Abstract
Environmental pollutants, such as bisphenol A (BPA) have recently been implicated in the development of adverse birth outcomes. However, the underlying teratogenic mechanisms remain unclear. We investigated the effects of BPA on the migration and invasion of human primary extravillous trophoblast HTR-8/SVneo cells. Our results indicated that BPA reduced cell migration and invasion. Moreover, it altered the ratio of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) by downregulating MMP-2 and MMP-9, and upregulating TIMP-1 and TIMP-2. Furthermore, BPA suppressed integrin β1, integrin α5, and vimentin. Interestingly, BPA-induced invasion was partially restored by G15, a membrane G-protein-coupled estrogen receptor 30 antagonist. We further revealed that 42 proteins were differentially expressed by mass spectrometry analysis, which could be divided into three categories based on gene ontology including biological process, cellular component, and molecular function. These results suggest that BPA reduces HTR-8/SVneo cell migration and invasion by downregulating MMP-2 and MMP-9, up-regulating TIMP-1 and TIMP-2, and suppressing adhesion molecules.
Keywords: bisphenol A, HTR8/SVneo cells, cell invasion, integrin β1
Introduction
Bisphenol A (BPA) is an environmental pollutant and an endocrine system modulator commonly used in the synthesis of polycarbonate plastics. It competitively binds to estrogen receptors (ERs) and G protein-coupled estrogen receptor 30 (GPR30) to induce estrogen-like effects[1]. Studies on BPA in body fluids, such as urine[2], serum[3], and amniotic fluid[4], have reported that it is harmful to multiple systems, including the neuroendocrine system[5], liver[6], and cardiovascular system[7]. Furthermore, BPA reportedly exerts cytotoxic effects on human placental cells[8], impairs embryonic implantation, increases the risk of spontaneous abortion in both humans and animals[9–10], and inhibits migration and invasion of HTR8/SVneo cells[11]. However, the mechanisms underlying BPA-induced toxicity during embryo implantation remain unclear.
Embryo implantation, wherein blastocysts adhere to the endometrium, occurs during the early stages of pregnancy and involves various changes in the endocrine and immune systems. The invasive and secretory properties of trophoblasts allow the blastocyst to anchor onto the endometrial surface and invade the uterine wall, allowing the embryo to be embedded in the decidua and generate the placenta, the fetal side of the maternal–fetal interface. Failed embryonic invasion can lead to an unsuccessful pregnancy or other complications, including placental hyperplasia, repeated miscarriage, pre-eclampsia, and preterm birth[12].
In a process similar to the epithelial–mesenchymal transition, first trimester placental cytotrophoblasts with epithelial characteristics differentiate into mesenchyme-like extravillous trophoblasts with invasive and migratory phenotypes that help them form the maternal-fetal interface[13]. As they penetrate the uterine wall, these differentiated trophoblasts differentially express numerous genes, including matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), and integrins, all of which are important regulators of trophoblast invasiveness[14–15]. Both the MMP and TIMP families include extracellular matrix-degrading proteinases. In particular, MMP-2 and MMP-9 degrade the extracellular matrix enabling trophoblasts to invade the uterine wall, while TIMP-1 and TIMP-2 inhibit MMP-9 and MMP-2, respectively, and therefore inhibit MMP activity to prevent excessive invasion[16]. Successful embryo implantation depends on the balance between MMP and TIMP activities, while disturbances caused by abnormal trophoblast invasion can result in abortion or other complications[17]. Integrins are a group of transmembrane glycoproteins that form heterodimeric receptors comprised of α- and β-subunits that are upregulated on the trophoblast surface where they facilitate trophoblast invasion[18]. Indeed, the inactivation of integrins α5 and β3 reduces the number of embryo implantation sites in mice[19]. However, the effects of BPA on trophoblast invasion and subsequent embryo implantation remain unclear.
This study aims to investigate the mechanism underlying the effect of BPA on embryo implantation. Our results suggest a potential mechanism by which BPA plays a role in the regulation of cell migration and invasion in human primary extravillous trophoblast HTR-8/SVneo cells.
Materials and methods
Reagents
BPA and ICI 182780 were purchased from Tokyo Chemical Industry (USA). G15 was purchased from Sigma-Aldrich (USA). Geneticin (G418) was purchased from InvivoGen (USA). Antibodies against integrin β1, integrin α5, MMP-2, MMP-9, TIMP-1, TIMP-2, CD97, N-cadherin, E-cadherin, vimentin, occludin, and β-actin were purchased from Abcam Biotechnology (USA). The cell counting kit-8 (CCK-8) was purchased from Dojindo Laboratories (Japan). The pcDNA3.1(+)-integrin β1 overexpression vector was cloned by Keygen Biotech (China).
Cell culture and drug administration
The human primary extravillous trophoblast cell line, HTR-8/SVneo, was generous gift from Fuheng Biology Company (China). HTR-8/SVneo cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS; Life Technologies, USA) at 37 °C in a 5% CO2 incubator. After 10 hours of incubation, the medium was replaced with fresh medium containing 0.4% FBS. After overnight culture, cells were treated with 10−9, 10−8, 10−7, or 10−6 mol/L of BPA dissolved in dimethyl sulfoxide (DMSO) for 48 hours.
RNA isolation and the quantitative reverse transcription PCR analysis
Total RNA was extracted from HTR-8/SVneo cells using TRIzol reagent (Thermo Fisher Scientific, USA) following the manufacturer's instructions. 1 mg of total RNA was reverse transcribed into cDNA using First Strand cDNA Synthesis SuperMix (Yeasen Biotech, China). SYBR green-based qRT-PCR was performed using StepOnePlus (Thermo Fisher Scientific) following the manufacturer's protocol. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. Quantitative reverse transcription PCR (qRT-PCR) assays were conducted in triplicate for each sample and data analyzed using the comparative Ct method. Gene-specific qRT-PCR primers were listed in Supplementary Table 1 (available online).
Transwell assay
To determine the effects of BPA on cell invasion, HTR-8/SVneo cells were treated with or without various concentrations of BPA (10−9, 10−8, 10−7, and 10−6 mol/L) for 48 hours before seeding in a transwell chamber (8 µm pore size) pre-coated with 50 µL of Matrigel (diluted 1:8 in serum-free RPMI-1640), and incubated at 37 °C for 4 hours to facilitate gelling. Pretreated cells were seeded in the upper chamber at 3×104 cells/well in 200 μL of RPMI-1640 containing 1% FBS. The lower chamber was filled with 800 μL of RPMI-1640 containing 10% FBS as the chemoattractant. After incubation for 24 hours, cells in the upper chamber were scraped off, and invading cells attached to the bottom of the transwell chamber were fixed with methanol for 20 minutes, stained with 0.1% crystal violet for 20 minutes, and washed three times in pure water. Cells in the bottom chamber were counted using an Olympus IX 71 inverted microscope (Olympus Corporation, Japan). Cell invasiveness was determined by enumerating stained cells in five random fields per well and was assessed using duplicate cultures in three independent experiments.
Wound healing assay
HTR-8/SVneo cells were seeded in 6-well plates and incubated to 80% confluence. The cell monolayer was scraped using a sterile pipette tip to create a wound, washed with phosphate-buffered saline (PBS) thrice to eliminate debris, and incubated with different concentrations of BPA for 48 hours. The control group was incubated with DMSO. Images of cells from each wound were acquired 0 and 48 hours after BPA treatment.
CCK-8 assay
Cell viability was determined using the CCK-8 assay following the manufacturer's instructions. Briefly, HTR-8/SVneo cells were seeded in a 96-well plate, treated with BPA (10–9–10–6 mol/L) for 48 hours, and incubated with 10 μL of CCK-8 reagent per well for an additional 2 hours at 37 °C. Absorbance was determined at 450 nm using a microplate reader (Bio-Tek, USA).
Transfection with pcDNA3.1(+)-integrin β1 and G418 selection
HTR-8/SVneo cells cultured up to 80% confluence in 60-mm culture dishes containing RPMI-1640 with 10% FBS were incubated for 16-18 hours, washed twice with PBS, and then incubated in Opti-MEM reduced serum medium (Life Technologies) at 37°C in a 5% CO2 incubator. The pcDNA3.1(+)-integrin β1 plasmid (1 µg) was mixed with 3 µL of PolyJet (SL100688; SignaGen, USA), added dropwise to the medium in the culture dishes, and then incubated for 6 hours. After transfection, fresh culture medium with 10% FBS was added and the cells were cultured for an additional 36 hours. Cells were selected at day 2 post-transfection using 600 µg/mL of G418 to obtain a pure population of transfected cells.
Mass spectrometry analysis
HTR-8/SVneo cells with or without BPA pretreatment were harvested, washed three times with cold PBS, and analyzed by mass spectrometry (MS) as described previously[20]. Peptides were subjected to reverse-phase separation using buffer A (2% acetonitrile, 0.5% acetic acid) and buffer B (80% acetonitrile, 0.5% acetic acid) with the following gradient: 4‒9% buffer B for 3 minutes, 9‒33% buffer B for 170 minutes, 33‒50% buffer B for 10 minutes, 50‒100% buffer B for 1 minute, 100% buffer B for 8 minutes, and 100‒4% buffer B for 1 minute. Samples were analyzed using a TripleTOF 5600+ mass spectrometer (AB Sciex, USA), configured to collect high resolution (R= 60 000 at m/z 400) broadband mass spectra (m/z 375–1 800) of the eluted peptides. Samples were electrosprayed into the spectrometer at 1.8 kV using the lock mass feature for the polydimethylcyclosiloxane ions generated (m/z 445.120 02). The most abundant peptides in the MS spectra were subjected to tandem MS with 35% relative collision-induced dissociation energy. Higher energy collision-induced dissociation-MS3 fragmentation was used to maximize efficiency.
Western blotting analysis
HTR-8/SVneo cells were lysed in 60 μL of radioimmunoprecipitation assay lysis buffer supplemented with 1 mmol/L of phenylmethylsulfonyl fluoride and 1 mmol/L of protein phosphatase inhibitors. Cell extracts were incubated for 30 minutes on ice and centrifuged at 13 800 g for 15 minutes at 4 °C. The supernatant was diluted in 5× sodium dodecyl sulfate polyacrylamide gel electrophoresis loading buffer, boiled at 100 °C for 5 minutes, and loaded into each well of a 10% polyacrylamide-sodium dodecyl sulfate gel. The separated proteins were transferred to polyvinylidene fluoride membranes (Millipore Sigma, USA) and probed with primary, then horseradish peroxidase-conjugated secondary antibodies. Signals were detected using an enhanced chemiluminescence reaction kit (Perkin Elmer, USA). Band intensities were quantified using Image J software (National Institutes of Health, USA).
Statistical analysis
Statistical analyses were performed using Prism 5 software (GraphPad Software, USA). All data were analyzed using unpaired two-tailed Student's t-tests or a one-way analysis of variance followed by Bonferroni's post-hoc multiple comparison test, as required. P-values <0.05 were considered statistically significant.
Results
Effects of BPA on spontaneous HTR-8/SVneo cell invasion and migration
To investigate the effect of BPA on the invasiveness and migration of HTR-8/SVneo cells, we performed transwell and scratch assays. BPA (10−8–10−6 mol/L) significantly reduced the spontaneous invasion and migration (P<0.05) of HTR-8/SVneo cells compared to DMSO (Fig. 1A–C). The CCK-8 assay revealed that BPA (10−9–10−6 mol/L) did not significantly reduce cell viability (Fig. 1D).
1.
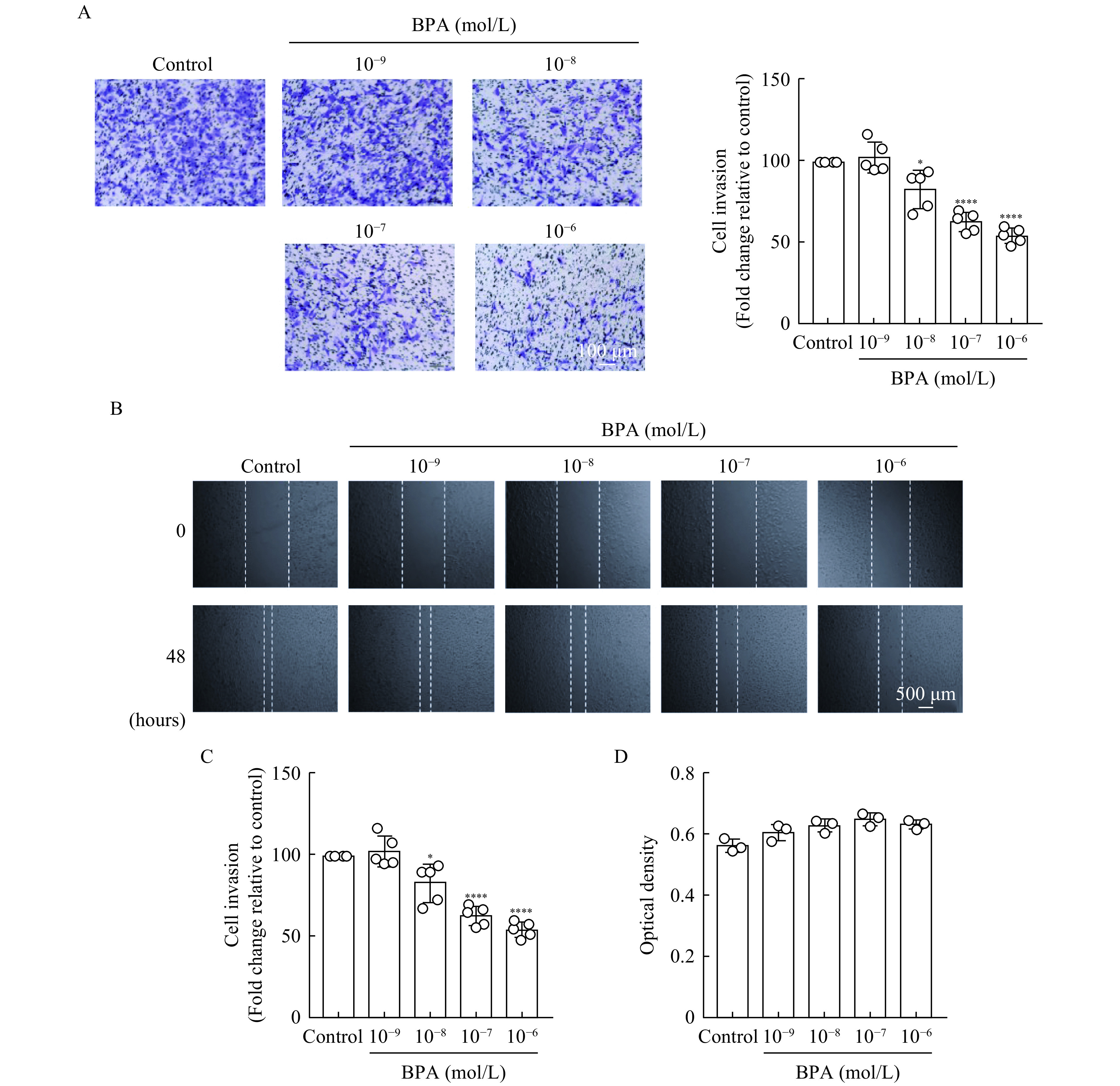
Effects of bisphenol A (BPA) on HTR-8/SVneo trophoblast invasiveness and migration.
A: HTR-8/SVneo cell invasiveness was evaluated using transwell assays. BPA-treated cells seeded into the upper chamber that invaded through to the bottom face of the transwell insert were quantified. The left panel shows representative images and the right panel denotes the quantitative findings. Scale bar: 100 μm. B: The migration of HTR-8/SVneo cells treated with various concentrations of BPA was analyzed via wound healing assays. Scale bar: 500 μm. C: The rate of wound closure was quantified following BPA treatment. D: The viability of HTR-8/SVneo cells treated with variousconcentrations of BPA was assessed using CCK-8 assays. Data represent means±SEM of at least three independent experiments. *P<0.05,**P<0.01,***P<0.001, and****P<0.0001.
Effects of BPA on MMP and TIMP protein levels in HTR-8/SVneo cells
BPA (10−6 mol/L) significantly reduced MMP-2 (P<0.05) and MMP-9 (P<0.01) protein levels (Fig. 2A–C), but elevated TIMP-1 (P<0.01) and TIMP-2 (P<0.05) protein levels at concentrations of 10−7–10−6 mol/L (Fig. 2A, D, and E), suggesting that BPA treatment may disrupt the balance between MMPs and TIMPs.
2.
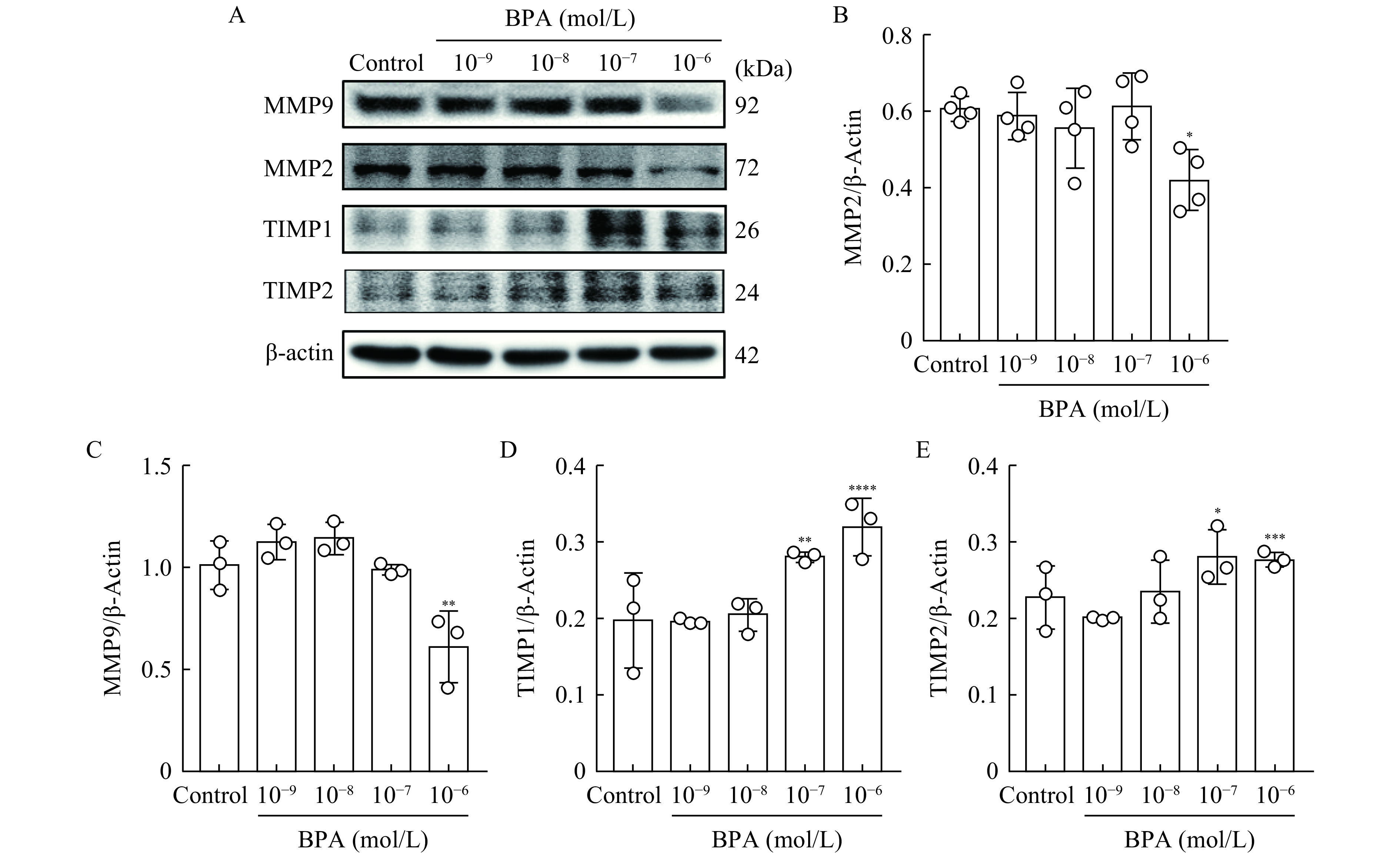
Effects of bisphenol A on matrix metalloproteinase (MMP)-2/9 and tissue inhibitors of MMPs (TIMP)-1/2 protein levels.
A: Western blotting was used to detect MMP-2, MMP-9, TIMP-1, and TIMP-2 protein levels in HTR-8/SVneo cells treated with BPA (10−9–10−6 mol/L) or an equivalent volume of dimethyl sulfoxide (control group) for 48 hours. β-Actin was used as the loading control. B-E: Relative abundances of MMP-2, MMP-9, TIMP-1, and TIMP-2 proteins were analyzed using Image J software. Data represent means±SEM of at least three independent experiments. *P<0.05,**P<0.01,***P<0.001, and****P<0.0001.
Effects of BPA on the expression of adhesion molecules in HTR-8/SVneo cells
Our results showed that BPA (10−7 mol/L) downregulated integrin α5/β1 and vimentin protein levels, but upregulated CD97. N-Cadherin, E-cadherin, and occludin protein levels remained unchanged upon BPA treatment (Fig. 3A). Upon treatment of HTR-8/SVneo cells with BPA (10−7 mol/L) or an equivalent volume of DMSO for 48 hours, mRNA expression levels were consistent with those of the proteins (Fig. 3B and C). The decrease in the integrin β1 mRNA level due to BPA treatment was highly statistically significant compared to that in the control group (P<0.01). Furthermore, overexpression of integrin β1 in HTR-8/SVneo cells reversed the reduction in invasiveness induced by BPA treatment (Fig. 3D). These results indicate that BPA reduces the migratory capabilities of HTR-8/SVneo cells by affecting integrin β1 expression.
3.
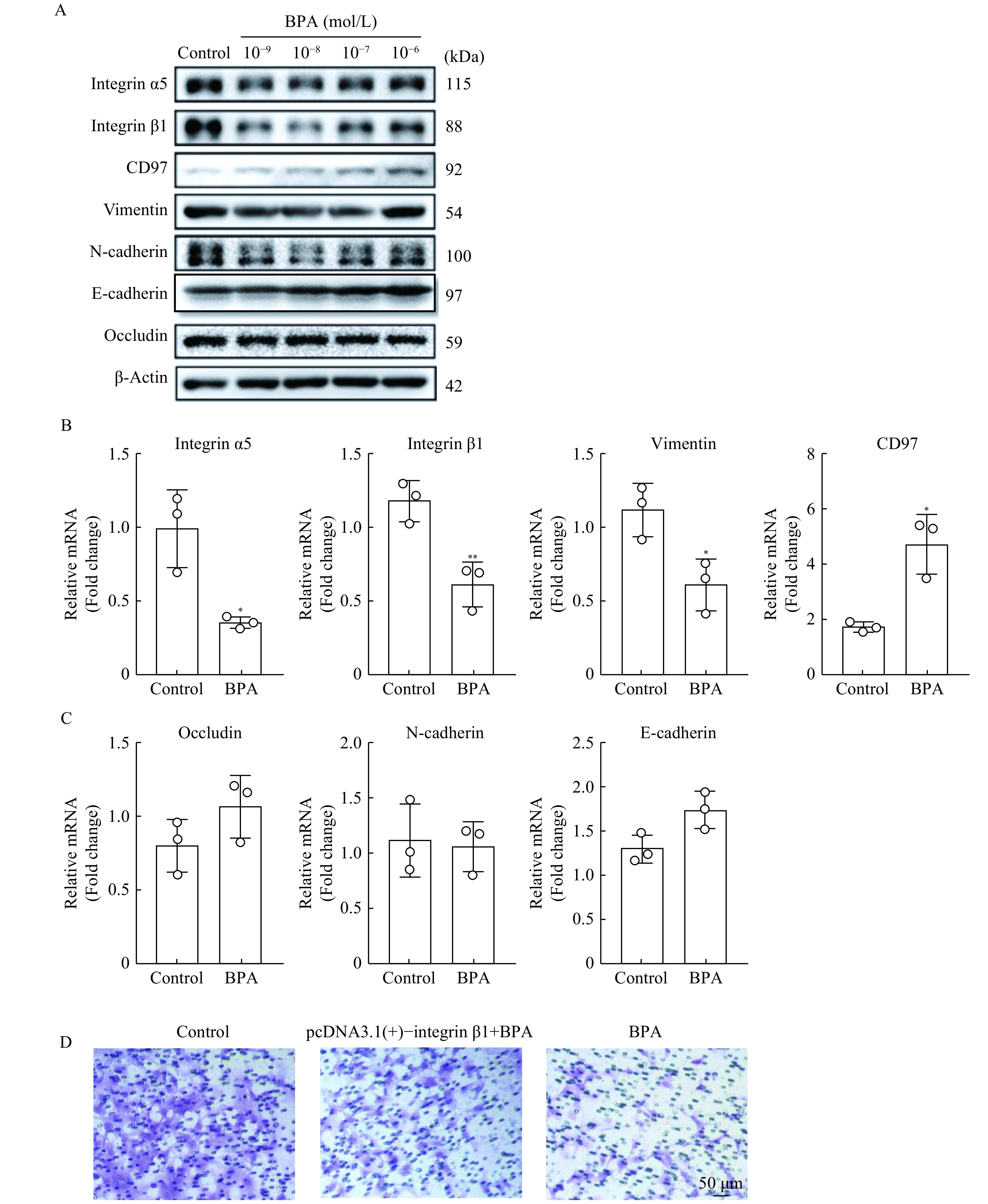
Effects of bisphenol A (BPA) on adhesion molecule expression.
HTR-8/SVneo cells were treated with BPA (10–9–10–6 mol/L) or an equivalent volume of dimethyl sulfoxide (DMSO) (control group) for 48 hours. A: Western blotting was used to detect integrin α5, integrin β1, CD97, vimentin, N-cadherin, E-cadherin, and occludin protein levels. β-Actin was used as the loading control. B and C:Quantitative reverse transcription PCR was used to measure integrin α5, integrin β1, CD97, vimentin, N-cadherin, E-cadherin, and occludin mRNA levels in control group and BPA (10–7 mol/L) group (n≥3 per group). D: Invasiveness of HTR-8/SVneo cells pre-transfected with integrin β1 and then treated with BPA (10–7 mol/L) (middle panel), or BPA (10–7 mol/L) alone (right panel) for 48 hours was determined using transwell assays. The control group (left panel) was treated with DMSO. Scale bar: 50 μm. Data represent means±SEM of at least three independent experiments. *P<0.05,**P<0.01,***P<0.001, and****P<0.0001.
ERs and GPR30 are involved in the inhibitory effects of BPA
We then aimed to determine whether ERs or GPR30 were involved in the inhibitory effects of BPA on HTR-8/SVneo cell migration, we pretreated cells with the nuclear ER antagonist, ICI-182780, or membrane ER GPR30 antagonist, G15. Remarkably, G15 exposure partially restored HTR-8/SVneo trophoblast migration (Fig. 4A and B), suggesting that BPA exerts its effects via GPR30 receptors (Fig. 4C).
4.
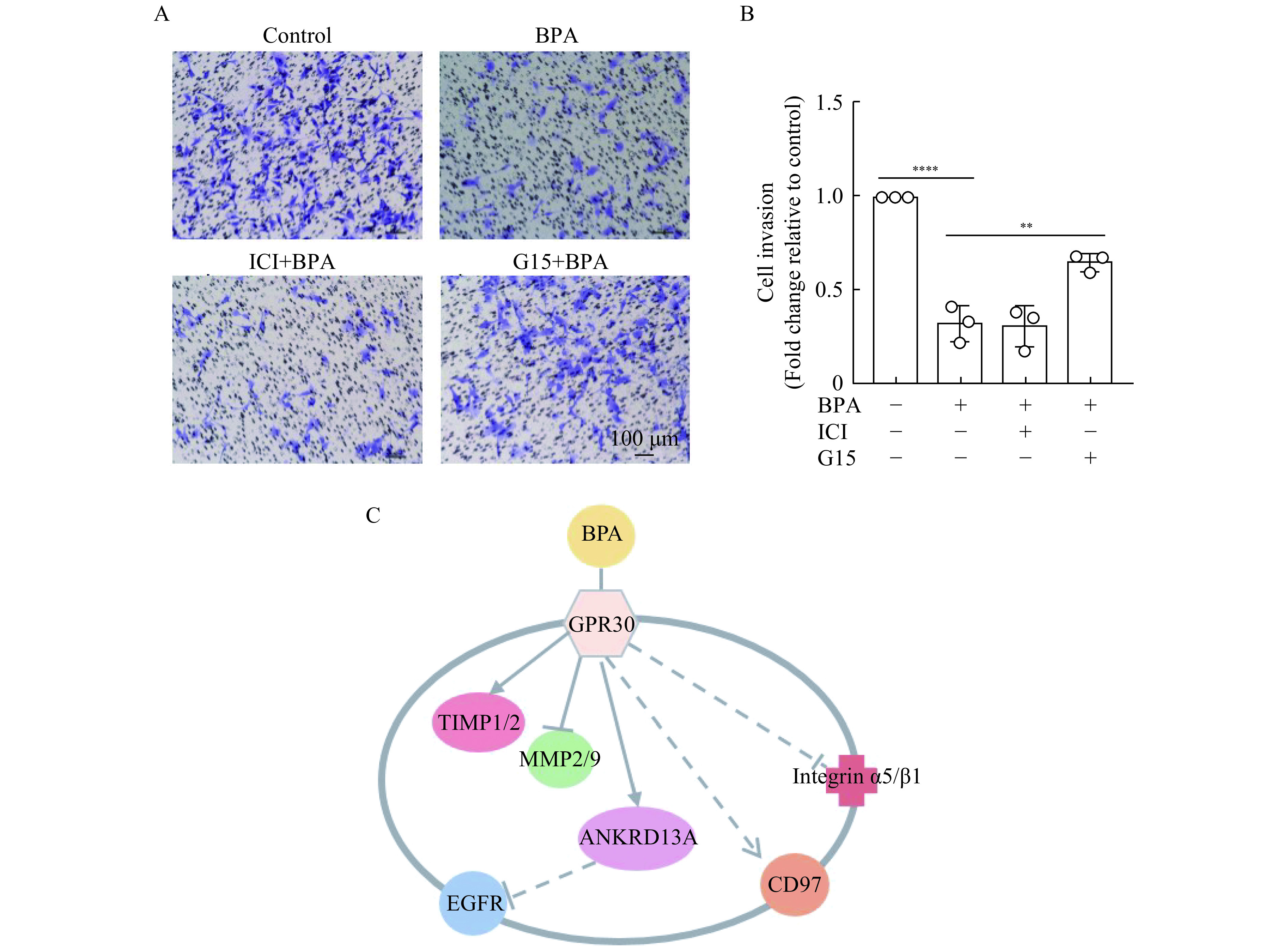
Estrogen receptors (ERs) and GPR30 are involved in bisphenol A (BPA)-induced inhibition of HTR-8/SVneo cell invasiveness.
A and B: Invasiveness of HTR-8/SVneo cells pretreated with 1 μmol/L ICI-182780 or G15 (ER and GPR30 antagonists, respectively) for 1 hour, and then with 10−7 mol/L BPA or an equal volume of dimethyl sulfoxide for 48 hours, was determined using transwell assays. Scale bar: 100 μm. C: Putative pathway(s) by which BPA influences HTR-8/SVneo cell function. Data represent means±SEM of at least three independent experiments. EGFR: epidermal growth factor receptor; ANKRD13A: ankyrin repeatdomain 13A. *P<0.05,**P<0.01,***P<0.001, and****P<0.0001.
Proteomic and bioinformatic analyses
In the present study, we identified 4258 proteins, of which 42 were differentially expressed with a fold change >1.5 ( P<0.05). Gene ontology enrichment analysis was performed to functionally categorize these proteins according to cellular components, molecular functions, and biological processes using the UniProt database (Fig. 5A). The heatmap of the 42 differentially expressed proteins is shown in Fig. 5B. According to previous reports, CD97, ankyrin repeat domain 13A (ANKRD13A), human beta-type platelet-derived growth factor receptor (PDGFRB), ADP-ribosylation factor guanine nucleotide-exchange factor 2 (ARFGEF2), and kinesin family member 16B (KIF16B) are all related to cell migration and invasiveness[21–25]. BPA treatment altered the expression of proteins involved in several canonical signaling pathways that regulate cell migration, the top of which were: sulfate activation for sulfonation, s-methyl-5-thio-α-D-ribose 1-phosphate degradation, PAK signaling, glioma signaling, assembly of the RNA polymerase complex, fatty acid activation, vitamin C transport, signaling at the adherens junction, γ-linolenate biosynthesis (animals), and the mitochondrial L-carnitine shuttle pathway (Fig. 5C).
5.
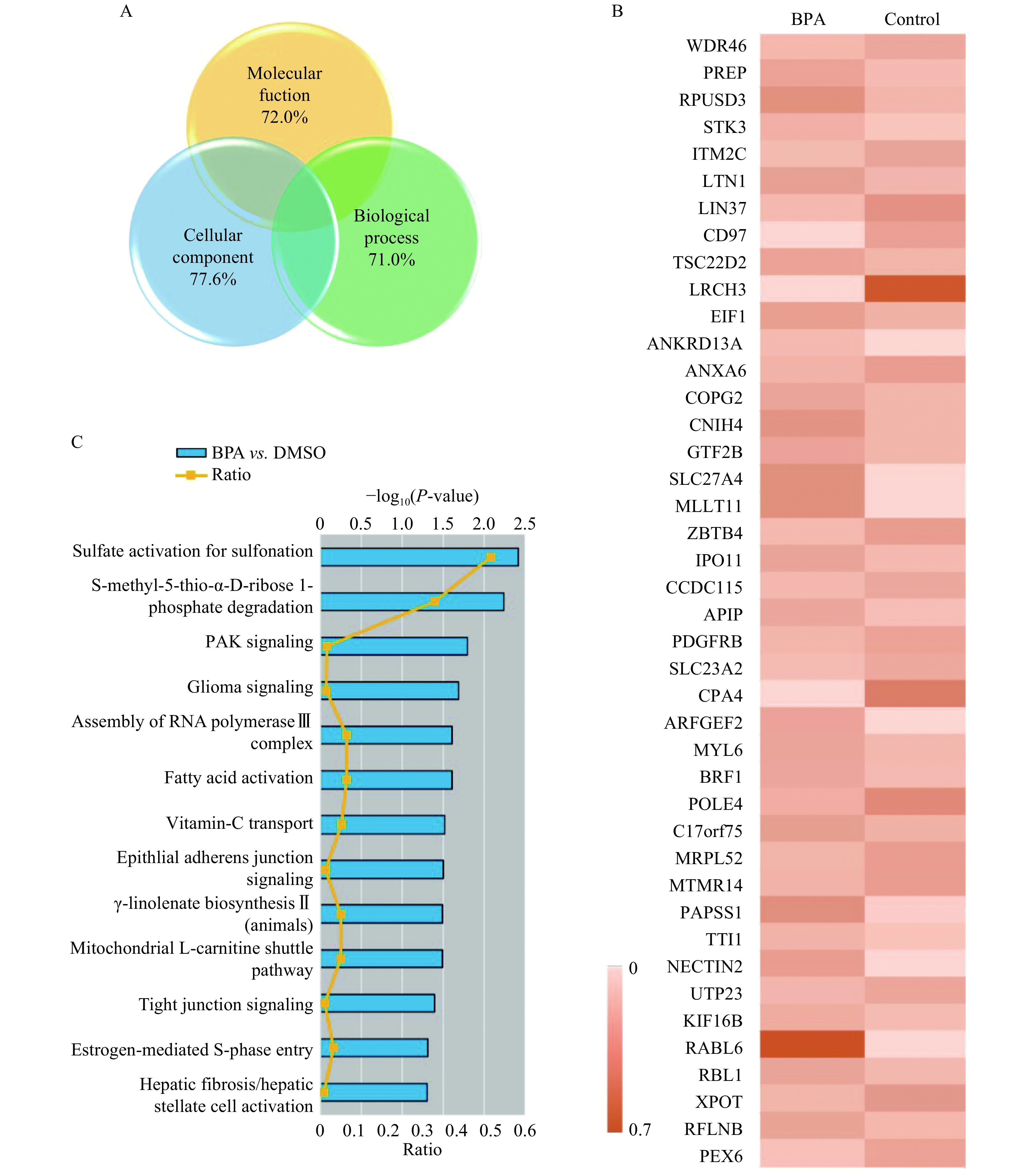
Mass spectrometry analysis of proteins in untreated and bisphenol A (BPA)-treated HTR-8/SVneo cells.
HTR-8/SVneo cells were treated with 10−7 mol/L BPA or an equivalent volume of dimethyl sulfoxide (DMSO) for 48 hours. A: Gene ontology enrichment analysis of the 42 differentially expressed proteins. B: Heatmap of the 42 differentially expressed proteins after BPA exposure. C: Ingenuity pathway analysis of proteins exhibiting significantly altered expression levels by BPA.
Discussion
BPA has been implicated recently in birth defects. However, its precise mechanisms of action have remained unclear. BPA was also reported to suppress CXCL8 expression to regulate trophoblast invasion in decidual stromal cells[26]. Moreover, BPA induced the differentiation of HTR-8/SVneo cells toward polyploidy through endoreduplication[11], and interfered with testis maturation by causing germ cell apoptosis and disrupting spermatogenesis[27]. The present study showed that BPA at 10−8–10−6 mol/L inhibited the invasiveness and migration of HTR-8/SVneo cells without affecting cell viability. BPA attenuated trophoblast function by altering the MMP:TIMP ratio and suppressing the expression of adhesion molecules, thereby directly and indirectly affecting trophoblast migration.
Adhesion proteins, chemokine receptors, MMPs, and TIMPs are essential for trophoblast migration and invasion[28–30]. Herein, our MS analysis revealed that BPA (10−9–10−6 mol/L) downregulated adhesion molecules including integrin α5 and CD97, while affecting ANKRD13A, PDGFRB, ARFGEF2, and KIF16B protein levels. The G-protein-coupled receptor, CD97, is involved in cell adhesion, transmembrane signaling, inflammatory responses, and cell migration[21]. Although CD97 is not directly involved in trophoblast invasion, it reportedly regulates the invasiveness and adhesion of cancer cells[31–32]. Indeed, another study reported that CD97 stimulated tumor migration via G-protein-coupled receptor-mediated signaling in hepatocellular carcinoma[33]. Furthermore, CD97 synergistically initiated endothelial cell invasion by co-engaging α5β1 integrin and chondroitin sulfate proteoglycan (CSPG)4[34]. Concurrently, CSPG4 is important for trophoblast function in humans; its depletion significantly affects the invasion of HTR-8/SVneo cells[35]. These results indicate that CD97, CSPG4, and their associated signaling pathways cooperatively regulate HTR-8/SVneo cell invasiveness.
In the present study, BPA downregulated integrin α5 and β1 expression levels and influenced sulfate activation, thereby affecting the activities of these molecules and suppressing the invasiveness of HTR-8/SVneo cells. Notably, Western blotting revealed that BPA altered MMP-2/9 and TIMP-1/2 protein levels. However, MMP-2/9 activity remained unchanged (unpublished data). Therefore, MMP-2/9 downregulation did not decrease the invasiveness of HTR-8/SVneo cells caused by BPA.
In conclusion, this study shows that BPA inhibits the invasiveness of trophoblast HTR-8/SVneo cells by downregulating integrin β1 expression. These results indicate that BPA inhibits the expression of adhesion proteins, alters the ratio between MMPs and TIMPs, and directly or indirectly influences the activity and expression of growth factor receptors. Our findings suggest a novel mechanism by which BPA interferes with trophoblast migration, thereby affecting the development of the maternal-fetal interface. This may, in turn, enhance our understanding of how BPA impairs embryonic development. Further study of the mechanisms involved may lead to therapeutic strategies that mitigate the negative effects of BPA exposure or policy recommendations to regulate acceptable BPA levels in the environment.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, No. 2014CB943303).
Contributor Information
Hong Zhou, Email: hongzhou@live.com.
Shuang Wen, Email: swen@njmu.edu.cn.
References
- 1.Cimmino I, Oriente F, D'Esposito V, et al Low-dose bisphenol-A regulates inflammatory cytokines through GPR30 in mammary adipose cells. J Mol Endocrinol. 2019;63(4):273–283. doi: 10.1530/JME-18-0265. [DOI] [PubMed] [Google Scholar]
- 2.Hines CJ, Christianson AL, Jackson MV, et al An evaluation of the relationship among urine, air, and hand measures of exposure to bisphenol A (BPA) in US manufacturing workers. Ann Work Expo Health. 2018;62(7):840–851. doi: 10.1093/annweh/wxy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koestel ZL, Backus RC, Tsuruta K, et al Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA. Sci Total Environ. 2017;579:1804–1814. doi: 10.1016/j.scitotenv.2016.11.162. [DOI] [PubMed] [Google Scholar]
- 4.Pinney SE, Mesaros CA, Snyder NW, et al Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod Toxicol. 2017;67:1–9. doi: 10.1016/j.reprotox.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Zhou LB, Bai YY, et al Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J Biomed Res. 2015;29(3):250–258. doi: 10.7555/JBR.29.20140058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martella A, Silvestri C, Maradonna F, et al Bisphenol A induces fatty liver by an endocannabinoid-mediated positive feedback loop. Endocrinology. 2016;157(5):1751–1763. doi: 10.1210/en.2015-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu FY, Wang XN, Wu NN, et al Bisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxia. Environ Pollut. 2017;231:1609–1620. doi: 10.1016/j.envpol.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 8.Chu PW, Yang ZJ, Huang HH, et al Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol Reprod. 2018;98(2):250–258. doi: 10.1093/biolre/iox162. [DOI] [PubMed] [Google Scholar]
- 9.Muller JE, Meyer N, Santamaria CG, et al Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci Rep. 2018;8:9196. doi: 10.1038/s41598-018-27575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng FL, Ji WL, Zhu F, et al A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of Chinese women with unexplained recurrent spontaneous abortion. Environ Res. 2016;150:622–628. doi: 10.1016/j.envres.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Spagnoletti A, Paulesu L, Mannelli C, et al Low concentrations of bisphenol A and para-nonylphenol affect extravillous pathway of human trophoblast cells. Mol Cell Endocrinol. 2015;412:56–64. doi: 10.1016/j.mce.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Teh WT, McBain J, Rogers P What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419–1430. doi: 10.1007/s10815-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aplin JD, Beristain A, Dasilva-Arnold S, et al IFPA meeting 2016 workshop report III: decidua-trophoblast interactions; trophoblast implantation and invasion; immunology at the maternal-fetal interface; placental inflammation. Placenta. 2017;60(S1):S15–S19. doi: 10.1016/j.placenta.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JH, Xiong K, Yang Y, et al Deleterious effects of benomyl and carbendazim on human placental trophoblast cells. Reprod Toxicol. 2015;51:64–71. doi: 10.1016/j.reprotox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Chen JJ, Khalil RA Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci. 2017;148:87–165. doi: 10.1016/bs.pmbts.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XX, Shi LL, Qiu MJ, et al Molecularly targeted anti-cancer drugs inhibit the invasion and metastasis of hepatocellular carcinoma by regulating the expression of MMP and TIMP gene families. Biochem Biophys Res Commun. 2018;504(4):878–884. doi: 10.1016/j.bbrc.2018.08.203. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y, Hu DN, Sun J, et al Correlations between MMPs and TIMPs levels in aqueous humor from high myopia and cataract patients. Curr Eye Res. 2017;42(4):600–603. doi: 10.1080/02713683.2016.1223317. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Mo HQ, Tian FJ, et al EIF5A1 promotes trophoblast migration and invasion via ARAF-mediated activation of the integrin/ERK signaling pathway . Cell Death Dis. 2018;9(9):926. doi: 10.1038/s41419-018-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YJ, Forbes K, Carver J, et al The role of the osteopontin-integrin αvβ3 interaction at implantation: functional analysis using three different in vitro models . Hum Reprod. 2014;29(4):739–749. doi: 10.1093/humrep/det433. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Xu J, Wang F, et al Mitochondrial proteomics approach reveals voltage-dependent anion channel 1 (VDAC1) as a potential biomarker of gastric cancer. Cell Physiol Biochem. 2015;37:2339–2354. doi: 10.1159/000438588. [DOI] [PubMed] [Google Scholar]
- 21.Tjong WY, Lin HH The role of the RGD motif in CD97/ADGRE5-and EMR2/ADGRE2-modulated tumor angiogenesis. Biochem Biophys Res Commun. 2019;520(2):243–249. doi: 10.1016/j.bbrc.2019.09.113. [DOI] [PubMed] [Google Scholar]
- 22.Avellino R, Carrella S, Pirozzi M, et al miR-204 targeting of Ankrd13A controls both mesenchymal neural crest and lens cell migration . PLoS One. 2013;8(4):e61099. doi: 10.1371/journal.pone.0061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CS, Li JC, Xiang XY, et al PDGF receptor-α promotes TGF-β signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-β receptors . Am J Physiol Gastrointest Liver Physiol. 2014;307(7):G749–G759. doi: 10.1152/ajpgi.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit MCY, de Coo IFM, Halley DJJ, et al Movement disorder and neuronal migration disorder due to ARFGEF2 mutation . Neurogenetics. 2009;10(4):333–336. doi: 10.1007/s10048-009-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlucci A, Porpora M, Garbi C, et al PTPD1 supports receptor stability and mitogenic signaling in bladder cancer cells. J Biol Chem. 2010;285(50):39260–39270. doi: 10.1074/jbc.M110.174706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XQ, Wang YN, Wei P, et al Bisphenol A affects trophoblast invasion by inhibiting CXCL8 expression in decidual stromal cells. Mol Cell Endocrinol. 2018;470:38–47. doi: 10.1016/j.mce.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Jin PP, Wang XL, Chang F, et al Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J Biomed Res. 2013;27(2):135–144. doi: 10.7555/JBR.27.20120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank SR, Köllmann CP, van Lidth de Jeude JF, et al The focal adhesion-associated proteins DOCK5 and GIT2 comprise a rheostat in control of epithelial invasion. Oncogene. 2017;36(13):1816–1828. doi: 10.1038/onc.2016.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes CE, Nibbs RJB A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui N, Hu M, Khalil RA Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Liu DR, Li GG, et al CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21(20):6215–6228. doi: 10.3748/wjg.v21.i20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang LY, Liu XF, Yang Y, et al Biochemical features of the adhesion G protein-coupled receptor CD97 related to its auto-proteolysis and HeLa cell attachment activities. Acta Pharmacol Sin. 2017;38(1):56–68. doi: 10.1038/aps.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin Y, Xu XL, Tang JW, et al CD97 promotes tumor aggressiveness through the traditional G protein-coupled receptor-mediated signaling in hepatocellular carcinoma. Hepatology. 2018;68(5):1865–1878. doi: 10.1002/hep.30068. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Ward Y, Tian LH, et al CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood. 2005;105(7):2836–2844. doi: 10.1182/blood-2004-07-2878. [DOI] [PubMed] [Google Scholar]
- 35.Van Sinderen M, Cuman C, Winship A, et al The chrondroitin sulfate proteoglycan (CSPG4) regulates human trophoblast function. Placenta. 2013;34(10):907–912. doi: 10.1016/j.placenta.2013.07.065. [DOI] [PubMed] [Google Scholar]


