Abstract
There is international variability in the determination of death. Death in donation after circulatory death (DCD) can be defined by the permanent cessation of brain circulation. Post‐mortem interventions that restore brain perfusion should be prohibited as they invalidate the diagnosis of death. Retrieval teams should develop protocols that ensure the continued absence of brain perfusion during DCD organ recovery. In situ normothermic regional perfusion (NRP) or restarting the heart in the donor's body may interrupt the permanent cessation of brain perfusion because, theoretically, collateral circulations may restore it. We propose refinements to current protocols to monitor and exclude brain reperfusion during in situ NRP. In abdominal NRP, complete occlusion of the descending aorta prevents brain perfusion in most cases. Inserting a cannula in the ascending aorta identifies inadequate occlusion of the descending aorta or any collateral flow and diverts flow away from the brain. In thoracoabdominal NRP opening the aortic arch vessels to atmosphere allows collateral flow to be diverted away from the brain, maintaining the permanence standard for death and respecting the dead donor rule. We propose that these hypotheses are correct when using techniques that simultaneously occlude the descending aorta and open the aortic arch vessels to atmosphere.
Keywords: donors and donation: donation after circulatory death (DCD), editorial/personal viewpoint, ethics, extracorporeal membrane oxygenation (ECMO), organ perfusion and preservation, organ procurement, organ procurement and allocation
Short abstract
The authors present techniques to prevent the restoration of brain perfusion during in situ normothermic regional perfusion by ensuring the diversion of any possible collateral supply.
Abbreviations
- A‐NRP
abdominal‐normothermic regional perfusion
- CARE
clinical audit risk and effectiveness
- cDCD
controlled donation after circulatory determination of death
- CT
computed tomography
- DCD
donation after circulatory determination of death
- DPP with A‐NRP
direct (hypothermic) procurement and perfusion with abdominal‐normothermic regional perfusion
- DPP
direct (hypothermic) procurement and perfusion
- ECMO
extracorporeal membrane oxygenation
- NHSBT
National Health Service Blood and Transplant
- NIHR BTRU
National Institute for Health Research Blood and Transplant Research Unit
- NRP
normothermic regional perfusion
- TA‐NRP
thoracoabdominal‐normothermic regional perfusion
- UK
United Kingdom
1. INTRODUCTION
In the United Kingdom and Canada, controlled donation after circulatory death (cDCD, DCD) has been responsible for the largest quantitative increase in organ donation and transplantation over the past decade.1, 2 The same has been seen in Belgium3 and in the Netherlands, where DCD accounts for over 40% of all deceased organ donors.4
One of the greatest challenges in the practice of DCD is to limit the damage caused to organs by warm ischemia.5 Any technique that can limit warm ischemia and provide post‐mortem organ reperfusion has the potential to improve transplant outcomes.6, 7 Organ reperfusion after death can be provided through normothermic regional perfusion (NRP) in situ (within the donor's body) or ex situ machine perfusion of individual organs (outside the donor's body). Both techniques are being increasingly used to improve organ assessment and transplant outcomes.
The key principle in deceased donation is respecting the dead donor rule,8 which stipulates that the donor must be dead before organ recovery begins; otherwise the recovery process could be considered to be the cause of death. In DCD, as in every day clinical practice, death is confirmed using circulatory criteria at the point of permanent loss of the circulation. This is the point at which the possibility of spontaneous return of the circulation (autoresuscitation) has passed and a decision had been made not to attempt to restore the circulation. This point is generally accepted to be achieved after 5 minutes of continuous apnea, loss of the circulation, and unresponsiveness.9 At this point permanent loss of the circulation also equates with permanent loss of brain functions, which will inevitably become irreversible if there is no restoration of brain perfusion.10, 11, 12
Countries vary in their policies and practices regarding whether the definition and determination of death in DCD is predicated on the cessation of whole‐body circulation or circulation to the brain. For example, Australian practices in DCD preclude the restoration of circulation after death and thus in situ NRP is not currently permissible.13 Canadian practices are in the process of analysis and reevaluation.14 The UK code of practice for the diagnosis and confirmation of death is clear on this point and permits restoration of circulation after death as long as brain perfusion does not resume: “It is obviously inappropriate to initiate any intervention that has the potential to restore cerebral perfusion after death has been confirmed."15
In order to maintain the permanence standard for death in DCD, our purpose was to answer the question, “What additional reassurance can we provide that brain perfusion is not restored during in situ NRP?” Any in situ NRP technique that restores perfusion to transplantable organs needs to respect this rule and ensure that the technique adopted cannot inadvertently restore brain perfusion. The prevention of brain perfusion during NRP has been previously ensured by occluding the descending thoracic aorta above the diaphragm using a vascular clamp or an intraluminal balloon during abdominal‐normothermic regional perfusion (A‐NRP) and in direct (hypothermic) procurement and perfusion of the heart combined with abdominal NRP (DPP with A‐NRP). During thoracoabdominal‐NRP (TA‐NRP), this was achieved by clamping or dividing the aortic arch vessels (right brachiocephalic, left common carotid, and left subclavian and any identified anomalous vessels) prior to starting reperfusion (see Table 1 for descriptions of the techniques). In the United Kingdom 90 DCD hearts were successfully transplanted by March 2019 with TA‐NRP being used in 22 occasions (data from National Health Service Blood and Transplant [NHSBT] Statistics and Clinical Studies). TA‐NRP has the advantage of allowing functional assessment of the heart unlike ex situ perfusion where functional assessment is more limited and based on serial assessment of lactate concentrations. It is also potentially more cost effective, particularly when NRP is followed by cold static storage, which has been used with success.
Table 1.
Description of the techniques used for in situ perfusion of organs in DCD organ retrieval
| In situ technique | Abbreviation | Description |
|---|---|---|
| Abdominal‐normothermic regional perfusion | A‐NRP |
Oxygenated blood pumped by an extracorporeal circuit via a cannula in the abdominal aorta or common iliac artery and drained via a cannula in the inferior vena cava or common iliac vein. Alternatively, cannulation may be via the femoral vessels where allowed pre‐mortem The descending thoracic aorta and the inferior vena cava are occluded above the diaphragm to confine the circulation to the organs in the abdominal cavity |
| Direct (hypothermic) procurement and perfusion of the heart combined with abdominal‐normothermic regional perfusion | DPP with A‐NRP | Cannulae inserted into the abdominal aorta and inferior vena cava or the common iliac vessels for extracorporeal circulation. The descending thoracic aorta is occluded before starting circulation to the abdominal organs. The ascending aorta is clamped distal to a cannula inserted proximally to allow the administration of cold preservation solution and excision of the heart without restarting it in situ |
| Thoraco abdominal‐normothermic regional perfusion | TA‐NRP | The extracorporeal circulation is provided by cannulae in the abdominal aorta or common iliac artery and in the right atrium or inferior vena cava. The descending thoracic aorta is not occluded to allow delivery of oxygenated blood to both the thoracic and abdominal organs |
Abbreviations: DCD, donation after circulatory death; A‐NRP, abdominal‐normothermic regional perfusion; TA‐NRP, thoraco‐abdominal‐ normothermic regional perfusion; DPP, direct (hypothermic) procurement and perfusion; NRP, normothermic regional perfusion.
Concerns have, however, been expressed previously regarding the use of extracorporeal membrane oxygenation (ECMO) techniques in DCD if brain and cardiac perfusion are not adequately excluded because of malfunction or misplacement of the supra‐diaphragmatic aortic occlusion balloon.16 A related concern is the possibility of the establishment of a perfusing collateral circulation to the brain despite interruption of the aortic arch vessels during TA‐NRP.17 This issue of how to ensure the absence of brain perfusion during A‐NRP and TA‐NRP was recently discussed at the Canadian DCD Heart Donation and Transplantation meeting held in Ottawa in October 2018.14 A collateral circulation that achieves brain blood perfusion may theoretically occur through a variety of thoracic arterial anastomoses,18 but may commonly be anticipated through anastomosis between the intercostal arteries with the anterior spinal artery and eventually the vertebrobasilar system, with the potential to supply the brain stem and, if the circle of Willis is patent, the supratentorial structures (Figure 1A). Other collateral circuits may arise between the inferior epigastric and internal thoracic (mammary) arteries or between the supreme (or superior) intercostal and the subclavian artery.
Figure 1.
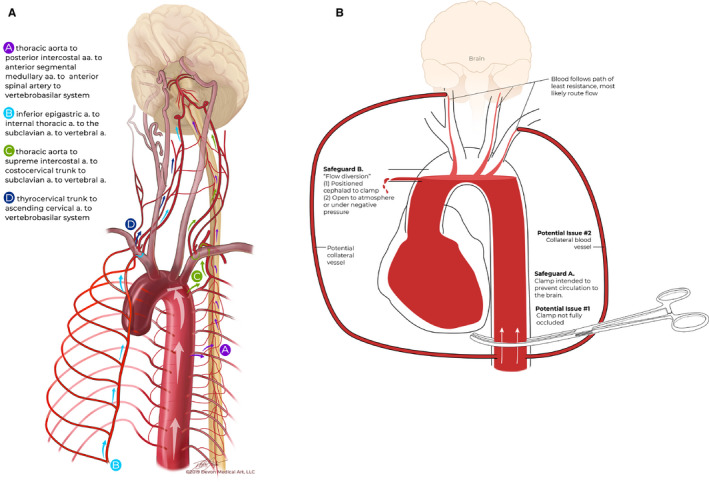
A, Potential collateral circulations A to D that could theoretically restore flow and/or perfusion to the brain. B, Proposed solution of flow diversion away from the brain by occluding the descending thoracic aorta and draining the aortic arch arteries to atmosphere either by inserting a large bore cannula into the ascending aorta or draining the arch arteries individually. Any potential collateral flow to the brain should be preferentially diverted to the low resistance large bore subclavian vessels open to atmospheric pressure
We propose refinements to current protocols to provide additional reassurance that there will be exclusion and maintenance of the absence of reperfusion of the brain during any of these in situ NRP techniques. We hypothesized that the adoption of techniques allowing free drainage of the aortic arch vessels (in TA‐NRP) or the ascending aorta (in A‐NRP and DPP with A‐NRP) to atmospheric or negative pressure in DCD organ recovery would divert any collateral flow away from the brain. This approach should allow any blood entering the spinal arteries or the vertebral arteries to preferentially flow back to atmosphere through the large diameter superior intercostal arteries, the costocervical trunk and subclavian artery (Figure 1B). In addition, we are considering simple methods of reducing warm ischemic time during DCD heart retrieval by reperfusing the heart in isolation while the arch vessels are cannulated to prevent brain collateral supply. These safeguards could address the risks of collateral circulation to the brain thus maintaining the permanence standard for death in DCD and respecting the dead donor rule.
The refinements in surgical technique described here were not introduced as a research project but rather as a quality improvement and service development initiative to provide additional reassurance against brain perfusion and to monitor for it. Publication of this anonymous case series was approved on behalf of the clinical audit risk and effectiveness (CARE) Committee of the Organ Donation and Transplant Directorate of NHSBT. Written consent for publication of the clinical photograph was obtained from the family of the deceased donor.
2. PROCEDURES
2.1. Abdominal‐NRP
After the confirmation of death, organ recovery proceeds with insertion of an arterial and venous cannula into either the iliac vessels or alternatively the abdominal aorta and inferior vena cava with ligation of the distal aorta/iliac vessels (Figure 2A). The latter approach excludes the potential of any anastomoses via the inferior epigastric artery (Figure 1A). The descending thoracic aorta is occluded above the diaphragm using a vascular clamp or an intraluminal balloon. Our additional recommended step is to insert a large (eg, 24Fr) cannula into the ascending aorta, measure the pressure, and leave it open to atmosphere before A‐NRP is commenced. The primary purpose of this cannula is to monitor for adequate occlusion of the descending aorta or detect and divert any potential collateral flow to the brain. Any drainage of blood with a significant pressure from the ascending aorta during A‐NRP will lead the retrieval team to check that the descending thoracic aorta is properly occluded. In the event of continued detectable blood flow, protocols must mandate that NRP is aborted immediately and in situ hypothermic perfusion and organ recovery initiated. Despite the diversion of any potential for brain flow through the aortic cannula, this caution is advocated to mitigate against any blood flow through the open arch vessels.
Figure 2.
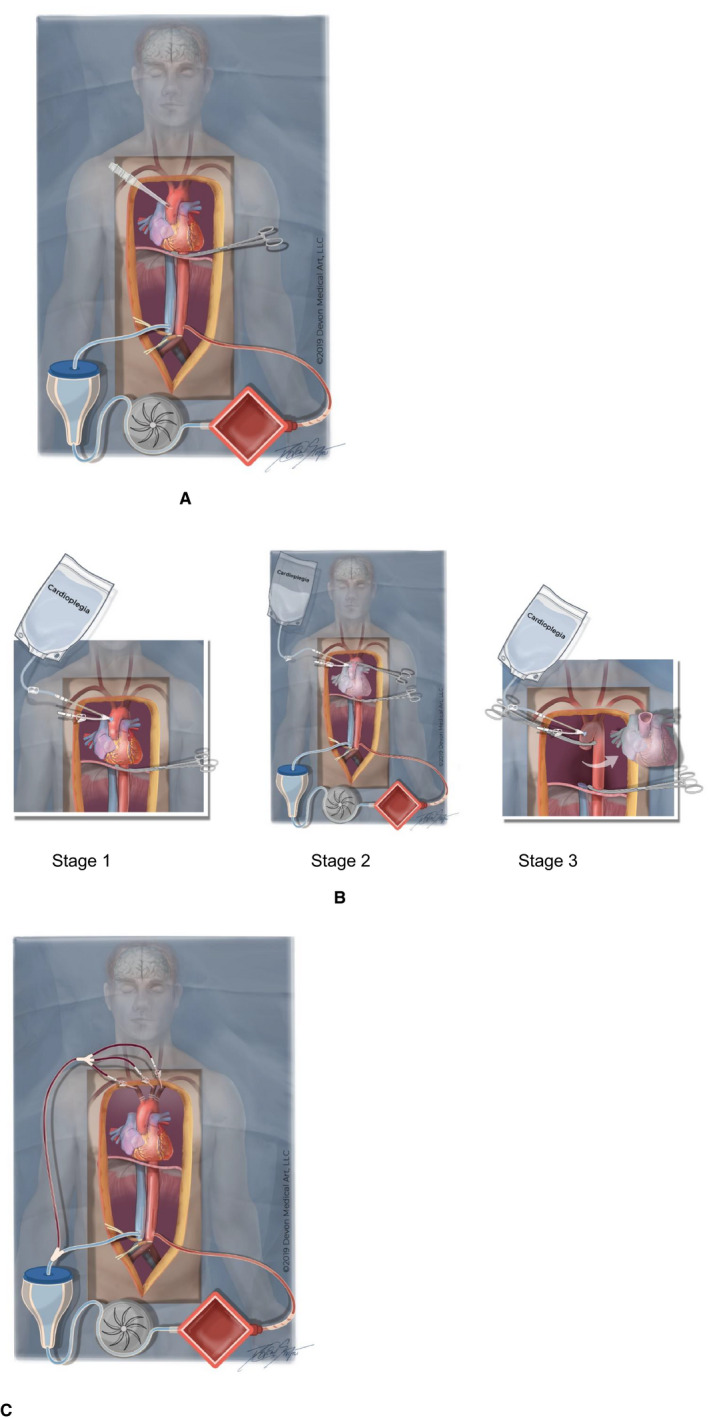
Proposed refinements to normothermic regional perfusion (NRP) techniques. A, Abdominal NRP. The procedure has been used in 12 donors with occlusion of the descending thoracic aorta and a large cannula in the ascending aorta. Delivery of oxygenated blood into the aorta rather than the iliac vessels excludes the possible collateral circulation between the inferior epigastric and internal thoracic arteries (collateral circulation B in Figure 1A). B, Direct (hypothermic) procurement and perfusion (DPP) with abdominal‐normothermic regional perfusion (A‐NRP). During the dissection, Stage 1, the descending thoracic aorta is occluded, a double lumen cannula inserted into the ascending aorta and left open to atmosphere, and A‐NRP commenced. In Stage 2 cardioplegia is administered rapidly before explantation of the heart by temporarily placing a clamp on the ascending aorta cephalad to double lumen cannula. After explantation of the heart, Stage 3, the ascending aortic clamp is repositioned proximal to the double lumen cannula to open the aortic arch vessels to atmosphere for the duration of A‐NRP. C, Thoraco‐abdominal NRP. The cephalad ends of each of the aortic arch vessels are cannulated and any drained blood returned to the venous reservoir for retransfusion
At the time of writing this approach has been used in 12 A‐NRP organ recoveries in the United Kingdom, the descending aorta being occluded with a clamp in 11 cases and with an intraluminal balloon in one (unpublished data). There was no proximal aortic flow in any patient and a maximum pressure of 0‐3 mm Hg in the ascending aorta cannula (Figure 3A) and, therefore no pressure or flow gradient for brain perfusion, supporting our conclusions that an adequately occluded descending aorta prevents collateral flow to the spinal and vertebral arteries, or the carotid circulation. When measured, the mean pressure in the abdominal aorta was 38‐65 mm Hg in all patients. There was no requirement to reposition the descending aortic clamp or intraluminal balloon in any patient. The patients were aged from 43‐64 years old and the diagnosis included intracerebral hemorrhage, subarachnoid hemorrhage, traumatic brain injury, hypoxic brain injury, and end‐stage respiratory failure.
Figure 3.
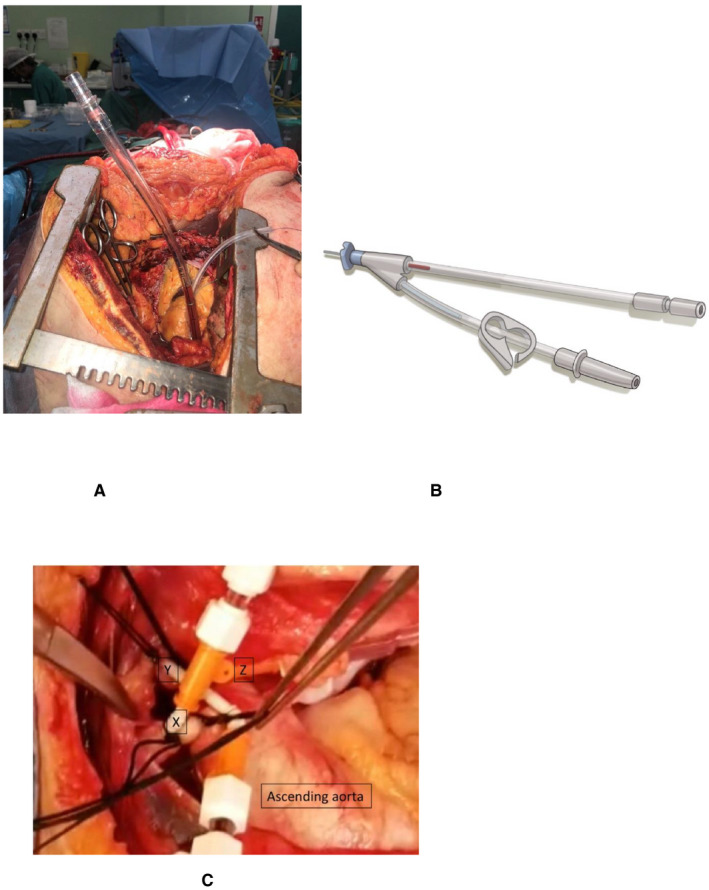
A, Abdominal normothermic regional perfusion (NRP). The descending aorta is clamped. Note the minimal pressure in the ascending aortic cannula that is open to atmosphere. (Image reproduced with the kind permission of the donor's family.) B, Double lumen cannula used in direct (hypothermic) procurement and perfusion (DPP) with abdominal‐normothermic regional perfusion (A‐NRP) to open the ascending aorta to atmosphere and allow the administration of cardioplegia before explantation of the heart. C, Intraoperative thoracoabdominal‐normothermic regional perfusion (TA‐NRP). 7Fr. cannula are inserted into the arch vessels and connected to hard‐shell reservoir during NRP. X ‐ brachiocephalic artery, Y – left carotid. Z – left subclavian artery arch vessels are ligated at origin, and second tie securing the cannula
We interpret these findings as strong evidence that excluding flow in the thoracic aorta by adequately occluding the descending aorta prevents any brain perfusion occurring during A‐NRP. The additional step of inserting a cannula in the ascending aorta allows the retrieval team to identify inadequate occlusion of the descending aorta or any potential collateral flow. It also diverts flow away from the brain while the measures described here are implemented.
2.2. DPP with abdominal NRP
DPP with A‐NRP has already been undertaken on a couple of occasions (Figure 2B). The procedure was similar to that for A‐NRP in inserting cannulae for perfusion into the abdominal aorta and inferior vena cava or the iliac vessels and clamping the descending thoracic aorta before starting NRP and rapid explanation of the heart. The new technique is similar to that described previously for A‐NRP in that it involves the additional step of inserting a single lumen or a double lumen aortic cannula, similar to the one in Figure 3B, high into the ascending aorta and left open to atmosphere while NRP is established. When the cardiac team is ready a clamp is placed just proximal to the aortic arch, and distal to the cannula, and cold cardioplegia is delivered into the root of the aorta. The clamp is then repositioned to the heart side of the cannula in the ascending aorta, which is now once again open to atmosphere for the duration of A‐NRP. The clamp across the descending thoracic aorta remains in place throughout the duration of perfusion. The procedure has not yet been undertaken this way for DPP heart retrieval during A‐NRP but has been successfully undertaken during a DCD lung retrieval with A‐NRP confirming that the technique is feasible. As in A‐NRP there was no flow or measurable pressure in the ascending aortic cannula. As in A‐NRP, any drainage of blood with a significant pressure from the ascending aorta during A‐NRP must mandate that the retrieval team checks that the descending thoracic aorta is properly occluded and if necessary, to stop NRP and begin immediate in situ hypothermic perfusion and organ recovery.
We suggest that, as the technique is identical to A‐NRP in excluding flow in the thoracic aorta, by adequate occlusion of the descending aorta and opening the ascending aorta to atmosphere brain perfusion is similarly prevented during DPP with A‐NRP.
2.3. TA‐NRP
In TA‐NRP there is no vascular clamp across the descending thoracic aorta, so a degree of collateral circulation between the intercostal vessels and spinal arteries is more likely (Figure 2C). During the organ recovery procedure the original practice was that the aortic arch vessels were ligated or stapled proximally. In order to provide additional reassurance that brain perfusion is not restored we trialed leaving the cephalad ends open to atmosphere or to a negative pressure before TA‐NRP is commenced via cannulae in the iliac artery and right atrium or inferior vena cava. Our hypothesis was that this would allow all collateral blood supply to preferentially flow down the lowest resistance circuit via the costocervical trunk and subclavian arteries.
This procedure has been undertaken in three patients in the UK. The first patient was a 43‐year‐old who died following a ruptured internal carotid aneurysm. Although the patient met all the clinical criteria for testing for the neurological determination of death, unresolved confounding conditions (electrolyte abnormalities, high dose sedation) precluded the clinical determination of brainstem death and withdrawal of life‐sustaining treatment and DCD was conducted. After the confirmation of death after circulatory arrest, the chest and abdomen were opened through a midline incision. A 2‐stage venous cannula was placed in the right atrium and an arterial cannula in the right common iliac artery. The left common iliac artery was clamped. A clamp was then placed across the descending thoracic aorta and NRP was established to the abdominal organs. In the chest the innominate, left common carotid and left subclavian arteries were stapled and divided. Once it was clear there were no anomalous arteries coming from the aortic arch, TA‐NRP was established by removing the descending thoracic aortic clamp. The cephalad ends of the brachiocephalic trunk and left common carotid and subclavian arteries were opened and allowed to drain. A TA‐NRP flow rate of 3.0‐3.6 L/min was delivered for the next 2 hours and the cephalad ends of the aortic arch vessels were left open to drainage. Deoxygenated blood was lost continuously through the procedure from the cut cephalad ends of the arteries. It was estimated that approximately 50 mL/min was drained from the open distal ends and from the chest, suggesting diversion of collateral flow away from the brain during the TA‐NRP. Bleeding from the innominate artery persisted with occlusion of the right common carotid branch but stopped on occlusion of the right subclavian artery. There was persistent bleeding through the left common carotid artery though it was impossible to identify if the flow was from the external or internal carotid. However, this finding raises the theoretical possibility that there could be a degree of vertebrobasilar flow into the brainstem and the circle of Willis.
It is difficult to speculate if such flow could have achieved any brain perfusion. As blood drained from open cephalad ends of the brain arteries, pressure was not measured. However, the adult human brain normally weighs 1300‐1400 g, has a blood flow of approximately 50 mL/100 g brain tissue/min and a threshold for loss of spontaneous and evoked electrical activity and function of 16‐18 mL/100 g brain tissue/min, and a threshold for loss of neuronal membrane integrity and function of 10‐12 mL/100 g brain tissue/min.19 Based on these data, the combined subclavian and carotid diverted flow rate of 50 mL/min equates to 3.9 mL/100 g brain/min and significantly less if the greater flow from the subclavian arteries is excluded. This flow is well below that required to support neuronal function and cerebral infarction would have likely continued.
In a second TA‐NRP procedure, the aortic arch vessels were ligated, and on this occasion a cannula was inserted separately in the cut cephalad ends of the right subclavian and right common carotid arteries and drained under negative pressure. The left common carotid artery and the left subclavian artery also had cannulae placed in and secured. There was no blood flow draining down from the carotids, only from the subclavian arteries. In the third case the open cephalad ends of the arch vessels were kept in continuity with the stapled proximal ends and cannulated to allow drainage to negative pressure and cell salvage for autotransfusion (Figure 3C). The presence of persisting drainage from the cut cephalad ends of the arch vessels during TA‐NRP (n = 3) confirms the potential for collateral blood flow and the techniques described here are proposed as methods to ensure such blood flow is diverted away from the brain.
Although TA‐NRP is attractive as it both achieves quick reperfusion of the thoraco‐abdominal organs and functional assessment of the heart, the approach described here is now slower and more complex, may challenge surgeons unfamiliar with the method, and, on current evidence, cannot be considered to provide absolute reassurance of the complete absence of brain blood flow or perfusion. Consequently, simple methods, of reducing warm ischemic time for the heart while allowing the surgeon to deal with the isolation of the arch vessels have been proposed by Messer and Large. These include perfusing the heart in isolation on opening the chest by placing a clamp across the ascending aorta and taking a 0.635 mm (1/4 inch) perfusion line from the NRP circuit to the aortic root. With the heart perfused in isolation the surgeon can then focus on dissecting and dividing the arch vessels with subsequent cannulation of the cephalic ends of the aortic arch vessels. This allows the surgeon time to perform these techniques safely with the knowledge that the ischemic time for the heart has ended. Following complete isolation of the aortic arch vessels the clamp on the ascending aorta can be released allowing the heart to undergo a full functional assessment with thermodilution cardiac output studies and transesophageal echocardiography. An alternative technique to continuous perfusion of the aortic root, again proposed by Messer and Large, is to deliver 1 L of cold blood oxygenated cardioplegia through the aortic root in isolation as soon as the chest is opened. Again, this will buy the surgeon additional time to isolate the aortic arch vessels, after which, the clamp can be released, and the heart will start beating allowing a full functional assessment.
3. DISCUSSION
The medico‐legal definition of death in DCD varies internationally. In countries such as the United Kingdom, the permanent cessation of a perfusing circulation to the brain is sufficient to diagnose death. In countries that accept this concept, maintaining the principle of permanence for death during abdominal and thoracoabdominal NRP should therefore be based on ensuring the absence of any potential for brain perfusion. As long as a perfusing circulation to the brain is excluded, restarting the circulation to the thoracic and/or abdominal cavities or restarting the heart in the donor's body after death remains consistent with the diagnosis of death during DCD organ retrieval. Similar to any post‐mortem surgical intervention for organ donation, we contend that there is no ethical dilemma in (nor any new ones created by) the surgical act of ligating or dividing the aortic arch vessels after the confirmation of death and before starting NRP.
Our currently practiced technique of in situ NRP, and that recently reported from Belgium,20 had been considered to preclude brain perfusion because any pressure or flow generated is too low, as long as complete occlusion of the descending aorta and/or the aortic arch vessels is achieved. Our findings suggest that the additional safeguards of occluding the descending thoracic aorta above the diaphragm and opening the ascending aorta or the arch vessels to atmosphere as proposed in this paper ensure that no brain perfusion is possible in A‐NRP, DPP with A‐NRP and very unlikely during in situ perfusion of the heart. An adequately occluded descending thoracic aorta effectively prevents any collateral flow to the brain in most cases, and the cannula in the ascending aorta allows the team to monitor the adequacy of occlusion of the descending thoracic aorta whether a clamp or an intraluminal device is used for this purpose. It also identifies any unexpected collateral flow and mitigates against the restoration of brain perfusion by diverting flow away from the brain while the occlusion device is checked or NRP stopped and in situ hypothermic perfusion and organ recovery initiated. The lack of any significant flow or pressure in the ascending aorta cannula during A‐NRP (n = 12) strongly supports the view that there is no significant collateral flow occurring along the spinal cord to the brain when a clamp is appropriately placed across the descending aorta just above the diaphragm.
Our findings in TA‐NRP currently cannot categorically confirm or refute the absence of some brain blood flow even if this is small. Equally it is impossible to speculate whether such flow would be at a pressure adequate to perfuse the brain. We suggest a pragmatic way of ensuring no brain perfusion during TA‐NRP based on vascular anatomy and sound physical principles, but they have not yet been tested experimentally. Our hypothesis is that the continued absence of brain perfusion can be achieved by the diversion of any potential collateral arterial brain blood flow to drain to atmosphere. In TA‐NRP, the aortic arch vessels (brachiocephalic trunk, left common carotid, and left subclavian) should be ligated, with the cephalad ends opened and allowed to drain, either to atmospheric or negative pressure. In this situation any collateral supply reaching the spinal arteries via the lower intercostal arteries should drain via the large superior intercostal arteries to the costocervical trunk and subclavian arteries to atmospheric pressure before any supply reaches the brainstem. Any blood reaching the vertebral artery will flow retrogradely to the open subclavian arteries. The presence of persisting drainage from the cut cephalad ends of the arch vessels during TA‐NRP (n = 3) confirms the potential for collateral blood flow that can be diverted away from the brain.
The approach described for DPP with A‐NRP is a national protocol endorsed by NHSBT and it is planned that the A‐NRP technique described will also be used by all UK retrieval teams. NHSBT will recommend that TA‐NRP be expanded more cautiously until further supporting evidence from the United Kingdom and elsewhere is available. Canada plans to proceed with DPP for heart DCD while addressing the issue of aligning Canadian death determination policies with the conditions required to permit NRP as per the logic model described in this manuscript. We accept that further evidence is required to confirm our hypothesis and we would encourage the donation and transplantation communities to address this. There may be variations in collateral connections and anatomical or functional differences in upstream and downstream vascular resistances that could theoretically affect the ability to freely divert brain flow. The scale of this challenge should not be underestimated. We would suggest undertaking computed tomography (CT) brain angiography or perfusion techniques during DCD organ recovery with NRP in theatre using mobile CT scanners. Demonstration of no flow would be the most reassuring evidence, but the presence of flow would not necessarily indicate perfusion, and finally the presence of some perfusion does not mean that brain functions can be restored.21 Neurophysiological demonstration of an isoelectric EEG and absent somatosensory potentials during NRP would also increase confidence, as would studies that exclude the potential of venous perfusion of the brain.
4. CONCLUSION
For those countries considering the use and acceptability of NRP in DCD, we suggest the following logical model:
If:
Death in DCD, as in everyday clinical practice, is defined by the permanent cessation of circulation to the brain, and
After the confirmation of death in DCD and prior to starting NRP, the surgical act of ligating or dividing the aortic arch vessels in TA‐NRP, or occluding the descending thoracic aorta in A‐NRP, is medically and ethically acceptable, and
In order to adhere to the principle of permanence for death, the absence of potential for brain perfusion can be ensured by the refinements outlined in this paper.
Then:
Restarting the circulation to the thoracic and/or abdominal cavities after death does not invalidate the definition of death in DCD organ retrieval,
Restarting the heart in the donor's body after death does not invalidate the definition of death when brain perfusion is excluded, and
Abdominal and thoracoabdominal NRP may be considered permissible.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr Healey reports a stipend income from Trillium Gift of Life Network as Chief Medical Officer‐Donation, outside the submitted work. Dr Large reports a patent Covering mOrgan2 organ perfuser. Dr Shemie reports being the Medical Advisor for deceased organ donation, Canadian Blood Services. Dr Messer reports proctoring fees from TransMedics, outside the submitted work. Dr Freed reports being founder and CSO for Tevosol, Inc, outside the submitted work. Dr Forsythe reports personal fees from NHS Blood and Transplant, during the conduct of the study. Ms Hornby reports personal fees from Canadian Blood Services, outside the submitted work. Dr Oniscu reports nonfinancial support from Maquet, grants from NHS Blood and Transplant, during the conduct of the study. The other authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
The study was funded in part by the National Health Service Blood and Transplant (NHSBT) Organ Donation and Transplant Directorate and the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHSBT. The views expressed are those of the authors and not necessarily those of the National Health Service, NHSBT, the NIHR or the UK Department of Health, or Canadian Blood Services.
Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. Am J Transplant. 2020;20:2017–2025. 10.1111/ajt.15775
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Canadian Organ Replacement Register . e‐Statistics On Organ Transplants, Waiting Lists And Donors. https://www.cihi.ca/en/e-statistics-on-organ-transplants-waiting-lists-and-donors. Published 2018. Accessed September 3, 2019.
- 2. NHS Blood and Transplant . Organ Donation and Transplantation Activity Report 2017/18. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/12300/transplant-activity-report-2017-2018.pdf. Published 2018. Accessed September 3, 2019.
- 3. Detry O, Van Deynse D, Van Vlierberghe H, Pirenne J. Organ procurement and transplantation in Belgium. Transplantation. 2017;101(9):1953‐1955. [DOI] [PubMed] [Google Scholar]
- 4. Leiden H, Haase‐Kromwijk B, Hoitsma A, Jansen N. Controlled donation after circulatory death in the Netherlands: more organs, more efforts. The Netherlands Journal of Medicine. 2016;74(7):285‐291. [PubMed] [Google Scholar]
- 5. Manara AR, Murphy PG, Ocallaghan G. Donation after circulatory death. Br J Anaesth. 2012;108(Suppl 1):i108‐i121. [DOI] [PubMed] [Google Scholar]
- 6. Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant. 2019;19(6):1745‐1758. [DOI] [PubMed] [Google Scholar]
- 7. Miñambres E, Suberviola B, Dominguez‐Gil B, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant. 2017;17(8):2165‐2172. [DOI] [PubMed] [Google Scholar]
- 8. Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29(6):6‐14. [PubMed] [Google Scholar]
- 9. Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med. 2014;40(6):788‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardiner D, Shemie S, Manara A, Opdam H. International perspective on the diagnosis of death. Br J Anaesth. 2012;108(Suppl 1):i14‐i28. [DOI] [PubMed] [Google Scholar]
- 11. Shemie SD, Gardiner D. Circulatory arrest, brain arrest and death determination. Frontiers in Cardiovascular Medicine. 2018;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manara AR. All human death is brain death: the legacy of the Harvard criteria. Resuscitation. 2019;138:210‐212. [DOI] [PubMed] [Google Scholar]
- 13. Australian and New Zealand Intensive Care Society (ANZICS) . The Anzics Statement on Death and Organ Donation. https://csds.qld.edu.au/sdc/Provectus/ELI/Module%202%20-%20Organ%20donation%20after%20brain%20death/files/ANZICS%20Statement%20on%20%20Death%20and%20Organ%20Donation%20Edition%203.2%20(3).pdf. Published 2013. Accessed September 3, 2019.
- 14. Shemie SD, Torrance S, Wilson LC, et al. Transplant of hearts after death by cardiac arrest in Canada. Can Med Assoc J. 2019. (in submission). [Google Scholar]
- 15. Academy of the Medical Royal Colleges . A Code of Practice for the Diagnosis and Confirmation of Death. http://aomrc.org.uk/wp-content/uploads/2016/04/Code_Practice_Confirmation_Diagnosis_Death_1008-4.pdf. Published 2008. Accessed September 3, 2019.
- 16. Dalle Ave AL, Shaw DM, Bernat JL. Ethical issues in the use of extracorporeal membrane oxygenation in controlled donation after circulatory determination of death. Am J Transplant. 2016;16(8):2293‐2299. [DOI] [PubMed] [Google Scholar]
- 17. Dalle Ave AL, Shaw D, Bernat JL. An analysis of heart donation after circulatory determination of death. J Med Ethics. 2016;42(5):312‐317. [DOI] [PubMed] [Google Scholar]
- 18. Prince EA, Ahn SH. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. Semin Intervent Radiol. 2013;30(3):234‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75(3):353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tchana‐Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38(6):593‐598. [DOI] [PubMed] [Google Scholar]
- 21. Sawicki M, Solek‐Pastuszka J, Chamier‐Cieminska K, Walecka A, Walecki J, Bohatyrewicz R. Computed tomography perfusion is a useful adjunct to computed tomography angiography in the diagnosis of brain death. Clin Neuroradiol. 2019;29(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


