Abstract
There were few studies of cumulative live birth rates (CLBRs) based on multicenter reproductive clinical data from the general Chinese population. Here we report a retrospective cohort study, including 14 311 women with 17 315 cycles, in three reproductive centers to evaluate two estimated parameters of CLBRs with multiple transfer cycles of in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) in a Chinese population. We found that CLBRs were related to female age and endometrial thickness. By the fourth transfer cycle, the conservative and optimal estimates of CLBRs were 52.95% and 77.30% in women under 30 years of age, and 18.17% and 26.51% in those 37 years of age or older, respectively. The two estimates were 44.70% and 63.15% in women with endometrial thickness more than 7 mm, and 32.05% and 46.18% in those with less than 7 mm, respectively. In addition, body mass index (BMI), duration of infertility, and infertility diagnoses may also be related to CLBRs on certain conditions. The findings from this study on CLBRs after multiple transfer cycles of IVF/ICSI treatment on different conditions in the Chinese population should be beneficial to both infertile couples and clinicians.
Keywords: cumulative live birth rate, in vitro fertilization , intracytoplasmic sperm injection
Introduction
Assisted reproductive technology (ART) has evolved rapidly since its invention 40 years ago[1], and up to now, more than 6 million people were estimated to have been born all over the world[2]. For a long time, one of the most commonly used indexes for ART success assessment is the probability of live birth of a single cycle[1,3–7]. However, with the development of embryo cryotherapy technology, thawed frozen embryo transfers (FETs) have been widely applied[8–9] and more times of embryo transplantation can be performed within a single ovarian stimulation and oocyte retrieval process. Therefore, researchers found cumulative live birth rates (CLBRs) based on complete treatment cycles might be more suitable to evaluate the efficacy of fresh stimulated cycle and all subsequent thawed cycles[10–11].
Several studies focusing on CLBRs have been reported, but results are inconsistent. A UK study showed that the conservative and optimal estimates of CLBRs after eight complete cycles of ART were 44.0% and 82.4%, respectively[12], while another study in Australia and New Zealand reported that the conservative and optimal estimates of CLBRs after eight cycles can reach 54.3% and 77.2%, respectively[13]. In China, one of the largest studies about CLBRs was conducted at the Peking University Third Hospital, in which the conservative and optimal estimates after eight cycles can reach up to 69.1% and 90.3%, respectively[14]. In most cases, CLBRs were proved to be steadily increased with the number of transfer cycles after ovarian stimulation[11,15]. Therefore, CLBRs were helpful for making ART clinical decisions. In addition, the reported influencing factors of CLBRs such as female age, weight decrease, low-calorie diet, and physical exercise also need validation in different populations[16–17].
In China, about 15% to 20% of women at reproductive age suffered from infertility[18]. The total number of ART treatment cycles is growing rapidly. According to the "Maternal and Child Health Development Report in China (2019)", the total number of ART cycles had exceeded 1 million annually in recent years[19]. However, no study has reported CLBRs based on multiple reproductive centers in a general Chinese population. Therefore, we conducted the study to estimate the CLBRs in Chinese women with complete ART cycles and to evaluate the CLBRs with different factors.
Materials and methods
Study population
All the patients in this study were recruited from three reproductive medicine centers of China (the First Affiliated Hospital of Nanjing Medical University, the Affiliated Nanjing Maternity and Child Health Hospital of Nanjing Medical University, and Shengjing Hospital of China Medical University) from 2013 to 2016. Patients who underwent their first cycle of IVF with or without ICSI were enrolled. All of our calculation and results were based on one complete oocyte retrieval cycle. A complete oocyte retrieval cycle is defined as all fresh and subsequent frozen-thawed embryo transfers after one episode of ovarian stimulation. In addition, the following exclusion criteria were applied: women whose first cycle used a frozen embryo; either of the infertile couples was a foreigner. Cycles for an individual woman were censored after a live birth. As few women underwent more than four transfer cycles, only data from the first four transfer cycles were used. As a result, 14 311 women with data of 17 315 cycles were included in the final analysis. The protocol was approved by the local institutional board at the authors' affiliated institutions and patient consent was not required because of the retrospective nature of the study.
Baseline characteristics and outcomes
Women's characteristics and ART treatment were retrieved from the hospitals' electronic medical records based on couples' last name, first name, and clinic ID. We considered the following characteristics for women at the start of their first treatment: female age (categorized as ≤30, 30–34, 34–37, and ≥37 years of age), duration of infertility (<2, 2–3, 3–5, and ≥5 years), body mass index (BMI; categorized as < l8.5, 18.5–24, 24–28, and ≥28 kg/m2), type of infertility (primary or secondary infertility), infertility diagnosis (categorized as tubal factor, endometriosis, ovulation disorder or polycystic ovarian syndrome, male factor, or unexplained factors), endometrial thickness (mm), and insemination method (ICSI vs. IVF). Live birth was defined as the birth of at least one infant with a gestation of at least 22 weeks and the birth weight of at least 300 g[20].
Statistical analysis
Two types of CLBRs were calculated as outcome variables. The two-type cumulative live birth rates and their standard errors were estimated as follows. A conservative estimate of CLBR was based on the assumption that none of the women who did not return for a subsequent cycle would have had a live birth, and an optimal estimate of CLBR was based on the assumption that women who did not return for a subsequent cycle would have the same success rates as those who did return. The conservative estimate of CLBR was calculated as the number of live births up to and including a specific cycle, divided by the number of women who ever received that treatment, while the optimal estimate of CLBR was based on the product-limit estimate[20]. The standard errors for both these CLBRs were computed with the use of the binomial distribution. The product-limit was the Kaplan-Meier estimates when all cycles were included in the analysis; in these cases, the patterns of cumulative live birth rates were compared with the use of the log-rank test.
Since there may be more than one infertility factor, women may be assigned to multiple groups when estimated according to factors. The primary data was presented graphically, and additional figures and the CLBRs and their standard errors are provided in Supplementary Table 2–8 (available online). The data were analyzed with the use of R software (version 3.6.1), using packages "survival" and "survminer" for the optimal estimate's calculation and log-rank test.
Results
Characteristics of the study population
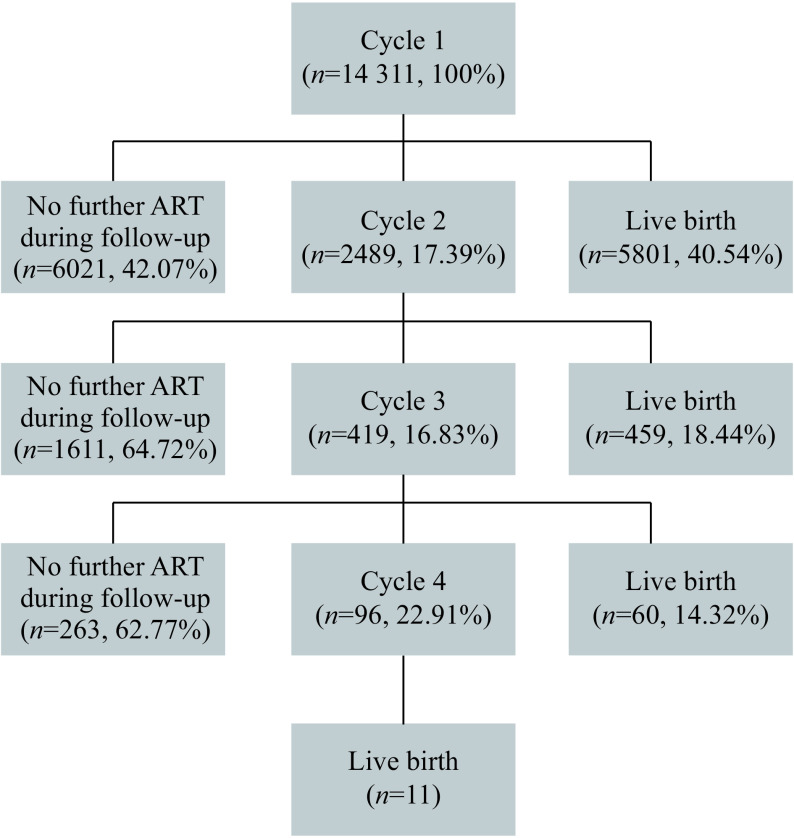
This study included 14 311 women, with 17 315 embryo transfer cycles and 6331 live births, and the overall live birth rate was 40.54% in the first cycle, rising by the fourth cycle up to 44.24% (Fig. 1). The characteristics are summarized in Table 1. About 48.84% of the women were younger than 30 years of age, and 8.80% were older than 37 years of age. Most of the women (61.16%) were at normal BMI. Tubal factor (62.48%) was the main cause of infertility.
1.
Flow chart over included cases and their course of treatments, women followed until their first live birth.
1. Baseline demographic and clinical characteristics of the study population [n (%)] .
| Characteristic | Women (n=14 311) |
|
*Multiple diagnoses were possible, so totals are greater than 100%; PCOS: polycystic ovarian syndrome; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection.
| |
| Age (year) | |
| Mean±SD | 30.95±4.65 |
| ≤30 | 6990 (48.84) |
| 30–34 | 4245 (29.66) |
| 34–37 | 1816 (12.69) |
| >37 | 1260 (8.80) |
| BMI (kg/m2) | |
| Mean±SD | 22.7±3.31 |
| <18.5 | 963 (6.73) |
| 18.5–24 | 8753 (61.16) |
| 24–28 | 3238 (22.63) |
| ≥28 | 996 (6.96) |
| Missing | 361 (2.52) |
| Duration of infertility (year) | |
| Mean±SD | 4.04±3.13 |
| <2 | 2693 (18.82) |
| 2–3 | 2684 (18.75) |
| 3–5 | 3911 (27.33) |
| ≥5 | 4447 (31.07) |
| Missing | 576 (4.02) |
| Type of infertility | |
| Primary infertility | 8387 (58.61) |
| Secondary infertility | 5761 (40.26) |
| Missing | 163 (1.14) |
| Infertility diagnosis* | |
| Male factor | 3169 (22.14) |
| Endometriosis | 1118 (7.81) |
| Ovulation disorder or PCOS | 1559 (10.89) |
| Tubal factor | 8941 (62.48) |
| Unexplained factor | 475 (3.32) |
| Insemination method | |
| IVF | 10 325 (72.15) |
| ICSI | 3063 (21.40) |
| IVF+ICSI | 602 (4.21) |
| Missing | 321 (2.24) |
| Endometrial thickness (mm) | |
| Mean±SD | 10.39±2.36 |
| <7 | 415 (2.90) |
| ≥7 | 10 602 (74.08) |
| Missing | 3294 (23.02) |
Live birth occurred in 36.56% of the cycles, and 44.24% of the women had a live birth. In the first cycle, of women who failed to get pregnant, 6021 (42.07%) quited the ART treatment and 2489 women (17.39%) went on with a second treatment cycle; in the second cycle, of women who were not pregnant, 1611 quitted and 419 continued; in the third cycle, just 96 chose a fourth cycle. The specific cumulative live birth rate of each cycle is detailed in Supplementary Table 1 (available online).
Live birth rates and female age
We found a progressive decline in both optimal and conservative estimates of the cumulative live birth rate with increasing female age (P<0.001) (Fig. 2A and B). By the fourth transfer cycle, the conservative and optimal estimates of live birth rates were 52.95% and 77.30% in women younger than 30 years of age and 18.17% and 26.51% in those 37 years of age or older, respectively.
2.
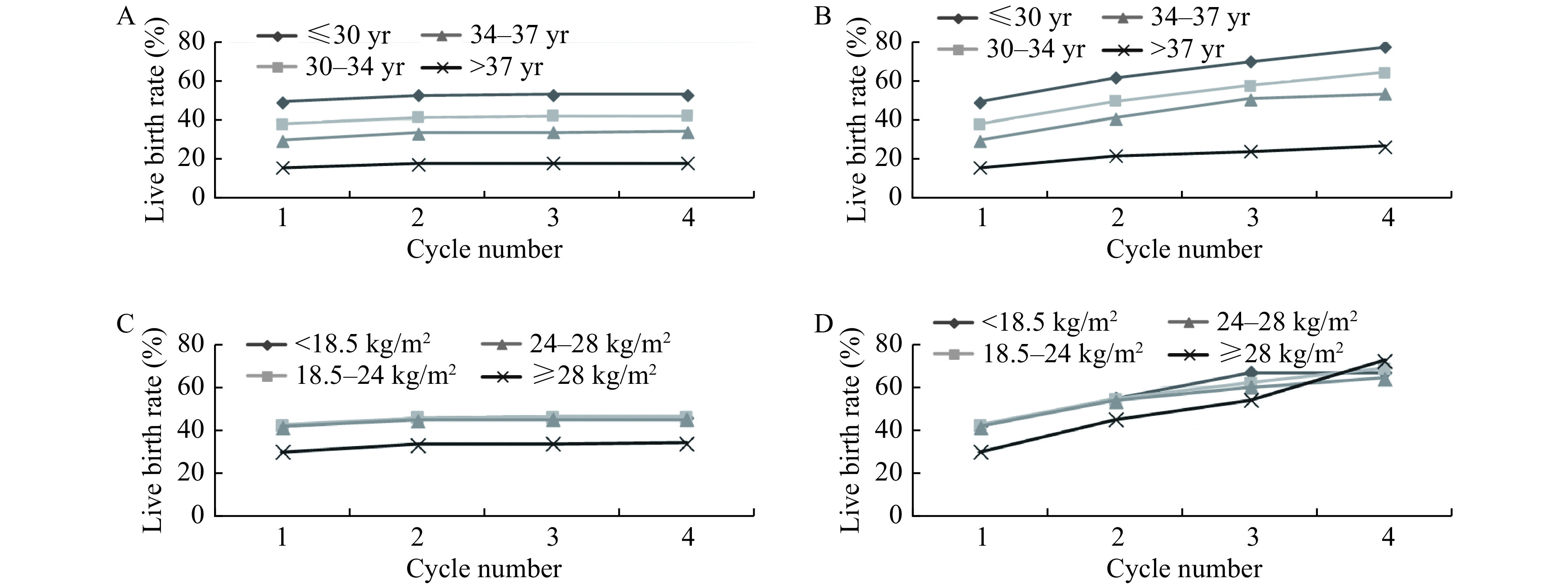
Cumulative live birth rates, according to female age and body mass index.
A and B: The conservative (A) and optimal (B)estimates of cumulative live birth rates, according to female age. C and D: The conservative (C) and optimal (D) estimates of cumulative live birth rates, according to body mass index.
Live birth rates and female BMI
Fig. 2C and D show the optimal and conservative live birth rates at different measures of female BMI. The conservative and optimal estimates of live birth rates after three transfer cycles were 46.22% and 61.97% in the normal BMI group (18.5–24 kg/m2), and 33.84% and 53.90% in the obese group (≥28 kg/m2), respectively. The contents of Fig. 2C and D indicate that obese women have lower optimal and conservative live birth rates than other women (P<0.001). Interestingly, as showed inFig. 2C, there was no statistical significance in the optimal estimates between those groups whose BMI lower than 28 kg/m2 (P>0.05).
Live birth rates and the duration of infertility
Fig. 3A and B show the correlations between duration of infertility and live birth rates. In the analysis of duration of infertility, there is a significant decrease in both optimal and conservative estimates of the cumulative live birth rate for those women with the duration of infertility exceeding 5 years. For instance, compared to those groups whose duration of infertility below 5 years, the conservative estimates declined by 7.96%, 8.19%, and 7.13%, while the optimal estimates declined by 5.63%, 13.51%, and 6.39%.
3.
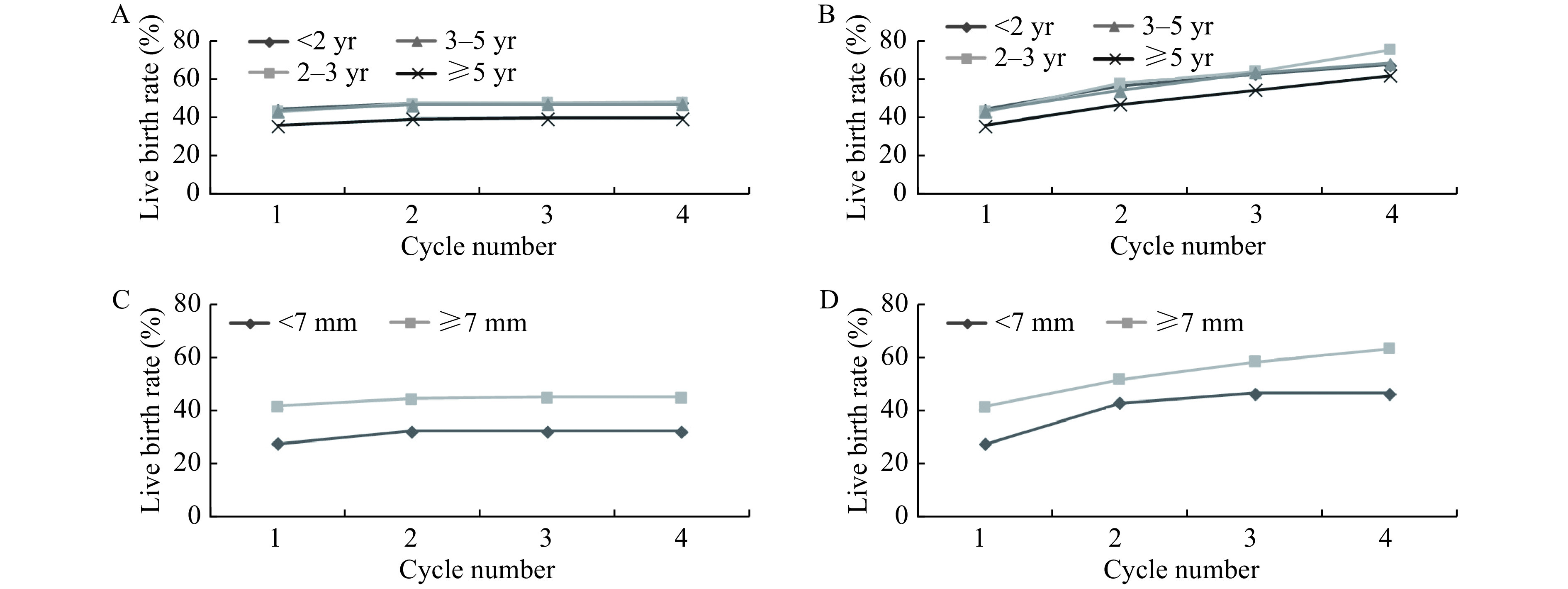
Cumulative live birth rates, according to duration of infertility and endometrial thickness.
A and B: The conservative (A) and optimal (B) estimates of cumulative live birth rates, according to duration of infertility. C and D: The conservative (C) and optimal (D)estimates of cumulative live birth rates, according to endometrial thickness.
Live birth rates and endometrial thickness
Fig. 3C and D show that, from the first cycle to the fourth, the live birth rate of women with endometrium thickness greater than 7 mm is about 1.5 times than those with endometrial thickness less than 7 mm (conservative estimate 44.70% vs. 32.05%, while optimal estimate 63.15% vs. 46.18%). By the fourth transfer cycle, the conservative and optimal estimates of live birth rates were both above 45% for women with endometrium thickness greater than 7 mm.
Live birth rates and type of infertility and infertility diagnosis
Fig. 4A and B show that the CLBRs of women with primary infertility was slightly higher than those with secondary infertility (4.14% and 7.80% respectively for conservative and optimal estimate) but showing no significant statistical difference. Apart from unexplained factor, women whose infertility is caused by male factor had the highest CLBRs of all types after the third complete cycle (the conservative and optimal estimates, 61.79% and 75.55%, repectively), while those with a diagnosis of tubal factor infertility had the lowest ones (42.10% and 60.54%, respectively) (Fig. 4C and D).
4.
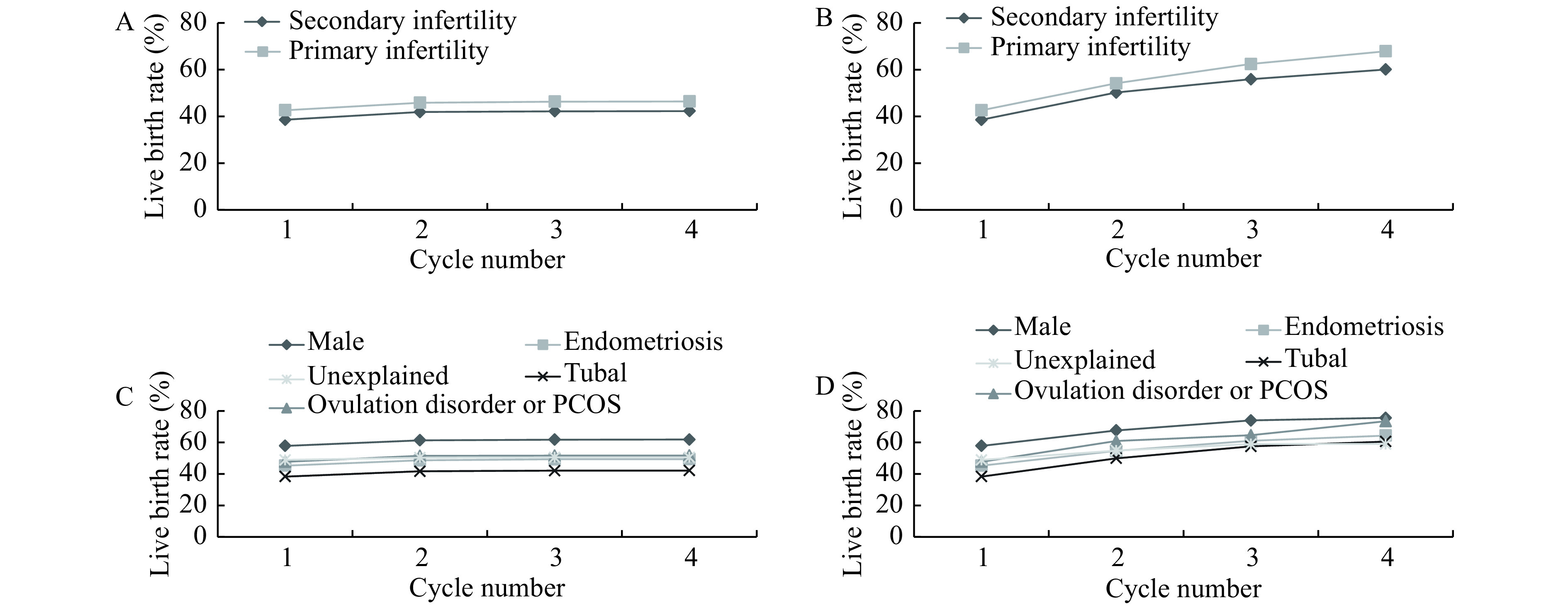
Cumulative live birth rates, according to type of infertility and infertility diagnosis.
A and B: The conservative (A) and optimal (B) estimates of cumulative live birth rates, according to type of infertility. C and D: The conservative (C) and optimal (D) estimates of cumulative live birth rates, according to infertility diagnosis.
Live birth rates and insemination method
Fig. 5 illustrates the conservative and optimal CLBRs stratified by different treatments in the first complete cycle. Among all groups, IVF plus ICSI had higher conservative and optimal estimates of CLBRs (49.00% and 72.26%). However, we can't investigate the real disparity among those groups because most of women had choosen the IVF and ICSI caused big population number difference between different methods.
5.
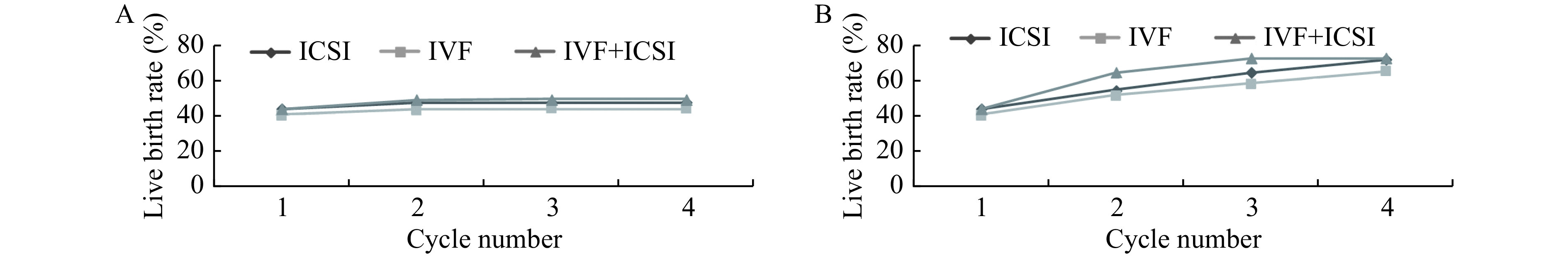
Cumulative live birth rates, according to insemination method.
The conservative (A) and optimal (B) estimates of cumulative live birth rates, according to insemination method. ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization.
Discussion
To date, this is one the of the largest reports of CLBRs based on ART treatment cycles derived from multiple clinic data in China. The CLBRs inform women about their chances of at least one live birth after a given number of repeated cycles. In our study, after four complete cycles, the conservative estimates of the CLBRs increased from 40.54% to 44.24% whilst optimal estimates increased to 63.21%, though the cycle-specific live birth rate declined to 11.46%. Age and endometrial thickness is the main influencing factor for CLBRs.
There were several studies on CLBRs in western countries. Recently, two studies from the UK[12,21] and one from Australia and New Zealand population[13] have reported their CLBRs. We cannot compare our results with those studies directly because of the different study periods and settings. However, conservative and optimal estamates of CLBRs in our study were both lower than theirs. These might result from the study design. All the western studies mentioned above were based on the national registration system, which covered the majority of cycles for the most people. For our study, the study duration is limited and many people may quit the treatment due to the high expenditure or other reasons, such as psychological factors[22–23].
As for domestic study, one of the largest was launched in Peking University Third Hospital based on its seven years' clinic data[14]. In the study, after four cycles, the conservative and optimal estimates can reach up to around 68% and 87%, respectively. Compared to the CLBRs, ours were significant lower than theirs. This may be due to better patient compliance in Peking University Third Hospital reputed for advanced assisted reproductive technology.
We also found several factors affecting live birth rates. The cycle-specific live birth rates and CLBRs declined significantly with age increasing, which is consistent with the results of previous studies[12,23–25]. The biological mechanism may lie in the diminished ovarian reserve which led to the poor quantity and quality of oocytes with increasing age[26]. Furthermore, increased incidence rates of aneuploid oocytes could explain the lower live birth rate partly as well[27].
We found that when BMI >28 kg/m 2, the CLBRs reached the lowest. Studies have shown that increased BMI correlates with reduced conception rates[28–29], suggesting that obesity may affect oocyte and/or embryo quality. Besides, low live birth rates with high BMI may be due to the action of a hormone named leptin[30]. What's more, high BMI may have an interaction with PCOS[31], which might influence the live birth rate partially.
We also found a correlation between endometrial thickness and CLBRs. Endometrial thickness is one of the most important factors in predicting pregnancy after IVF[32]. It is generally accepted that an endometrial thickness below a minimum value of 6 to 8 mm showed negative predictive value for IVF outcomes. As for the optimal thickness for ART outcomes, Gallos et al concluded that the optimal endometrial thickness threshold of 10 mm or more maximized live birth rates[33].
In addition to all the above factors, we also found that the duration of infertility as well as its causes may be related to CLBRs. As for the duration of infertility, we found that when the duration of infertility exceeded 5 years, the CLBRs reached the lowest. This might be partially due to the increasing psychological burden and severity of infertility, which may significantly influence the ART outcomes.
Tubal factor was associated with the lowest CLBRs, whereas male factor the highest. Reasons for highest CLBRs with male factor may lie in that generally ICSI is applied for male infertility and provides an effective improvement of ART treatment outcomes of male infertility[34]. As for the lowest rates for the tubal facor, one of the assumption is that it may be related to salpingitis, which is believed to account for >50% of these tubal-factor cases and can easily lead to hydrosalpinx [35]. The hydrosalpinx fluid may act on two different target systems: directly on the transferred embryos or on the endometrium and its receptivity for implantation, or both[36]. However, the actual mechanism still needs to be clarified.
There are several limitations about our study. First of all, for the limitation of study duration, we can't include all the related treatment cycles in our study. Second, some important factors such as stimulation protocol, and aspirated oocytes weren't included in our study either. Third, we did not take the interval between transferring into consideration. Therefore, the findings from our study should be interpreted within the context of limitations.
In summary, we reported the trend between CLBRs and the repeated treatment cycles, and several prognostic factors that may affect subfertility couples' ART treatment success rates. These findings may be helpful for making the ART clinical decisions for both the clinician and the subfertile couples.
Acknowledgments
The authors thank all of medics in Reproductive Medicine Center of the Affiliated Nanjing Maternity and Child Health Hospital of Nanjing Medical University, Reproductive Medicine Center of the First Affiliated Hospital of Nanjing Medical University and Reproductive Medicine Center of Shengjing Hospital Affiliated to China Medical University for recording all the data in this study through the years. This work was supported by National Key Research & Development Program (Grant No. 2016YFC1000200, No. 2016YFC1000204, and No. 2018YFC1004200), the State Key Program of National Natural Science of China (Grant No. 31530047), National Natural Science Foundation of China (Grant No. 81602927), Innovation Fund of State Key Laboratory of Reproductive Medicine (Grant No. SKLRM-GC201802), and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (Grant No. PPZY2015A067).
Contributor Information
Zhibin Hu, Email: zhibin_hu@njmu.edu.cn.
Jichun Tan, Email: tjczjh@163.com.
Feiyang Diao, Email: phenix_y@163.com.
References
- 1.Dyer S, Chambers GM, de Mouzon J, et al International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology 2008, 2009 and 2010. Hum Reprod. 2016;31(7):1588–1609. doi: 10.1093/humrep/dew082. [DOI] [PubMed] [Google Scholar]
- 2.The European IVF-Monitoring Consortium, European Society of Human Reproduction and Embryology, Kupka MS, et al Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod. 2016;31(2):233–248. doi: 10.1093/humrep/dev319. [DOI] [PubMed] [Google Scholar]
- 3.International Committee for Monitoring Assisted Reproductive Technology, Adamson GD, de Mouzon J, et al World collaborative report on in vitro fertilization, 2000 . Fertil Steril. 2006;85(6):1586–1622. doi: 10.1016/j.fertnstert.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 4.International Committee for Monitoring Assisted Reproductive Technology, de Mouzon J, Lancaster P, et al World collaborative report on assisted reproductive technology, 2002. Hum Reprod. 2009;24(9):2310–2320. doi: 10.1093/humrep/dep098. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan EA, Zegers-Hochschild F, Mansour R, et al International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod. 2013;28(5):1375–1390. doi: 10.1093/humrep/det036. [DOI] [PubMed] [Google Scholar]
- 6.Mansour R, Ishihara O, Adamson GD, et al International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology 2006. Hum Reprod. 2014;29(7):1536–1551. doi: 10.1093/humrep/deu084. [DOI] [PubMed] [Google Scholar]
- 7.Adamson GD, de Mouzon J, Chambers GM, et al International committee for monitoring assisted reproductive technology: world report on assisted reproductive technology, 2011. Fertil Steril. 2018;110(6):1067–1080. doi: 10.1016/j.fertnstert.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Wong KM, Mastenbroek S, Repping S Cryopreservation of human embryos and its contribution to in vitro fertilization success rates . Fertil Steril. 2014;102(1):19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Kushnir VA, Barad DH, Albertini DF, et al Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15(1):6. doi: 10.1186/s12958-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari A, McLernon D, Bhattacharya S Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30(12):2703–2707. doi: 10.1093/humrep/dev263. [DOI] [PubMed] [Google Scholar]
- 11.Malchau SS, Henningsen AA, Forman J, et al Cumulative live birth rate prognosis based on the number of aspirated oocytes in previous ART cycles. Hum Reprod. 2019;34(1):171–180. doi: 10.1093/humrep/dey341. [DOI] [PubMed] [Google Scholar]
- 12.McLernon DJ, Maheshwari A, Lee AJ, et al Cumulative live birth rates after one or more complete cycles of IVF: a population-based study of linked cycle data from 178 898 women. Hum Reprod. 2016;31(3):572–581. doi: 10.1093/humrep/dev336. [DOI] [PubMed] [Google Scholar]
- 13.Chambers GM, Paul RC, Harris K, et al Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success. Med J Aust. 2017;207(3):114–118. doi: 10.5694/mja16.01435. [DOI] [PubMed] [Google Scholar]
- 14.Chen LX, Li R, Ye RW Cumulative live birth rate in vitro fertilization in China: a population-based study . Chin J Reprod Health (in Chinese) 2017;28(2):101–105. [Google Scholar]
- 15.Polyzos NP, Drakopoulos P, Parra J, et al Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ~15 000 women . Fertil Steril. 2018;110(4):661–670. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Raz N, Shalev A, Horowitz E, et al Cumulative pregnancy and live birth rates through assisted reproduction in women 44-45 years of age: is there any hope? J Assist Reprod Genet. 2018;35(3):441–447. doi: 10.1007/s10815-017-1094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinós JJ, Polo A, Sánchez-Hernández J, et al Weight decrease improves live birth rates in obese women undergoing IVF: a pilot study. Reprod BioMed Online. 2017;35(4):417–424. doi: 10.1016/j.rbmo.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Qiao J, Feng HL Assisted reproductive technology in China: compliance and non-compliance. Transl Pediatr. 2014;3(2):91–97. doi: 10.3978/j.issn.2224-4336.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese National Department of Maternal and Child Health. Maternal and child health development report in China (2019) (in Chinese)[EB/OL]. [2019-05-27]. http://www.nhc.gov.cn/fys/s7901/201905/bbd8e2134a7e47958c5c9ef032e1dfa2.shtml.
- 20.Luke B, Brown MB, Wantman E, et al Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012;366(26):2483–2491. doi: 10.1056/NEJMoa1110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith ADAC, Tilling K, Nelson SM, et al Live-birth rate associated with repeat in vitro fertilization treatment cycles . JAMA. 2015;314(24):2654–2662. doi: 10.1001/jama.2015.17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimzadeh M, Salsabili N, Akbari Asbagh F, et al Psychological disorders among iranian infertile couples undergoing Assisted Reproductive Technology (ART) Iran J Public Health. 2017;46(3):333–341. [PMC free article] [PubMed] [Google Scholar]
- 23.Ramezanzadeh F, Aghssa MM, Abedinia N, et al A survey of relationship between anxiety, depression and duration of infertility. BMC Womens Health. 2004;4(1):9. doi: 10.1186/1472-6874-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moragianni VA, Penzias AS Cumulative live-birth rates after assisted reproductive technology. Curr Opin Obstet Gynecol. 2010;22(3):189–192. doi: 10.1097/GCO.0b013e328338493f. [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Liu F, Guan YC, et al A nomogram predicting clinical pregnancy in the first fresh embryo transfer for women undergoing in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments . J Biomed Res. 2019;33(6):422–429. [Google Scholar]
- 26.Broekmans FJ, Knauff EAH, te Velde ER, et al Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.te Velde ER, Pearson PL The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 28.van der Steeg JW, Steures P, Eijkemans MJC, et al Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–328. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- 29.Jensen TK, Scheike T, Keiding N, et al Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422–428. doi: 10.1097/00001648-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum M, Leibel RL The role of leptin in human physiology. N Engl J Med. 1999;341(12):913–915. doi: 10.1056/NEJM199909163411211. [DOI] [PubMed] [Google Scholar]
- 31.Yuan C, Liu XQ, Mao YD, et al Polycystic ovary syndrome patients with high BMI tend to have functional disorders of androgen excess: a prospective study. J Biomed Res. 2016;30(3):197–202. doi: 10.7555/JBR.30.20140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craciunas L, Gallos I, Chu J, et al Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(2):202–223. doi: 10.1093/humupd/dmy044. [DOI] [PubMed] [Google Scholar]
- 33.Gallos ID, Khairy M, Chu J, et al Optimal endometrial thickness to maximize live births and minimize pregnancy losses: Analysis of 25, 767 fresh embryo transfers. Reprod BioMed Online. 2018;37(5):542–548. doi: 10.1016/j.rbmo.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Palermo G, Joris H, Devroey P, et al Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 35.Honoré GM, Holden AEC, Schenken RS Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71(5):785–795. doi: 10.1016/S0015-0282(99)00014-X. [DOI] [PubMed] [Google Scholar]
- 36.Strandell A, Lindhard A Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod. 2002;17(5):1141–1145. doi: 10.1093/humrep/17.5.1141. [DOI] [PubMed] [Google Scholar]