Abstract
Aims
Severe deficiency of the essential trace element selenium can cause myocardial dysfunction although the mechanism at cellular level is uncertain. Whether, in clinical practice, moderate selenium deficiency is associated with worse symptoms and outcome in patients with heart failure is unknown.
Methods and results
BIOSTAT‐CHF is a multinational, prospective, observational cohort study that enrolled patients with worsening heart failure. Serum concentrations of selenium were measured by inductively coupled plasma mass spectrometry. Primary endpoint was a composite of all‐cause mortality and hospitalization for heart failure; secondary endpoint was all‐cause mortality. To investigate potential mechanisms by which selenium deficiency might affect prognosis, human cardiomyocytes were cultured in absence of selenium, and mitochondrial function and oxidative stress were assessed. Serum selenium concentration (deficiency) was <70 μg/L in 485 (20.4%) patients, who were older, more often women, had worse New York Heart Association class, more severe signs and symptoms of heart failure and poorer exercise capacity (6‐min walking test) and quality of life (Kansas City Cardiomyopathy Questionnaire). Selenium deficiency was associated with higher rates of the primary endpoint [hazard ratio (HR) 1.23; 95% confidence interval (CI) 1.06–1.42] and all‐cause mortality (HR 1.52; 95% CI 1.26–1.86). In cultured human cardiomyocytes, selenium deprivation impaired mitochondrial function and oxidative phosphorylation, and increased intracellular reactive oxygen species levels.
Conclusions
Selenium deficiency in heart failure patients is independently associated with impaired exercise tolerance and a 50% higher mortality rate, and impaired mitochondrial function in vitro, in human cardiomyocytes. Clinical trials are needed to investigate the effect of selenium supplements in patients with heart failure, especially if they have low plasma concentrations of selenium.
Keywords: Selenium, Malnutrition, Heart failure, Mitochondrial function, Cardiomyocytes, All‐cause mortality
Introduction
An aberrant equilibrium of circulating molecules like minerals and trace elements (for example iron, iodine and zinc) in the patients' blood is closely involved in the development and progression of heart failure.1, 2, 3, 4 It was shown that up to 50% of patients with heart failure suffer from some form of malnutrition, like micronutrient insufficiencies.5, 6
Selenium is an essential micronutrient and sufficient concentrations are essential for numerous biological functions including thyroid hormone metabolism, antioxidant defenses, the immune system and in certain types of cancer.7, 8 Severe selenium deficiency in humans is associated with a rare but fatal form of dilated cardiomyopathy that is restricted to specific geographic regions (Keshan disease)9 that have a very low amount of selenium in the soil and therefore in food. Keshan disease is reversible with selenium supplementation.1 Selenium soil content is very variable and this affects dietary selenium intake. For example, intakes are high in Venezuela, Canada, the USA, and Japan (>100 μg/day), and much lower in some parts of Europe (∼40 μg/day).7 Observational studies in the general population7, 10 suggest that selenium deficiency might be common, however data on serum selenium concentrations in heart failure are scarce.11, 12, 13
On a molecular level selenium is incorporated into, and is essential for the enzymatic activity of 25 different selenoproteins.13 Selenium depletion deprives the cell of its ability to synthesize enzymatically active selenoproteins, but the effects on mitochondrial function, biogenesis and oxidative stress in human cardiomyocytes are unknown.
Accordingly, we conducted both in vitro experiments on the effects of selenium deprivation on human cardiomyocytes and investigated associations between serum concentrations of selenium and the clinical characteristics and outcomes of a large cohort of patients with heart failure.
Materials and methods
Abbreviated methods are displayed below, for the full materials and methods section, see online supplementary Methods S1 .
BIOSTAT‐CHF patient population
We used information and samples from the BIOSTAT‐CHF index cohort, which consists of 2516 patients with heart failure from 69 centres in 11 European countries.14, 15, 16, 17 Inclusion criteria included: age > 18 years, symptoms of new‐onset or worsening heart failure and at least one of the following: a left ventricular ejection fraction of ≤ 40%, a plasma B‐type natriuretic peptide of >400 pg/mL or a plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) of >2000 pg/mL. Patients could only be included if their treatment did not conform to guideline recommendations [i.e. ≤ 50% of angiotensin‐converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB) and/or beta‐blockers] and were anticipated to have treatment with ACEi/ARBs and/or beta‐blockers initiated or up‐titrated. The BIOSTAT‐CHF study was approved by the medical ethics committee for each centre and complies with the Declaration of Helsinki. All participants provided written informed consent prior to any study‐related procedures.
For additional details on the BIOSTAT‐CHF study definitions, measurements and outcome analyses and statistical analysis, see online supplementary Methods S1 .
Serum selenium determination
Selenium was measured using a validated inductively coupled plasma mass spectrometry (ICP‐MS) method with a lower limit of quantitation of 20 μg/L serum. The 78Se isotope concentration was measured using a Varian 820‐MS ICP mass spectrometer. Reference ranges for serum selenium vary between coutries because of differences in dietary sources of selenium. For caucasians of European ancestry, adult reference ranges are between 70 and 130 μg/L.18 Deficiency of selenium was therefore set at serum levels < 70 μg/L.
For additional details on serum selenium determination, see online supplementary Methods S1 .
Cell culture, cardiomyocyte differentiation, selenium depletion and cardiac stress model
Differentiation of HUES9 human pluripotent stem cells (hPSC, Harvard Stem Cell Institute) to cardiomyocytes was achieved as described previously.19, 20, 21, 22 For the experiments, cells were grown either in CDM3 medium supplemented with 100 nM sodium selenite23 (Control; S5261, Sigma‐Aldrich) or in CDM3 medium without added selenium (selenium deficient cells) for 2 weeks.
For additional details on the functional characterisation of selenium depletion of cardiomyocytes (protein extraction and western blot, RNA extraction and qRT‐PCR, immunofluorescence, reactive oxygen species detection and Seahorse experiments), see online supplementary Methods S1 and Table S1 .
Statistical analyses
Differences between clinical characteristics across quartiles of selenium levels were compared using one‐way analysis of covariance (ANOVA), the Kruskal–Wallis test or the Chi square test where appropriate. Multivariable logistic regression was used to investigate independent associations with selenium deficiency. The association with outcome was investigated using Kaplan–Meier curves and the log‐rank test. For multivariable analyses, Cox regression analysis was performed on selenium deficiency and standardized selenium levels, correcting for relevant clinical confounders independently associated with selenium levels (online supplementary Table S2 ) and the BIOSTAT‐CHF risk models, which were previously published.15 The baseline model included, age, daily protein intake, country, haemoglobin, C‐reactive protein, high‐density lipoprotein cholesterol, albumin, NT‐proBNP, sodium and estimated glomerular filtration rate (eGFR by the Chronic Kidney Disease Epidemiology Collaboration equation), all independently associated with selenium levels. The BIOSTAT‐CHF risk model for predicting mortality included, age, blood urea nitrogen, NT‐proBNP, haemoglobin and the use of a beta‐blocker at time of inclusion. The BIOSTAT‐CHF risk model for predicting mortality or heart failure hospitalization included age, NT‐proBNP, haemoglobin, the use of a beta‐blocker at time of inclusion, a heart failure hospitalization in the year before inclusion, peripheral oedema, systolic blood pressure, high‐density lipoprotein cholesterol and sodium.
Experimental groups consisted of at least three independent biological replicates and technical duplicates were used. Data shown are expressed as means ± standard error of the mean (SEM). Differences were assessed by Student's t‐test. A P‐value of < 0.05 was considered statistically significant.
All tests and analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX, USA) or GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
Results
BIOSTAT‐CHF baseline characteristics
Median serum selenium was 87 μg/L [interquartile range (IQR) 73–103] and 485 (20.4%) patients were selenium‐deficient (<70 μg/L). Patients with lower serum selenium concentrations were older, more often women, had worse symptoms and signs of heart failure, were more likely to have anaemia, iron deficiency and chronic kidney disease (eGFR <60 mL/min/1.73 m2) and had lower serum albumin and cholesterol (Table 1). In multivariable analyses, country of residence, higher age, lower protein intake and lower haemoglobin and albumin concentrations were most strongly associated with selenium deficiency (baseline model; online supplementary Table S2 ). There was considerable geographic variation in serum selenium concentrations (online supplementary Figure S1 ).
Table 1.
Baseline characteristics
| Normal selenium (≥ 70 μg/L) | Selenium‐deficient (< 70 μg/L) | P‐value | |
|---|---|---|---|
| n | 1897 | 485 | |
| Demographics | |||
| Age (years) | 67.8 (12.0) | 72.6 (11.3) | <0.001 |
| Female sex | 456 (24.0%) | 158 (32.6%) | <0.001 |
| BMI (kg/m2) | 28.0 (5.5) | 27.5 (5.5) | 0.10 |
| Protein intake (g/day) | 55.8 (11.5) | 52.2 (9.9) | <0.001 |
| Ischaemic aetiology (%) | 870 (46.7%) | 215 (44.9%) | 0.47 |
| LVEF (%) | 30.5 (9.9) | 32.9 (12.7) | <0.001 |
| HF subgroup | <0.001 | ||
| HFrEF | 1430 (83.6%) | 303 (71.8%) | |
| HFmrEF | 194 (11.3%) | 73 (17.3%) | |
| HFpEF | 86 (5.0%) | 46 (10.9%) | |
| NYHA class | <0.001 | ||
| I | 198 (11.9%) | 27 (6.2%) | |
| II | 896 (54.0%) | 201 (46.4%) | |
| III | 512 (30.9%) | 179 (41.3%) | |
| IV | 52 (3.1%) | 26 (6.0%) | |
| Systolic BP (mmHg) | 124.5 (21.5) | 124.6 (22.7) | 0.93 |
| Diastolic BP (mmHg) | 75.3 (13.2) | 73.4 (12.9) | 0.005 |
| Heart rate (bpm) | 79.6 (19.5) | 80.1 (18.9) | 0.63 |
| Smoking | 0.074 | ||
| No | 670 (35.4%) | 196 (40.5%) | |
| Past | 943 (49.8%) | 229 (47.3%) | |
| Current | 282 (14.9%) | 59 (12.2%) | |
| Signs and symptoms | |||
| Extent of peripheral oedema | <0.001 | ||
| Not present | 706 (44.9%) | 100 (24.9%) | |
| Ankle | 456 (29.0%) | 121 (30.2%) | |
| Below knee | 320 (20.4%) | 121 (30.2%) | |
| Above knee | 90 (5.7%) | 59 (14.7%) | |
| Elevated JVP | 384 (30.0%) | 138 (46.9%) | <0.001 |
| Hepatomegaly | 252 (13.3%) | 81 (16.8%) | 0.049 |
| Orthopnoea | 605 (32.0%) | 215 (44.4%) | <0.001 |
| Pulmonary congestion | 943 (51.3%) | 291 (61.0%) | <0.001 |
| Co‐morbidities | |||
| Anaemia | 567 (33.2%) | 222 (47.9%) | <0.001 |
| Atrial fibrillation | 839 (44.2%) | 238 (49.1%) | 0.056 |
| Diabetes mellitus | 599 (31.6%) | 170 (35.1%) | 0.14 |
| COPD | 313 (16.5%) | 98 (20.2%) | 0.054 |
| CKD | 487 (25.7%) | 170 (35.1%) | <0.001 |
| Hypertension | 1178 (62.1%) | 310 (63.9%) | 0.46 |
| Peripheral arterial disease | 197 (10.4%) | 56 (11.5%) | 0.46 |
| Stroke | 167 (8.8%) | 50 (10.3%) | 0.30 |
| PCI | 429 (22.6%) | 98 (20.2%) | 0.25 |
| CABG | 320 (16.9%) | 90 (18.6%) | 0.38 |
| Medication | |||
| Loop diuretics | 1889 (99.6%) | 482 (99.4%) | 0.57 |
| ACEi/ARB | 1391 (73.3%) | 333 (68.7%) | 0.040 |
| Aldosterone antagonist | 1031 (54.3%) | 243 (50.1%) | 0.094 |
| Beta‐blocker | 1608 (84.8%) | 382 (78.8%) | 0.001 |
| Antiplatelets | 983 (51.8%) | 252 (52.0%) | 0.96 |
| Laboratory | |||
| Haemoglobin (g/dL) | 13.4 (1.8) | 12.4 (1.9) | <0.001 |
| Ferritin (μg/L) | 107 (52–199) | 76 (41–159) | <0.001 |
| Transferrin saturation (%) | 17.7 (11.7–25.6) | 13.3 (9.0–21.2) | <0.001 |
| Albumin (g/L) | 33.1 (8.6) | 28.9 (8.6) | <0.001 |
| C‐reactive protein (mg/L) | 12.1 (5.1–24.2) | 17.6 (8.4–32.3) | <0.001 |
| Total cholesterol (mmol/L) | 4.2 (3.4–5.1) | 3.8 (3.1–4.6) | <0.001 |
| HDL cholesterol (mmol/L) | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | 0.001 |
| LDL cholesterol (mmol/L) | 2.6 (1.9–3.2) | 2.2 (1.5–3.0) | <0.001 |
| HbA1c (%) | 6.3 (5.7–7.2) | 6.3 (5.8–6.9) | 0.96 |
| NT‐proBNP (ng/L) | 3845 (2242–7681) | 5506 (3045–9727) | <0.001 |
| Sodium (mmol/L) | 140 (137–142) | 139 (136–141) | <0.001 |
| Potassium (mmol/L) | 4.2 (3.9–4.6) | 4.3 (3.9–4.6) | 0.81 |
| Creatinine (μmol/L) | 100 (82–126) | 106 (83–141) | 0.007 |
| eGFR (mL/min/1.73 m2) | 61.0 (45.9–80.0) | 53.7 (37.7–74.4) | <0.001 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (by Chronic Kidney Disease‐Epidemiology Collaboration equation); HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; JVP, jugular venous pressure; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Selenium‐deficient patients were less likely to complete a 6‐min walking test (6MWT) (54% vs. 67%, P < 0.001) and those that did had a shorter 6MWT distance [151 (IQR 0–276) m vs. 225 (IQR 0–364) m; P < 0.001]. Selenium‐deficient patients also had a lower overall score on the Kansas City Cardiomyopathy Questionnaire (KCCQ) [41 (IQR 25–57) vs. 51 (IQR 34–69)] (Figure 1 A and 1 B). These associations remained after multivariable correction for baseline associations with serum selenium (coefficient −30, beta −0.07, P = 0.001 and coefficient −4, beta −0.06, P = 0.001, for 6MWT and KCCQ, respectively).
Figure 1.
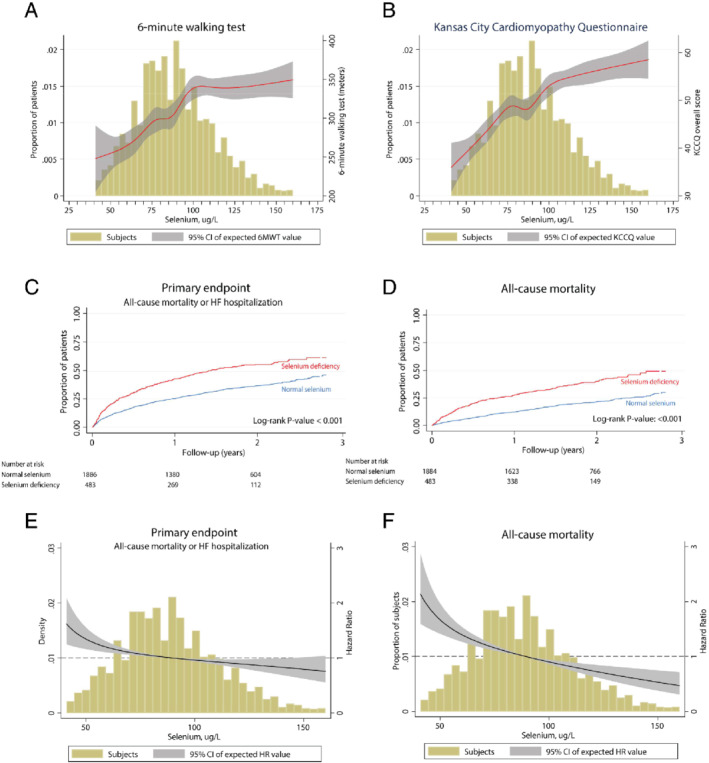
Selenium and clinical outcome parameters. Association of selenium levels with (A) 6‐min walking test (6MWT) and (B) Kansas City Cardiomyopathy Questionnaire (KCCQ) results. Kaplan–Meier curves showing the increased risk of chronic heart failure patients with selenium deficiency (selenium <70 μg/L) for (C) the composite endpoint [all‐cause mortality and hospitalization due to heart failure (HF)] and (D) all‐cause mortality. Selenium levels as a continuous variable for hazard ratio (HR) plots using fractional polynomials, showing increasing HR with decreasing selenium concentrations for both the composite endpoint (E) and all‐cause mortality (F). CI, confidence interval.
Selenium deficiency associates with higher all‐cause mortality and rehospitalisation for heart failure
Overall, 963 (40%) patients died or were hospitalized for heart failure during a median follow‐up of 21 (IQR 16–27) months. Selenium defiency was strongly associated [hazard ratio (HR) 1.78; 95% confidence interval (CI) 1.54–2.09] with the primary composite endpoint (Figure 1 C and Table 2). This association remained significant (HR 1.21; 95% CI 1.02–1.43) after correction for baseline variables associated with serum selenium or variables included in the BIOSTAT‐CHF risk model (HR 1.23; 95% CI 1.06–1.42). Similar associations were found for all‐cause mortality (HR 1.52; 95% CI 1.26–1.86) (Figure 1 D and Table 2). In sensitivity analyses, we analysed selenium levels as a continuous variable for HR plots using fractional polynomials, which showed an increasing HR with decreasing selenium concentrations for both the composite endpoint (Figure 1 E) and all‐cause mortality (Figure 1 F). These results were supported by the Kaplan–Meier plots of selenium concentration quartiles, showing gradual increased risk with lower concentrations (online supplementary Figure S2 ). Selenium concentrations were strongly inversely associated with higher rates of the primary combined outcome (HR 0.87; 95% CI 0.81–0.94) and mortality alone (HR 0.82; 95% CI 0.76–0.88) in the multivariable BIOSTAT‐CHF risk model. For patients with serum selenium ≥70 μg/L, higher concentrations were independently associated with a better outcome (primary endpoint: HR: 0.90; 95% CI 0.82–0.99 and all‐cause mortality: HR: 0.79; 95% CI 0.70–0.90).
Table 2.
Cox regression analyses
| HR (95%CI) | P‐value | |
| Combined outcome | ||
| Univariable | 1.78 (1.54–2.09) | <0.001 |
| Baseline modela | 1.21 (1.02–1.43) | 0.028 |
| BIOSTAT‐CHF risk modelb | 1.23 (1.06–1.42) | 0.007 |
| All‐cause mortality | ||
| Univariable | 2.17 (1.83–2.58) | <0.001 |
| Baseline modela | 1.46 (1.19–1.79) | <0.001 |
| BIOSTAT‐CHF risk modelc | 1.52 (1.26–1.86) | <0.001 |
CI, confidence interval; HR, hazard ratio.
Age, daily protein intake, country, haemoglobin, C‐reactive protein, high‐density lipoprotein cholesterol, albumin, N‐terminal pro‐B‐type natriuretic peptide, sodium and estimated glomerular filtration rate (by Chronic Kidney Disease‐Epidemiology Collaboration equation).
Age, N‐terminal pro‐B‐type natriuretic peptide, haemoglobin, use of a beta‐blocker at time of inclusion, heart failure hospitalization in the year before inclusion, peripheral oedema, systolic blood pressure, high‐density lipoprotein cholesterol and sodium.
Age, blood urea nitrogen, N‐terminal pro‐B‐type natriuretic peptide, haemoglobin and use of a beta‐blocker at time of inclusion.
Low selenium reduces mitochondrial functionality in human cardiomyocytes
Generated human cardiomyocytes from hPSCs (hPSC‐CMs) were stained for cardiac markers, and cardiac‐specific gene expression was determined for characterization purposes as described before by Hoes et al.20
hPSC‐CMs cultured for 2 weeks in selenium‐free conditions showed reduced mitochondrial function (Figure 2 A). Selenium depleted hPSC‐CMs showed a basal levels of oxygen consumption rate of 49% (P < 0.00001) compared to control hPSC‐CMs cultured in the presence of selenium (Figure 2 A and 2B). Addition of oligomycin inhibited ATP synthase‐linked respiration resulted in 48% less ATP synthase‐linked respiration (P < 0.001) (Figure 2 B). Subsequent addition of the uncoupler FCCP induced mitochondria to function at maximum capacity, which showed a 29% lower maximal capacity (P < 0.005) in selenium‐depleted hPSC‐CMs (Figure 2 B). Respiration reserve capacity, an estimate of the potential bioenergetic reserve, was not significantly different between selenium‐depleted and control cells (Figure 2 B). The same was true for non‐mitochondrial oxygen consumption, proton leak and coupling efficiency (data not shown).
Figure 2.
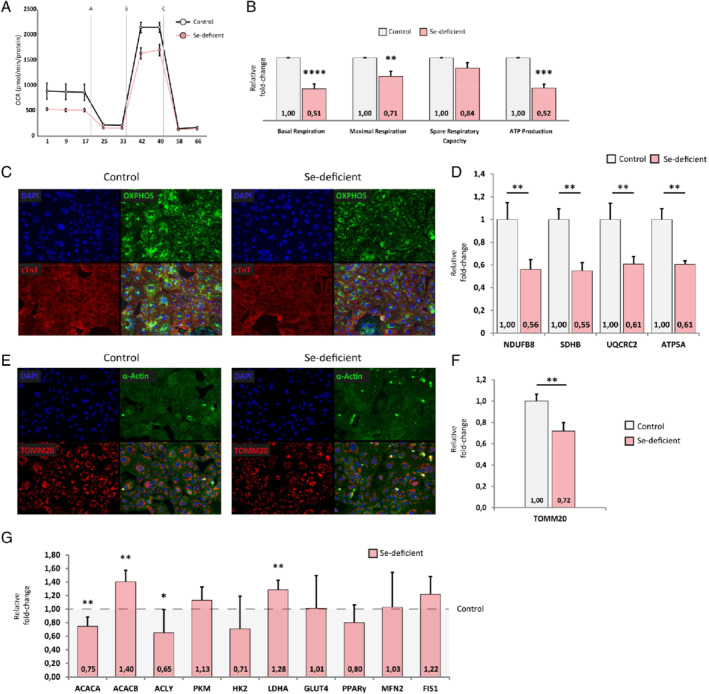
Effects of selenium (Se) depletion on mitochondrial respiration and metabolic function. (A) Representative traces for control cardiomyocytes and Se‐deficient cardiomyocytes in a Mito Stress Test. (B) Effects of Se deficiency on basal respiration, maximal respiration, respiratory reserve and ATP‐linked respiration (n = 5). Representative immunofluorescent staining for (C) DAPI, OXPHOS and cardiac troponin T (cTnT) and (E) DAPI, TOMM20 and α‐actin in control and Se‐deficient cardiomyocytes (n = 3). Western blot quantification of (D) NDUFB8, SDHB, UQCRC2 and ATP5A (mitochondrial complexes 1 to 3 and 5, respectively) (n = 6) and (F) TOMM20 (n = 6). (G) Relative fold‐change of genes involved in (anaerobic) glycolysis or fatty acid metabolism and mitochondrial fusion and fission (n = 6). OCR, oxygen consumption rate. *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001.
Mitochondria in selenium‐depleted hPSC‐CMs also had decreased expression of OXPHOS proteins [NDUFB8 (FC = 0.56), SDHB (FC = 0.55), UQCRC2 (FC = 0.61) and ATP5A (FC = 0.61) representing mitochondrial complexes 1 to 3 and 5, respectively] in hPSC‐CMs with immunofluorescence (Figure 2 C) and western blot (Figure 2 D). To determine mitochondrial abundance, hPSC‐CMs were checked for TOMM20, a mitochondrial membrane marker. Based on immunofluorescence (Figure 2 E) and western blot results (Figure 2 F) TOMM20 was reduced in selenium‐depleted cardiomyocytes (FC = 0.72; P = 0.004), albeit to a lesser extent than OXPHOS. Furthermore, expression of MFN2 (mitochondrial fusion) and FIS1 (mitochondrial fission) did not change with selenium deficiency (Figure 2 G).
mRNA expression levels of genes involved in (anaerobic) glycolysis or fatty acid metabolism were assessed (Figure 2 G) to determine whether mitochondrial dysfunction was accompanied by a switch in metabolic substrate. Selenium‐depleted hPSC‐CMs had decreased expression of ACACA and ACLY, but expression of ACACB. LDHA was upregulated as the result of low selenium, but not GLUT4, PKM, HK2.
Low selenium promotes stress‐induced increase of reactive oxygen species levels in human cardiomyocytes
Selenium depletion was associated with more oxidative stress. Under normal culture conditions, we found a 33% increase of reactive oxygen species (ROS) levels (P < 0.0001) in selenium‐depleted compared to selenium supplemented cardiomyocytes. Induction of oxidative stress by adding rotenone to hPSC‐CMs resulted in an additional 20% increase of ROS levels only in selenium‐depleted cells (P < 0.005) (Figure 3 A). Under oxidative stress conditions, the total increase of ROS levels between selenium‐depleted and control cells was 52% (P < 0.0001). Addressing the expression of oxidative stress markers at a transcriptional level in selenium‐depleted hPSC‐CMs showed that, upon stress induction with rotenone, expression of PGC‐1α, NOS2, AP‐1 and RELA are increased two‐fold or more (P < 0.05) (Figure 3 B). Subsequently, under selenium replete conditions, induction of oxidative stress with rotenone, did not result in a significant induction of the stress markers NOS2, AP‐1 and RELA.
Figure 3.
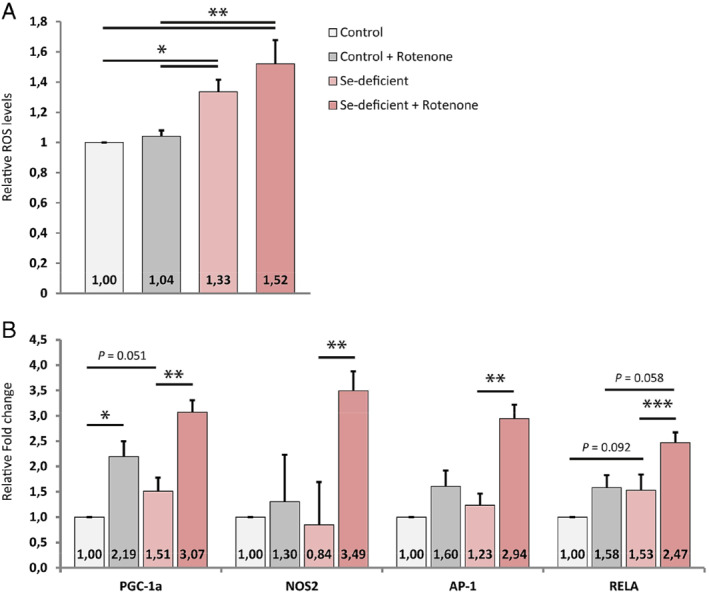
Effects of selenium (Se) depletion on reactive oxygen species (ROS) levels and transcriptional stress markers. (A) Relative ROS levels in control and Se‐deficient cardiomyocytes, either with or without the addition of rotenone (n = 5). (B) Relative fold‐change of genes involved in cardiomyocyte stress response upon oxidative stress (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
This analysis shows that many patients with worsening heart failure have a serum selenium concentration < 70 μg/L and that this is associated with poorer quality of life, exercise capacity and prognosis. Serum concentrations of 70–100 μg/L appeared to have similar adverse associations, suggesting that values <100 μg/L, found in >50% of this cohort, might be considered abnormal.24, 25 In vitro experiments on cultured human cardiac myocytes suggest that selenium deficiency disrupts mitochondrial electron transport chain function leading to less efficient production of ATP, increased production of ROS and intracellular oxidative damage. However, selenium may not only be a marker of greater disease severity and worse outcome but also a therapeutic target. We found that the relationship between selenium deficiency and signs, symptoms and prognosis were similar or even more pronounced than for iron deficiency. Clinical trials of intravenous iron supplements have shown improvements in symptoms and well‐being, and trials investigating effects on morbidity and mortality are underway. Unlike iron, selenium is readily absorbed orally. Trials of selenium supplementation are now required.
Selenium accumulates in biological tissue and is generally known as an antioxidant due to its presence in selenoproteins as selenocysteine. Furthermore, it is known to affect production of active thyroid hormone T3 26 and anti‐inflammatory and anti‐apoptotic capacity.27 With the occurrence of modest deficiency, selenoprotein concentrations and activities are preferentially lost based on their demand.28 Serum selenium concentrations are reduced in many other age‐related diseases, including cancer and heart failure.7, 28 Appetite and therewith food intake may be reduced because of dietary advice on salt and calorie intake, social isolation, possible circulatory congestion and inflammation‐induced malabsorption, although evidence on the latter is conflicting.12, 29 Age‐matched healthy controls showed higher selenium levels than patients with worsening heart failure (online supplementary Table S3 ), with only 5% of controls <70 μg/L. Selenium toxicity can occur with ingestion of excess selenium and symptoms include nausea, vomiting, nail brittleness and loss, hair loss, fatigue, irritability and foul breath odour as found in a study investigating a misformulated liquid dietary supplement that resulted in 201 cases of selenium poisoning [the median estimated amount of selenium ingested was 989 mg (range: 41–5875 mg)].30 Nonetheless, selenium supplementation is considered to be safe with a recommended dietary allowance of 55 μg/day for persons ≥14 years, with a tolerable upper intake limit of 400 μg/day.30
A meta‐analysis of 25 observational studies on selenium supplementation showed a moderate association between increasing serum selenium concentration and reduced risk of coronary heart disease.10 However, randomised trials of selenium supplementation did not show a profound reduction in cardiovascular events or mortality,10 but these where often performed in small cohorts and contain several confounding factors (e.g. age and baseline selenium levels) hampering interpretation and generalization. One major confounding factor in these trials is the high mean serum selenium levels, especially in cohorts including subjects from countries with relatively high intake (USA, Canada).31 Beyond a specific selenium concentration, there may be no further advantage of selenium supplementation in reducing cardiovascular mortality.32 This is supported by our data on exercise capacity and quality of life, showing only for selenium concentrations <100 μg/L a negative effect. Furthermore, selenium is often given in combination with other vitamins or minerals (e.g. zinc, vitamin C and E and β‐carotene) making it difficult to identify selenium specific effects.10 A recently reported trial of older people (aged 70–80 years), many of whom were not known to have serious cardiac disease, randomly assigned participants to placebo or supplements of selenium 200 μg/day and coenzyme Q10 for a median of 5.1 years.33, 34 Daily selenium supplements increased serum selenium concentrations from 45–87 μg/L at baseline to 185–245 μg/L at 48 months, whereas it did not change in those assigned to placebo.33 Those receiving supplements had lower plasma concentrations of NT‐proBNP at 24 and 48 months and improved echocardiographic function.34 Furthermore, participants with a serum selenium concentration <65 μg/L had a higher cardiovascular mortality compared to those with concentrations >85 μg/L.33 It is unclear whether there is a synergistic effect of selenium and coenzyme Q10 supplements. No randomized trial has evaluated the effect of selenium supplements alone in patients with heart failure.
The effects of selenium deprivation was assessed for the first time in human PSC‐derived cardiomyocytes and showed to impair mitochondrial function, biogenesis and oxidative stress. Mitochondria of selenium‐deprived cardiomyocytes had a two‐fold lower basal oxygen consumption rate and ATP‐linked respiration. Furthermore, on gene expression level, we found decreased expression ACACA and ACLY, but increased expression of ACACB indicating decreased de novo lipogenesis and increased inhibition of fatty acid and glucose oxidation, respectively. LDHA was up‐regulated as the result of low selenium, indicative of anaerobic glycolysis. This was in concordance with lower levels of oxidative phosphorylation, implicating a more glycolytic metabolism profile similar to failing cardiomyocytes.35 We found no evidence for induced mitochondrial fission or fusion. One potential mechanism of benefit from selenium supplementation is the reduction of oxidative stress. ROS levels were increased in selenium‐depleted cardiomyocytes under normal culture conditions. Upon inhibiting mitochondrial complex I, ROS levels increased even more with low selenium status, but not in cardiomyocytes with a sufficient selenium status, implying non‐optimal working antioxidant machinery. In the acute phase following myocardial infarction and especially subsequent reperfusion, oxidative damage is predominant. One major source for myocardial ROS lies within the mitochondria and its production is linked to glutathione.33, 36, 37 Glutathione is a selenium‐containing enzyme and an important antioxidant in human. Among the 25 selenoproteins in mammals, the family of glutathione peroxidases is a major group whose function is well understood.1 Nevertheless, much is still unknown of the effect of the other selenoproteins and more research regarding the fundamental mechanisms of these proteins on cardiac homeostasis is necessary.
Limitations
In this retrospective study we only investigated the associations of selenium serum levels with clinical characteristics and outcomes. No data were currently available on other proteomic markers of selenium‐dependent pathways, nor oxidative stress. As a result, other signalling components such as selenoprotein P or gluthatione peroxidase were not investigated next to serum selenium. Furthermore, reference ranges for serum selenium vary between coutries because of differences in dietary sources of selenium. For Caucasians of European ancestry, adult reference ranges are between 70 and 130 μg/L. Deficiency of selenium was therefore set at serum levels <70 μg/L. Finally, although numerous associations were identified between selenium and clinical measurements/outcomes, more research regarding the fundamental mechanisms of selenium on cardiac homeostasis and the potential benefits of selenium supplements in patients with heart failure and low serum selenium concentrations is necessary.
Conclusions
In conclusion, selenium is required to maintain health including normal mitochondrial and cardiac function. Many older people, with and without heart failure, have low serum concentrations of selenium, suggesting deficiency. In patients with heart failure, low serum concentrations are associated with more severe symptoms and signs and a worse prognosis. Selenium supplements safely correct low serum concentrations and in a trial of older people, many of whom did not have serious cardiovascular disease, a combination of selenium and coenzyme Q10 supplements improved outcome. Selenium is readily available, inexpensive and has been thoroughly tested for toxicity, making it a potentially important ‘nutraceutical’. Having said this, we would like to state that by using an observational study we are unable to attribute cause and effect of selenium deficiency. Although we show significant effects on mitochondrial function and ROS of in vitro cultured cardiomyocytes, these functional data are suggestive and do not constitute such proof. Therefore, therapeutic trials are needed to achieve conclusive evidence of a causative role of selenium deficiency in heart failure patients and to determine the benefits of selenium supplements in patients with heart failure and low serum selenium concentrations.
Supporting information
Methods S1. Supplementary methods.
Table S1. Primer sequences as used for RT‐qPCR expression analyses.
Table S2. Multivariable logistic regression analysis between the prevalence of selenium deficiency (< 70 μg/L) and baseline characteristics.
Table S3. Baseline characteristics of healthy controls against BIOSTAT‐CHF patients.
Figure S1. Geographic variation in serum selenium concentrations.
Figure S2. Kaplan–Meier plots by quartile of selenium concentration for the primary endpoint of all‐cause mortality or heart failure hospitalization and the secondary endpoint of all‐cause mortality.
Acknowledgements
The authors thank Silke Oberdorf‐Maass for her excellent technical assistance.
Funding
BIOSTAT‐CHF was funded by a grant from the European Commission (FP7‐242209‐BIOSTAT‐CHF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
Contributor Information
Nils Bomer, Email: n.bomer@umcg.nl.
Peter van der Meer, Email: p.van.der.meer@umcg.nl.
References
- 1. Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med 2014;370:1756–1760. [DOI] [PubMed] [Google Scholar]
- 2. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582.e3. [DOI] [PubMed] [Google Scholar]
- 3. Yoshihisa A, Abe S, Kiko T, Kimishima Y, Sato Y, Watanabe S, Kanno Y, Miyata‐Tatsumi M, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y. Association of serum zinc level with prognosis in patients with heart failure. J Card Fail 2018;24:375–383. [DOI] [PubMed] [Google Scholar]
- 4. Roberts CG, Ladenson PW. Hypothyroidism. Lancet 2004;363:793–803. [DOI] [PubMed] [Google Scholar]
- 5. Hughes CM, Woodside JV, McGartland C, Roberts MJ, Nicholls DP, McKeown PP. Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 2012;22:376–382. [DOI] [PubMed] [Google Scholar]
- 6. McKeag NA, McKinley MC, Harbinson MT, McGinty A, Neville CE, Woodside JV, McKeown PP. Dietary micronutrient intake and micronutrient status in patients with chronic stable heart failure: an observational study. J Cardiovasc Nurs 2017;32:148–155. [DOI] [PubMed] [Google Scholar]
- 7. Rayman MP. Selenium and human health. Lancet 2012;379:1256–1268. [DOI] [PubMed] [Google Scholar]
- 8. Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, Stoppe C. Selenium and its supplementation in cardiovascular disease – what do we know? Nutrients 2015;7:3094–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang GQ, Ge KY, Chen JS, Chen XS. Selenium‐related endemic diseases and the daily selenium requirement of humans. World Rev Nutr Diet 1988;55:98–152. [DOI] [PubMed] [Google Scholar]
- 10. Flores‐Mateo G, Navas‐Acien A, Pastor‐Barriuso R, Guallar E. Selenium and coronary heart disease: a meta‐analysis. Am J Clin Nutr 2006;84:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lorgeril M, Salen P, Accominotti M, Cadau M, Steghens JP, Boucher F, de Leiris J. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail 2001;3:661–669. [DOI] [PubMed] [Google Scholar]
- 12. Witte KKA, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, Clark AL, Cleland JG. The effect of micronutrient supplementation on quality‐of‐life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J 2005;26:2238–2244. [DOI] [PubMed] [Google Scholar]
- 13. Alexanian I, Parissis J, Farmakis D, Pantziou C, Ikonomidis I, Paraskevaidis I, Ioannidou S, Sideris A, Kremastinos D, Lekakis J, Filippatos G. Selenium contributes to myocardial injury and cardiac remodeling in heart failure. Int J Cardiol 2014;176:272–273. [DOI] [PubMed] [Google Scholar]
- 14. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy study to TAilored treatment in chronic heart failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 15. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017;19:627–634. [DOI] [PubMed] [Google Scholar]
- 16. Santema BT, Kloosterman M, Van Gelder IC, Mordi I, Lang CC, Lam CS, Anker SD, Cleland JG, Dickstein K, Filippatos G, Van der Harst P, Hillege HL, Ter Maaten JM, Metra M, Ng LL, Ponikowski P, Samani NJ, Van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, Van der Meer P, Rienstra M, Voors AA. Comparing biomarker profiles of patients with heart failure: atrial fibrillation vs. sinus rhythm and reduced vs. preserved ejection fraction. Eur Heart J 2018;39:3867–3875. [DOI] [PubMed] [Google Scholar]
- 17. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 18. Moffat A C, Osselton MD, Widdop B, Watts J. Clarke's Analysis of Drugs and Poisons, 4th ed. London: Pharmaceutical Press; 2011. [Google Scholar]
- 19. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BN, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018;20:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β‐catenin signaling under fully defined conditions. Nat Protoc 2013;8:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ovchinnikova E, Hoes M, Ustyantsev K, Bomer N, de Jong TV, van der Mei H, Berezikov E, van der Meer P. Modeling human cardiac hypertrophy in stem cell‐derived cardiomyocytes. Stem Cell Reports 2018;10:794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C, Deng Y, Lei Y, Zhao J, Wei W, Li Y. Effects of selenium on myocardial apoptosis by modifying the activity of mitochondrial STAT3 and regulating potassium channel expression. Exp Ther Med 2017;14:2201–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS; Cardiovascular Outcomes Research Consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 25. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res 2008;52:1235–1246. [DOI] [PubMed] [Google Scholar]
- 27. Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 2005;37:1234–1241. [DOI] [PubMed] [Google Scholar]
- 28. McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J 2011;25:1793–1814. [DOI] [PubMed] [Google Scholar]
- 29. Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol 2001;37:1765–1774. [DOI] [PubMed] [Google Scholar]
- 30. MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, Burk RF, Dunn JR, Green AL, Hammond R, Schaffner W, Jones TF. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med 2010;170:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bleys J. Serum selenium levels and all‐cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med 2008;168:404. [DOI] [PubMed] [Google Scholar]
- 32. Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, Farinaro E, Clark LC, Reid ME. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol 2006;163:694–699. [DOI] [PubMed] [Google Scholar]
- 33. Alehagen U, Alexander J, Aaseth J. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. PLoS One 2016;11:e0157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N‐terminal‐proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5‐year prospective randomized double‐blind placebo‐controlled trial among elderly Swedish citizens. Int J Cardiol 2013;167:1860–1866. [DOI] [PubMed] [Google Scholar]
- 35. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018;15:457–470. [DOI] [PubMed] [Google Scholar]
- 36. Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ; AtheroGene Investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 2003;349:1605–1613. [DOI] [PubMed] [Google Scholar]
- 37. van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019;21:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Supplementary methods.
Table S1. Primer sequences as used for RT‐qPCR expression analyses.
Table S2. Multivariable logistic regression analysis between the prevalence of selenium deficiency (< 70 μg/L) and baseline characteristics.
Table S3. Baseline characteristics of healthy controls against BIOSTAT‐CHF patients.
Figure S1. Geographic variation in serum selenium concentrations.
Figure S2. Kaplan–Meier plots by quartile of selenium concentration for the primary endpoint of all‐cause mortality or heart failure hospitalization and the secondary endpoint of all‐cause mortality.


