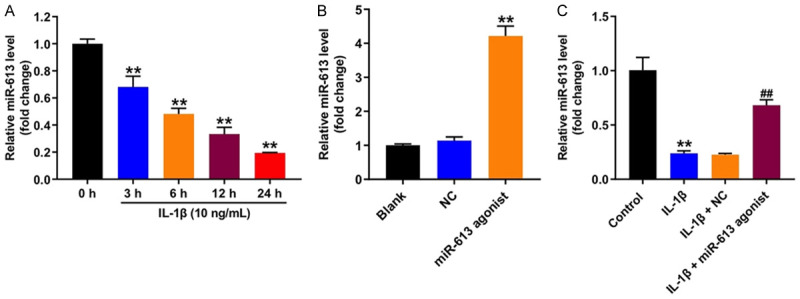
MiR-613 was downregulated in IL-1β-stimulated CHON-001 cells. A. CHON-001 cells were stimulated with 10 ng/mL IL-1β for 0, 3, 6, 12 and 24 h. CCK-8 assay was applied to determine the cell viability. **P < 0.01 compared with the control group. B. CHON-001 cells were transfected with miR-613 agonist or NC for 48 h. RT-qPCR was used to measure the level of miR-613 in cells. **P < 0.01 compared with the NC group. C. CHON-001 cells were transfected with miR-613 agonist or NC for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. RT-qPCR was used to measure the level of miR-613 in cells. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β + NC group.
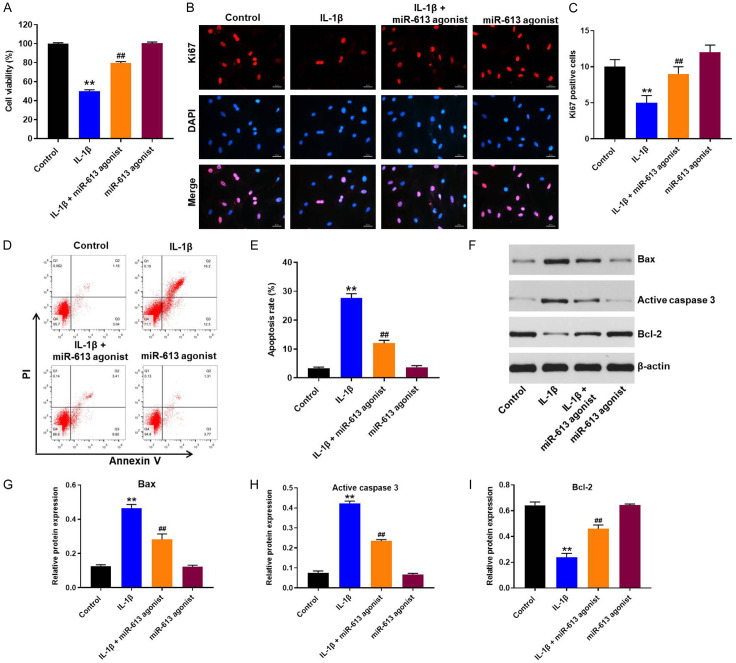
Overexpression of miR-613 inhibited IL-1β-induced apoptosis in CHON-001 cells
To determine the role of miR-613 on proliferation of CHON-001 cells, CCK-8 and immunofluorescence assays were applied. As indicated in Figure 2A-C, IL-1β treatment obviously suppressed the proliferation of CHON-001 cells, and that effect was markedly reversed by miR-613 overexpression. In addition, flow cytometry assay indicated that IL-1β significantly induced apoptosis of CHON-001 cells, and that effect was largely reversed by miR-613 overexpression (Figure 2D and 2E). Moreover, IL-1β notably upregulated the expressions of Bax and active caspase 3, and downregulated the expression of Bcl-2 in CHON-001 cells; however, these IL-1β-induced changes in protein expressions were reversed following transfection with miR-613 agonist (Figure 2F-I, Supplementary Figure 1). These results indicated that overexpression of miR-613 could inhibit IL-1β-induced apoptosis in CHON-001 cells.
Figure 2.
Overexpression of miR-613 inhibited IL-1β-induced apoptosis in CHON-001 cells. CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. A. CCK-8 assay was applied to determine the cell viability. B, C. Relative fluorescence expressions were quantified by Ki67 and DAPI staining. D, E. Apoptotic cells were measured by flow cytometry. F-I. Expression levels of Bax, active caspase 3, and Bcl-2 in CHON-001 cells were detected with western blot assay. Relative expression levels were quantified by normalization to β-actin. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β group.
Overexpression of miR-613 suppressed IL-1β-induced inflammatory response in CHON-001 cells
To investigate whether miR-613 could regulate IL-1β-induced inflammatory injury in CHON-001 cells, western blot was used. As shown in Figure 3A-C, IL-1β treatment notably increased the expressions of TLR4 and p-p65 in CHON-001 cells, and these IL-1β-induced changes were reversed by miR-613 overexpression. In addition, IL-1β significantly increased the levels of TNF-α, IL-6, and IL-8 in the supernatants of CHON-001 cells (Figure 3D-F). However, these IL-1β-induced changes were reversed by miR-613 overexpression (Figure 3D-F). These results illustrated that overexpression of miR-613 could suppress IL-1β-induced inflammatory response in CHON-001 cells.
Figure 3.
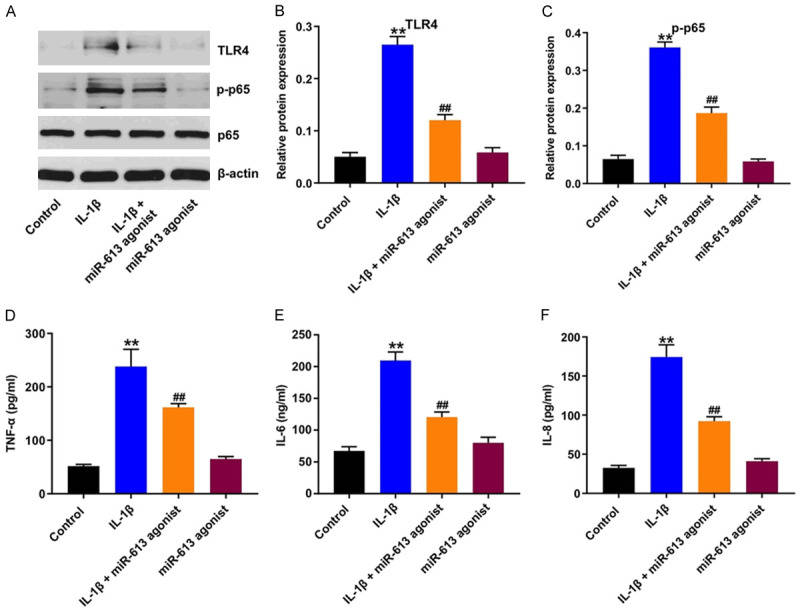
Overexpression of miR-613 suppressed IL-1β-induced inflammatory response in CHON-001 cells. CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. A-C. Expression levels of TLR4, p-p65 in CHON-001 cells were detected with western blot assay. Relative expression levels were quantified by normalization to β-actin and p65 respectively. D-F. ELISA assay was applied to measure the production of TNF-α, IL-6, and IL-8 in the supernatants of CHON-001 cells. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β group.
Overexpression of miR-613 alleviated IL-1β-induced cartilage matrix degradation in CHON-001 cells
To further investigate the role of miR-613 on IL-1β-stimulated CHON-001 cells, ECM-related proteins were detected by western blot. As shown in Figure 4A-E, IL-1β significantly downregulated the level of aggrecan, and upregulated the expressions of ADAMTS5, MMP13 and Runx2 in CHON-001 cells; however, these IL-1β-induced changes were reversed by miR-613 overexpression. These data indicated that overexpression of miR-613 could alleviate IL-1β-induced cartilage matrix degradation in CHON-001 cells.
Figure 4.
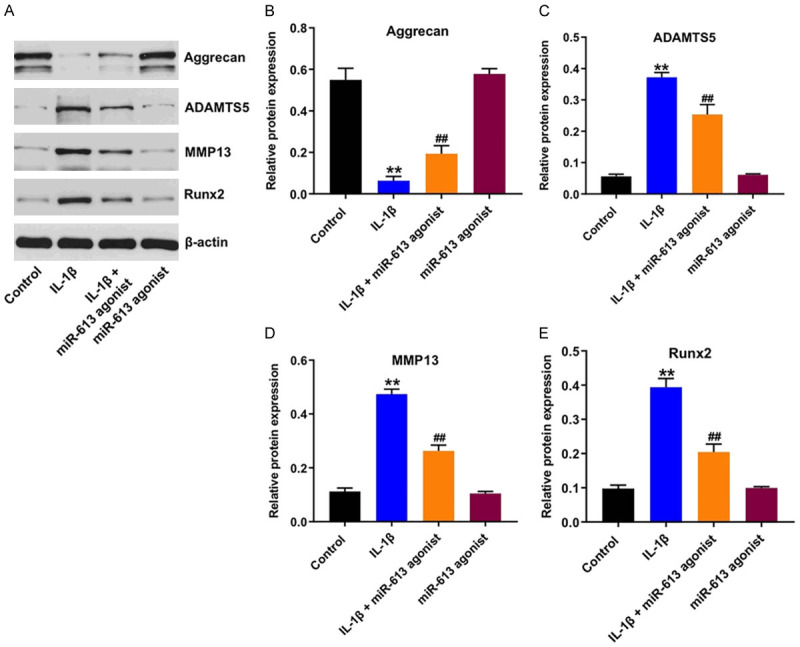
Overexpression of miR-613 alleviated IL-1β-induced cartilage matrix degradation in CHON-001 cells. CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. A-E. Expression levels of aggrecan, ADAMTS5, MMP13 and Runx2 in CHON-001 cells were detected with western blot assay. Relative expression levels were quantified by normalization to β-actin. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β group.
Fibronectin 1 was a directly binding target of miR-613
Next, targetScan dataset (http://www.targetscan.org/vert_71/) was used to predict the potential binding targets of miR-613. The data indicated that fibronectin 1 might be a potential target of miR-613 (Figure 5A). In addition, dual luciferase reporter assay validated that miR-613 agonist suppressed the luciferase activity of psiCHECK-2-fibronectin 1-WT in CHON-001 cells, but it did not affect the luciferase activity of psiCHECK-2-fibronectin 1-MT (Figure 5B). Moreover, overexpression of miR-613 notably suppressed the level of fibronectin 1 in CHON-001 cells (Figure 5C). Moreover, miR-613 antagonist markedly downregulated the level of miR-613 in CHON-001 cells, compared with the NC group (Figure 5D). Meanwhile, CCK-8 assay indicated that IL-1β or miR-613 antagonist treatment significantly inhibited the viability of CHON-001 cells (Figure 5E). As expected, the inhibitory effect of IL-1β on the viability of CHON-001 cells were further enhanced in the presence of miR-613 antagonist (Figure 5E). These results illustrated fibronectin 1 was a directly binding target of miR-613.
Figure 5.
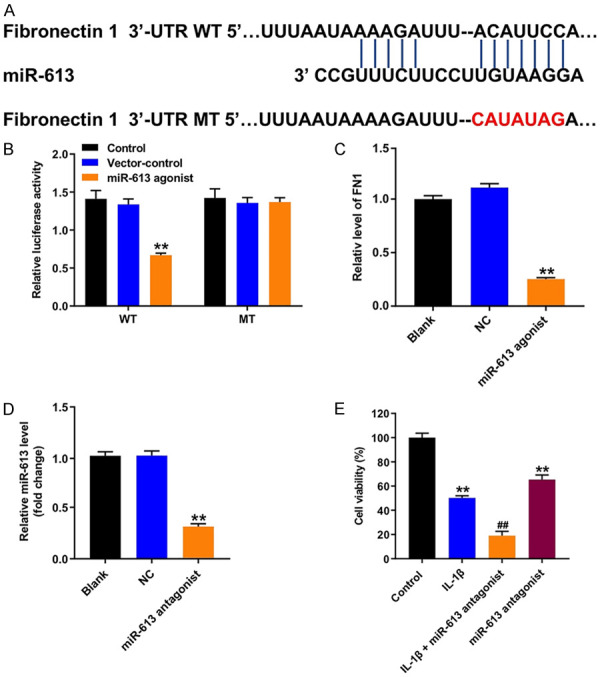
Fibronectin 1 was a directly binding target of miR-613. A. Sequence alignment of miR-613 with the binding sites within the WT or MT regions of fibronectin 1. B. Luciferase activity in CHON-001 cells following co-transfection with fibronectin 1-WT/MT 3’-UTR plasmid and miR-613 agonist measured using dual luciferase reporter assay. **P < 0.01 compared with the vector-control group. C. RT-qPCR analysis of fibronectin 1 level in CHON-001 cells transfected with miR-613 agonist. **P < 0.01 compared with the NC group. D. RT-qPCR analysis of miR-613 level in CHON-001 cells transfected with miR-613 antagonist. **P < 0.01 compared with the NC group. E. CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. CCK-8 assay was applied to determine the cell viability. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β group.
Overexpression of miR-613 alleviated IL-1β-induced injury in CHON-001 cells via downregulation of fibronectin 1
To explore the molecular mechanism by which miR-613 affects the progress of OA, the expression of fibronectin 1 in IL-1β-stimulated CHON-001 cells was evaluated. As indicated in Figure 6A-D, IL-1β markedly upregulated the expressions of fibronectin 1 and N-cadherin, and significantly downregulated the expression of E-cadherin in CHON-001 cells, but, these IL-1β-induced changes were reversed by miR-613 overexpression. These data indicated that overexpression of miR-613 could alleviate IL-1β-induced injury in CHON-001 cells via downregulation of fibronectin 1.
Figure 6.
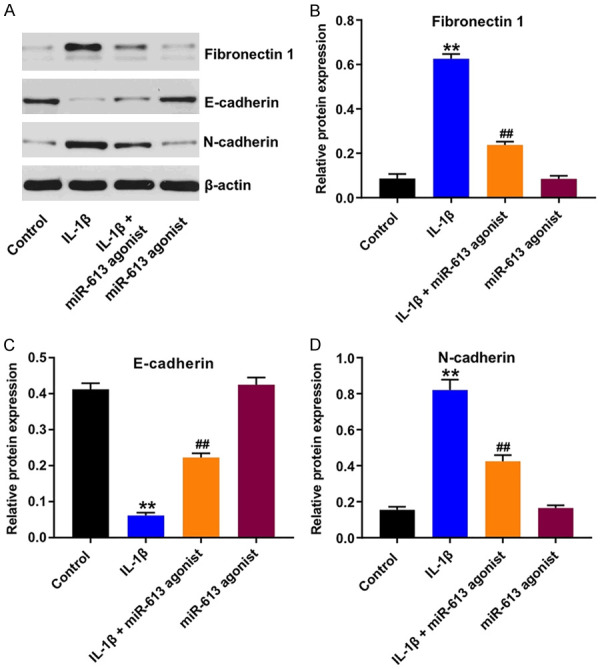
Overexpression of miR-613 alleviated IL-1β-induced injury in CHON-001 cells via downregulation of fibronectin 1. CHON-001 cells were transfected with miR-613 agonist for 48 h, and then exposed to 10 ng/mL IL-1β for 24 h. A-D. Expression levels of fibronectin 1, E-cadherin and N-cadherin in CHON-001 cells were detected with western blot assay. Relative expression levels were quantified by normalization to β-actin. **P < 0.01 compared with the control group; ##P < 0.01 compared with the IL-1β group.
Overexpression of miR-613 attenuated OA progression in a mouse model of OA
To investigate the role of miR-613 in progression of OA in vivo, a mouse model of OA was established. As shown in Figure 7A, the surface of the cartilage was undamaged and complete in the control group, whereas massive proteoglycan loss and worsening, and reduction in articular cartilage thickness were observed in the OA group. In contrast, miR-613 agonist obviously reduced the cartilage degradation and erosion (Figure 7A). Additionally, overexpression of miR-613 significantly reduced subchondral bone thickness and synovitis scores, compared to OA group (Figure 7B and 7C). Moreover, overexpression of miR-613 markedly reduced OARSI scores, compared to OA group (Figure 7D). These data illustrated that overexpression of miR-613 could attenuate OA progression in a mouse model of OA.
Figure 7.
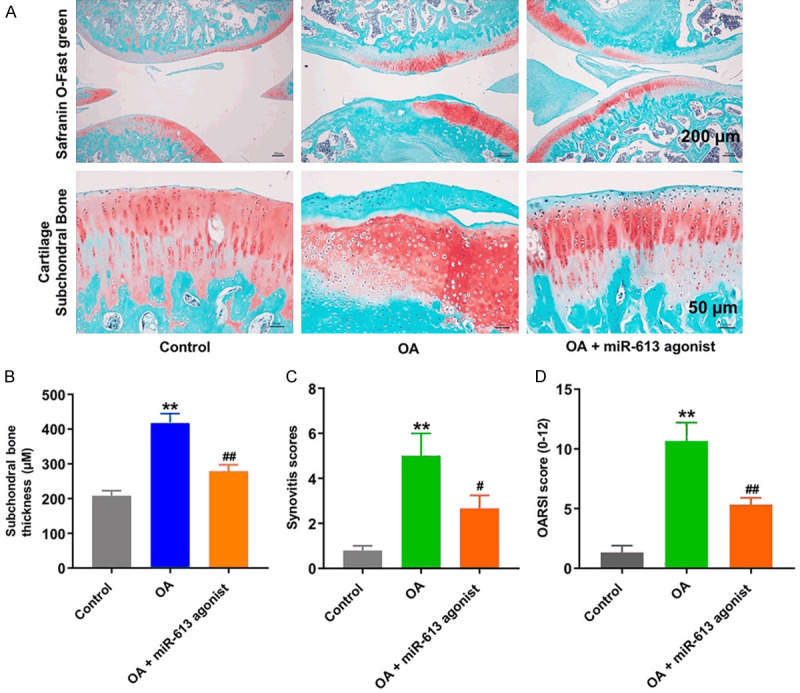
Overexpression of miR-613 attenuated OA progression in a mouse model of OA. (A) Histological analysis of OA was measured by Safranin O staining. (B) Subchondral bone thickness, (C) synovitis scores and (D) OARSI scores were used to measure the progression of OA. **P < 0.01 compared with the control group; ##P < 0.01 compared with OA group.
Discussion
OA is a kind of joint diseases, which could lead to joint dysfunction [20]. Evidence has been shown that a high rate of apoptosis in chondrocytes could contribute to matrix degradation [21]. In addition, inflammation also plays an important role in the development of OA, which lead to joint swelling and joint tissue degradation [22]. Chen et al found that IL-1β could induce apoptosis and inflammatory response in chondrocytes, which was consistent with our results [23]. Therefore, inhibition of chondrocyte apoptosis and inflammatory response might be the treatment options for OA [24].
It has been shown that several miRNAs exert anti-apoptotic and anti-inflammatory effects on OA [25,26]. However, the biological role of miR-613 in OA remains unclear. In recent years, miR-613 has attracted considerable interest in last several years due to its ability to regulate cell growth functions in different tissues of the human [27,28]. In this study, we found that upregulation of miR-613 could alleviate IL-1β-induced apoptosis in CHON-001 cells, as shown by the decreased expressions of Bax and active caspase 3. Meanwhile, upregulation of miR-613 could inhibit IL-1β-induced inflammatory response in CHON-001 cells, as evidenced by the decreased signals of TLR4, p-p65, TNF-α, IL-6, and IL-8. Papathanasiou et al indicated that miR-140-5p and miR-146a exhibited an anti-inflammatory effect in OA chondrocytes via suppressing the TLR4/NF-κB signaling [29]. These data indicated that overexpression of miR-613 could alleviate IL-1β-induced apoptosis and inflammatory response on CHON-001 cells. In addition, our data indicated that overexpression of miR-613 could attenuate OA progression in a mouse model of OA. These results suggested that overexpression of miR-613 could attenuate the progression of OA in vitro and in vivo, indicating that miR-613 may be employed as a therapeutic biomarker for OA.
Previous study indicated that type II collagen and aggrecan are the two main components of the ECM of cartilage [30]. In addition, MMPs and ADAMTS family play key roles in OA via degrading cartilage and ECM [31]. Moreover, the expression of Runx2 is increased in chondrocytes during OA, and Runx2 could induce the production of ADAMTS5 and MMP13 in chondrocytes [32]. IL-1β has been indicated to induce MMP-13, ADAMTS5 and Runx2 in chondrocytes [33-35]. Our data indicated that IL-1β significantly reduced the expression of aggrecan and increased the expressions of ADAMTS5, MMP13 and Runx2 in CHON-001 cells, which were consistent with previous study. However, these IL-1β-induced changes were markedly reversed by miR-613 overexpression. Our data found that overexpression of miR-613 alleviated IL-1β-induced upregulation of Runx2, and further decreased IL-1β-induced production of MMP-13 and ADAMTS5, and then reversed IL-1β-induced downregulation of aggrecan in CHON-001 cells. These results illustrated that overexpression of miR-613 could play a chondro-protective effect by promoting chondrocyte ECM synthesis.
It is believed that miRNAs exert their function primarily via suppression of target genes [36]. To explore the underlying mechanism of miR-613 in the progression of OA, bioinformatics analysis and luciferase reporter assay were used to identify potential binding targets of miR-613. The data indicated that fibronectin 1 was identified as a binding target of miR-613. Fibronectin 1 is a class of cleave ECM components [37]. In addition, the expression of N-cadherin was upregulated and the expression of E-cadherin was downregulated in patients with OA [38,39]. Our data indicated that IL-1β significantly upregulated the levels of fibronectin 1 and N-cadherin, and downregulated the expression of E-cadherin in CHON-001 cells, but, these changes were markedly reversed by miR-613 overexpression. Collectively, these results suggested that overexpression of miR-613 could alleviate IL-1β-induced injury in CHON-001 cells via downregulation of fibronectin 1.
Conclusion
Taken together, this study demonstrated that overexpression of miR-613 could inhibit apoptosis, inflammatory response and cartilage matrix degradation in IL-1β-stimulated CHON-001 cells via targeting fibronectin 1. In addition, overexpression of miR-613 could alleviate cartilage degradation as well as synovitis in a mouse model of OA. These data indicate that miR-613 might be regarded as a promising therapeutic target for the treatment of OA.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gao Y, Zhao H, Li Y. LncRNA MCM3AP-AS1 regulates miR-142-3p/HMGB1 to promote LPS-induced chondrocyte apoptosis. BMC Musculoskelet Disord. 2019;20:605. doi: 10.1186/s12891-019-2967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mobasheri A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr Rheumatol Rep. 2013;15:364. doi: 10.1007/s11926-013-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X, Jiang S, Du Z, Ke A, Liang Q, Li X. KLF2 protects against osteoarthritis by repressing oxidative response through activation of Nrf2/ARE signaling in vitro and in vivo. Oxid Med Cell Longev. 2019;2019:8564681. doi: 10.1155/2019/8564681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang X, Ni B, Xi Y, Chu X, Zhang R, You H. Protease-activated receptor 2 (PAR-2) antagonist AZ3451 as a novel therapeutic agent for osteoarthritis. Aging (Albany NY) 2019;11:12532–12545. doi: 10.18632/aging.102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asjid R, Faisal T, Qamar K, Khan SA, Khalil A, Zia MS. Platelet-rich plasma-induced inhibition of chondrocyte apoptosis directly affects cartilage thickness in osteoarthritis. Cureus. 2019;11:e6050. doi: 10.7759/cureus.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nees TA, Rosshirt N, Reiner T, Schiltenwolf M, Moradi B. Inflammation and osteoarthritis-related pain. Schmerz. 2019;33:4–12. doi: 10.1007/s00482-018-0346-y. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–49. doi: 10.1016/j.tvjl.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Yao J, Hao Q, Duan Z. miRNA-103 promotes chondrocyte apoptosis by down-regulation of Sphingosine kinase-1 and ameliorates PI3K/AKT pathway in osteoarthritis. Biosci Rep. 2019;39:BSR20191255. doi: 10.1042/BSR20191255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Shen J, Chan MT, Wu WK. MicroRNA-379 suppresses osteosarcoma progression by targeting PDK1. J Cell Mol Med. 2017;21:315–323. doi: 10.1111/jcmm.12966. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhang Y, Wang G, Ma L, Wang C, Wang L, Guo Y, Zhao X. miR-137 suppresses cell growth and extracellular matrixdegradation through regulating ADAMTS-5 in chondrocytes. Am J Transl Res. 2019;11:7027–7034. [PMC free article] [PubMed] [Google Scholar]
- 13.Asahara H. Current status and strategy of microrna research for cartilage development and osteoarthritis pathogenesis. J Bone Metab. 2016;23:121–127. doi: 10.11005/jbm.2016.23.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Yan X, Zhang M, Ji F, Wang S. miR-21-5p protects IL-1beta-induced human chondrocytes from degradation. J Orthop Surg Res. 2019;14:118. doi: 10.1186/s13018-019-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164–172. doi: 10.1016/j.lfs.2019.03.071. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Zuo Y, Xu Y, Zhang Z, Li Y, Pang J. MiR-613 inhibits proliferation and invasion and induces apoptosis of rheumatoid arthritis synovial fibroblasts by direct down-regulation of DKK1. Cell Mol Biol Lett. 2019;24:8. doi: 10.1186/s11658-018-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Ji ML, Zhang XJ, Shi PL, Wu H, Wang C, Im HJ. MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther. 2017;25:2676–2688. doi: 10.1016/j.ymthe.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Fan J, Ding X, Sun Y, Cui Z, Liu W. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Devel Ther. 2019;13:3559–3568. doi: 10.2147/DDDT.S216596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin W, Lei Y. Leonurine inhibits IL-1beta induced inflammation in murine chondrocytes and ameliorates murine osteoarthritis. Int Immunopharmacol. 2018;65:50–59. doi: 10.1016/j.intimp.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res. 2017;35:735–739. doi: 10.1002/jor.23471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Gu YT, Xie JJ, Wu CC, Xuan J, Guo WJ, Yan YZ, Chen L, Wu YS, Zhang XL, Xiao J, Wang XY. Gastrodin reduces IL-1beta-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed Pharmacother. 2018;97:642–651. doi: 10.1016/j.biopha.2017.10.067. [DOI] [PubMed] [Google Scholar]
- 24.Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z, Zhang J, Pan J. Geniposide suppresses interleukin-1beta-induced inflammation and apoptosis in rat chondrocytes via the PI3K/Akt/NF-kappaB signaling pathway. Inflammation. 2018;41:390–399. doi: 10.1007/s10753-017-0694-2. [DOI] [PubMed] [Google Scholar]
- 25.Tu Y, Ma T, Wen T, Yang T, Xue L, Cai M, Wang F, Guan M, Xue H. MicroRNA-377-3p alleviates IL-1beta-caused chondrocyte apoptosis and cartilage degradation in osteoarthritis in part by downregulating ITGA6. Biochem Biophys Res Commun. 2019;23:46–53. doi: 10.1016/j.bbrc.2019.11.186. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. Corrigendum to “miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis” [Life Sci. 226 (2019) 164-172] . Life Sci. 2020;240:117135. doi: 10.1016/j.lfs.2019.117135. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Qi Y, Guo Z, Li P, Zhou D. miR-613 suppresses ischemia-reperfusion-induced cardiomyocyte apoptosis by targeting the programmed cell death 10 gene. Biosci Trends. 2016;10:251–257. doi: 10.5582/bst.2016.01122. [DOI] [PubMed] [Google Scholar]
- 28.Gao R, Feng Q, Tan G. microRNA-613 exerts anti-angiogenic effect on nasopharyngeal carcinoma cells through inactivating the AKT signaling pathway by down-regulating FN1. Biosci Rep. 2019;39:BSR20182196. doi: 10.1042/BSR20182196. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Papathanasiou I, Balis C, Trachana V, Mourmoura E, Tsezou A. The synergistic function of miR-140-5p and miR-146a on TLR4-mediated cytokine secretion in osteoarthritic chondrocytes. Biochem Biophys Res Commun. 2019;522:783–791. doi: 10.1016/j.bbrc.2019.11.168. [DOI] [PubMed] [Google Scholar]
- 30.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 31.Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9:247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-García S, Carrión M, Villanueva-Romero R, Hermida-Gómez T, Fernández-Moreno M, Mellado M, Blanco FJ, Juarranz Y, Gomariz RP. Wnt and RUNX2 mediate cartilage breakdown by osteoarthritis synovial fibroblast-derived ADAMTS-7 and -12. J Cell Mol Med. 2019;23:3974–3983. doi: 10.1111/jcmm.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-García S, Gutiérrez-Cañas I, Seoane IV, Fernández J, Mellado M, Leceta J, Tío L, Villanueva-Romero R, Juarranz Y, Gomariz RP. Healthy and osteoarthritic synovial fibroblasts produce a disintegrin and metalloproteinase with thrombospondin motifs 4, 5, 7, and 12: induction by IL-1β and fibronectin and contribution to cartilage damage. Am J Pathol. 2016;186:2449–2461. doi: 10.1016/j.ajpath.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Ma S, Su H, Cheng J. Isoliquiritigenin inhibits IL-1β-induced production of matrix metalloproteinase in articular chondrocytes. Mol Ther Methods Clin Dev. 2018;9:153–159. doi: 10.1016/j.omtm.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei Y, Harvey A, Yu XP, Chandrasekhar S, Thirunavukkarasu K. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: potential role of Runx2 in mediating p38 effects. Osteoarthritis Cartilage. 2006;14:749–758. doi: 10.1016/j.joca.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Davis BN, Hata A. Regulation of microRNA biogenesis: a miRiad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-García S, Carrión M, Gutiérrez-Cañas I, Villanueva-Romero R, Castro D, Martínez C, González-Álvaro I, Blanco FJ, Juarranz Y, Gomariz RP. Profile of matrix-remodeling proteinases in osteoarthritis: impact of fibronectin. Cells. 2019;9:40. doi: 10.3390/cells9010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruedel A, Stark K, Kaufmann S, Bauer R, Reinders J, Rovensky J, Blazicková S, Oefner PJ, Bosserhoff AK. N-cadherin promoter polymorphisms and risk of osteoarthritis. FASEB J. 2014;28:683–691. doi: 10.1096/fj.13-238295. [DOI] [PubMed] [Google Scholar]
- 39.Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, Stuchin SA, Patel IR, Abramson SB. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231–1237. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.