Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread worldwide from epicenter of Wuhan, China since December 2019. The aim of our study was to describe the clinical characteristics and outcome of hospitalized patients with SARS-CoV-2 pneumonia at the Toulouse university hospital, France.
Patients and methods
We selected the patients included from March 7, 2020 to April 20, 2020 in the retrolective Covid-clinic-Toul cohort that follows all hospitalized patients with SARS-CoV-2 infection at the Toulouse Hospital. Cases were confirmed by real-time reverse transcriptase polymerase chain reaction. We report demographics, clinical, biological and radiological features, as well as unfavorable outcome at Day 14 after admission (admission in an intensive care unit, mechanical ventilation, death).
Results
Among 263 hospitalized patients, the median age was 65 years and 155 (58.9%) were males. Two hundred and twenty-seven patients (86.3%) had at least one comorbidity. The median time from first symptom to hospital admission was 7.0 days (interquartile range: 4–10). On day 14 after admission, 111 patients (42.2%) had been transferred to intensive care unit (ICU), including 50 (19.0%) on Day 1; 61 (23.1%) needed mechanical ventilation and 19 patients (7.2%) had died. Patients admitted to ICU at Day 1 of admission (n = 50) were more frequently men (66.0% vs 57.3%), smokers (25.0% vs 7.1%), with obesity (42.0% vs 24.7%) and had a higher mean level of C-reactive protein (median: 110.9 mg/L vs 46.2 mg/L).
Conclusion
This cohort provides epidemiological data on SARS-CoV-2 in hospitalized patients in a University hospital in the South of France.
Keywords: COVID-2019, SARS-CoV-2, Epidemiology
Résumé
Introduction
L’infection à SARS-CoV-2 s’est répandue dans le monde entier à partir du foyer de Wuhan depuis décembre 2019. Le but de notre étude était de décrire l’épidémiologie des patients hospitalisés pour une infection à SARS-CoV-2 au CHU de Toulouse.
Méthodes
La population était l’ensemble des patients inclus du 7 mars 2020 au 20 avril 2020 dans la cohorte rétrolective Covid-clinic-Toul qui suit l’ensemble des patients hospitalisés pour une infection à SARS-CoV-2 au CHU de Toulouse. Les cas étaient confirmés par reverse transcriptase polymerase chain reaction et leurs données démographiques, cliniques, biologiques et radiologiques étaient analysées. La survenue à j14 de l’admission d’un transfert en unité de soins intensifs ou réanimation, le besoin de ventilation mécanique et les décès ont été décrits.
Résultats
Parmi les 263 patients de l’étude, l’âge médian était de 65 ans, et 155 patients (58,9 %) étaient des hommes. Deux cent vingt-sept patients (86,3 %) avaient au moins une comorbidité. Le délai médian entre le premier symptôme et l’admission était de 7,0 jours (Q1 : 4 ; Q3 : 10). À j14 de l’admission, 111 patients (42,2 %) avaient été transférés en service de soins intensifs ou réanimation, dont 50 (19,0 %) à j1 ; 61 (23,1 %) avaient eu besoin de ventilation mécanique et 19 patients (7,2 %) étaient décédés. Les patients admis en soins intensifs ou réanimation à j1 de l’admission (n = 50) étaient plus fréquemment des hommes (66,0 % vs 57,3 %), fumeurs (25,0 % vs 7,1 %), obèses (42,0 % vs 24,7 %) et avec une proteine C-réactive plus élevée (médiane : 110,9 mg/L vs 46,2 mg/L).
Conclusion
Cette cohorte permet de décrire l’épidémiologie de l’infection à SARS-CoV-2 des patients hospitalisés au sein d’un centre hospitalier universitaire du sud de la France.
Mots clés: COVID-19, SARS-CoV-2, Épidémiologie
1. Introduction
The Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been first reported in Wuhan, China, in December 2019 [1], [2]. It has subsequently spread to other provinces of China and then worldwide. The World Health Organization declared COVID-19 outbreak a pandemic on March 11, 2020. SARS-CoV-2 causes viral pneumonia that can lead to severe acute respiratory distress syndrome (ARDS) and even death. Previous studies have reported the clinical, biological and radiological characteristics of the first infected patients in Wuhan area [3], [4], [5], and other authors have compared the epidemiological and clinical features of patients with COVID-19 in Wuhan and other regions in China [6], [7], [8]. Especially, disease evolution and risk factors of complications, essentially ARDS, have been described in this population [9], [10]. Due to a lack of consensual hospitalization criteria, data about hospitalized patients are heterogeneous. To date, clinical characteristics and outcomes of patients outside China have been reported mostly in Asia, Italy and in the Unites States of America, and mostly for patients admitted to intensive care units (ICU) [11], [12], [13], [14], [15], [16], [17], [18]. Descriptive data about clinical presentation, comorbidities, treatment, and outcomes of hospitalized patients in France are lacking [19]. Moreover, prognosis factors specific to European hospitalized patients must be identified. Therefore, we developed the Covid-clinic-Toul cohort, recording data about all patients hospitalized for a SARS-CoV-2 infection at the Toulouse University hospital, South of France (2800 beds, unique tertiary hospital covering an area of about 3 million inhabitants).
The aim of this study was to describe demographics, clinical, biological and radiological features, as well as outcome at Day 14 after admission (admission in an intensive care unit, mechanical ventilation, death) in patients included in the Covid-clinic-Toul cohort until April 20, 2020.
2. Methods
2.1. Study design and participants
The Covid-clinic-Toul cohort records data about all patients hospitalized for SARS-CoV-2 at the Toulouse University hospital. First patients (from March 7 to April 1) were included retrospectively and data from subsequent patients hospitalized after April 1 were collected prospectively. All the patients primarily admitted at the Toulouse University hospital (not transferred from another hospital) were included. Exclusion criteria was the opposition to data collection. All patients, or their representatives for those not able to understand the purpose of the study, were informed by a letter given at admission to hospital and/or sent to their place of residency. This cohort has been approved by institutional review board (no RnIPH 2020-31), in accordance with the French data protection authority (MR004, Commission nationale de l’informatique et des libertés, CNIL).
In the present study, we selected the patients included in the Covid-Clinic-Toul up to April 20, 2020 and with a SARS-CoV-2 infection proven by reverse transcriptase polymerase chain reaction (RT-PCR). All patients enrolled in this cohort were diagnosed according to interim guidance of theWorld Health Organization [20].
2.2. Data collection
We collected demographics, clinical, laboratory, radiological (description of chest computed tomography [CT] scans), and treatment data within the first 24 hours after admission, as well as outcome at Day 14 after admission (see below). Data were extracted from electronic medical records and recorded in an electronic data collection form.
The date of disease onset was defined by the day on which the first symptom was noticed. Duration of symptoms was defined by the time between first symptoms and admission at the hospital. Chest-CT scans were classified as mild, moderate, severe, or critical by the extent of lung lesions, according to the severity score of the French Society of Radiology that assesses the extent of lesions [21].
2.3. Outcome on Day 14 after admission
The primary outcome was composite, including admission to ICU, need of mechanical ventilation and death occurring during the 14 days after admission to the hospital.
2.4. Statistical analysis
We described the characteristics of the whole population, and then by two categories of subgroups: first, the patients admitted to ICU at Day 1 of admission to the hospital versus others; then, among the patients not admitted to ICU at Day 1, those with occurrence of the composite outcome versus others. Continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR) depending on their distribution. Categorical variables were presented as numbers of patients and percentages. We described, using Kaplan-Meier curves, the occurrence of the composite outcome and of death within the 14 days after admission in the subgroup of patients not admitted to ICU at Day 1, as well as the occurrence of death within the 14 days after admission in the whole population.
3. Results
3.1. Patient characteristics (whole population)
A total of 263 patients were included in this study. Data are summarized in Table 1 . The median age was 65 years (IQR: 54–76), and 155 patients (58.9%) were male. Among these, 227 (86.3%) had one or more chronic medical illness. Overweight (36.1%), obesity (28.3%), hypertension (39.5%), chronic pulmonary disease (21.6%) and diabetes (19.8%) were the most common coexisting medical conditions. Immunosuppression was found in 9.1% of the patients. Only one patient had infection by the human immunodeficiency virus (HIV), with normal lymphocyte count. Thirty-one (11.8%) patients were exposed to angiotensin converting enzyme inhibitors (ACEis), 45 (17.1%) to angiotensin receptor blockers (ARBs), 19 (7.2%) to corticosteroids and 6 (2.3%) to non-steroidal anti-inflammatory drugs (NSAIDs).
Table 1.
Characteristics of the 263 patients hospitalized for a SARS-CoV-2 infection proven by RT-PCR included in the Covid-Clinic-Toul cohort until April 20, 2020.
| Variables | All patients (n = 263) | Patients admitted to the ICU at Day 1 of hospitalization |
Among the patients not admitted to the ICU at D1 of hospitalization (n = 213) |
||
|---|---|---|---|---|---|
| Yes (n = 50; 19.0%) | No (n = 213; 81.0%) | Admission to ICU, mechanical ventilation or death at Day 14 (n = 72; 33.8%) | No admission to ICU, mechanical ventilation or death at Day 14 (n = 141; 66.2%) | ||
| Age, median (IQR), years | 65 (54–76) | 67 (56–73) | 64 (53–76) | 69 (60–79) | 63 (50–74) |
| Men, n (%) | 155 (58.9) | 33 (66.0) | 122 (57.3) | 49 (68.1) | 73 (51.8) |
| Comorbidities | |||||
| ≥ 1 comorbidity, n (%) | 227 (86.3) | 42 (84.0) | 185 (86.9) | 67 (93.1) | 118 (83.7) |
| Overweight (BMI: 25–30 kg/m2), n (%) | 88/244 (36.1) | 14/50 (28) | 74/194 (38.1) | 29/68 (42.7) | 45/126 (35.7) |
| Obesity (BMI > 30 kg/m2), n (%) | 69/244 (28.3) | 21/50 (42.0) | 48/194 (24.7) | 19/68 (27.9) | 29/126 (23.0) |
| Hypertension, n (%) | 104 (39.5) | 19 (38.0) | 85 (39.9) | 37 (51.4) | 48 (34.0) |
| Diabetes, n (%) | 52 (19.8) | 11 (22.0) | 41 (19.2) | 19 (26.4) | 22 (15.6) |
| Cardiovascular disease, n (%) | 35 (13.3) | 6 (12.0) | 29 (13.6) | 13 (18.1) | 16 (11.3) |
| Cerebrovascular disease, n (%) | 17 (6.5) | 2 (4.0) | 15 (7.0) | 6 (8.3) | 9 (6.4) |
| Chronic lung disease, n (%) | 57 (21.6) | 11 (22.0) | 46 (21.6) | 19 (26.4) | 27 (19.1) |
| Chronic kidney disease, n (%) | 24 (9.1) | 3 (6.0) | 21 (9.8) | 9 (12.5) | 12 (8.5) |
| Chronic liver disease, n (%) | 2 (0.8) | 0 (0) | 2 (0.9) | 2 (2.7) | 0 (0) |
| Malignancy < 5 years, n (%) | 27 (10.3) | 5 (10.0) | 22 (10.3) | 10 (13.9) | 12 (8.5) |
| Immunosuppression, n (%) | 24 (9.1) | 4 (8.0) | 20 (9.4) | 10 (13.9) | 10 (7.1) |
| Current smokers, n (%)a | 11/115 (9.3) | 4/16 (25.0) | 7/99 (7.1) | 3/35 (8.6) | 4/64 (6.3) |
| Exposure to selected drugs | |||||
| NSAIDs, n (%) | 6 (2.3) | 1 (2.0) | 5 (2.3) | 1 (1.4) | 4 (2.8) |
| Corticosteroids, n (%) | 19 (7.2) | 3 (6.0) | 16 (7.5) | 8 (11.1) | 8 (5.7) |
| ACE inhibitors, n (%) | 31 (11.8) | 4 (8.0) | 27 (12.7) | 11 (15.3) | 16 (11.3) |
| ARBs, n (%) | 45 (17.1) | 12 (24.0) | 33 (15.5) | 15 (20.8) | 18 (12.8) |
| Signs and symptoms | |||||
| Cough, n (%) | 179/241 (74.3) | 30/41 (73.2) | 149/200 (74.5) | 45/62 (72.6) | 104/138 (75.4) |
| Crackling, n (%) | 156/248 (62.9) | 33/44 (75.0) | 123/204 (60.3) | 44/69 (63.8) | 79/135 (58.5) |
| Confusion, n (%) | 21/255 (8.2) | 3/45 (6.7) | 18/210 (8.6) | 6/70 (8.6) | 12/140 (8.6) |
| Diarrhea, n (%) | 83/234 (35.5) | 14/37 (37.8) | 69/197 (35.0) | 19/65 (29.2) | 50/132 (37.9) |
| Anosmia, n (%) | 27/87 (31.0) | 5/14 (35.7) | 22/73 (30.1) | 4/22 (18.2) | 18/51 (35.3) |
| Dysgeusia, n (%) | 33/83 (39.8) | 5/14 (35.7) | 28/69 (40.6) | 4/21 (19.0) | 24/48 (50.0) |
| Time from first symptoms to admissiona, median (IQR), days | 7 (4 –10) | 8 (6 –11) | 7 (4 –10) | 7 (4–8) | 7 (4–10) |
| Vital signs at admissiona | |||||
| Temperature, median (IQR), Celsius degrees | 37.8 (37.0–38.5) | 37.8 (37.1–38.5) | 37.8 (37.0–38.5) | 38.0 (37.2–38.8) | 37.6 (37.0–38.3) |
| Heart rate, median (IQR), by minute | 90 (77–100) | 90 (80–100) | 90 (76–101) | 90 (81–100) | 88 (75–101) |
| Respiratory rate, median (IQR), by minute | 22 (19–17) | 25 (20–30) | 22 (18–26) | 24 (19–28) | 22 (18–25) |
| Systolic arterial pressure, median (IQR), mmHg | 130 (120–140) | 128 (120–135) | 130 (119–140) | 130 (119–140) | 130 (119–140) |
| Diastolic arterial pressure, median (IQR), mmHg | 76 (67–85) | 78 (70–81) | 76 (67–86) | 74 (62–87) | 77 (70–85) |
| Oxygen saturation ≤ 92% (without oxygen therapy), n (%) | 58/202 (28.7) | 19/22 (86.4) | 39/180 (21.6) | 22/56 (39.3) | 17/124 (13.7) |
| With oxygen therapy before admission, n (%) | 59/261 (22.6) | 28/49 (57.1) | 31/212 (14.6) | 15/71 (21.1) | 16/140 (11.4) |
| Laboratory findings at admissiona | |||||
| Lymphocyte count, median (IQR), × 109/L | 0.9 (0.7–1.3) | 0.8 (0.7–1.1) | 1.0 (0.7–1.3) | 0.8 (0.6–1.1) | 1.1 (0.8–1.5) |
| Platelet count, median (IQR), × 109/L | 186 (150–233) | 184 (140–233) | 186 (154–236) | 175 (134–211) | 192 (160–244) |
| C-reactive protein level, median (IQR), mg/L | 52.4 (27.0–107.6) | 110.9 (50.3–220.5) | 46.2 (22.0–88.8) | 73.7 (30.5–107.9) | 39.5 (19.3–74.3) |
| Creatinine level, median (IQR), μmol/L | 80 (66–97) | 80.0 (67.0–112.0) | 79 (65–95) | 86 (73–109) | 76 (63–92) |
| Serum ferritin, median (IQR), μg/L | 972 (399–1820) | 1570 (792–2484) | 863 (305–1622) | 1326 (594–1986) | 661 (280–1622) |
| D-dimers, median (IQR), μg/L | 780 (555–1300) | 1200 (1040–2870) | 740 (540–1170) | 765 (670–1790) | 710 (460–1170) |
| Troponin > 14 ng/L, n (%) | 43/113 (38.1) | 15/29 (51.7) | 28/84 (33.3) | 15/31 (48.4) | 13/53 (24.5) |
| Lactate dehydrogenase (UI/L), median (IQR) | 332 (258–428) | 389 (338–590) | 295 (236–351) | 308 (252–635) | 285 (228–336) |
| Chest CT scan at admission | 253 (96.2) | 49 (98.0) | 204 (95.8) | 71 (98.6) | 133 (94.3) |
| Chest CT scan severity score | |||||
| No typical sign of COVID-19, n (%) | 10 (4.0) | 2 (4.1) | 8 (3.9) | 2 (2.8) | 6 (4.5) |
| Mild, n (%) | 34 (13.4) | 1 (2.0) | 33 (16.2) | 12 (16.9) | 21 (15.8) |
| Moderate, n (%) | 139 (54.9) | 17 (34.7) | 122 (59.8) | 29 (40.8) | 93 (69.9) |
| Severe, n (%) | 64 (25.3) | 24 (49.0) | 40 (19.6) | 27 (38.0) | 13 (9.8) |
| Critical, n (%) | 6 (2.4) | 5 (10.2) | 1 (0.5) | 1 (1.4) | 0 (0) |
| Treatment administered during the first 24 hours after admission | |||||
| Oxygen therapy, n (%) | 212 (80.6) | 50 (100) | 162 (76.1) | 66 (91.7) | 96 (68.1) |
| Antibiotics, n (%) | 165 (62.7) | 48 (96.0) | 117 (54.9) | 55 (76.4) | 62 (44.0) |
| Hydroxychloroquine, n (%) | 33 (12.5) | 19 (38.0) | 14 (6.6) | 9 (12.5) | 5 (3.5) |
| Lopinavir/ritonavir, n (%) | 9 (3.4) | 5 (10.0) | 4 (1.9) | 4 (5.6) | 0 (0) |
| Remdesivir | 1 (1.9) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) |
| Detailed outcomes at Day 14 after admission | |||||
| Admission to ICU, n (%) | 111 (42.2) | 50 (100) | 61 (28.6) | 61 (84.7) | – |
| Mechanical ventilation, n (%) | 61 (23.2) | 29 (58.0) | 32 (15.0) | 32 (45.7) | – |
| Death, n (%) | 19 (7.2) | 4 (8.0) | 15 (7.0) | 15 (20.8) | – |
| Discharged, n (%) | 173 (65.8) | 21 (42.0) | 152 (71.4) | 32 (44.4) | 120 (85.1) |
ACE: angiotensin converting enzyme; ARBs: angiotensin receptor blockers; BMI: body mass index; CT: computed tomography; ICU: intensive care unit; IQR: interquartile range; NSAIDs: non-steroidal anti-inflammatory drugs; RT-PCR: reverse transcriptase polymerase chain reaction.
Missing values: current smoker, n = 148; time from first symptoms to admission, n = 2; temperature, n = 7; heart rate, n = 7; respiratory rate, n = 12; arterial pressure, systolic/diastolic, n = 6; oxygen saturation, n = 2; lymphocyte count, n = 35; platelet count, n = 10; C-reactive protein, n = 9; creatinine level, n = 6; serum ferritin, n = 200; d-dimers, n = 227; troponin, n = 150, lactate dehydrogenase, n = 213.
The median duration of symptoms at admission was 7.0 days (IQR: 4.0–10.0). On admission, most patients had experienced cough (74.3%) and presented with fine crackles at pulmonary auscultation (62.9%). Less frequent symptoms were diarrhea (35.5%), anosmia (31.0%), dysgeusia (39.8%) and confusion (8.2%).
On admission, patients had moderate lymphopenia (median: 0.9 × 109/L, IQR: 0.7–1.3) and moderate elevation of C-reactive protein (median: 52.4 mg/L, IQR: 27.0–107.6). Platelet count was normal in most patients.
Most patients (96.2%) had a chest CT-scan at admission, with a “moderate” and “severe” score in 139 (54.9%) and 64 (25.3%) patients, respectively.
Thirty-three patients (12.5%) had received hydroxychloroquine, 9 (3.4%) lopinavir/ritonavir and only one patient (1.9%) remdesivir within the first 24 hours after admission. Most patients (62.7%) were given antibiotic treatment, mostly cephalosporins, amoxicillin/clavulanic acid and macrolides.
3.2. Outcome
Of the 263 patients, 111 (42.2%) were admitted to ICU because of the development of organ dysfunction, ARDS or need of oxygen therapy ≥ 5 L/minute during the 14 first days of hospitalization. The median time from onset of symptoms to ICU admission was 8.0 days (IQR: 5.0–11.0). Mechanical ventilation was required in 61 patients (23.2%).
Nineteen patients (7.2%) had died within the 14 days after admission, including 7 in ICU. Characteristics of these 19 patients are presented in Table 2 . All of them were aged > 65 years and had one or more chronic medical illness, except one patient who was 49 year-old with no other comorbidity than obesity and died of thrombo-embolic complication, sepsis and ARDS. Limitation of life-sustaining treatment was made in 14 patients (73.7%). Causes of death was ARDS for all the patients, plus pulmonary embolism (n = 3), acute heart failure (n = 4) and sepsis (n = 3).
Table 2.
Characteristics of the patients included in the Covid-Clinic-Toul cohort until April 20, 2020 who died within the first 14 days of hospitalization.
| Patient | Age | Sex | Comorbidities | Time from first symptom to hospital admission | At hospital admission |
Limitation of life-sustaining treatments | ICU | Mechanical ventilation | Time from hospital admission to death | Cause of death | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory parameters | Biological findingsa | Chest CT-scan severity score | ||||||||||
| #1 | 81 | Male | Arterial hypertension, coronary disease, cerebrovascular disease, diabetes, immunosuppression (giant cell arteritis treated by corticosteroids) | 4 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | No | No | 5 | ARDS |
| #2 | 90 | Male | Arterial hypertension, heart failure, coronary disease, current smoker | 7 | Need for oxygen, respiratory rate ≥ 22/min | Thrombocytopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | No | No | 6 | ARDS |
| #3 | 77 | Male | Glioblastoma, immunosuppression (chemotherapy, corticosteroids) | 4 | – | Lymphopenia | Moderate | Yes | No | No | 8 | ARDS |
| #4 | 85 | Female | Obesity, arterial hypertension, chronic lung disease (dermatomyositis, pulmonary fibrosis), cancer < 5 years, immunosuppression (corticosteroids) | 7 | Need for oxygen | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | No | No | 3 | ARDS |
| #5 | 87 | Male | Arterial hypertension, cerebrovascular disease | 3 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Lymphopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | Yes | No | 3 | ARDS |
| #6 | 49 | Male | Obesity | 8 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Moderate | No | Yes | Yes | 9 | ARDS, pulmonary embolism, sepsis |
| #7 | 79 | Female | Overweight, arterial hypertension, multiple myeloma, immunosuppression (chemotherapy) | 3 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Moderate | No | Yes | Yes | 8 | ARDS |
| #8 | 89 | Male | Arterial hypertension, COPD, chronic kidney disease | 3 | Respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Moderate | Yes | No | No | 9 | ARDS, acute heart failure |
| #9 | 83 | Female | Obesity, arterial hypertension, heart failure, coronary disease, sleep apnea, chronic kidney disease, diabetes, giant cell arteritis, immunosuppression (corticosteroids) | 4 | Need for oxygen | Lymphopenia, C-reactive protein ≥ 50 mg/L | Moderate | Yes | Yes | No | 3 | ARDS, sepsis |
| #10 | 93 | Male | Obesity, arterial hypertension, heart failure, sleep apnea, chronic respiratory disease | 1 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | – | Moderate | Yes | No | No | 7 | ARDS, acute heart failure |
| #11 | 85 | Male | Overweight, arterial hypertension, coronary disease | 8 | – | Lymphopenia, C-reactive protein ≥ 50 mg/L | Mild | No | Yes | Yes | 8 | ARDS |
| #12 | 67 | Male | Arterial hypertension | 2 | Need for oxygen | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | No typical sign | No | Yes | Yes | 5 | ARDS |
| #13 | 92 | Female | Cerebrovascular disease | 2 | – | Lymphopenia | No typical sign | Yes | No | No | 11 | ARDS, sepsis, acute heart failure |
| #14 | 92 | Female | Overweight, heart failure, cerebrovascular disease, pulmonary arterial hypertension, chronic kidney disease | 7 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Thrombocytopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | No | No | 4 | ARDS |
| #15 | 78 | Female | Overweight, arterial hypertension, asthma, pulmonary arterial hypertension | 2 | SaO2 ≤ 92%, respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Moderate | Yes | No | No | 4 | ARDS, pulmonary embolism |
| #16 | 79 | Female | Obesity, arterial hypertension, chronic kidney disease, diabetes | 1 | Need for oxygen | Lymphopenia, thrombocytopenia | – | No | Yes | Yes | 9 | ARDS |
| #17 | 68 | Female | Obesity, arterial hypertension, breast cancer, immunosuppression (chemotherapy) | 7 | Need for oxygen, respiratory rate ≥ 22/min | Lymphopenia, C-reactive protein ≥ 50 mg/L | Moderate | Yes | No | No | 5 | ARDS, pulmonary embolism, acute heart failure |
| #18 | 61 | Female | Obesity, arterial hypertension, COPD, sarcoidosis, diabetes, immunosuppression (immunosuppressants) | 4 | Respiratory rate ≥ 22/min | Lymphopenia | Mild | Yes | No | No | 12 | ARDS |
| #19 | 86 | Female | Overweight, arterial hypertension | NA | Respiratory rate ≥ 22/min | Lymphopenia, thrombocytopenia, C-reactive protein ≥ 50 mg/L | Severe | Yes | No | No | 8 | ARDS |
ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; NA: not available.
Lymphopenia was defined by lymphocyte count < 1.5 G/L; thrombocytopenia was defined by platelet count < 150 × 109/L.
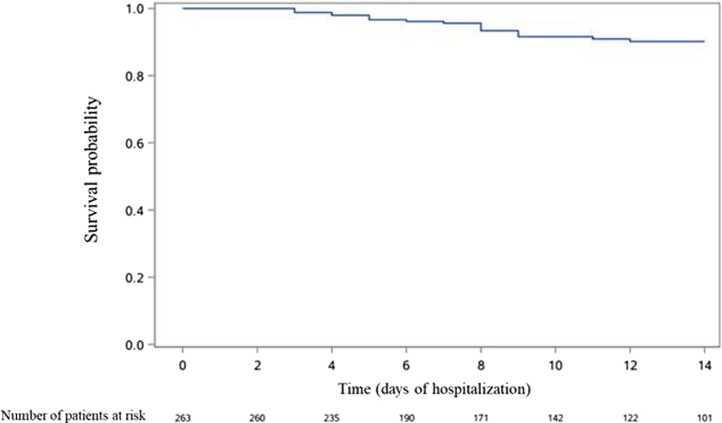
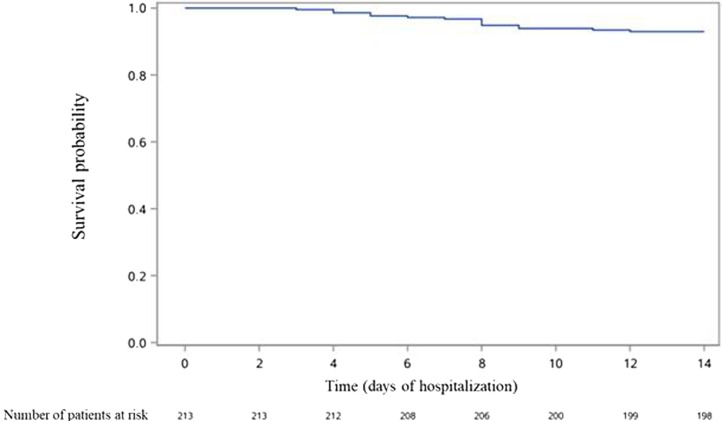
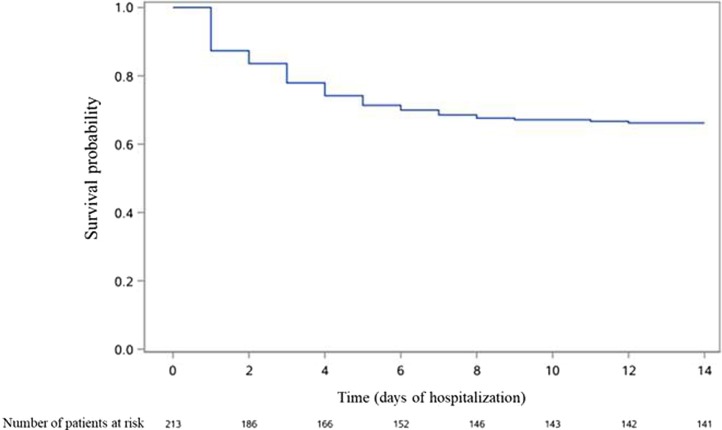
Occurrence over time of death in the whole population, as well as occurrence of death and of the composite outcome in patients not admitted to ICU at Day 1 are presented in Fig. 1, Fig. 2, Fig. 3 .
Fig. 1.
Occurrence over time of death in the whole population of patients admitted to hospital for COVID-19 (n = 263).
Fig. 2.
Occurrence of death in patients not admitted to ICU at Day 1 of admission (n = 213).
Fig. 3.
Occurrence of admission to ICU, need for mechanical ventilation or death in patients not admitted to ICU at Day 1 of admission (n = 213).
3.3. Patient characteristics in the subgroup of patients admitted to the ICU at Day 1 versus others
On admission, 213 patients (81.0%) were admitted to general wards and 50 patients (19.0%) were admitted to the ICU at Day 1. In the subgroup of those admitted to the ICU, patients were more frequently men (66.0% vs 57.3%) and smokers (25.0% vs 7.1%). Except for obesity (42.0% vs 24.7%), there was no other predominance in comorbidities. They were not more frequently exposed to ACEis, corticosteroids or NSAIDs but were more frequently exposed to ARBs (24.0% vs 15.5%). Clinical features and time from first symptoms to admission were similar. Laboratory results showed higher serum concentrations of ferritin (median: 1570 μg/L vs 863 μg/L), C-reactive protein (median: 110.9 mg/L vs 46.2 mg/L) and higher levels of troponin (51.7% with troponin > 14 ng/L vs 33.3%) in patients hospitalized in the ICU at Day 1. Chest CT-scan results werecorrelated with clinical severity in these patients (49.0% and 10.2% of “severe” and “critical” CT-scans in ICU patients vs 19.6% and 0.5%, respectively). Most of these patients (96.0%) received an antibacterial therapy during the first day of hospitalization and 38.0% received hydroxychloroquine.
3.4. Characteristics in the subgroup of patients not admitted to the ICU at Day 1, according to the outcome at Day 14
On Day 14, among the 213 patients who were initially hospitalized in regular medical wards, 72 patients (33.8%) had an unfavorable outcome, and 15 died. These patients, being compared with those who were still alive without ICU admission on day 14, were more frequently men (68.1% vs 51.8%) with a history of hypertension (51.4% vs 34.0%) or diabetes (26.4% vs 15.6%). They were more frequently exposed to ARBs (20.8% vs 12.8%). Anosmia and dysgeusia were less frequently reported in patients with unfavorable outcome (18.2% and 19.0% vs 35.3% and 50.0% respectively). These patients were given more frequently antibiotic and hydroxychloroquine treatments during the first 24 hours after admission (76.4% and 12.5% vs 44.0% and 3.5%, respectively).
4. Discussion
We report here a case series of 263 hospitalized patients with laboratory-confirmed SARS-CoV-2 infection. One hundred and eleven patients (42.2%) needed ICU and nineteen patients died (7.2%) within 2 weeks after their admission.
The median age of this cohort (65 years, IQR 54–76) is higher than in the three largest cohorts from China (median ages between 47 and 56 years). We also observed a higher prevalence of comorbidities [8], [9], [10]. Among hospitalized patients with COVID-19 in China, the percentage of patients who required ICU care ranged from 5 to 32% [8], less than in our cohort which included both “critical care unit” and “intensive care unit” patients. Compared to national epidemiological data, median age of the patients included in the Covid-Clinic-Toul cohort was slightly higher (65 years versus 61.5 years). However, characteristics of the ICU-hospitalized patients were consistent with national data with a male predominance and high prevalence of chronic medical illnesses [22]. Mortality rate among hospitalized patients ranged from 0 to 11% in the first reports [4], [5], [11]. Mortality rate in our study may have been underestimated due to an initially broad hospitalization criteria. Moreover, we censored the follow-up at Day 14 after admission. All patients who died had comorbidities and most of them were aged over 65 years. Causes of death were ARDS for all the patients. We also observed pulmonary embolism in 15.8% of the deceased patients, in accordance with previous reports [23].
We observed more smokers and overweight patients among those admitted to the ICU at Day 1, which is concordant with previous studies [24], [25], [26]. In our cohort, we observed a predominance of male gender, hypertension, and diabetes in patients with poor outcome within the 14 days after their admission. Grasseli et al. also reported a high prevalence of men and patients with chronic hypertension in their cohort of ICU patients [12]. Zhou et al. and Whu et al. also identified hypertension as a risk factor of ARDS [9], [10]. This may explain the higher proportion of patients exposed to ARBs among those with unfavorable outcome [27], [28]. However, we found no difference in proportion of older and other chronic medical illnesses between ICU patients and non-ICU patients. Severe patients had also higher biological inflammatory parameters and troponin levels at admission. Myocardial injury has been described and associated with severity [5], [10], [24], [29].
We did not observe a more frequent outcome occurrence in patients who had taken NSAIDs or corticosteroids before admission, but few patients were exposed. This may be explained by public information before the onset of the epidemic in France.
Anosmia and dysgeusia were less frequently reported in critically ill patients, which is consistent with previous studies reporting these symptoms as associated with mild cases [30]. However, this finding must be taken with caution due to missing data regarding these symptoms.
This study has several limitations. First, only patients with laboratory-confirmed COVID-19 were included; suspected but undiagnosed cases were ruled out, especially those with typical clinical and radiological presentation but with negative RT-PCR (10% of the COVID-19 patients admitted to the emergency department at the Toulouse University hospital; unpublished data). However, chest-CT has higher sensitivity than RT-PCR assay [31], [32]. The characteristics of patients with compatible CT-scan and negative RT-PCR need to be further described. Second, some data were missing. In particular, smoking status, anosmia and dysgeusia were not systematically searched in the beginning of the pandemics. Third, the first patients were retrospectively included. Fourth, criteria for admission to ICU may have varied over time during the study period with improved knowledge of the disease by physicians. Lastly, we presented patient characteristics at admission and outcome at Day 14. This is useful for predictive models, but we did not assess in detail what happened between Day 1 and Day 14 (including treatments administered after the first 24 hours following admission [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]).
5. Conclusion
This cohort provides data on patients hospitalized for COVID-19 in a University hospital in the south of France, the Toulouse University hospital, and confirms some epidemiological findings: a high frequency of comorbidities in hospitalized patients, a high frequency of male gender and obesity for initial severity; and important rates of admission to ICU, mechanical ventilation, and death.
Collaborators: Covid-Clinic-Toul investigators
Muriel Alvarez, Jacques Amar, Michel Attal, Laurent Balardy, Frédéric Balen, Odile Beyne-Rauzy, Ourdia Bouali, Fanny Bounes, Christophe Bureau, Louis Buscail, Lionel Calviere, François Chollet, Isabelle Claudet, Arnaud Constantin, Alexa Debard, Arnaud Del Bello, Karen Delavigne, Julien Delrieu, Alain Didier, Stanislas Faguer, Olivier Fourcade, Bernard Georges, Étienne Grunenwald, Sophie Guyonnet, Hélène Hanaire, Christophe Hein, Nassim Kamar, Olivier Lairez, Pierre Mansat, Jérémie Pariente, Carle Paul, Pierre Payoux, Marie-Léa Piel-Julian, Grégory Pugnet, Agnès Ribes, Christian Recher, Yves Rolland, Adeline Ruyssen-Witrand, Jean Sabatier, Laurent Sailler, Jean-Pierre Salles, Nicolas Sans, Marion Secher, Annick Sevely, Olivier Toulza.
Credit authors statement
A.J., M.L., G.M.B, G.M. and A.S. designed the study. A.J. and M.L. performed data analysis. A.J., M.L., and G.M. wrote the manuscript. All the authors contributed to data acquisition and gave final approval for submission.
Funding
The Covid-clinic-Toul cohort is funded by the Toulouse University hospital.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors deeply thank Christophe Morin, Gaëlle Barencourt, Caroline Hurault-Delarue, Charlotte Vert and Julien Jacquot for their help in data acquisition and Isabelle Olivier for reglementary aspects.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Eng J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wuhan Municipal Health Commission . 2019. Report of clustering pneumonia of unknown etiology in Wuhan City.http://www.wuhan.gov.cn/front/web/showDetail/2019123108989 [Accessed July 28, 2020] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Wu X., Jiang X., Xu K., Ying L., Ma C. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [published correction appears in BMJ. 2020 Feb 27;368:m792] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Z., Cao H., Jie Y., Huang Z., Guo X., Chen J. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis. 2020;35:101664. doi: 10.1016/j.tmaid.2020.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young B.E., Xiang Ong S.W., Kalimuddin S., Low J., Yen Tan S., Loh J. Epidemiological features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasseli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu C.P., Marin A., Melinte V., Gherlan G.S., Banicioui F.C., Dogaru A. COVID-19 in a tertiary hospital from Romania: epidemiology, preparedness and clinical challenges. Travel Med Infect Dis. 2020;35:101662. doi: 10.1016/j.tmaid.2020.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019–COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S., Hirsch J., Narasimhan M., Crawford J., McGinn T., Davidson K. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers L., Parodi S., Escobar G., Liu V. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings M., Baldwin M., Abrams D., Jacobson S., Meyer B., Balough E. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plaçais L., Richier Q. COVID-19: clinical, biological and radiological characteristics in adults, infants and pregnant women. An up-to-date review at the heart of the pandemic. Rev Med Interne. 2020;41:308–318. doi: 10.1016/j.revmed.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCov) infection is suspected: interim guidance.https://www.apps.who.int/iris/handle/10665/330854 [Accessed July 28, 2020] [Google Scholar]
- 21.French Society of Radiology . 2020. Chest CT severity score of the French Society of Radiology.http://www.sfrnet.org/rc/org/sfrnet/nws/News/2020/20200316-155630-175/src/nws_fullText/fr/CR%20TYPE%20COVID-19%20LAST.pdf [Accessed July 28, 2020] [Google Scholar]
- 22.Sante Publique France . 2020. COVID-19 point épidémiologique.https://www.santepubliquefrance.fr/recherche/#search=COVID%2019%20%20%20point%20epidemiologique&publications=donn%C3%A9es®ions=National&sort=date.%20Accessed%20Jul%2019,%202020 [Accessed July 28, 2020] [Google Scholar]
- 23.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARSCoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardavas C., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;28:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarcho J., Ingelfinger J., Hamel M., D’Agostino R., Harrington D. Inhibitors of the Renin–Angiotensin–Aldosterone System and Covid-19. N Engl J Med. 2020;382:2462–2464. doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fosbol E., Butt J., Ostergaard L., Andersson C., Selmer C., Kragholm K. Association of Angiotensin Converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020:ciaa270. doi: 10.1093/cid/ciaa270. [Published online on March 16, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long C., Xu H., Shen Q., Zhang X., Fan B., Wang C. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fadel R., Morrison A., Vahia A., Smith Z., Chaudhry Z., Bhargava P. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020:ciaa601. doi: 10.1093/cid/ciaa601. [Published online on May 19, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.05.021. [S0091-6749(20)30740-5; Published online on May 29, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [Published online on May 14, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [Published online on May 14, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M., Li M., Xiao F., Pang P., Liang J., Tang T. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci Rev. 2020:nwaa113. doi: 10.1093/nsr/nwaa113. [Published online on May 28, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Eng J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon J.H., Udy A., Peleg A.Y. Remdesivir for the treatment of Covid-19 — Preliminary report. N Engl J Med. 2020;383 doi: 10.1056/NEJMc2022236. [10.1056/NEJMc2022236#sa2. Published online on July 10, 2020] [DOI] [PubMed] [Google Scholar]