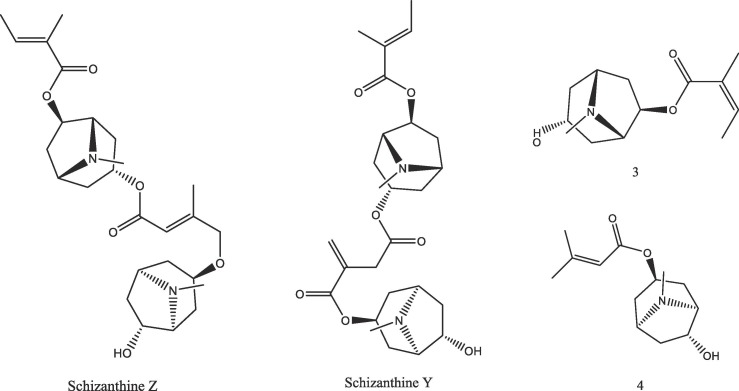
Graphical abstract

Keywords: SARS-CoV-2, Tropane alkaloids, Schizanthus porrigens, Molecular docking
Abstract
This paper presents identification of potential inhibitors of SARS-CoV-2 papain-like protease from tropane alkaloids from Schizanthus porrigens, using molecular docking method. Binding affinities were compared with those obtained with Lopinavir as a SARS-CoV-2 papain-like protease inhibitor. Overall, our findings indicate that Schizanthine Z binds to the SARS-CoV-2 papain-like protease with relatively high affinity and favorable ADME properties. Therefore, Schizanthine Z may represent an appropriate compound for further evaluation in antiviral assays.
1. Introduction
Coronavirus disease 2019 COVID-19 causes severe acute respiratory syndrome (SARS), and therefore this new virus has been given the name severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. Coronaviruses non-structural polyprotein is processed by two proteases, the main protease (Mpro) and the papain-like protease (PLpro), inhibition of PLpro affects virus replication through poor viral protein processing and can also affect activities of other PLpro [2]. Other PLpro that can be affected are desubiquitination proteases, which recognize ubiquitin through an enzymatic ubiquitin binding site and can specifically bind and cleave polyubiquitin by binding to diubiquitin through the ubiquitin active site, in contrast, SARS PLpro recognizes Lys48 bound polyubiquitin through ubiquitin binding sites and is, therefore, capable of directly removing Lys48 bound diubiquitin from substrates [3]. Maiti (2020) reports that the PLpro encoded by SARS-CoV-2 has a labile Zn site formed by the amino acids Cys189, Cys192, Cys224 and Cys226 and a classic catalytic site formed by the amino acids Cys111, His272 and Asp286, which play key functions for viral replication and represents promising drug targets, because sulfur-based drugs such as peptide-based inhibitors can block Cys residues at the catalytic site or Zn site of CoV-2-PLpro, which leads to CoV-2-PLpro dysfunction and thus stops viral replication [4]. Moreover, de-ISGylation proteases [5] and innate anti-host immunity reactions can also be affected, PLpro comprises a central catalytic domain and the ubiquitin-like domain, which interferes with the host's antiviral pathways and modulates the immune response of the host, furthermore PLpro subdomains showed different reaction patterns in presence or absence of ubiquitin, lastly, it should be mentioned that PLpro was an important drug target against several Coronaviruses, including MERS CoV [2]. Freitas et al (2020) reported that naphthalene-based PLpro inhibitors are effective against SARS-CoV-2 [6], other researchers have also reported that a naphthalene derivative inhibited SARS-CoV-2 PLpro [3], [7]. Due to the above, the search for new drug prospects is the most reliable option to design an efficient therapy for infected patients without delay [8], because the development of vaccines against the SARS-CoV-2 virus can take many months. Furthermore, virus-encoded peptide-based vaccines may not be effective against future coronavirus epidemics, as virus mutations could render them useless [9]. In the pharmaceutical industry the development and discovery of new drugs is the main driver of it. In the past, the design of drugs was directly related to Chemistry of natural products, which were later modified by organic synthesis. Currently, the development of more powerful and efficient computers has allowed the generation of methodologies and simulations that have optimized this process [10]. However, Natural Products Chemistry continues to be a source for obtaining drugs, for example, S. porrigens Graham is an herbaceous plant that grows only in humid soils in northern Chile (IV Region) [11]. Studies on Schizanthus have shown that a series of tropane-derived alkaloids accumulate in this genus [12], which have anticholinergic activity [13], antiemetic [14], anesthetics [15], mydriatics [16], antispasmodics [17], bronchodilators [18] and antivirals [19]. Species Schizanthus porrigens, native to Chile and Argentina [20], was chosen as source of potential inhibitors for SARS-CoV-2 papain-like protease, which have not yet been investigated, choosing molecular docking for carry out the investigation in silico of tropane alkaloids of Schizanthus porrigens since this method predicts the orientation of a ligand that binds to a protein forming a stable complex and the strength of the bonds generated between them [21], [22]. Molecular docking is one of the most frequently used methods in the design of structure-based drugs, due to the ability to predict the conformation of the ligand to the binding site [23]. Kandeel et al (2020) investigated PLpro use of SARS-CoV-2 to select virtually 1,697 FDA-approved clinical drugs, finding that Losartan, Montelukast Sodium, Quercitrin, Zanamivir, NAD1, Candesartan, Chlorhexidine chlorhydrate and Fostamatinib disodium exhibited binding energy superiors to −7.5 kcal/mol [2], and within the natural compounds Laskar and Choudhury (2020) repot Baicalin, Quercetin, Licoleafol, Biopterin, Luteolin and Rutin with binding energy equal to or greater than −25 kcal/mol [24]. The characterization of the protein ligand bond in the cases of viral proteins plays an important role in the search for drugs [25]. Thus the objective of this work was the identification of potential inhibitors of SARS-CoV-2 papain-like protease from tropane alkaloids from Schizanthus porrigens using molecular docking.
2. Materials and methods
SARS-CoV-2 papain-like protease is essential for virus maturation and infectivity [26], [27]. The crystalline structure of the papain-like protease was obtained from the Protein Data Bank (https://www.rcsb.org/) code 6WX4 [28]. The water molecules together with the VIR251 ligand were removed and then the hydrogen atoms were added to the protein to correct the ionization of the amino acid residues of the protein using the Discovery Studio 2020 Client program [29], in order to prepare the protein to effect molecular docking. The obtained protein was saved in pdb format and imported into the PyRx program [30] to perform the molecular docking, which was carried out with the Autodock Vina program [30]. Fig. 1 shows complex between SARS-CoV-2 papain-like protease and inhibitor VIR251. Fig. 2 shows the 2D structure of tropane alkaloids from Schizanthus porrigens, which were imported from the ChemDraw 3D program [31] in sdf format. The most stable conformer was determined with the PyPx program [30], then the ADME properties (Absorption, Distribution, Metabolism, and Excretion) were analyzed using the SwissADME database (http://www.swissadme.ch/) [32]. Since the main objective in molecular docking is to evaluate protein–ligand interactions to predict binding affinity [33], Autodock Vina [34] and PyRx [30] were used to determine the orientations of the ligands in the active site of SARS-CoV-2 papain-like protease, according to those reported by Rut et al. (2020), deep hydrophobic pocket formed by the residues Asp164, Met208, Pro247, Pro248, Tyr264, Tyr268, Tyr273 y Thr301 was considered as active site [28]. Discovery Studio 2020 Client software [29] was used to determine and illustrate the intermolecular interactions between tropane alkaloids and residues of active site SARS-CoV-2 papain-like protease. The molecular dynamics simulation was performed using NAMD v.2.14 [35], [36] with CHARMM36 force field [37], [38], [39]. Then each structure was converted to a psf protein structure file by automatic psf generation plugin within the VMD v program. 1.9.3 [40]. The simulation was carried out with 5000 steps that were carried out for all the atoms of the system to ensure the elimination of any residual steric shock [41]. Then, the structure was simulated at a constant temperature of 310 K and a pressure of 1 atm using Langevin dynamics [42].
Fig. 1.
Complex between the papain-like protease of SARS-CoV-2 and the crystallized inhibitor VIR251. Source Protein Data Bank (https://www.rcsb.org/) code 6WX4.
Fig. 2.
2D structure of tropane alkaloids from Schizanthus porrigens.
3. Results and discussion
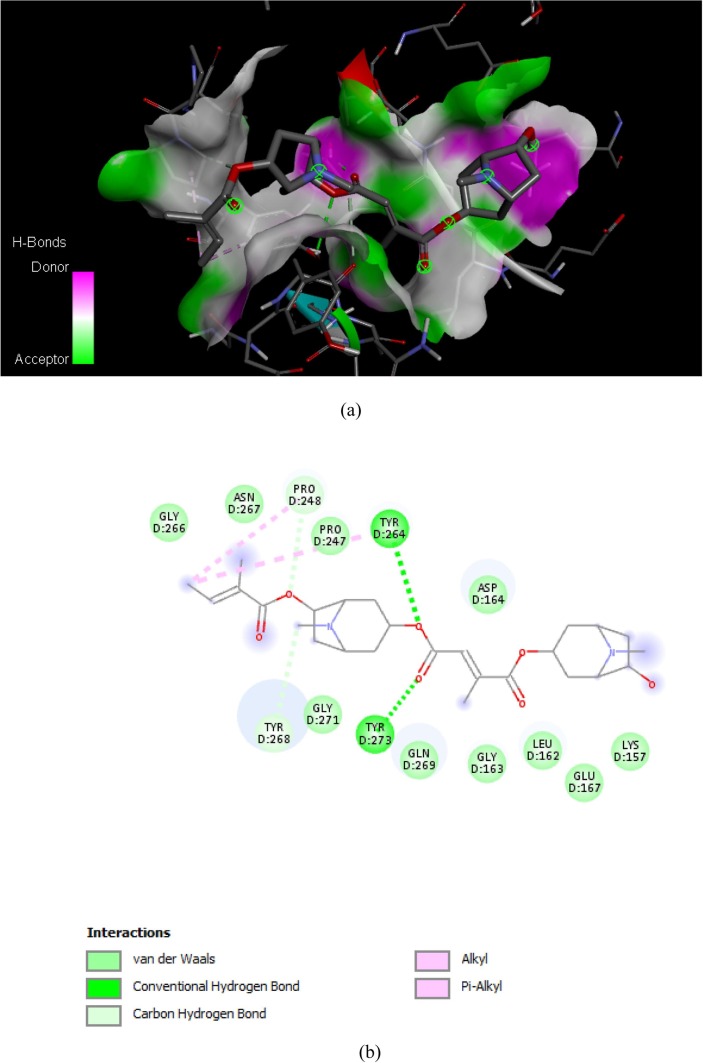
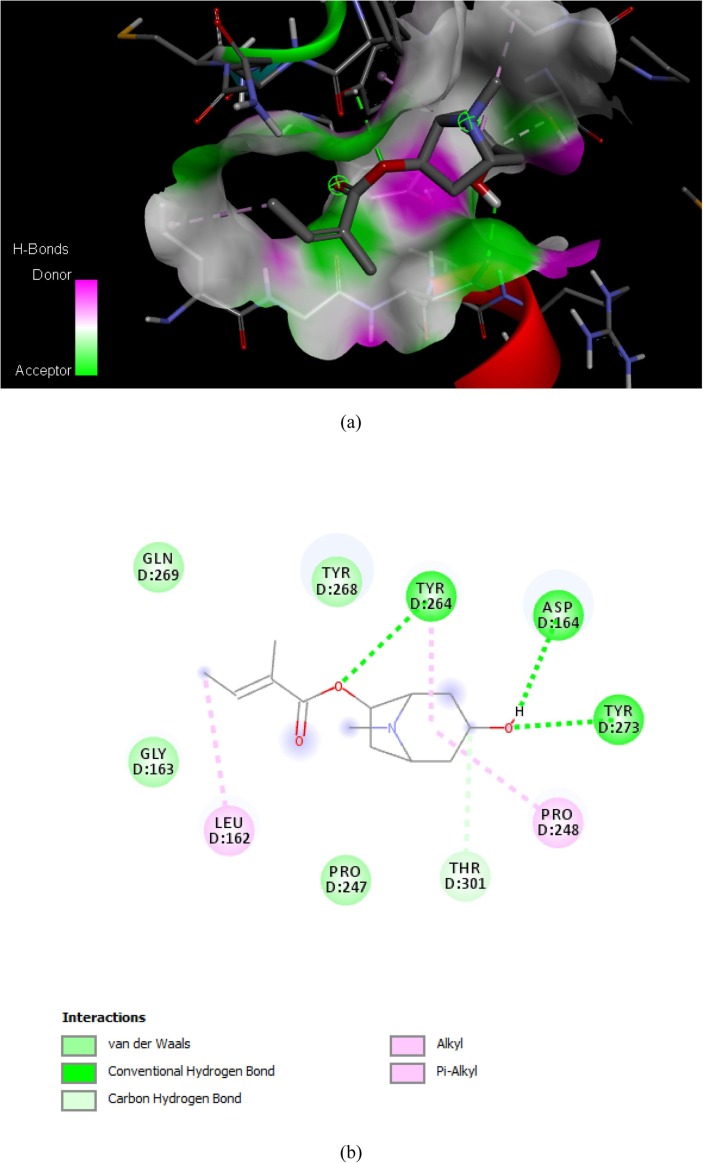
Binding affinities and major interactions between SARS-CoV-2 papain-like protease and tropane alkaloids from Schizanthus porrigens were obtained using Autodock Vina. Binding affinities were compared with those obtained with Lopinavir as inhibitor of the SARS-CoV-2 papain-like protease. The binding affinity obtained from 6WX4 binding with the Lopinavir and tropane alkaloids from Schizanthus porrigens are shown in Table 1 . The binding affinity values vary from −5.6 to −7.5 kcal/mol. Discovery Studio 2020 Client software was used to illustrate the molecular interactions between active site SARS-CoV-2 papain-like protease and the ligands. The ligands showed the expected interactions with the amino acids present in active site of protein, which implies powerful antagonistic properties towards SARS-CoV-2 papain-like protease. Exhibiting the highest binding affinity Schizantina Z with a value of −7.5 kcal/mol followed by Schizantina Y with a binding energy value of −7.1 kcal/mol. The ligands with the lowest values obtained in this study correspond to ligands 3 and 4 with binding affinity that −5.7 kcal/mol and −5.6 kcal/mol respectively. In general, the binding affinity values of Schizantin Z and Schizantin Y obtained from the set of 4 tropane alkaloids from Schizanthus porrigens suggest that they can be used as potential inhibitors of SARS-CoV-2 papain-like protease. The binding energies of all compounds are close to that of the reference ligand, except for compounds 3 and 4. As shown in Fig. 3, Fig. 4, Fig. 5, Fig. 6 complexes between ligands and receptor are stabilized mainly by hydrogen bonds, where the ligand can act as electron acceptor, like in the case of Schizanthine Z or a simultaneous donor and acceptor as in the case of Schizanthine Y, however, other interactions such as hydrophobic interactions also contribute to the stability of the complexes [33]. Table 2 shows that all of tropane alkaloids from Schizanthus porrigens that form hydrogen bonds with the key amino acid residues of SARS-CoV-2 papain-like protease. Natural products provide a source for the development of new antiviral drugs [43]. Identifying the antiviral mechanisms of these natural agents has shed light on where they interact with the virus life cycle, such as viral entry, replication, assembly, and release, as well as on the goal of virus-specific interactions and host [33]. In the present work, we summarize the antiviral activity of tropane-derived alkaloids present in the shrub plant Schizanthus porrigens that grows in soils in northern Chile (IV Region) [11] and perform molecular docking of these compounds. (four ligands) with the SARS-CoV-2 papain-like protease to identify possible inhibitors of this, as well as the ADME analysis [32]. This is vital since several proteins have recently been described that play a key role in SARS-CoV-2 virus infection and that are considered possible pharmacological targets. These include in addition to the SARS-CoV-2 papain-like protease considered in the present study, the SARS-CoV-2 main protease Mpro [7], RNA-dependent RNA polymerase (NPS12) [44], spike glycoprotein (S) [16], transmembrane protease serine 2 (TMPRSS2) [45] and angiotensin-converting enzyme 2 (ACE2) [46] among others. Therefore, it should be borne in mind that the best candidate for a drug against SARS-CoV-2 is the molecule that can specifically bind to one of the pharmacological targets mentioned above to form a thermodynamically stable complex, this is a compound with the energy of highest possible binding expressed in terms of Gibbs free energy variation (ΔG) [33]. Lipinski's rule of five [47] and ADME properties [47] which were obtained from SwissADME see Table 3, Table 4 . The results of virtual detection of two tropane alkaloids from Schizanthus porrigens based on molecular docking scores, hydrogen bridge interactions, and Lipinski's rule of five suggest that they are potential inhibitors of the major protease SARS-CoV-2 papain-like protease, which is essential for virus maturation and infectivity [26], [27]. Schizanthine Z is the best drug candidate between the two successes since, in addition to fully obeying Lipinski's rules, it exhibits the highest binding energy. Schizanthine Z, being the one with the highest docking score and the most favorable pharmacokinetics was subjected to a molecular dynamics simulation. Schizanthine Y was also subjected to a molecular dynamics simulation since it was the next best candidate. Fig. 7 shows a water box simulations using molecular dynamics simulation of Schizanthine Z and Schizanthine Y papain-like protease complexes of SARS-CoV-2 [2]. Fig. 8 shows the mean square deviation (RMSD), which was used to examine the structural changes of the Schizanthine Z and Schizanthine Y papain-like protease complexes of SARS-CoV-2, both graphs show a similar deviation and fluctuation, validating the consistency of the simulation trajectories [41].
Table 1.
Binding affinity (kcal/mol) of 6WX4 and Ligands.
| Receptor PDB ID | Ligands | Binding Affinity (ΔG in kcal/mol) |
|---|---|---|
| 6WX4 | Schizanthine Y | −7.1 |
| Schizanthine Z | −7.5 | |
| 3 | −5.7 | |
| 4 | −5.6 | |
| Lopinavir (Ref. ligand) | −7.0 |
Fig. 3.
(a) H-bonding interactions between the Schizanthine Z with papain-like protease of SARS-CoV-2 target; (b) All types of interactions between the Schizanthine Z with papain-like protease of SARS-CoV-2.
Fig. 4.
(a) H-bonding interactions between the Schizanthine Y with papain-like protease of SARS-CoV-2 target; (b) All types of interactions between the Schizanthine Y with papain-like protease of SARS-CoV-2.
Fig. 5.
(a) H-bonding interactions between the Compound 3 with papain-like protease of SARS-CoV-2 target; (b) All types of interactions between the Compound 3 with papain-like protease of SARS-CoV-2.
Fig. 6.
(a) H-bonding interactions between the Compound 4 with papain-like protease of SARS-CoV-2 target; (b) All types of interactions between the Compound 4 with papain-like protease of SARS-CoV-2.
Table 2.
Amino acids residues involved in H-bonds in both ligands and receptors.
| Ligands | Amino acids with H-bonds |
|---|---|
| Schizanthine Y | TYR164, TYR168, TYR173 |
| Schizanthine Z | TYR164, TYR173, |
| 3 | ASP164, TYR164, TYR173 |
| 4 | ASP164 |
| Lopinavir | TYR168, GLN269 |
Table 3.
Lipinski parameters for dataset from SwissADME.
| Inhibitor | MW | Log P | HBD | HBA | Violation | Yes/No | Solubility | Log S |
|---|---|---|---|---|---|---|---|---|
| Schizanthine Y | 490,59 | 4,52 | 1 | 9 | 0 | Yes | M.S. | −4.62 |
| Schizanthine Z | 490.59 | 4.33 | 1 | 9 | 0 | Yes | M.S. | −4.71 |
| 3 | 239.31 | 2,51 | 1 | 4 | 0 | Yes | S | −2.20 |
| 4 | 239.31 | 2,51 | 1 | 4 | 0 | Yes | S | −2.46 |
MW: Molecular weight (Da), Log P: Octanol-water partition coefficient, HBD: Hydrogen bond donors, HBA: Hydrogen bond acceptors Log S: 10-based logarithm of the solubility measured in mol/l unit.
Table 4.
Pharmacokinetics of the four potential inhibitors.
| Inhibitor | GIa | BBBp | P-gps | 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | Log Kp |
|---|---|---|---|---|---|---|---|---|---|
| Schizanthine Y | High | No | No | No | No | No | No | No | −7.34 |
| Schizanthine Z | High | No | No | No | No | No | No | No | −7.28 |
| 3 | High | Yes | No | No | No | No | No | No | −6.65 |
| 4 | High | Yes | No | No | No | No | No | No | −6.48 |
Fig. 7.
(a) Water box simulation using molecular dynamics simulation of complex between the papain-like protease of SARS-CoV-2 and the Schizanthine Z. (b) Water box simulation using molecular dynamics simulation of complex between the papain-like protease of SARS-CoV-2 and the Schizanthine Y.
Fig. 8.
(a) The Root Mean Square Deviation (RMSD) curve of complex between the papain-like protease of SARS-CoV-2 and the Schizanthine Z. (b) The Root Mean Square Deviation (RMSD) curve of complex between the papain-like protease of SARS-CoV-2 and the Schizanthine Y.
4. Conclusions
The development of new drugs to treat COVID-19 is of vital importance to design an efficient therapy, because development of vaccines against SARS-CoV-2 virus can take many months. Furthermore, virus-encoded peptide-based vaccines may not be effective against future coronavirus epidemics, as virus mutations could render them useless. While researchers are looking for molecules with anti-SARS-CoV-2 activity using organic synthesis techniques, another way is to promote chemistry research on natural products. Considering the enormous amount of existing phytocomposites, we can refer to computational chemistry to help in the study of different plant activities such as Schizanthus porrigens candidates from the set of 4 tropane alkaloids from Schizanthus porrigens using molecular docking and ADME properties. The reactivity of SARS-CoV-2 papain-like protease with 4 tropane alkaloids from Schizanthus porrigens showed that the most stable complex is obtained with Schizanthine Z with a binding affinity value of −7.5 kcal/mol, followed by Schizanthine Y with −7.1 kcal/mol. Finally, Lipinski's rule of five based on ADME analysis and molecular dynamics simulation confirms that Schizanthine Z is the best candidate for the drug, which is why it is recommended for in vitro tests.
CRediT authorship contribution statement
Marco Alfaro: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Ignacio Alfaro: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Writing - original draft, Writing - review & editing. Constanza Angel: Resources, Software, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cplett.2020.138068.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chhikara F., Rathi B.S., Singh B., Poonam J. Corona virus SARS-CoV-2 disease COVID-19: infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem. Biol. Lett. 2020;7(1):63–72. [Google Scholar]
- 2.Kandeel M., Abdelrahman A.H.M., Oh-hashi K., Ibrahim A., Katharigatta N. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J. Biomol. Struct. Dyn. 2020:1–8. doi: 10.1080/07391102.2020.1784291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemm T., et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV- 2. EMBO J. 2020;39:1–17. doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.B. K. Maiti, Can Papain-like Protease Inhibitors Halt SARS-CoV ‑ 2 Replication?, (1) (2020), 2–4. [DOI] [PMC free article] [PubMed]
- 5.K. Ratia, A. Kilianski, Y. M. Baez-santos, S. C. Baker, A. Mesecar, Structural basis for the ubiquitin-linkage specificity and deISGylating activity of SARS-CoV papain-like protease, 10 (5) (2014). [DOI] [PMC free article] [PubMed]
- 6.B.T. Freitas, et al., Characterization and noncovalent inhibition of the deubiquitinase and deISGylase activity of SARS-CoV - 2 papain-like protease, 15 (2020). [DOI] [PubMed]
- 7.Jin Y., Du Z., Xu X., Deng Y., Liu Y., Zhao M., Zhang Y., Li B., Zhang X., Peng L., Duan C. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 8.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents J. 2020 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020:2–5. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prieto-Martínez J.L., Medina-Franco F.D. Diseño de fármacos asistido por computadora: cuando la informática, la química y el arte se encuentran. TIP Rev. Espec. en Ciencias Químico-Biológicas. 2018;21(2):124–134. [Google Scholar]
- 11.Muñoz O., Cortés S. Tropane Alkaloids from Schizanthus porrigens. Pharm. Biol. ISSN. 1998;36(3):162–166. [Google Scholar]
- 12.Jordan O., Humam M., Bieri M., Christen S., Poblete P., Munoz E. In vitro shoot and root organogenesis, plant regeneration and production of tropane alkaloids in some species of Schizanthus. Phytochemistry. 2006;67(6):570–578. doi: 10.1016/j.phytochem.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Holzman R.S. The legacy of Atropos, the fate who cut the thread of life. Anesthesiol. J. Am. Soc. Anesthesiol. 1998;89(1):241–249. doi: 10.1097/00000542-199807000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Grynkiewicz G., Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008;60(4):439–463. [PubMed] [Google Scholar]
- 15.R.L. Clarke, The tropane alkaloids, in: The Alkaloids: Chemistry and Physiology, vol. 16, Elsevier, 1977, pp. 83–180.
- 16.Padoan M., Sciacovelli A., Basso L., Negrini D., Zuin D., Cosma S., Faggian C., Matricardi D., Plebani P. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Bazaoui A., Bellimam A., Lançar M.A., Soulaymani I.T. Gas-liquid chromatography-mass spectrometry investigation of tropane alkaloids in Hyoscyamus albus L. from Morocco Ahmed. Zeitschrift für Naturforsch. C. 2012;67(9–10):461–465. doi: 10.1515/znc-2012-9-1003. [DOI] [PubMed] [Google Scholar]
- 18.Kohnen-johannsen K.L., Kayser O. Tropane alkaloids: chemistry, pharmacology, biosynthesis and production. Molecules. 2019;24(4):1–23. doi: 10.3390/molecules24040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Özçelik I., Kartal B., Orhan M. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49(4):396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Schick A., Moreira-Muñoz M. El género Schizanthus (Solanaceae) en Chile. Rev. Chagual. 2008:21–32. [Google Scholar]
- 21.Agarwal R., Mehrotra S. An overview of molecular docking. JSM Chem. 2016;4(2):1024–1028. [Google Scholar]
- 22.Sanghani R., Ganatra H.V., Pande S.H. Molecular – docking studies of potent anticancer agent. J. Comput. Sci. Syst. Biol. 2020;5(1):12–15. [Google Scholar]
- 23.Ferreira A.D., Dos Santos L.G., Oliva R.N., Andricopulo G. Molecular docking and structure-based drug design strategies. Molecules. 2015;20(7):13384–13421. doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M.D. Laskar, M.A. Choudhury, Search for therapeutics against COVID 19 targeting SARS-CoV-2 papain-like protease : an in silico study, (2020) 1–28.
- 25.Zhou F., Huang P., Tian J. Specific noncovalent interactions at protein-ligand interface: implications for rational drug design. Curr. Med. Chem. 2012;19(2):226–238. doi: 10.2174/092986712803414150. [DOI] [PubMed] [Google Scholar]
- 26.Chen D., Liu Y., Guo Q. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rut S.K., Lv W., Zmudzinski Z., Patchett M., Nayak S., Snipas D., El Oualid S.J., Bekes F., Huang M., Drag T.T., Olsen M. Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design. bioRxiv. 2020 doi: 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D.S. Biovia, Discovery studio visualizer, vol. 936, San Diego, CA, USA, 2017.
- 30.A.J. Dallakyan, S. Olson, Small molecule library screening by docking with PyRx, in: H.C. Hempel, J. Williams, (Eds.) Chemical Biology, Humana Press, New York, 2015, pp. 243–250. [DOI] [PubMed]
- 31.Li P., Wan Z., Shi H., Ouyang Y. Personal experience with four kinds of chemical structure drawing software: review on ChemDraw, ChemWindow, ISIS/Draw, and ChemSketch. J. Chem. Inf. Comput. Sci. 2004;44(4):1886–1890. doi: 10.1021/ci049794h. [DOI] [PubMed] [Google Scholar]
- 32.Daina V., Michielin A., Zoete O. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;42717:1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mpiana D.D., Tshibangu P.T., Kilembe D.S., Gbolo J.T., Mwanangombo B.Z., Inkoto D.T., Lengbiye C.L., Mbadiko E.M., Matondo C.M., Bongo A., Tshilanda G.N. Identification of potential inhibitors of SARS-CoV-2 main protease from Aloe vera compounds: a molecular docking study. Chem. Phys. Lett. 2020;754 doi: 10.1016/j.cplett.2020.137751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trott A.J., Olson O. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalé S.K.L., Skeel R., Bhandarkar M., Brunner R., Gursoy A., Krawetz N., Phillips J., Shinozaki A., Varadarajan K. NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 1999;151:283–312. [Google Scholar]
- 36.Phillips K., Braun J.C., Wang R., Gumbart W., Tajkhorshid J., Villa E., Chipot E., Skeel C., Kale R.D., Schulten L. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2008;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.B.R. Brooks, R.E. Bruccoleri, B.D. Olafson, D.J. States, S. Swaminathan, M. Karplus, Program for macromolecular energy, minimization, and dynamics calculations, 4 (2) (1983) 187–217.
- 38.Lee S., Cheng J., Swails X., Yeom J.M., Eastman M.S., Lemkul P.K., Wei J.A., Buckner S., Jeong J., Qi J.C., Jo Y. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2014;12(1):405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.A.D. Mackerell, et al., All-atom empirical potential for molecular modeling and dynamics studies of proteins, 1998. [DOI] [PubMed]
- 40.Humphrey K., Dalke W., Schulten A. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee S., Dasgupta S., Adhikary T., Panja S.S. Structural insight to hydroxychloroquine-3C- like proteinase complexation from SARS-CoV-2: inhibitor modelling study through molecular docking and MD-simulation study. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1804458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gullingsrud J., Kosztin D., Schulten K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys. J. 2001;80(5):2074–2081. doi: 10.1016/S0006-3495(01)76181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.S. Antonio, S. Moreira, Natural products’ role against COVID-19, (2020) 23379–23393. [DOI] [PMC free article] [PubMed]
- 44.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting : an in silico perspective. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vipul K., et al. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1775704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang A.S., Penninger H., Li J.M., Zhong Y., Slutsky N. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.