Abstract
Aim of the study
Patients with cancer might have an increased risk for severe outcome of coronavirus disease 2019 (COVID-19). To identify risk factors associated with a worse outcome of COVID-19, a nationwide registry was developed for patients with cancer and COVID-19.
Methods
This observational cohort study has been designed as a quality of care registry and is executed by the Dutch Oncology COVID-19 Consortium (DOCC), a nationwide collaboration of oncology physicians in the Netherlands. A questionnaire has been developed to collect pseudonymised patient data on patients' characteristics, cancer diagnosis and treatment. All patients with COVID-19 and a cancer diagnosis or treatment in the past 5 years are eligible.
Results
Between March 27th and May 4th, 442 patients were registered. For this first analysis, 351 patients were included of whom 114 patients died. In multivariable analyses, age ≥65 years (p < 0.001), male gender (p = 0.035), prior or other malignancy (p = 0.045) and active diagnosis of haematological malignancy (p = 0.046) or lung cancer (p = 0.003) were independent risk factors for a fatal outcome of COVID-19. In a subgroup analysis of patients with active malignancy, the risk for a fatal outcome was mainly determined by tumour type (haematological malignancy or lung cancer) and age (≥65 years).
Conclusion
The findings in this registry indicate that patients with a haematological malignancy or lung cancer have an increased risk of a worse outcome of COVID-19. During the ongoing COVID-19 pandemic, these vulnerable patients should avoid exposure to severe acute respiratory syndrome coronavirus 2, whereas treatment adjustments and prioritising vaccination, when available, should also be considered.
Keywords: Coronavirus, COVID-19, Pandemic, Cancer, Cancer treatment
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, leading to coronavirus disease 2019 (COVID-19) [1,2], has major impact on healthcare [3,4]. In particular, the consequences for oncological care are extensive, as the effects of malignancy or cancer treatments on the outcome of COVID-19 are yet unknown [[5], [6], [7], [8], [9], [10]]. In addition, hospital visits for anticancer therapies may put patients at even more risk of getting infected with SARS-CoV-2 [7,11]. Consequently, oncological treatment was frequently adjusted during the COVID-19 pandemic, even in regions with relatively low COVID-19 incidence [12]. These treatment adjustments were made according to COVID-19 guidelines of (inter)national oncological societies, which were primarily based on expert opinions [[13], [14], [15], [16]].
Awaiting the development of vaccines against SARS-CoV-2, new outbreaks are expected worldwide. A nationwide registry was initiated by the Dutch Oncology COVID-19 Consortium (DOCC). It aims to identify characteristics of patients with cancer and/or their treatments associated with a worse outcome of COVID-19 to facilitate evidence-based decisions in oncological care during this ongoing pandemic. In the Netherlands, all patients have equal access to medical care and open discussions with patients and their families about treatment restrictions, i.e. do-not-intubate or do-not-resuscitate orders, are daily practice.
2. Methods
2.1. Study design
The registry is executed by DOCC, which is a nationwide consortium of oncology physicians (haematologists, medical oncologists, neuro-oncologists and pulmonologists) in the Netherlands. This observational cohort study was designed as a national quality of care registry to support rapid clinical decision-making in oncological practice. A questionnaire was developed to collect pseudonymised patient data on four topics: baseline patient characteristics, diagnosis and treatment of cancer, characteristics of COVID-19 and treatment and outcome of COVID-19 (appendix 2). Some patients with COVID-19 were transferred to another hospital because of capacity issues. Therefore, data of transfer of patients between hospitals were requested to avoid duplicates. This registry was approved by the ethics committee and the Privacy Knowledge Office at Erasmus Medical Centre. According to local hospital guidelines, additional approval was obtained by local committees when needed.
2.2. Inclusion criteria of DOCC registry
All patients with COVID-19 and a cancer diagnosis or cancer treatment in the past 5 years were eligible for inclusion in the DOCC registry. Besides, patients with a diagnosis or treatment more than 5 years ago could be included if the diagnosis or treatment was expected to have had an impact on COVID-19 outcome (e.g. bone marrow transplantation, chest radiation therapy). The diagnosis of COVID-19 was defined as a positive test for SARS-CoV-2 using reverse transcription polymerase chain reaction (RT-PCR) and/or radiological findings on computed tomography (CT) and/or clinical symptoms of COVID-19. However, as a diagnosis of COVID-19 based solely on clinical symptoms is insecure and subject to bias, it was decided to restrict eligibility to a PCR and/or CT-based COVID-19 diagnosis for this analysis.
2.3. Collection of data
The DOCC registry was initiated on March 27th, 2020, and supported by the Dutch societies of medical oncologists, pulmonologists and neuro-oncologists [[17], [18], [19]]. Dutch oncology physicians in all 69 hospital organisations in the Netherlands were informed about the registry by communications via different cancer societies. Physicians were encouraged to identify cancer patients with COVID-19 and to collect pseudonymised data using the questionnaire. Subsequently, the data provided were centrally entered into an electronic clinical record form (eCRF) using a secured digital database (ALEA Clinical).
2.4. Data processing
For the first analysis, an update on the course and outcome of COVID-19 was requested for all patients diagnosed with COVID-19 ≥ 4 weeks before May 4th, 2020. Also, all clinical data in eCRFs were checked for inconsistencies by experienced oncology physicians (D.D., P.M., A.V.), and the queries generated were sent to the participating hospitals. The returned queries and updated data were processed in eCRFs. Clinical data were both annotated and cleaned, including the processing of transfer data to avoid duplicates.
2.5. Distribution of COVID-19 in the Netherlands
In the Netherlands, the COVID-19 pandemic is monitored by The National Institute for Public Health and the Environment [20]. All patients with a positive RT-PCR test for SARS-CoV-2 are centrally registered. The 12 geographic regions of the Netherlands were classified according to the number of COVID-19 positive patients per 100,000 inhabitants. This allows evaluation of the national coverage of the DOCC registry according to regional incidence of COVID-19.
2.6. Statistical analysis
The characteristics of patients with resolved COVID-19 versus a fatal outcome of COVID-19 were analysed. Descriptive statistics were used for baseline characteristics. To analyse the risk for different age categories, patients were categorised into three age groups; i.e. <65 years, ≥65–75 years and ≥75 years. Pearson's chi-square test was used to identify univariable risk factors for a fatal outcome of COVID-19, and odds ratios were presented with 95% confidence intervals. Variables with p ≤ 0.10 in univariable analysis were included in multivariable logistic regression analyses. This was done with backward selection with a threshold of p < 0.05. All statistical tests were performed two-sided. Data were analysed using IBM SPSS statistics 25.
As patients with metastatic disease and/or active cancer treatment could be more susceptible to a severe course of COVID-19, a separate analysis was performed for this subgroup of patients. Active malignancy was defined as metastatic disease in patients with solid tumours and/or recent cancer treatment (<90 days before diagnosis of COVID-19). In patients with an active malignancy, the impact of cancer treatment on COVID-19 severity was also evaluated. For this group, treatment was defined as any cancer treatment 30 days before COVID-19 diagnosis. Finally, the impact of steroid use was analysed as a possible risk factor for fatal outcome of COVID-19. For this specific analysis, steroid use (30 days before COVID-19 diagnosis) as supportive medication for cytotoxic treatment (e.g. part of the chemotherapeutic regime or anti-emetic medication) versus steroid use not related to cancer treatment was analysed.
3. Results
3.1. COVID-19 in the Netherlands
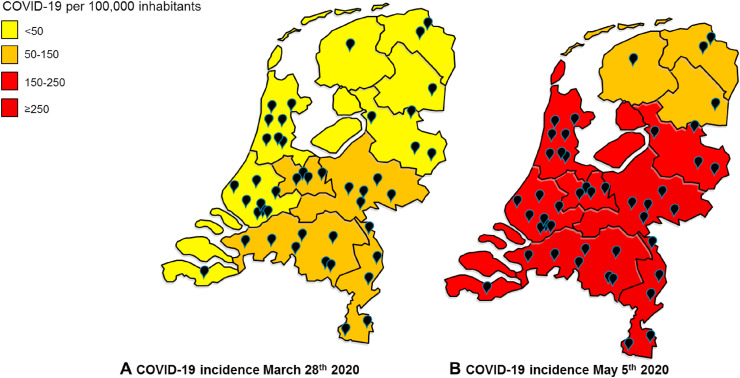
At initiation of the registry, March 27th 2020, all Dutch regions experienced an outbreak of COVID-19. At that time, the Southern region of the Netherlands had the highest incidence of COVID-19. Forty-five out of the 69 Dutch hospital organisations participated in the registry. All hospitals that provided care for the majority of patients with COVID-19 participated. The distribution of COVID-19 and the location of participating hospitals show nationwide coverage of this registry (Figure 1 ).
Fig. 1.
Prevalence of COVID-19 in the Netherlands. Patients with a positive test for SARS-CoV-2 at start of the DOCC registry March 28th, 2020 (a) and one day after the database lock on (b) May 5th, 2020. The black bullets indicate the hospitals that participated in the registry (n = 45).
3.2. Characteristics of COVID-19 patients with cancer
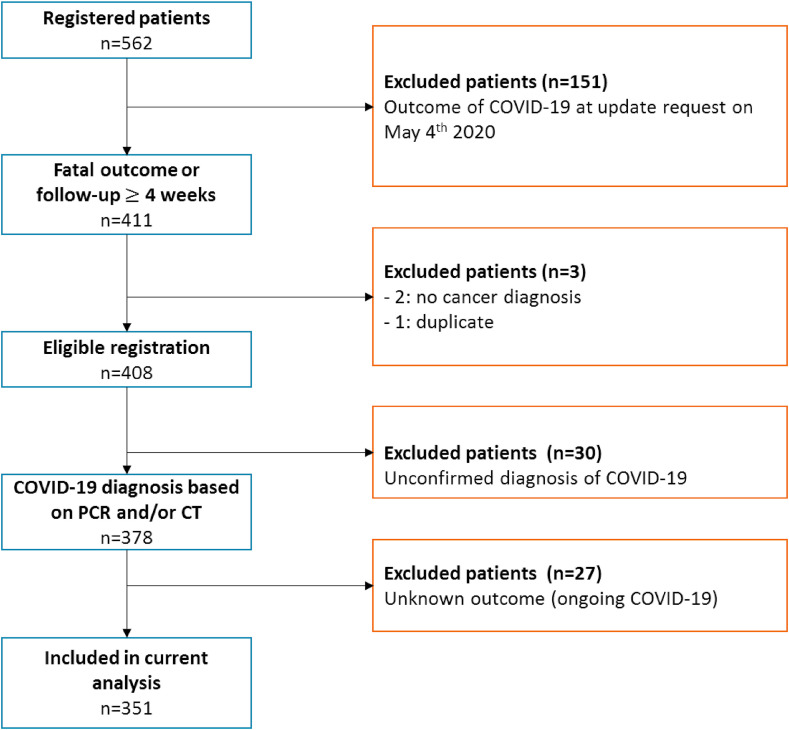
Between March 27th and May 4th, 442 patients were registered. Data from 409 cancer patients were complete for the current analysis. In addition, the following patients were excluded form analyses: one duplicate case, 30 patients because of unconfirmed diagnosis of COVID-19 and 27 patients because of ongoing COVID-19 with unknown outcome. For this first analysis, 351 patients were included (Figure 2 ).
Fig. 2.
Patient selection. Flowchart of patient selection for the current analysis.
Detailed baseline characteristics are presented in Table 1 . Overall, the median age was 70 years (interquartile range [IQR] 61–77) and 187 (53.3%) patients were male. The main cancer diagnoses were non-small cell lung cancer (13.4%), breast cancer (13.4%) and chronic lymphocytic leukaemia (8.8%). Metastatic disease was present in 112 (47.1%) out of 238 patients with solid tumours. In more than half of all patients (53.6%), the last cancer treatment was with non-curative intent. Besides cancer diagnosis, most patients had one or more relevant comorbidities, and 51% of the patients had a history of smoking.
Table 1.
Patients' characteristics.
| Variable | Resolved (n = 237) | Fatal (n = 114) | Total group (n = 351) |
|---|---|---|---|
| Sex—n (%) | |||
| Male | 112 (47.3) | 75 (65.8) | 187 (53.3) |
| Female | 125 (52.7) | 39 (34.2) | 164 (46.7) |
| Age | |||
| Median age in years (interquartile range) | 68 (59–76) | 74 (68–80) | 70 (61–77) |
| <65 years—n (%) | 99 (41.8) | 12 (10.5) | 111 (31.6) |
| ≥65 years < 75 years—n (%) | 71 (30.0) | 46 (40.4) | 117 (33.3) |
| ≥75 years—n (%) | 67 (28.3) | 56 (49.1) | 123 (35.0) |
| Smoking—n (%) | |||
| All smokers | 112 (47.3) | 67 (58.5) | 179 (51.0) |
| Current smoker | 12 (5.1) | 12 (10.5) | 24 (6.8) |
| History of smoking | 100 (42.2) | 55 (48.2) | 155 (44.2) |
| Comorbidities—n (%) | |||
| Cardiovascular disease | 119 (50.2) | 71 (62.3) | 190 (54.1) |
| BMI ≥ 30 | 48 (20.3) | 16 (14.0) | 64 (18.2) |
| COPD | 26 (11.0) | 20 (17.5) | 46 (13.1) |
| Diabetes mellitus | 34 (14.3) | 21 (18.4) | 55 (15.7) |
| Autoimmune disease | 13 (5.5) | 9 (7.9) | 22 (6.3) |
| Prior/other malignancy | 31 (13.1) | 32 (28.1) | 63 (17.9) |
| Use of steroids at COVID-19 diagnosis | 53 (22.4) | 40 (35.1) | 93 (26.5) |
| As part of cancer treatment (<1 week) | 32 (13.5) | 23 (20.2) | 55 (15.7) |
| Use >1 week (not related to cancer treatment) | 21 (8.9) | 17 (14.9) | 38 (10.8) |
| Cancer type—n (%) | |||
| Non-small-cell lung cancer | 25 (10.5) | 22 (19.3) | 47 (13.4) |
| Breast cancer | 40 (16.9) | 7 (6.1) | 47 (13.4) |
| Chronic lymphocytic leukaemia | 22 (9.3) | 9 (7.9) | 31 (8.8) |
| Colorectal cancer | 26 (11.0) | 5 (4.4) | 31 (8.8) |
| Prostate cancer | 19 (8.0) | 10 (8.8) | 29 (8.3) |
| Multiple myeloma | 14 (5.9) | 14 (12.3) | 28 (8.0) |
| Non-Hodgkin lymphoma | 17 (7.2) | 11 (9.6) | 28 (8.0) |
| Urinary cell cancer | 8 (3.4) | 5 (4.4) | 13 (3.7) |
| Myeloproliferative neoplasms | 7 (3.0) | 3 (2.6) | 10 (2.8) |
| Myelodysplastic syndrome | 4 (1.7) | 5 (4.4) | 9 (2.6) |
| Renal cell cancer | 6 (2.5) | 3 (2.6) | 9 (2.6) |
| Melanoma | 7 (3.0) | 1 (0.9) | 8 (2.3) |
| Endometrial cancer | 6 (2.5) | 1 (0.9) | 7 (2.0) |
| Neuro-endocrine tumour | 6 (2.5) | 1 (0.9) | 7 (2.0) |
| Oesophageal cancer | 1 (0.4) | 5 (4.4) | 6 (1.7) |
| Chronic myeloid leukaemia | 4 (1.7) | 1 (0.9) | 5 (1.4) |
| Ovarian cancer | 4 (1.7) | 0 (0) | 4 (1.1) |
| Pancreatic cancer | 4 (1.7) | 0 (0) | 4 (1.1) |
| Small-cell lung cancer | 1 (0.4) | 3 (2.6) | 4 (1.1) |
| Other | 14 (5.9) | 8 (7.0) | 24 (6.8) |
| Last oncological treatment—n (%) | |||
| Surgery | 25 (10.5) | 17 (14.9) | 42 (12.0) |
| Radiotherapy | 43 (18.1) | 24 (21.1) | 67 (19.1) |
| Thoracic radiotherapy | 27 (11.4) | 16 (14.0) | 43 (12.3) |
| Chemotherapy | 104 (43.9) | 49 (43.0) | 153 (43.6) |
| Immunotherapy | 41 (17.3) | 16 (14.0) | 57 (16.2) |
| Targeted therapy | 39 (16.5) | 17 (14.9) | 56 (16.0) |
| Hormonal therapy | 35 (14.8) | 13 (11.4) | 48 (13.7) |
| Disease stage solid tumours—n (%) | |||
| Metastatic | 81 (34.2) | 31 (27.2) | 112 (47.1) |
| Intention most recent cancer treatment given—n (%) | |||
| Curative | 105 (44.3) | 45 (39.5) | 150 (42.7) |
| Non-curative | 122 (51.5) | 66 (57.9) | 188 (53.6) |
| Unknown | 10 (4.2) | 3 (2.6) | 13 (3.7) |
| Treatment restrictions—n (%) | |||
| Do-not-intubate | 82 (34.6) | 95 (83.3) | 177 (50.4) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Before the COVID-19 diagnosis, cancer treatment had been completed in 108 (30.8%) patients. In 101 (28.8%) patients, cancer treatment was not adjusted during the COVID-19 outbreak. Adjustments before the COVID-19 diagnosis included dose reduction (n = 4, 1.1%), premature withdrawal of treatment (n = 14, 4.0%), administration of higher dose (e.g. immunotherapy or radiotherapy) at longer interval (n = 16, 4.6%), cancellation of recent treatment cycle (n = 35, 10.0%) and/or temporarily interruption of treatment (n = 70, 19.9%).
3.3. Outcome of COVID-19 in patients with cancer
In total, 114 (32.3%) of the patients died from COVID-19. Patients with a fatal outcome of COVID-19 had a higher median age as compared with patients with non-fatal outcome (74 [IQR 68–80] versus 68 [IQR 59–76] years). Patients with age ≥65 years had an increased risk of fatal outcome (p < 0.001). In univariable analyses (Table 2 ), male gender, smoking, cardiovascular disease, chronic obstructive pulmonary disease, prior or other malignancy, use of steroids at COVID-19 diagnosis, a current diagnosis of haematologic malignancy and lung cancer were associated with fatal outcome of COVID-19.
Table 2.
Univariable analysis of features of patients related to a fatal outcome of COVID-19.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Sex (male) | 2.15 (1.35–3.41) | 0.001 |
| Age (years) | ||
| <65 years | – | – |
| ≥65 years < 75 years | 5.35 (2.64–10.81) | <0.001 |
| ≥75 years | 6.90 (3.44–13.84) | <0.001 |
| Smoking | ||
| All smokers | – | – |
| History of smoking | 1.72 (1.03–2.88) | 0.040 |
| Active smoker | 3.13 (1.28–7.64) | 0.012 |
| Comorbidities | ||
| Cardiovascular disease | 1.64 (1.04–2.58) | 0.034 |
| BMI ≥ 30 | 0.64 (0.35–1.19) | 0.158 |
| COPD | 1.73 (0.92–3.25) | 0.087 |
| Diabetes mellitus | 1.35 (0.74–2.45) | 0.325 |
| Autoimmune disease | 1.48 (0.61–3.56) | 0.383 |
| Prior/other malignancy | 2.59 (1.49–4.52) | 0.001 |
| Use of steroids at COVID-19 diagnosis | – | – |
| As part of cancer treatment (<1 week) | 1.94 (1.06–3.57) | 0.033 |
| Use >1 week (not related to cancer treatment) | 2.18 (1.08–4.41) | 0.029 |
| Cancer type | ||
| Other | – | – |
| Haematological malignancy | 2.15 (1.30–3.57) | 0.003 |
| Lung cancer | 3.13 (1.64–5.95) | 0.001 |
| Last oncological treatment | ||
| Surgery | 1.49 (0.77–2.88) | 0.238 |
| Radiotherapy | 1.20 (0.69–2.10) | 0.516 |
| Thoracic radiotherapy | 1.27 (0.65–2.47) | 0.479 |
| Chemotherapy | 0.96 (0.61–1.51) | 0.874 |
| Immunotherapy | 0.78 (0.42–1.46) | 0.437 |
| Targeted therapy | 0.89 (0.48–1.65) | 0.712 |
| Hormonal therapy | 0.74 (0.38–1.47) | 0.390 |
| Disease stage | ||
| Metastatic | 0.87 (0.54–1.41) | 0.575 |
| Intention most recent cancer treatment given | ||
| Non-curative | 1.30 (0.83–2.03) | 0.259 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CI, confidence interval.
In multivariable analyses, age ≥65 years (p < 0.001), male gender (p = 0.035), prior or other malignancy (p = 0.045) and an active diagnosis of haematological malignancy (p = 0.046) or lung cancer (p = 0.003) remained independent risk factors for a fatal outcome of COVID-19 (Table 3 ).
Table 3.
Multivariable analysis of features of patients related to a fatal outcome of COVID-19.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Sex (male) | 1.84 (1.04–3.23) | 0.035 |
| Age (median age in years) | ||
| <65 years | – | – |
| ≥65 years < 75 years | 4.26 (1.89–9.58) | <0.001 |
| ≥75 years | 5.75 (2.56–12.92) | <0.001 |
| Comorbidities | ||
| Prior/other malignancy | 2.02 (1.02–4.02) | 0.045 |
| Cancer type | ||
| Other | – | – |
| Haematological malignancy | 1.89 (1.01–3.53) | 0.046 |
| Lung cancer | 3.40 (1.51–7.64) | 0.003 |
CI, confidence interval.
Treatment restrictions with a do-not-intubate order were reported in 117/351 (50.4%) patients and in 95/114 (83.3%) patients with fatal COVID-19 outcome.
3.4. Active malignancy
A subgroup analysis was performed in 227 patients with active malignancy. The characteristics and results of the univariable analysis are shown in Table 4 . Patients with a haematological malignancy or lung cancer had an increased risk of a fatal outcome of COVID-19 compared with patients with other cancer types. In addition, male patients, age ≥65 years, smoking, cardiovascular disease and use of steroids as part of anticancer treatment remained risk factors for fatal outcome in univariable analysis. In this subgroup analysis, treatment in non-curative setting was also associated with fatal outcome.
Table 4.
Univariable analysis for the subgroup of patients with active malignancy and COVID-19.
| Variable | Total group (n = 227) |
||
|---|---|---|---|
| Frequency n (%) | Odds ratio (95% CI) | p value | |
| Sex (male) | 115 (50.7) | 1.79 (1.01–3.17) | 0.045 |
| Age (median age in years) | |||
| <65 years | 84 (37.0) | – | – |
| ≥65 years < 75 years | 77 (33.9) | 4.72 (2.12–10.55) | <0.001 |
| ≥75 years | 66 (29.1) | 6.55 (2.89–14.86) | <0.001 |
| Smoking | |||
| All smokers | 115 (50.7) | – | – |
| History of smoking | 99 (43.6) | 1.20 (0.64–2.26) | 0.579 |
| Active smoker | 16 (7.0) | 2.63 (0.89–7.78) | 0.082 |
| Comorbidities | |||
| Cardiovascular disease | 107 (47.1) | 1.86 (1.06–3.29) | 0.031 |
| BMI ≥ 30 | 39 (17.2) | 0.61 (0.27–1.36) | 0.225 |
| COPD | 23 (10.1) | 1.47 (0.61–3.58) | 0.392 |
| Diabetes mellitus | 30 (13.2) | 1.12 (0.49–2.52) | 0.794 |
| Autoimmune disease | 10 (4.4) | 1.49 (0.41–5.46) | 0.543 |
| Prior/other malignancy | 38 (16.7) | 1.77 (0.87–3.63) | 0.115 |
| Use of steroids at COVID-19 diagnosis | 134 (59.0) | – | – |
| As part of cancer treatment (<1 week) | 53 (23.3) | 2.26 (1.16–4.40) | 0.017 |
| Use >1 week (not related to cancer treatment) | 25 (11.0) | 1.65 (0.67–4.09) | 0.275 |
| Cancer type | |||
| Other | 127 (55.9) | – | – |
| Haematological malignancy | 62 (27.3) | 3.64 (1.89–7.04) | <0.001 |
| Lung cancer | 38 (16.7) | 2.53 (1.16–5.53) | 0.020 |
| Last oncological treatment | |||
| Surgery | 15 (6.6) | 1.51 (0.52–4.41) | 0.451 |
| Radiotherapy | 49 (21.6) | 0.85 (0.42–1.70) | 0.645 |
| Thoracic radiotherapy | 31 (13.7) | 0.88 (0.39–2.03) | 0.772 |
| Chemotherapy | 117 (51.5) | 0.88 (0.50–1.54) | 0.648 |
| Immunotherapy | 46 (20.3) | 0.84 (0.41–1.71) | 0.621 |
| Targeted therapy | 49 (21.6) | 1.22 (0.63–2.38) | 0.560 |
| Hormonal therapy | 39 (17.2) | 0.72 (0.33–1.57) | 0.404 |
| Disease stage for solid tumours | |||
| Metastatic | 118 (52.0) | 0.93 (0.53–1.63) | 0.795 |
| Intention most recent cancer treatment given | |||
| Non-curative | 148 (65.2) | 1.89 (1.01–3.53) | 0.044 |
| Treatment restrictions | |||
| Do-not-intubate | 121 (53.3) | – | – |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CI, confidence interval.
The above-mentioned characteristics were all included in the multivariable analysis. The risk for a fatal outcome was mainly determined by tumour type and age, as older patients (≥65 years) and patients with a haematological malignancy or lung cancer had a worse outcome of COVID-19 (Table 5 ).
Table 5.
Multivariable analysis for the subgroup of patients with active malignancy and COVID-19.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age (median age in years) | ||
| <65 years | – | – |
| ≥65 years < 75 years | 4.09 (1.70–9.89) | 0.002 |
| ≥75 years | 5.56 (2.21–14.02) | <0.001 |
| Cancer type | ||
| Other | – | – |
| Haematological malignancy | 3.60 (1.72–7.53) | 0.001 |
| Lung cancer | 3.01 (1.20–7.59) | 0.019 |
CI, confidence interval.
In total, 165 patients were on active treatment (i.e. ≤30 days between the last treatment and date of COVID-19 diagnosis). In this group, there were no differences in the risk of a fatal outcome of COVID-19 between the different cancer therapies. The disease setting (non-metastatic versus metastatic) and treatment setting (curative versus non-curative) were not associated with an increased risk of fatal outcome of COVID-19.
4. Discussion
The DOCC registry was initiated to identify clinical characteristics of patients with cancer related to an increased risk of fatal outcome of COVID-19. An active diagnosis of haematological malignancy or lung cancer, age (≥65 years), male gender and diagnosis of a prior or other malignancy were independent risk factors for a fatal outcome of COVID-19. In the subgroup of patients with active malignancy, age (≥65 years) and a diagnosis of a haematological malignancy or lung cancer remained independent risk factors for increased mortality of COVID-19.
Although chemotherapy has previously been identified as a risk factor for mortality of COVID-19 in cancer patients [21], this could not be confirmed in our registry. This is supported by data from a UK registry [9]. However, steroid use at the time of COVID-19 diagnosis was associated with an increased risk of fatal outcome of COVID-19 in univariable analysis. This result is of particular interest, as a recent randomised clinical trial showed that dexamethasone decreases mortality of COVID-19 in patients requiring respiratory support [22]. Steroids may contribute to an increased viral load of SARS-CoV-2 by an increase in viral replication and a delay of viral clearance [23]. Steroid co-medication is usually prescribed as supportive medication for haematological treatment and/or highly emetogenic chemotherapy regimens. Therefore, systemic treatment or disease itself cannot be excluded as confounding factor.
Apart from the current DOCC registry, other international registries have been published to identify the clinical characteristics of cancer patients with severe COVID-19 [[5], [6], [7],9,21,[24], [25], [26], [27], [28]]. As the design and data collection of these registries are significantly different, a comparison between results is challenging. Therefore, for appropriate interpretation of data published by these registries, attention should be paid to the different designs and patient selections (Table 6 ).
Table 6.
Overview of previously published registries.
| Author | Variable | Dai [5] | Liang [6]c | Zhang [7] | Lee [9] | Garassino (TERAVOLT) [21] | Kuderer (CCC1S) [24] | Scarfo [25] | Pinato (OnCovid) [26] | Lara [27] | Robilotti [28] | Joode (DOCC) |
| Country | China | China | China | UK | 8 countries | USA, Canada and Spain | Europe (mainly Italy and Spain) | Europe (UK, Spain, Italy, Germany) | New York | Memorial Sloan Kettering Cancer Center New York | The Netherlands | |
| Registry (hospital and/or general practitioner) | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | Hospital only | |
| Number of patients with cancer | 105 | 18 out of 1590 COVID-19 patients had cancer | 26 | 800 | 200 | 928 | 190 | 890 | 121 | 423 | 442 | |
| Number of hospitals | 14 | 575 | 3 | 55 | 87 | Not reported | 118 | 19 | 6 | 1 | 45 | |
| covid-19 diagnosis | WHO interim guidance | PCR | PCR | PCR | WHO interim guidance | PCR | PCR | PCR | Laboratory confirmation (PCR and/or serology) and/or radiological (X-ray or CT) and/or high clinical suspicion | Laboratory confirmation (PCR and/or serology) and/or symptomatic | PCR and/or CT | |
| Study design | Multicentre prospective cohort study | Prospective cohort study | Retrospective cohort study | Prospective cohort study | Multicentre observational study | Retrospective cohort study | Multicentre retrospective study | Multicentre retrospective observational study | Multicentre retrospective observational study | Retrospective cohort study | Observational cohort study | |
| Informed consent patients | No | Not reported | No | Not reported | According to local need | Not reported | Yes | Not reported | Not reported | Not reported | No | |
| Monitoring of the data | Reviewed by > 2 oncologists | Not reported | Reviewed by two physicians | Not reported | Yes (by REDCap) | Not reported | Not reported | Not reported | Not reported | Not reporter | Data cleaned by experienced oncology physicians | |
| Population | Cancer diagnosis from | Ever, distributed in several cohortsb | Ever | Ever | Last 12 months | Not reported | Not reported | Ever | Ever | Ever | Not reported | Last 5 yeard |
| Lung cancer | 22 (21%) | 5/18 (28%) | 7 (25%) | 90 (11%) | Only thoracic malignancies | 91 (10%); thoracic cancer | 0 | 119 (13%) | 0 | 35 (8%) | 51 (15%) | |
| Haematologic cancer | 9 (9%) | 1/18 (6%) (lymphoma) | 0 | 169 (21%) | 0 | 204 (22%) | All haematologic cancer | 137 (15%) | 0 | 102 (24%) | 111 (32%) | |
| Other solid tumours | Not reported | 12/18 (67%) | 21 (75%) | 494 (62%) | Only thoracic malignancies | 667 (72%) | 0 | 634 (71%) | Only gynaecological cancer | 286 (68%) | 165 (47%) | |
| Treatment status | Definition of ‘recent’ | Within 40 days | Within 1 month | Within 14 days | Within 4 weeks | Not reported | Within 4 weeks | Within 12 months | Within 4 weeks | Not reported | Within 30 days | Within 30 days |
| Recent chemotherapy | 17 | 4 (chemotherapy or surgery) | 3 | 281 | 68 | 160 | Not reported | 206 | 35 | 191 | 117 | |
| Recent surgery | 8 | 4 (chemotherapy or surgery) | 0 | 29 | 0 | 2 | Not reported | 0 | 11 | 31 | 15 | |
| Recent radiotherapy | 13 | 0 | 1 | 76 | 0 | 12 | Not reported | 33 | 9 | Not reported | 49 | |
| Recent immunotherapy | 6 | 0 | 1 | 44 | 54 | 38 | Not reported | 56 | 8 | 31 | 46 | |
| Recent hormonal therapy | 0 | 0 | 0 | 0 | 0 | 0 | Not reported | 92 | 9 | Not reported | 39 | |
| In follow-up | Not reported | 12 | 12 | Not reported | 52 (26%) | Not reported | 73 | 403 | 52 | Not reported | 108 | |
| Treatment restrictions | Not reported | Not reported | Not reported | Not reported | Yes | Not reported | Not reported | Not reported | Not reported | Not reported | With a do-not-intubate order | |
| Data registered | Baseline characteristicsa | Yes | Yes | Yes | Yes (including covid-19 severity) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Laboratory examination | Not reported | Not reported | Yes | Not reported | Yes | Not reported | Not reported | Yes | Yes | Yes | Yes | |
| Abnormalities at baseline on X-ray or CT | Not reported | Yes | Yes | Not reported | Yes | Not reported | Not reported | Not reported | Not reported | Yes | Yes | |
| Use of antibiotics | Yes | Not reported | Yes | Not reported | Yes | Not reported | Not reported | Yes | Yes | Yes | Yes | |
| Use of antiviral s | Yes | Not reported | Yes | Not reported | Yes | Not reported | Yes | Yes | Yes | Yes | Yes | |
| Use of hydroxychloroquine | Not reported | Not reported | Not reported | Not reported | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Use of glucocorticoids | Yes | Not reported | Yes | Not reported | Yes | Not reported | Not reported | Yes | Yes | Yes | Yes | |
| Use of anti-IL6 | Not reported | Not reported | Not reported | Not reported | Yes | Not reported | Yes | Yes | Yes | Yes | Yes | |
| Use of anticoagulants | Not reported | Not reported | Not reported | Not reported | Yes | Not reported | Yes | Not reported | Yes | Not reported | Not reported | |
| Admission to ICU | Yes | Yes | Yes | Yes | Yes | Yes | Not reported | Yes | Yes | Yes | Not reported | |
| Invasive ventilation | Yes | Yes | Yes | Not reported | Not reported | Yes | Not reported | Yes | Yes | Yes | Not reported | |
| Death | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Other | Length of hospital stay | COVID-19 management at home, COVID-19 resolution | Occurrence of complicated SARS-Cov-2 infection | Adjustment of oncological treatment, treatment restrictions regarding mechanical ventilation and admission to ICU |
DOCC, Dutch Oncology COVID-19 Consortium; ICU, intensive care unit; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; CT, computed tomography.
Age, smoking, comorbidity, cancer type, cancer treatment, COVID-19 symptoms.
<3 months, 1–3 months, 3–6 months, 6–12 months, 1–3 years, >3 year.
On behalf of the National Clinical Research Center for Respiratory Disease.
Or longer if the cancer treatment is expected to have an impact on COVID-19 outcome, for example after bone marrow transplantation or thoracic radiotherapy.
At the beginning of the COVID-19 outbreak in the Netherlands, both international and national oncological guidelines were published [[13], [14], [15], [16]]. In summary, the national guidelines were rather reluctant to start or continue oncological therapies. In addition, treating physicians were encouraged to discuss treatment restrictions regarding intubation and ICU admission with their patients. Owing to these conservative guidelines, adjustments in oncological treatment were rather common [12] and probably even more frequent in vulnerable patients. Therefore, the lack of effect of oncological treatments on fatal outcomes of COVID-19 should be interpreted cautiously in the current study, and the impact of anticancer therapies on the course of COVID-19 cannot be excluded.
Moreover, discussing treatment restrictions with patients in the outpatient clinic was already common practice in the Netherlands prior to COVID-19, especially for patients with cancer in the non-curative setting. In the DOCC registry, more than 50% of patients had a do-not-intubate order prior to infection with SARS-CoV-2. Among patients with fatal outcome of COVID-19, more than 80% had a do-not-intubate order. In addition, in the Netherlands, patients with COVID-19 are almost solely admitted to the ICU when mechanical ventilation is required, whereas most other supportive treatments are given outside the ICU. As a result, <20% of patients with fatal outcome of COVID-19 was admitted to the ICU in the current study, despite the lack of capacity issues of ICUs in the Netherlands. Although discussing treatment restrictions is common practice in the Netherlands and probably more common as compared to other countries, the percentage of patients with a fatal outcome is comparable to other countries [6,7,9,21,24]. Therefore, early discussion of treatment restrictions with vulnerable patients is preferred during this ongoing pandemic.
As the DOCC registry is only executed by oncology physicians in hospitals, a limitation of this study is the potential selection bias. As a result, particular groups of patients may have been underreported. For instance, patients who already had completed oncological treatment, patients who were not admitted to the hospital or patients who died in an out-hospital setting, may not have been registered. Next, the Dutch testing policy for SARS-CoV-2 was restrictive in the beginning of the pandemic, which initially resulted in an underestimation of the total number of patients with COVID-19. Although a potential selection bias may have occurred, this does not directly affect the results of this analysis, as the potentially underreported patient groups mainly included patients without active malignancy and/or recent cancer treatment. In addition, the Dutch healthcare system provides equal access to medical care and cancer treatment decisions are based on the same national guidelines. Therefore, the results of the current study seem to be representative of a national cancer patient population.
As the COVID-19 pandemic overwhelmed healthcare systems worldwide, non-evidence–based decisions had to be made about the treatment of patients with non-COVID-19 diseases such as cancer. Therefore, it is essential to combine data from several international registries and to ensure the collection of new and more comprehensive data during this ongoing pandemic. In particular, more data concerning cancer treatment and supportive medication (e.g. steroids) should be collected.
In conclusion, the findings of the DOCC registry in cancer patients confirm previous findings that older, male patients with comorbidities have an increased risk of a fatal outcome of COVID-19 [29]. Besides, the results of this registry indicate that patients with a haematological malignancy or lung cancer have an increased risk of a poorer outcome. During the ongoing COVID-19 pandemic, these vulnerable patients should avoid exposure to SARS-CoV-2, whereas treatment adjustments and prioritising vaccination, when available, should be considered as well.
Conflict of interest statement
D.D. reports personal fees from speakers fee MSD, personal fees from speakers fee Roche, personal fees from speakers fee AstraZeneca, personal fees from speakers fee BMS, personal fees from speakers fee Novartis, personal fees from speakers fee Pfizer, outside the submitted work; H.W. reports honoraria from Astellas and Roche and travel expenses from Ipsen, outside the submitted work; K.S. reports personal fees and advisory role for Novartis, personal fees from Roche, personal fees and advisory role for MSD, advisory role BMS, advisory role Pierre Fabre, advisory role Abbvie, outside the submitted work; L.H. reports other from Boehringer Ingelheim, other from BMS, other from Roche Genentech, other from BMS, grants from Roche Genentech, grants from Boehringer Ingelheim, other from AstraZeneca, personal fees from Quadia, grants from Astra Zeneca, other from Eli Lilly, other from Roche Genentech, other from Pfizer, other from MSD, other from Takeda, non-financial support from AstraZeneca, non-financial support from Novartis, non-financial support from BMS, non-financial support from MSD/Merck, non-financial support from GSK, non-financial support from Takeda, non-financial support from Blueprint Medicines, non-financial support from Roche Genentech, other from Amgen, outside the submitted work; A.D. reports personal fees from Roche, personal fees from Eli Lily, personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from BMS, personal fees from Novartis, personal fees from Takeda, personal fees from Pharmamar, non-financial support from Abbvie, grants from BMS, grants from Amgen, outside the submitted work; A.V. reports advisory board of BMS, MSD, Merck, Pfizer, Ipsen, Eisai, Pierre Fabre, Roche, Novartis, Sanofi, outside the submitted work.
All remaining authors declare no competing interests.
Acknowledgements
The authors thank all the oncology physicians and healthcare staff for their participation in the DOCC registry during this COVID-19 pandemic. They would like to thank S. Aammari and the Clinical Trial Center Rotterdam for the design and implementation of the study and S. Jeup for administrative support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.09.027.
Contributor Information
DOCC Investigators:
C.J. van Loenhout, C.H. van der Leest, A. Becker-Commissaris, J.S.W. Borgers, F. Terhegggen, B.E.E.M. van den Borne, L.J.C. van Warmerdam, L. van Leeuwen, F.S. van der Meer, M.A. Tiemessen, D.M. van Diepen, Y. Klaver, A.P. Hamberg, E.J. Libourel, L. Strobbe, M. Cloos, E.J. Geraedts, J.C. Drooger, R. Heller, J.W.B. de Groot, J.A. Stigt, V.J.A.A. Nuij, C.C.M. Pitz, M. Slingerland, F.J. Borm, B.C.M. Haberkorn, S.C. van 't Westeinde, M.J.B. Aarts, J.W.G. van Putten, M. Youssef, G.A. Cirkel, G.J.M. Herder, C.R. van Rooijen, E. Citgez, N.P. Barlo, B.M.J. Scholtes, R.H.T. Koornstra, N.J.M. Claessens, L.M. Faber, C.H. Rikers, R.A.W. van de Wetering, G.L. Veurink, B.W. Bouter, I. Houtenbos, M.P.L. Bard, K.H. Herbschleb, E.A. Kastelijn, P. Brocken, G. Douma, M. Jalving, T.J.N. Hiltermann, O.C.J. Schuurbiers-Siebers, K.P.M. Suijkerbuijk, A.S.R. van Lindert, A.J. van de Wouw, V.E.M. van den Boogaart, S.D. Bakker, E. Looysen, A.L. Peerdeman, W.K. de Jong, E.J.M. Siemerink, A.J. Staal, B. Franken, W.H. van Geffen, and G.P. Bootsma
Appendix 1.
Dutch Oncology COVID-19 Consortium (DOCC) contributors list.
C.J. van Loenhout1, C.H. van der Leest2, A. Becker-Commissaris3, J.S.W. Borgers4, F. Terhegggen5, B.E.E.M. van den Borne6, L.J.C. van Warmerdam7, L. van Leeuwen8, F.S. van der Meer9, M.A. Tiemessen10, D.M. van Diepen10, Y. Klaver11, A.P. Hamberg12, E.J. Libourel13, L. Strobbe14, M. Cloos15, E.J. Geraedts16, J.C. Drooger17, R. Heller18, J.W.B. de Groot19, J.A. Stigt20, V.J.A.A. Nuij21, C.C.M. Pitz22, M. Slingerland23, F.J. Borm24, B.C.M. Haberkorn25, S.C. van ‘t Westeinde26, M.J.B. Aarts27, J.W.G. van Putten28, M. Youssef29, G.A. Cirkel30, G.J.M. Herder31, C.R. van Rooijen32, E. Citgez33, N.P. Barlo34, B.M.J. Scholtes35, R.H.T. Koornstra36, N.J.M. Claessens37, L.M. Faber38, C.H. Rikers39, R.A.W. van de Wetering40, G.L. Veurink41, B.W. Bouter42, I. Houtenbos43, M.P.L. Bard44, K.H. Herbschleb45, E.A. Kastelijn46, P. Brocken47, G. Douma48, M. Jalving49, T.J.N. Hiltermann50, O.C.J. Schuurbiers-Siebers51, K.P.M. Suijkerbuijk52, A.S.R. van Lindert53, A.J. van de Wouw54, V.E.M. van den Boogaart55, S.D. Bakker56, E. Looysen57, A.L. Peerdeman58, W.K. de Jong59, E.J.M. Siemerink60, A.J. Staal61, B. Franken62, W.H. van Geffen63, G.P. Bootsma.64
1Department of Pulmonology, Admiraal de Ruijter Hospital, Goes, the Netherlands; 2Department of Pulmonology, Amphia Hospital, Breda, the Netherlands; 3Department of Pulmonary Diseases, Cancer Center Amsterdam, Amsterdam Medical Center, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; 4Department of Medical Oncology, The Netherlands Cancer Institute (NKI), Amsterdam, The Netherlands; 5Department of Internal Medicine, Bravis Hospital, Bergen op Zoom, The Netherlands; 6Department of Pulmonary Diseases, Catharina Hospital, Eindhoven, Netherlands; 7Department of Internal Medicine, Catharina-Hospital, Eindhoven, The Netherlands; 8Department of Internal Medicine, Diakonessenhuis, Utrecht, The Netherlands; 9Department of Pulmonology, Diakonessenhuis, Utrecht, The Netherlands; 10Department of Pulmonology, Dijklander Hospital, Purmerend, The Netherlands; 11Department of Internal Medicine, Elisabeth-Tweesteden hospital, Tilburg, The Netherlands; 12Department of Oncology, Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands; 13Department of Internal Medicine, Franciscus Hospital, Rotterdam, the Netherlands.; 14Department of Internal Medicine, Gelre Hospital, Zutphen, The Netherlands; 15Department of Internal Medicine, Groene Hart Hospital, Gouda, The Netherlands; 16Department of Pulmonology, Groene Hart Hospital, Gouda, The Netherlands; 17Department of Medical Oncology, Ikazia Hospital, Rotterdam, The Netherlands; 18Department of Pulmonology, Ikazia hospital, Rotterdam, The Netherlands; 19Department of Medical Oncology, Isala Oncology Center, Zwolle, The Netherlands; 20Department of Respiratory Medicine, Isala Hospital, Zwolle, The Netherlands; 21Department of Internal Medicine, Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands; 22Department of Pulmonology, Laurentius Hospital, Roermond, The Netherlands; 23Department of Medical Oncology, Leiden University Medical Center, Leiden, The Netherlands; 24Department of Pulmonology, Leiden University Medical Center, Leiden, The Netherlands; 25Department of Medical oncology, Maasstad Hospital, Rotterdam, The Netherlands; 26Department of Pulmonology, Maasstad Hospital, Rotterdam, The Netherlands; 27Department of Medical Oncology, Maastricht University Medical Centre, Maastricht, The Netherlands; 28Department of Pulmonary Diseases, Martini Hospital, Groningen, The Netherlands; 29Department of Respiratory Medicine, Máxima Medical Centre, Veldhoven, The Netherlands; 30Department of Internal Medicine, Meander Medical Center, Amersfoort, The Netherlands; 31Department of Pulmonary Medicine, Meander Medical Center, Amersfoort, The Netherlands; 32Department of Internal Medicine, Medisch Spectrum Twente, Enschede, The Netherlands; 33Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, The Netherlands; 34Department of Respiratory Medicine, Noordwest Ziekenhuisgroep, Alkmaar, the Netherlands; 35Department of Internal Medicine, Maasziekenhuis Pantein, Beugen, The Netherlands; 36Department of Internal Medicine, Rijnstate ziekenhuis, Arnhem, The Netherlands; 37Department of Respiratory Medicine, Rijnstate ziekenhuis, Arnhem, The Netherlands; 38Internal Medicine, Rode Kruis Hospital, Beverwijk, The Netherlands; 39Department of Pulmonology, Rode Kruis Hospital, Beverwijk, The Netherlands; 40Department of Internal Medicine, Slingeland Hospital, Doetinchem, The Netherlands; 41Department of Medical Oncology, Saxenburgh, Hardenberg, The Netherlands; 42Department of Pulmonology, Saxenburgh, Hardenberg, The Netherlands; 43Department of Internal Medicine, Spaarne Gasthuis, Haarlem, The Netherlands; 44Department of Pulmonology, Spaarne Gasthuis, Haarlem, The Netherlands; 45Department of Internal Medicine, St. Antonius Hospital Utrecht/Nieuwegein, Utrecht, The Netherlands; 46Department of Pulmonology, St. Antonius Hospital Utrecht/Nieuwegein, Utrecht, The Netherlands; 47Department of Pulmonary Diseases, Haga Ziekenhuis, den Haag, The Netherlands; 48Department of Pulmonary Diseases, Treant Zorggroep, Scheper hospital, Emmen, The Netherlands; 49Department of Medical Oncology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; 50Department of Pulmonary Diseases, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 51Department of Pulmonary Diseases, Radboud university medical center, Nijmegen, The Netherlands; 52Department of Medical Oncology, University Medical Center Utrecht Cancer Center, Utrecht, The Netherlands; 53Department of Respiratory Medicine, University Medical Centre Utrecht, Utrecht, The Netherlands; 54Department of Internal Medicine, VieCuri Medical Center, Venlo, The Netherlands; 55Department of Respiratory Medicine, VieCuri Medical Center Venlo, The Netherlands; 56Department of Internal Medicine, Zaans Medical Center, Zaandam, The Netherlands; 57Department of Pulmonology, Zaans Medical Center, Zaandam, The Netherlands; 58Department of Internal Medicine, Bernhoven, Uden, The Netherlands; 59Department of Pulmonology, Hospital Gelderse Vallei, Ede, The Netherlands; 60Department of Internal Medicine, Ziekenhuis Groep Twente (ZGT), Hengelo, The Netherlands; 61Department of Pulmonary Diseases, ZGT Almelo/Hengelo, Hengelo, The Netherlands; 62Department of Hematology, Medical Center Leeuwarden, Leeuwarden, The Netherlands; 63Department of Respiratory Medicine, Medical Center Leeuwarden, Leeuwarden, The Netherlands; 64Department of Pulmonology, Zuyderland Medical Center, Heerlen, The Netherlands.
The role of the funding source
This study was supported by a grant from the Dutch Cancer Society, a non-profit organisation. The Dutch Cancer Society had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Author contributions
K.J., D.D., J.T., H.W., L.B., F.B., P.M., N.D., O.V., E.O., H.B., H.L., L.H., J.H., E.V., A.D. and A.V. have contributed to the design of the study. All authors except for E.O. contributed to data collection. K.J., D.D., A.D., A.V. have contributed to literature search, data analysis, data interpretation and writing of the manuscript. D.D., P.M. and A.V. have checked all clinical data for inconsistencies. K.J. and E.O. have contributed to statistical analysis of the data. K.J., D.D., J.T., H.W., L.B., F.B., P.M., N.D., O.V., E.O., H.B., H.L., L.H., J.H., E.V., A.D. and A.V. participated in drafting the article and revising it critically for important intellectual content. All authors reviewed the manuscript and have given final approval of the submitted version.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) outbreak 2020.
- 4.World Health Organization. Coronavirus disease (COVID-2019) situation reports.
- 5.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Canc Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Team UKCCMP. Kerr R., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosain R., Abdou Y., Singh A., Rana N., Puzanov I., Ernstoff M.S. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22:53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Joode K., Dumoulin D.W., Engelen V., Bloemendal H.J., Verheij M., van Laarhoven H.W.M., et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients' perspective. Eur J Canc. 2020;136:132–139. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whisenant J.G., Trama A., Torri V., De Toma A., Viscardi G., Cortellini A., et al. TERAVOLT: thoracic cancers international COVID-19 collaboration. Canc Cell. 2020;37:742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.the European Society for Medical Oncology (ESMO). Cancer patient managment during the COVID-19 pandemic 2020.
- 15.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingemans A.C., Soo R.A., Jazieh A.R., Rice S.J., Kim Y.T., Teo L.L.S., et al. Treatment guidance for patients with lung cancer during the coronavirus 2019 pandemic. J Thorac Oncol. 2020;15:1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nederlandse Vereniging voor Medische Oncologie (NVMO). Dutch oncology COVID-19 consortium.
- 18.Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose (NVALT). [PubMed]
- 19.Landelijke werkgroep neuro-oncologie (LWNO). Dutch oncology COVID-19 consortium.
- 20.Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Actuele informatie over het nieuwe coronavirus (COVID-19).
- 21.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J.W., Fan L.C., Miao X.Y., Mao B., Li M.H., Lu H.W., et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:956–963. doi: 10.1016/j.cmi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarfo L., Chatzikonstantinou T., Rigolin G.M., Quaresmini G., Motta M., Vitale C., et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinato D.J., Lee A.J.X., Biello F., Segui E., Aguilar-Company J., Carbo A., et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12 doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lara O.D., O'Cearbhaill R.E., Smith M.J., Sutter M.E., Knisely A., McEachron J., et al. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020 doi: 10.1002/cncr.33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.