Abstract
Objectives
This study sought to investigate the clinical outcomes of patients with and without peripheral artery disease (PAD) in the BRAVO‐3 trial with respect to the effect of bivalirudin versus unfractionated heparin (UFH).
Background
PAD is found frequently in patients undergoing transcatheter aortic valve replacement (TAVR) and is reported to confer an increased risk of adverse events. It is unknown whether patients with and without PAD may demonstrate a differential response to bivalirudin versus UFH.
Methods
BRAVO‐3 was a randomized multicenter trial comparing transfemoral TAVR with bivalirudin versus UFH (31 centers, n = 802). Major adverse cardiovascular events (MACE) were a composite of 30‐day death, myocardial infarction, or cerebrovascular accidents (CVA). Net adverse cardiovascular events (NACE) were a composite of major bleeding or MACE.
Results
The total cohort included 119 patients with PAD. Vascular complications occurred significantly more frequently in patients with PAD both in‐hospital (25.2 vs. 16.7%; OR 1.68) and at 30 days (29.4 vs. 17.3%; OR 1.99). No significant differences were observed regarding mortality, NACE, MACE, major bleeding or CVA with bivalirudin versus UFH among patients with or without PAD. In patients with PAD, bivalirudin was associated with an increased risk of minor vascular complications at 30 days.
Conclusions
Patients with PAD undergoing transfemoral TAVR did not exhibit an increased risk of any major adverse events, according to the procedural anticoagulant randomization. However, patients treated with Bivalirudin had significantly higher rates of minor vascular complications.
Keywords: bivalirudin, heparin, peripheral artery disease, TAVR
Abbreviations list
- AS
aortic stenosis
- BARC
Bleeding Academic Research Consortium
- CAD
coronary artery disease
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- CVA
cerebrovascular accidents
- eGFR
estimated glomerular filtration rate
- EuroSCORE
European System for Cardiac Operative Risk Evaluation score
- LVEF
left ventricular ejection fraction
- MACE
Major adverse cardiovascular events
- MI
myocardial infarction
- NACE
Net adverse cardiovascular events
- PAD
peripheral artery disease
- PCI
percutaneous coronary intervention
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- UFH
unfractionated heparin
1. INTRODUCTION
Aortic stenosis (AS) is the most prevalent heart valve disease and its incidence is expected to further increase with the growing life expectancy in the western world.1, 2 While surgical aortic valve replacement (SAVR) was the only treatment option available for decades, transcatheter aortic valve replacement (TAVR) is a rapidly emerging treatment option in patients with severe AS at high,3 intermediate4 or low risk.5, 6 The insertion of large‐size arterial sheaths during TAVR carries the risk of severe procedure‐related vascular complications and identifying patients at risk or mitigating strategies remains an important area of research.7
This can be especially relevant in patients with peripheral arterial disease (PAD), who form an important subset of the AS population at significant operative risk including risk of vascular injury and bleeding.8, 9, 10 The use of unfractionated heparin (UFH) has been the standard anticoagulant,11 having the potential benefit of being reversible with protamine in case of severe bleeding or major vascular injury. Bivalirudin, a nonreversible, short acting, direct thrombin inhibitor, has been demonstrated to provide adequate anticoagulation while reducing access and nonaccess hemorrhagic complications when compared to UFH in patients undergoing percutaneous coronary interventions (PCI).12, 13 The BRAVO‐3 (Effect of Bivalirudin on Aortic Valve Intervention Outcomes‐3) trial14 was able to demonstrate noninferiority of bivalirudin compared with UFH in TAVR procedures. The aim of the present study was to investigate the impact of the presence of PAD on clinical outcomes in the BRAVO‐3 trial.
2. METHODS
As previously detailed, the BRAVO‐3 trial was a multicenter randomized controlled trial comparing bivalirudin with UFH in high‐risk patients undergoing TAVR, across 31 sites in Europe and North America.14 The study was approved by the ethics committee at each site. All clinical endpoint events were adjudicated by an independent, centralized clinical events committee. The current study was a prespecified subgroup analysis according to the presence of PAD.
2.1. Patient population
Patients with severe aortic stenosis who were ≥ 18 years of age, at high surgical risk defined as a European System for Cardiac Operative Risk Evaluation score (EuroSCORE) of ≥18, or deemed inoperable by the heart team, and scheduled for TAVR via transfemoral approach were eligible for enrollment. The key exclusions were planned surgical cut down for access; iliofemoral artery minimal luminal diameter < 6.5 mm; presence of a previous mechanical or mitral bioprosthetic valve; left ventricular ejection fraction <15%; severe aortic or mitral regurgitation; concomitant percutaneous coronary intervention; recent bleeding or neurological event; and dialysis dependence.
2.2. Study medications
Bivalirudin was administered as an initial bolus of 0.75 mg/kg, followed by a continuous infusion at a rate of 1.75 mg kg−1 hr−1 in patients with an estimated glomerular filtration rate (eGFR) ≥60 ml/min that was stopped after successful valve implantation. Patients with eGFR < 60 ml/min had an adjusted lower infusion rate. Heparin administration was recommended to be titrated to a target activated clotting time of >250 s; the decision for reversal with protamine and its dosage in the end of the procedure were at the treating team's discretion. Patients underwent TAVR according to the standard practices at each site, including the use of preprocedural medications and the selection of a commercially available valve system. After TAVR, all patients were recommended to receive oral therapy with low dose aspirin 75–100 mg/day and clopidogrel 75 mg/day for a period defined by local practice.
2.3. Study endpoints and definitions
The BRAVO‐3 trial endpoints have been previously described in detail. For the present analysis we assessed the occurrence of major bleeding defined as BARC ≥3b, the composite of major adverse cardiovascular events (MACE: all‐cause mortality, myocardial infarction [MI], or stroke) and net adverse events (NACE: either MACE or major bleeding).
All the endpoints were assessed at 48 hr or hospital discharge, whichever occurred first, and at 30 days.
2.4. Statistical analysis
Patients were grouped by the presence or absence of PAD. Categorical variables were reported as frequencies and percentages and tested using the chi‐square test. Continuous variables were reported as mean ± SD and tested using Student's t test. Tabulated event rates were tested using the chi‐square test. Using Cox‐regression proportional hazards model, adjusted hazard ratios for outcomes in patients with versus without PAD were generated accounting for the following variables: age, sex, weight, diabetes mellitus, coronary artery disease (CAD), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), left ventricular ejection fraction (LVEF), Sheath size of valve system, and country. Comparisons were performed for bivalirudin versus UFH among men and women, with interaction testing for the effect of treatment and sex. p‐values < .05 were considered statistically significant. All analyses were performed using Stata version 15.0 (StatCorp, College Station, Texas).
3. RESULTS
The patient population included 15% (n = 119) patients with PAD and 85% (n = 682) without PAD (Figure 1). Table 1 shows the baseline characteristics between PAD and no‐PAD groups. Patients with PAD had higher prevalence of diabetes mellitus (42 vs. 27%, p = .001) and coronary arterial disease (CAD) (64 vs. 48%, p = .001) but similar EuroSCORE I (17.3 vs. 17.0%, p = .72) compared with patients without PAD.
Figure 1.
Study flow
Table 1.
Baseline patient characteristics
| No PAD 682 (85%) | PAD 119 (15%) | p‐value | |
|---|---|---|---|
| Age, (years) mean ± SD | 82.4 ± 6.2 | 81.9 ± 6.1 | .45 |
| Women | 338 (49.6%) | 53(44.5%) | .31 |
| Weight | 73.9 ± 16.5 | 75.0 ± 17.5 | .51 |
| Logistic EuroSCORE (%) mean ± SD | 17.0 ± 10.1 | 17.3 ± 10.9 | .72 |
| CKD | |||
| No CKD | 305 (44.7%) | 59 (49.6%) | |
| GFR 30–59 ml/min | 343 (50.3%) | 54 (45.4%) | .60 |
| GFR <30 ml/min | 34 (5.0%) | 6 (5.0%) | |
| Diabetes | 188 (27.6%) | 50 (42.0%) | .001 |
| Prior CVA/TIA | 67 (9.9%) | 16 (13.5%) | .24 |
| COPD | 127 (18.6%) | 28 (23.5%) | .82 |
| CAD | 328 (48.1%) | 76 (64.4%) | .001 |
| Prior MI | 97 (14.4%) | 19 (16.1%) | .62 |
| Prior AF | 399 (58.5%) | 71 (59.7%) | .81 |
| Prior VT | 17 (2.6%) | 3 (2.7%) | .95 |
| Previous CABG | 99 (14.5%) | 17 (14.3%) | .95 |
| Previous BAV | 52 (7.6%) | 8 (6.7%) | .73 |
| LVEF | |||
| ≤35% | 89 (13.1%) | 15 (12.6%) | |
| 35–49% | 110 (16.2%) | 13 (10.9%) | .31 |
| ≥50% | 480 (70.7%) | 91 (76.5%) |
Abbreviations: AF, atrial fibrillation; BAV, balloon aortic valvuloplasty; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM,diabetes mellitus; EuroSCORE, European System for Cardiac Operative Risk Evaluation score; LVEF, left ventricular ejection fraction; MI, myocardial infarction; N/A, not available; NDM, no diabetes mellitus; PAD, peripheral arterial disease; SD, standard deviation; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack; VT, ventricular tachycardia.
During the procedure, PAD patients were less likely to be treated with an additional TAVR device (0 vs. 4%, p = .025) (Table 2); but there were no other significant differences between the groups. Among PAD and non‐PAD patients, there were no significant baseline differences between bivalirudin and UFH treated patients (Table 3).
Table 2.
Procedural characteristics
| No PAD 682 (85%) | PAD 119 (15%) | p‐value | |
|---|---|---|---|
| Procedural success | 664 (97.4%) | 116 (97.5%) | .94 |
| Balloon‐expanding valve | 427 (64.1%) | 73 (63.5%) | .90 |
| Duration of procedure (min) median (IQR) | 24 (17–34) | 26 (18–36) | .33a |
| Sheath size of valve system | |||
| <18 | 215 (32.3%) | 40 (34.5%) | .86 |
| 18 | 363 (54.5%) | 60 (51.7%) | |
| >18 | 88 (13.2%) | 16 (13.79) | |
| Valve type | |||
| Balloon expandable | 472 (62.6%) | 73 (61.3%) | .65 |
| Self‐expanding | 198 (29.0%) | 33 (27.7%) | |
| Other valve | 57 (8.4%) | 13 (10.9%) | |
| Valve size | |||
| ES 23 mm | 125 (18.7%) | 25 (21.7%) | .38 |
| ES 26 mm | 178 (26.7%) | 22 (19.1%) | |
| ES 29 mm | 79 (11.8%) | 16 (13.9%) | |
| MCV 26 mm | 61 (9.1%) | 8 (7.0%) | |
| MCV 29 mm | 76 (11.4%) | 12 (10.4%) | |
| MCV 31 mm | 41 (6.1%) | 12 (10.4%) | |
| Other | 108 (16.2%) | 20 (17.4%) | |
| Valvuloplasty performed | 554 (80.2%) | 93 (79.5%) | .85 |
| Additional TAVR device used | 28 (4.1%) | 0 (0.0%) | .025 |
| Embolic protection device used | 7 (1.0%) | 3 (2.6%) | .17 |
| Successful deployment of access site closure | 620 (92.1%) | 105 (91.3%) | .43 |
| Post‐dilation | 170 (25.1%) | 29 (24.8%) | .95 |
| Temporary pacemaker | 649 (97.5%) | 108 (93.9%) | .043 |
| Prior loading with clopidogrel | 254 (37.4%) | 39 (33.01%) | .71 |
| Post‐procedure anti‐thrombotic therapy | |||
|---|---|---|---|
| No PAD 682 (85%) | PAD 119 (15%) | p‐value | |
| Aspirin | 587 (86.5%) | 104 (88.1%) | .62 |
| P2Y12 inhibitor | 182 (26.8%) | 21 (17.8%) | .038 |
| ASA and P2Y12 inhibitor | 438 (64.4%) | 87 (73.1%) | .064 |
| Oral anticoagulant | |||
| Coumadin | 121 (17.7%) | 20 (16.8%) | .81 |
| Dabigatran | 9 (1.3%) | 2 (1.7%) | .76 |
| Rivaroxaban | 20 (2.9%) | 4 (3.4%) | .80 |
Abbreviations: DM, diabetes mellitus; NDM, no diabetes mellitus; SD, standard deviation; TAVR, transcatheter aortic valve replacement.
Median test.
Table 3.
Baseline characteristics in patients with and without PAD by anticoagulant type
| No PAD 682 (85%) | PAD 119 (15%) | |||
|---|---|---|---|---|
| Bivalirudin 344 (50.4%) | UFH 338 (49.6%) | Bivalirudin 60 (50.4%) | UFH 59 (49.6%) | |
| Age, (years) mean ± SD | 82.4 ± 6.6 | 82.4 ± 6.6 | 81.6 ± 6.2 | 82.2 ± 6.0 |
| Female | 170 (49.4) | 168 (49.7) | 25 (41.6) | 28 (47.5) |
| Weight | 74.2 ± 17.2 | 73.7 ± 15.7 | 76.2 ± 17.1 | 73.8 ± 17.9 |
| Logistic EuroSCORE (%) mean ± SD | 16.4 ± 10.3 | 16.2 ± 9.7 | 21.4 ± 12.0 | 21.1 ± 10.5 |
| CKD | ||||
| No CKD | 153 (44.5) | 152 (45.0) | 28 (46.7) | 31 (52.5) |
| GFR 30–59 ml/min | 176 (51.2) | 167 (49.4) | 29 (48.3) | 25 (42.4) |
| GFR <30 ml/min | 15 (4.4) | 19 (5.6) | 3 (5.0) | 3 (5.1) |
| Diabetes | 99 (28.8) | 89 (26.3) | 26 (43.3) | 24 (40.7) |
| Prior CVA/TIA | 38 (11.1) | 29 (8.6) | 7 (11.7) | 9 (15.3) |
| COPD | 53 (15.4) | 74 (21.9) | 15 (25.0) | 13 (22.0) |
| CAD | 174 (50.6) | 154 (45.6) | 35 (58.3) | 41 (70.7) |
| Prior MI | 50 (14.7) | 47 (14.1) | 13 (22.0) | 6 (10.2) |
| Prior AF | 149 (43.3) | 134 (39.6) | 28 (46.7) | 20 (33.9) |
| Prior VT | 10 (3.0) | 7 (2.2) | 1 (1.8) | 2 (3.6) |
| Previous CABG | 50 (14.5) | 49 (14.5) | 11 (18.3) | 6 (10.2) |
| Previous BAV | 26 (7.6) | 26 (7.7) | 3 (5.0) | 5 (8.5) |
| LVEF | ||||
| ≤35% | 39 (11.4) | 50 (14.9) | 8 (13.3) | 7 (11.9) |
| 35–49% | 60 (17.5) | 50 (14.9) | 8 (13.3) | 5 (8.5) |
| ≥50% | 244 (71.1) | 236 (70.2) | 44 (73.3) | 47 (80.0) |
The in‐hospital and 30‐day clinical outcomes are presented in Table 4, and Figures 2 and 3. No significant differences were observed in the in‐hospital or 30‐day occurrences of death, NACE, MACE, CVA or bleeding events between the groups.
Table 4.
In‐hospital and 30‐day clinical outcomes
| OUTCOMES | No PAD 682 (85%) | PAD 119(15%) | Odds ratio (95% CI) | p‐value | Adjusteda odds ratio (95% CI) | p‐value |
|---|---|---|---|---|---|---|
| Death | ||||||
| In‐hospital | 3 (2.5) | 1.58 (0.43–5.7) | .49 | 1.79 (0.44–7.3) | .42 | |
| 30‐days | 5 (4.2) | 0.86 (0.33–2.26) | .76 | 1.08 (0.39–3.02) | .89 | |
| NACE | ||||||
| In‐hospital | 70 (10.3) | 15 (12.6) | 1.26 (0.70–2.29) | .45 | 1.39 (0.75–2.59) | .30 |
| 30‐days | 102 (15.0) | 18 (15.1) | 1.01 (0.59–1.75) | .96 | 1.16 (0.66–2.04) | .61 |
| CVA | ||||||
| In‐hospital | 13 (1.9) | 2 (1.7) | 0.88 (0.20–3.95) | .87 | 1.38 (0.27–6.89) | .70 |
| 30‐days | 20 (2.9) | 4 (3.4) | 1.15 (0.39–3.43) | .80 | 1.48 (0.46–4.75) | .50 |
| AKI | ||||||
| In‐hospital | 50 (7.3) | 16 (13.5) | 1.96 (1.08–3.58) | .028 | 1.60 (0.84–3.04) | .149 |
| 30‐days | 110 (16.1) | 22 (18.5) | 1.18 (0.71–1.96) | .52 | 1.04 (0.61–1.76) | .90 |
| VASC COMP | ||||||
| In‐hospital | 114 (16.7) | 30 (25.2) | 1.68 (1.06–2.66) | .027 | 1.80 (1.10–2.93) | .018 |
| 30‐days | 118 (17.3) | 35 (29.4) | 1.99 (1.28–3.10) | .002 | 2.15 (1.34–3.44) | .001 |
| MAJOR VASC | ||||||
| In‐hospital | 57 (8.4) | 14 (11.8) | 1.46 (0.79–2.72) | .23 | 1.80 (0.83–3.48) | .080 |
| 30‐days | 60 (8.8) | 15 (12.6) | 1.50 (0.82–2.73) | .19 | 1.87 (0.98–3.55) | .056 |
| MINOR VASC | ||||||
| In‐hospital | 57 (8.4) | 16 (13.5) | 1.70 (0.94–3.08) | .078 | 1.54 (0.83–2.88) | .1.73 |
| 30‐days | 60 (8.8) | 20 (16.8) | 2.09 (1.21–3.63) | .008 | 1.89 (1.06–3.37) | .030 |
| BARC | ||||||
| In‐hospital | 51 (7.5) | 12 (10.1) | 1.39 (0.72–2.69) | .33 | 1.48 (0.74–2.97) | .27 |
| 30‐days | 63 (9.2) | 13 (10.9) | 1.20 (0.64–2.27) | .56 | 1.37 (0.70–2.64) | .36 |
Abbreviations: AKI, acute kidney injury; BARC, bleeding academic research consortium criteria; C‐death, cardiovascular death; CVA, cerebrovascular accident; DM, diabetes mellitus; LIFE BLEED, life threatening bleeding (VARC‐2 criteria); MAJOR VASC, major vascular complications (VARC ‐2 criteria); MI, myocardial infarction; MINOR VASC, minor vascular complications (VARC‐2 criteria); NACE, net adverse cardiovascular events; NDM, no diabetes mellitus; VASC COMP, all vascular complications (VARC‐2 criteria).
Adjusted for: age, sex, weight, diabetes mellitus, coronary artery disease (CAD), CKD, COPD, LVEF, Sheath size of valve system, and country.
Figure 2.
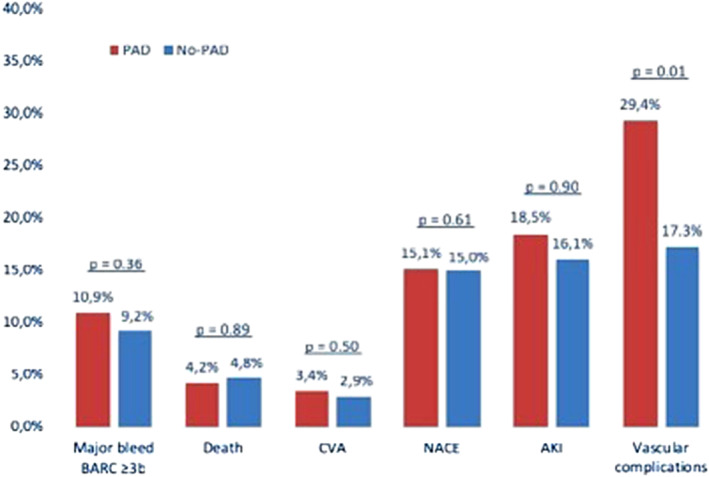
Incidence of 30‐day clinical outcomes and adjusted risk associated with PAD compared with no‐PAD
Figure 3.
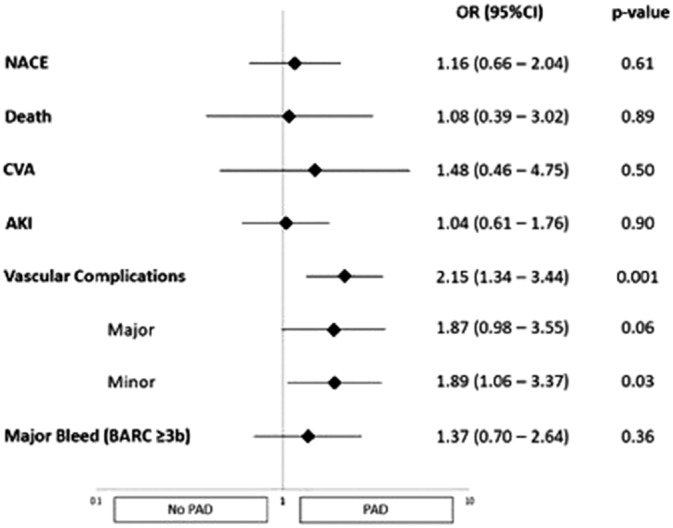
Adjusted risk associated with PAD compared with no‐PAD
Vascular complications occurred significantly more frequently in patients with PAD both in‐hospital (25.2 vs. 16.7%; OR 1.68, 95% CI 1.06–2.66, p = .027) and at 30 days (29.4 vs. 17.3%; OR 1.99, 95% CI 1.28–3.10, p = .002). This was also sustained after adjustment, both in‐hospital (OR 1.80, 95% CI 1.10–2.93, p = .018) and at 30 days (OR 1.80, 95% CI 1.10–2.93, p = .018). While no significant differences were observed in the occurrence of major vascular complications, the 30‐day rate of minor vascular complications was higher in patients with PAD (16.8 vs. 8.8%; OR 2.09, 95% CI 1.21–3.63, p = .008), which also held true after adjustment (OR 1.89, 95% CI 1.06–3.37, p = .030). A higher rate of acute kidney injury (AKI) was observed in patients with PAD in the unadjusted analysis (13.5 vs. 7.3%; OR 1.96, 95% CI 1.08–3.58, p = .028) but was attenuated after adjustment (OR 1.60, 95% CI 0.84–3.04, p = .149).
When outcomes were examined for the effect of anticoagulant randomization (Table 5), vascular complications occurred less frequently in patients with PAD treated with UFH compared to bivalirudin (20.3 vs. 38.3%, p = .031). However, this effect did not reach significance after testing for interaction with patients without PAD (p‐interaction = .18). The risk of minor vascular complications was significantly attenuated in PAD patients treated with UFH both in‐hospital (3.4 vs. 23.3%, p = .001) and at 30 days (5.1 vs. 28.3%, p = .001). No such differences between UFH and bivalirudin were observed in patients without PAD; hence, significant interactions were noted (p‐interaction = .035 and .027, respectively).
Table 5.
30‐day clinical outcomes in patients with and without PAD by anticoagulant type
| OUTCOMES | No PAD 682 (85%) | PAD 119(15%) | p‐value for interaction | ||||
|---|---|---|---|---|---|---|---|
| Bivalirudin 344 (50.4%) | UFH 338 (49.6%) | p‐value | Bivalirudin 60 (50.4%) | UFH 59 (49.6%) | p‐value | ||
| Death | 14 (4.1%) | 19 (5.6%) | .35 | 4 (6.7%) | 1 (1.7%) | .18 | .14 |
| NACE | 47 (13.7%) | 55 (16.3%) | .34 | 8 (13.3%) | 10 (17.0%) | .58 | .89 |
| CVA | 11 (3.2%) | 9 (2.7%) | .68 | 2 (3.3%) | 2 (3.4%) | .99 | .85 |
| AKI | 61 (17.7%) | 49 (14.5%) | .25 | 15 (25.0%) | 7 (11.9%) | .07 | .22 |
| VASC COMP | 66 (19.2%) | 52 (15.4%) | .19 | 23 (38.3%) | 12 (20.3%) | .031 | .181 |
| MAJOR VASC | 31 (9.0%) | 29 (8.6%) | .84 | 6 (10.0%) | 9 (15.3%) | .39 | .39 |
| MINOR VASC | 36 (10.5%) | 24 (7.1%) | .12 | 17 (28.3%) | 3 (5.1%) | .001 | .027 |
| BARC | 30 (8.7%) | 33 (9.8%) | .64 | 4 (6.7%) | 9 (15.3%) | .133 | .24 |
There were no differences in mortality, NACE, MACE, or CVA with bivalirudin versus UFH among patients with or without PAD. With respect to bleeding, there were numerically more major in‐hospital bleeding events (BARC ≥3b) in PAD patients on UFH versus bivalirudin (5.7 vs. 1.9%), but this was not statistically significant (p = .063).
At discharge post TAVR, P2Y12 inhibitors were prescribed less often in PAD patients (18 vs. 27%, p = .038) compared with no‐PAD patients (Table 6). DAPT was prescribed in 73 vs. 64% (p = .064) of PAD versus no‐PAD patients. No significant differences were observed in the use of oral anticoagulants.
Table 6.
Procedural characteristics in patients with and without PAD by anticoagulant type
| No PAD 682 (85%) | PAD 119 (15%) | |||
|---|---|---|---|---|
| Bivalirudin 344 (50.4%) | UFH 338 (49.6%) | Bivalirudin 60 (50.4%) | UFH 59 (49.6%) | |
| Anesthesia type | ||||
| General | 136 (40.1) | 134 (40.1) | 17 (29.3) | 22 (37.9) |
| Local | 189 (55.8) | 183 (54.8) | 39 (67.2) | 33 (56.9) |
| Other | 14 (4.1) | 17 (5.1) | 2 (3.5) | 3 (5.2) |
| Procedural success | 309 (91.2) | 311 (93.1) | 50 (87.7) | 55 (94.8) |
| Balloon‐Expanding valve | 212 (61.6) | 215 (63.6) | 39 (65.0) | 34 (57.6) |
| Duration of procedure (min) median (IQR) | 24 (16–35) | 25 (17–34) | 28 (17–38) | 25 (18–35) |
| Sheath size of valve system | ||||
| <18 | 107 (31.9) | 108 (32.6) | 21 (36.2) | 19 (23.8) |
| 18 | 187 (55.8) | 176 (53.2) | 29 (50.0) | 31 (53.5) |
| >18 | 41 (12.2) | 47 (14.2) | 8 (13.8) | 8 (13.8) |
| Valve type | ||||
| Balloon expandable | 212 (61.3) | 215 (63.6) | 39 (65.0) | 34 (57.6) |
| Self‐expanding | 99 (28.8) | 99 (29.3) | 16 (26.7) | 17 (28.8) |
| Other valve | 33 (5.6) | 24 (7.1) | 5 (8.3) | 8 (13.6) |
| Valve size | ||||
| ES 23 mm | 65 (19.3) | 60 (18.1) | 15 (26.3) | 10 (17.2) |
| ES 26 mm | 91 (27.0) | 87 (26.3) | 12 (21.1) | 10 (17.2) |
| ES 29 mm | 35 (10.4) | 44 (13.3) | 6 (10.5) | 10 (17.2) |
| MCV 26 mm | 30 (8.9) | 31 (9.4) | 3 (5.3) | 5 (8.6) |
| MCV 29 mm | 39 (11.6) | 37 (11.2) | 6 (10.5) | 6 (10.3) |
| MCV 31 mm | 23 (6.8) | 18 (5.4) | 6 (10.5) | 6 (10.3) |
| Other | 54 (16.0) | 54 (16.3) | 9 (15.8) | 11 (19.0) |
| Valvuloplasty performed | 275 (80.4) | 269 (80.1) | 50 (84.8) | 43 (74.1) |
| Additional TAVR device used | 16 (4.8) | 12 (3.6) | 0 (0.0) | 0 (0.0) |
| Embolic protection device used | 5 (1.5) | 2 (0.6) | 2 (3.5) | 1 (1.7) |
| Successful deployment of access site closure | 309 (91.2) | 311 (93.1) | 50 (87.7) | 55 (94.8) |
| Post‐dilation | 97 (28.4) | 73 (21.7) | 15 (24.4) | 14 (24.1) |
| Temporary pacemaker | 323 (96.7) | 326 (98.2) | 54 (93.1) | 54 (94.7) |
| Prior loading with clopidogrel | 131 (38.2) | 123 (36.5) | 21 (35.6) | 18 (30.5) |
| Post‐procedure anti‐thrombotic therapy in patients with and without PAD by anticoagulant type | ||||
|---|---|---|---|---|
| No PAD 682 (85%) | PAD 119 (15%) | |||
| Bivalirudin 344 (50.4%) | UFH 338 (49.6%) | Bivalirudin 60 (50.4%) | UFH 59 (49.6%) | |
| Aspirin | 293 (85.7) | 294 (12.8) | 50 (84.8) | 54 (91.5) |
| P2Y12 inhibitor | 248 (72.5) | 249 (73.9) | 51 (86.4) | 46 (78.0) |
| ASA and P2Y12 inhibitor | 219 (63.7) | 220 (65.1) | 45 (75.0) | 42 (71.2) |
| Oral anticoagulant | ||||
| Coumadin | 62 (18.0) | 59 (17.5) | 12 (20.0) | 8 (13.6) |
| Dabigatran | 2 (0.6) | 7 (2.1) | 2 (3.3) | 0 (0.0) |
| Rivaroxaban | 8 (2.3) | 12 (3.6) | 3 (5.0) | 1 (1.7) |
4. DISCUSSION
In patients undergoing transfemoral TAVR in the randomized BRAVO‐3 trial of UFH versus bivalirudin, we observed the following:
The rates of mortality, NACE, MACE, CVA, or bleeding events did not differ significantly between patients with and without PAD: While in the general population, PAD has been observed to be associated with increased cardiovascular mortality and stroke risk,15, 16 the mortality rates of patients with and without PAD did not differ significantly in the present analysis, thus contrasting recently reported data from the Society of Thoracic Surgeons/Transcatheter Valve Therapy Registry, reporting excess mortality in patients with PAD undergoing transfemoral TAVR.17 While these findings might be explained in part by the limited sample size and the possible inclusion of patients with lesser‐degree PAD, they might also be reflective of steady advances in TAVR technology as well as the learning curves due to increased procedure volumes of operating physicians. Possibly due to the same reasons, counterintuitively, stroke rates did not differ between the two groups in the present analysis. Since PAD and AS share common comorbidities such as diabetes mellitus, hyperlipidemia, smoking, and chronic renal insufficiency,18, 19 they frequently exist concomitantly. While previous studies have reported a substantial prevalence of PAD in patients undergoing TAVR, ranging from 27% in the PARTNER B trial20 to 41% in the CoreValve US study,21 this rate was lower in the BRAVO‐3 trial (15%). This might be explained by the fact that only patients undergoing transfemoral TAVR were included and patients in whom a surgical cutdown was planned were excluded from the trial. In the recent low‐risk TAVR trials, the rate of PAD was even lower with 8 and 7.1% in patients undergoing TAVR with self‐expanding and balloon‐expandable valves, respectively.5, 6
PAD patients had higher vascular complications and borderline high AKI compared to patients without PAD: As might be expected, the rate of vascular injury in patients with diseased vessels was higher than in patients with unaffected peripheral vasculature. Accordingly, smaller luminal diameters, extensive calcification, and tortuosity of the iliofemoral arteries have been found to be associated with a higher incidence of arterial injury the setting of a transfemoral approach.22, 23 Postprocedural AKI can have a significant impact on survival, even when only subtle decreases in GFR are present.24, 25 The association between PAD and AKI might be explained by the possible athero‐embolization of cholesterol debris to the renal vasculature during cannulation26 as well as the fact that preexisting chronic kidney disease is a major risk factor for the developement of PAD,27 which in turn might beget worsening renal failure through ischemia, in particular when the renal vasculature is involved.28
In PAD patients undergoing TAVR, the use of bivalirudin led to a higher rate of (minor) vascular complications: While thrombin inhibition was assumed to more effectively suppress the highly thrombogenic processes initiated by the exposure of tissue factor to the circulation during TAVR and thus explain the absence of early myocardial infarctions in patients treated with bivalirudin in the BRAVO‐3 trial, vascular complications, including peripheral embolization, occurred more frequently in the present subanalysis of patients with PAD treated with bivalirudin compared to UFH. At the same time, numerically lower major bleeding rates (≥3b BARC) were noted with bivalirudin, albeit not statistically significant, possibly owing to the limited sample size and power to detect a difference in our subgroup of interest, particularly given the lower risk for bleeding events with smaller caliber contemporary devices as well as high rate of successful closure device deployment. The present findings emphasize the dilemma of counterbalancing anticoagulation benefits in the prevention of ischemic events with bleeding risks in patients undergoing TAVR. In particular, since the dosage experience of coronary interventions was largely utilized in the BRAVO‐3 trial, TAVR‐specific dose regimens might be warranted in order to achieve optimal anticoagulation with minimal bleeding risk. Furthermore, the UFH activity reversal with protamine was not tracked or examined in any in the present study; a quite variable practice of complete, partial or nonreversal was followed by the different operators.
5. STUDY LIMITATIONS
This is a retrospective sub‐analysis of the BRAVO‐3 trial and therefore has intrinsic limitations of nonrandomized comparisons such as allocation bias, different distribution of clinical risk factors and the possibility of confounding variables. Secondly, BRAVO‐3 was a trial comparing anticoagulation therapy in TAVR procedures and as such, was not powered to evaluate outcomes according to the presence of PAD. The relatively small sample size might as well be viewed as a limitation, however, to the best of our knowledge, BRAVO‐3 is the only, and thus the largest trial to address this particular problem. Results are not generalizable to other TAVR studies that utilized both transfemoral and nontransfemoral approaches. The use of protamine was not recorded and may have had an impact on bleeding outcomes in heparin treated patients. Data on frailty status at baseline and change in New York Heart Association class at 30 days were not available to analyze for differences by the presence of PAD and potential associations with outcomes.
6. CONCLUSION
In this analysis of the BRAVO‐3 trial, patients with PAD undergoing contemporary transfemoral TAVR did not exhibit an increased risk of any major adverse events, according to the procedural anticoagulant randomization. However, patients treated with Bivalirudin had significantly higher rates of minor vascular complications.
DISCLOSURES
Dr. Hengstenberg has received proctoring fees from Boston Scientific and Edwards.
Dr. Dangas has received consulting fees from GE HealthCare, Janssen Pharmaceuticals, Inc. and Medtronic, Inc.; has <1% equity with Claret Medical and Elixir Medical; has delivered industry‐sponsored lectures for The Medicines Company; and is on the scientific advisory board for AstraZeneca.
Dr. Mehran has received institutional research funding from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol‐Myers Squibb/Sanofi, CSL Behring, Eli Lilly/Daiichi Sankyo, Medtronic, Novartis and OrbusNeich; is a consultant to Boston Scientific, Abbott Vascular, Medscape, Siemens Medical Solutions, Regeneron Pharmaceuticals Inc. (no fees), Roivant Sciences Inc, and Sanofi; is an institution consultant (payment to institution) with Abbott Vascular and Spectranetics/Phillips/Volcano Corporation; is on executive committee for Janssen Pharmaceuticals and BMS; receives institutional (payment to institution) advisory board funding from Bristol Myers Squibb and Novartis; has received DSMB membership funding (payment to institution) from Watermark Research; and, has <1% equity with Claret Medical and Elixir Medical.
Dr. Anthopoulos is a former employee of The Medicines Company, currently Head of Medical, Europe for Arena Pharmaceuticals.
Dr. ten Berg has received advisory/consulting/speakers fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, Accumetrics, Boehringer Ingelheim, BMS, Pfizer, Bayer, Ferrer.
Zilberszac R, Chandiramani R, Hengstenberg C, et al. Clinical outcomes after TAVR with heparin or bivalirudin as periprocedural anticoagulation in patients with and without peripheral arterial disease: Results from the BRAVO‐3 randomized trial. Catheter Cardiovasc Interv. 2020;96:E377–E386. 10.1002/ccd.28642
REFERENCES
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368(9540):1005‐1011. 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2. Berry C, Lloyd SM, Wang Y, Macdonald A, Ford I. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Eur Heart J. 2013;34(21):1538‐1547. 10.1093/eurheartj/ehs339. [DOI] [PubMed] [Google Scholar]
- 3. Mack MJ, Leon MB, Smith CR, et al. 5‐year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477‐2484. 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 4. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374(17):1609‐1620. 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380(18):1695‐1705. 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 6. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706‐1715. 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 7. Généreux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64(24):2605‐2615. 10.1016/j.jacc.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 8. Martinez‐Selles M, Barrio JM, Hortal J, Ruiz M, Bueno H. Prevalence of peripheral arterial disease and prior stroke in octogenarians with symptomatic severe aortic stenosis or severe coronary artery disease: influence in management and outcome. Int Angiol. 2007;26(1):33‐37. http://www.ncbi.nlm.nih.gov/pubmed/17353886 Accessed January 9, 2019. [PubMed] [Google Scholar]
- 9. Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734‐735. 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 10. O'Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88(1):S23‐S42. 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 11. Holmes DR, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200‐1254. 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12. Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218‐2230. 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 13. Kastrati A, Neumann F‐J, Schulz S, et al. Abciximab and heparin versus bivalirudin for non‐ST‐elevation myocardial infarction. N Engl J Med. 2011;365(21):1980‐1989. 10.1056/NEJMoa1109596. [DOI] [PubMed] [Google Scholar]
- 14. Dangas GD, Lefèvre T, Kupatt C, et al. Bivalirudin versus heparin anticoagulation in transcatheter aortic valve replacement the randomized BRAVO‐3 trial. J Am Coll Cardiol. 2015;66:2860‐2868. 10.1016/j.jacc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 15. Ankle Brachial Index Collaboration , FGR F, Murray GD, et al. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality. Jama. 2008;300(2):197‐208. 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerjee A, Fowkes FG, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke. 2010;41(9):2102‐2107. 10.1161/STROKEAHA.110.582627. [DOI] [PubMed] [Google Scholar]
- 17. Fanaroff AC, Manandhar P, Holmes DR, et al. Peripheral artery disease and transcatheter aortic valve replacement outcomes. Circ Cardiovasc Interv. 2017;10(10):e005456. 10.1161/CIRCINTERVENTIONS.117.005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99(6):396‐400. 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 19. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29‐e322. 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 20. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597‐1607. 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 21. Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;371(10):967‐968. 10.1056/NEJMc1408396. [DOI] [PubMed] [Google Scholar]
- 22. Murray D, Ghosh J, Khwaja N, Murphy MO, Baguneid MS, Walker MG. Access for endovascular aneurysm repair. J Endovasc Ther. 2006;13(6):754‐761. 10.1583/06-1835.1. [DOI] [PubMed] [Google Scholar]
- 23. Tops LF, Kapadia SR, Tuzcu EM, et al. Percutaneous valve procedures: an update. Curr Probl Cardiol. 2008;33(8):417‐457. 10.1016/J.CPCARDIOL.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24. Palant CE, Amdur RL, Chawla LS. Long‐term consequences of acute kidney injury in the perioperative setting. Curr Opin Anaesthesiol. 2016;30:10.1097/ACO.0000000000000428. 10.1097/ACO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 25. Ram P, Mezue K, Pressman G, Rangaswami J. Acute kidney injury post‐transcatheter aortic valve replacement. Clin Cardiol. 2017;40(12):1357‐1362. 10.1002/clc.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thongprayoon C, Cheungpasitporn W, Srivali N, et al. AKI after transcatheter or surgical aortic valve replacement. J Am Soc Nephrol. 2016;27(6):1854‐1860. 10.1681/ASN.2015050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(2):629‐636. 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 28. Chonchol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol. 2006;1(2):172‐181. 10.2215/CJN.00940905. [DOI] [PubMed] [Google Scholar]


