Abstract
This position statement describes the recommendations of the Clinical Oncology Society of Australia (COSA) regarding management of cancer‐related malnutrition and sarcopenia. A multidisciplinary working group completed a review of the literature, focused on evidence‐based guidelines, systematic reviews and meta‐analyses, to develop recommendations for the position statement. National consultation of the position statement content was undertaken through COSA members. All people with cancer should be screened for malnutrition and sarcopenia in all health settings at diagnosis and as the clinical situation changes throughout treatment and recovery. People identified as “at risk” of malnutrition or with a high‐risk cancer diagnosis or treatment plan should have a comprehensive nutrition assessment; people identified as “at risk” of sarcopenia should have a comprehensive evaluation of muscle status using a combination of assessments for muscle mass, muscle strength and function. All people with cancer‐related malnutrition and sarcopenia should have access to the core components of treatment, including medical nutrition therapy, targeted exercise prescription and physical and psychological symptom management. Treatment for cancer‐related malnutrition and sarcopenia should be individualised, in collaboration with the multidisciplinary team (MDT), and tailored to meet needs at each stage of cancer treatment. Health services should ensure a broad range of health care professionals across the MDT have the skills and confidence to recognise malnutrition and sarcopenia to facilitate timely referrals and treatment. The position statement is expected to provide guidance at a national level to improve the multidisciplinary management of cancer‐related malnutrition and sarcopenia.
Keywords: cancer, exercise, malnutrition, nutrition, sarcopenia
1. BACKGROUND
Malnutrition is characterised by loss of weight, loss of muscle and loss of subcutaneous body fat. 1 Cancer‐related malnutrition can occur due to the presence of the cancer itself, the effect of cancer treatment on the consumption or absorption of nutrients, or patients undertaking “restrictive” cancer diets. 2 Research over several decades has shown cancer‐related malnutrition is associated with serious adverse consequences, including reduced survival and ability to complete treatment, poorer quality of life and higher costs to the health care system. 3 , 4 , 5 , 6 , 7 It is a common condition, occurring in 30% to 40% of people with cancer, yet it is under‐recognised and under‐treated. 7 Australian studies have found that almost 50% of people with cancer‐related malnutrition are not under the care of a dietitian. 8 Malnutrition can occur in people with any cancer diagnosis, although certain cancers, including head and neck, gastrointestinal and lung cancers, have up to a 4‐fold increased risk of malnutrition compared to breast cancer. 9 Malnutrition can occur in people of any body mass index (BMI) category, including those classified as overweight or obese. 10 , 11 However, with the rising incidence of obesity in Australia the identification of malnutrition and underlying muscle loss is becoming more complex and concurrently under‐diagnosis is likely to rise.
Sarcopenia is characterised by loss of skeletal muscle mass and strength with an impact on physical performance, and is a key component of cancer‐related malnutrition. 12 , 13 This is reflected in recent international definitions of malnutrition where the assessment of muscle mass is a criterion for the diagnosis of malnutrition. 14 , 15 Sarcopenia associated with ageing is known as primary sarcopenia, as opposed to secondary sarcopenia which is associated with disease (including cancer), sedentary lifestyle or inadequate nutrition. 12 Research in cancer‐related sarcopenia has largely focused on the presence of low muscle mass rather than the presence of both low muscle mass and low muscle function (strength or performance). 16 , 17 , 18 Similar to malnutrition, low muscle mass can occur across all BMI categories. 16 Cancer‐related sarcopenia is associated with similar adverse consequences to cancer‐related malnutrition and is also a common condition occurring in up to 60% of people with cancer, depending on cancer type. 18 , 19 , 20 Furthermore, sarcopenic obesity, a unique phenotype where low muscle mass and obesity occur simultaneously, is independently associated with reduced survival and increased complications in multiple cancer diagnoses and treatment modalities. 21
Loss of muscle mass in cancer‐related malnutrition and sarcopenia may simply be related to reduced nutritional intake. 2 Alternatively, muscle loss may develop as a result of tumour‐related metabolic alterations or the presence of systemic inflammation. 2 This is particularly evident in cancer cachexia, a multifactorial syndrome with a complex underlying pathology. 22 While there are similarities across these conditions, it is generally accepted that weight and muscle loss occurring in cancer cachexia cannot be reversed by conventional nutrition intervention. 23
Evidence from national and international guidelines strongly supports the identification and treatment of cancer‐related malnutrition and sarcopenia prior to, during and post‐treatment in order to improve outcomes for people with cancer. 3 , 6 , 24 , 25 Despite this, considerable variation in screening, diagnosis and intervention occurs across Australian health care settings. Barriers to improving care largely include inadequate time and services and varying perception of responsibility for identification and treatment. 26 Nevertheless, international studies demonstrate failure to improve care has a considerable financial burden that is likely to translate to a similar impact on the Australian health care system. 27 , 28
This document outlines the position of Clinical Oncology Society of Australia (COSA) on the role of health professionals and health services in recognising and treating patients with cancer‐related malnutrition and sarcopenia, with a focus on practice tips to support implementation of optimal management. It is intended for use by clinicians and health services to advocate for the resources and services required to support optimal management of cancer‐related malnutrition and sarcopenia. Malnutrition and muscle loss also occur in adolescents and young adults with cancer, however this document is intended to address the management of these conditions in adults.
2. METHODS
A multidisciplinary expert working group of dietitians, exercise physiologists, nurses, medical oncologists and general practitioners was convened to develop the position statement. A comprehensive literature review of PubMed and Conchrane databases from 1 July 2013 to 3 June 2019 was undertaken by the working group using search terms from the European Society of Clinical Nutrition and Metabolism (ESPEN) guidelines on nutrition in cancer patients. 3 This was to capture literature not included in the ESPEN guidelines, for which the literature search was conducted up to 30 June 2013. A focus was placed on using evidence‐based guidelines, systematic reviews and meta‐analyses identified from the literature search to inform the recommendations. Consensus of the working group was used where evidence was limited. Subsequent national consultation was undertaken with COSA members and key stakeholders, with relevant feedback incorporated into the position statement.
3. RECOMMENDATIONS
Table 1 summarises COSA's position on cancer‐related malnutrition and sarcopenia. Table 2 contains suggestions for the implementation of recommendations into health services. A pathway for the management of cancer‐related malnutrition and sarcopenia, applicable across all health settings, is outlined in Figure 1.
TABLE 1.
Clinical Oncology Society of Australia position on cancer‐related malnutrition and sarcopenia
| All people with cancer should be screened for malnutrition and sarcopenia in all health settings at diagnosis and as the clinical situation changes throughout treatment and recovery. |
| All people with cancer identified as being “at risk” of malnutrition following screening or with a cancer diagnosis or treatment plan known to lead to high risk of malnutrition should have comprehensive nutrition assessment using a tool validated in the oncology population. |
| All people with cancer identified as being “at risk” of sarcopenia following appropriate screening should have a comprehensive evaluation of muscle status using a combination of assessments for muscle mass, muscle strength and function. |
| All people with cancer‐related malnutrition and sarcopenia should have access to the core components of treatment including medical nutrition therapy, targeted exercise prescription and physical activity advice, and physical and psychological symptom management. |
| Treatment for cancer‐related malnutrition and sarcopenia should be individualised, in collaboration with the multidisciplinary team (MDT), and tailored to meet needs at each stage of cancer treatment. |
| Health services should ensure a broad range of health care professionals across the MDT have the skills and confidence to recognise malnutrition and sarcopenia to facilitate timely referrals and treatment. |
TABLE 2.
Tips for implementing recommendations into practice
| Identification |
| Consider incorporating screening for malnutrition and sarcopenia into existing multidisciplinary and/or supportive care screening processes or patient‐reported outcomes to aid ease of completion and compliance, reduce the need for additional resources and to support the initiation of appropriate assessment and care. |
| Screening should focus on early identification using a systematised model of care or pathway that defines the tools to be used, who will conduct screening, the timing and frequency of screening, and pathways for treatment referrals appropriate to the setting. |
| Assessment |
| Malnutrition assessment should be incorporated into the appropriate nutrition care policy directives with local governance, management committees and performance review processes embedded to support successful and sustainable implementation. |
| A measure of muscle mass should be a component of assessment of malnutrition and sarcopenia and incorporated into routine clinical practice. |
| Identification of barriers and enablers to malnutrition and sarcopenia assessment at individual, team and system levels is the first step to facilitate adherence to evidence‐based nutrition care recommendations and policies. |
| Treatment |
| Models of care to treat malnutrition and sarcopenia should provide consistent information regarding cancer‐related malnutrition and sarcopenia across disciplines and throughout phases of treatment to ensure reinforcement of a clear treatment plan. |
| Consider the use of a care pathway, or similar process, developed by MDT members and people with cancer to support implementation of optimal care for cancer‐related malnutrition and sarcopenia. |
| Multidisciplinary care |
| Engage consumers in the development and evaluation of MDT services across the continuum of care. |
| Utilise a framework, for example team mental model, to develop and refine MDT services to optimise the success of the team, and importantly clinical and patient‐reported outcome and experience measures. |
FIGURE 1.
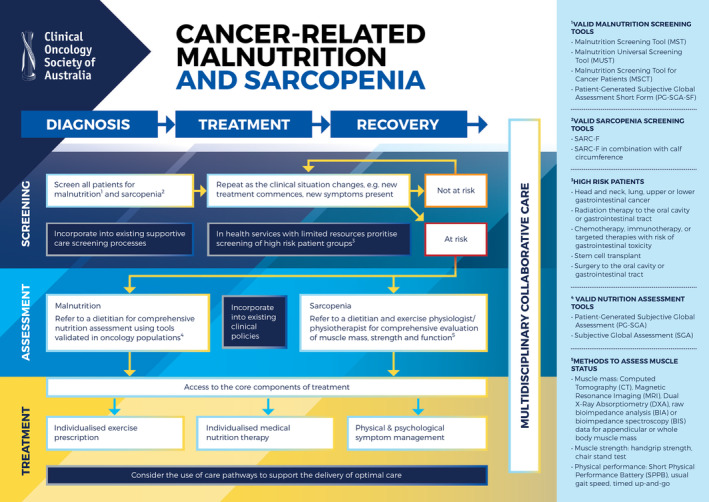
Pathway for the management of cancer‐related malnutrition and sarcopenia within acute, community and primary care health services
3.1. Identifying cancer‐related malnutrition and sarcopenia
All people with cancer should be screened for malnutrition in all health settings at diagnosis and repeated as the clinical situation changes, using a screening tool that is valid and reliable in the setting in which it is intended for use.
All people with cancer should be screened for sarcopenia at diagnosis and repeated as the clinical situation changes, using the validated screening tool SARC‐F or SARC‐F in combination with calf circumference.
Cancer‐related malnutrition and sarcopenia are under‐recognised and therefore under‐treated conditions. Neither malnutrition nor sarcopenia should be associated exclusively with being underweight, as these conditions can be present at any body weight or BMI category including obesity. 18 , 29 Timely and accurate identification of malnutrition and sarcopenia in all clinical practice settings is essential to support the initiation of optimal multidisciplinary care for people with these conditions, and a subsequent reduction in disability, mortality and health care costs. 3 , 7 , 30 This identification process, commonly known as screening, enables recognition of people with cancer as “at risk” or “not at risk” of these conditions.
Screening for malnutrition should be undertaken on all people with cancer at diagnosis, and repeated as clinically indicated throughout each modality of treatment (eg, surgery, radiation therapy, chemotherapy), post‐treatment and surveillance. 3 , 24 , 25 Where limited resources restrict the ability to screen all patients, resources should be devoted to ensuring those at highest risk are screened (Table 3). Malnutrition screening should be undertaken using a malnutrition screening tool validated in the setting in which it is intended for use. 6 , 24 A number of malnutrition screening tools have been shown to be valid and reliable for identifying malnutrition in people with cancer including the Malnutrition Screening Tool (MST), Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool for Cancer Patients (MSTC) and the Patient‐Generated Subjective Global Assessment Short Form (PG‐SGA SF). 3 The MST and MUST are most commonly used in Australia and can be self‐administered or completed by any health professional. 31 , 32 Screening for malnutrition can be bypassed for people with a cancer diagnosis or treatment plan known to lead to high risk of malnutrition, for example, radiation therapy with or without chemotherapy to the gastrointestinal tract or head and neck area. 6 , 25 All people with cancer identified as being “at risk” of malnutrition following screening, or with a diagnosis or treatment plan that places them at high risk of malnutrition, should be referred to an accredited practising dietitian for a comprehensive nutritional assessment and initiation of appropriate treatment. 3 , 6 , 24
TABLE 3.
Factors indicative of high risk of malnutrition in people with cancer
| Cancer diagnosis | Treatment | Other |
|---|---|---|
| Head and neck | Radiation therapy to oral cavity or gastrointestinal tract | Advanced stage disease |
| Upper or lower gastrointestinal | Chemotherapy, immunotherapy or targeted therapy with risk of gastrointestinal toxicity | Older age (>65 years) |
| Thoracic | Stem cell transplant | |
| Acute leukaemia (myeloid and lymphoid) | Surgery to the oral cavity or gastrointestinal tract | |
| Steroid use with treatment |
Screening for sarcopenia should be completed in all people with cancer at diagnosis and as clinically indicated throughout each modality of treatment (eg, surgery, radiation therapy, chemotherapy), post‐treatment and surveillance. The SARC‐F is a sarcopenia screening tool that has been well studied and validated in older adults. 33 The SARC‐F has recently been validated for use in people with cancer, either alone or in combination with measurement of calf circumference for greater sensitivity. 34 All people identified as “at risk” of sarcopenia should be referred to an accredited practising dietitian and exercise specialist that is, accredited exercise physiologist and/or physiotherapist, experienced in the cancer setting and with knowledge of body composition science, for assessment of muscle strength, muscle mass and physical performance and initiation of appropriate treatment.
3.2. Assessing cancer‐related malnutrition and sarcopenia
All people with cancer identified as being “at risk” of malnutrition following screening or with a cancer diagnosis or treatment plan known to lead to high risk of malnutrition should have a comprehensive nutrition assessment using a tool validated in the oncology population.
All people with cancer identified as being “at risk” of sarcopenia following appropriate screening should have a comprehensive evaluation of muscle status using a combination of assessments for muscle mass, muscle strength and function.
- Interpretation of diagnostic criteria for sarcopenia should be applied recognising that:
- Threshold values for assessing muscle mass, muscle strength and physical performance are variable.
- Care should be taken to determine the appropriate cut‐off values in the population in which they are being applied.
- Most data regarding muscle strength and performance comes from older populations.
- The applicability of diagnostic criteria in different ethnicities is uncertain.
Nutrition assessment is recommended for all people with cancer identified as “at risk” of malnutrition following screening or with a cancer diagnosis or treatment plan known to lead to high risk of malnutrition for example, patients receiving radiation therapy to the oral cavity or gastrointestinal tract. 6 , 25 Nutrition assessment and malnutrition diagnosis should be completed by an appropriately trained health care professional such as an accredited practising dietitian or if not available, other allied health or medical professional. Nutrition assessment should be repeated as the clinical situation changes and incorporated into routine clinical consultations. 3 Nutrition assessment should incorporate measures of involuntary weight loss, BMI, body composition, food intake and nutrient absorption, functional status and inflammation. 3 The 2019 Global Leadership Initiative on Malnutrition (GLIM) has produced a consensus statement outlining the recommended assessment domains for a diagnosis of malnutrition including etiologic (reduced food intake or assimilation; inflammation) and phenotypic criteria (weight loss; low BMI; reduced muscle mass). 15 The Patient‐Generated Subjective Global Assessment (PG‐SGA) 35 and Subjective Global Assessment (SGA) 36 are validated assessment tools that align with the GLIM criteria for diagnosing malnutrition and can be used to assess and diagnose malnutrition in people with cancer. 6
Sarcopenia assessment is recommended for all people identified as “at risk” of sarcopenia following screening and is ideally performed at the time of cancer diagnosis and before an initial treatment plan is implemented. Reassessment should occur as the clinical situation changes and incorporated into routine clinical consultations during active treatment and then ongoing surveillance throughout the course of care. Sarcopenia assessment and diagnosis should be completed by an appropriately trained health care professional such as an accredited practising dietitian, accredited exercise physiologist, physiotherapist or other allied health or medical professional. Assessment should consider whether it is primary sarcopenia (age‐related) or secondary sarcopenia (other causal factors). 13 These causal factors may include: (a) Disease—secondary to a systemic disease especially where inflammation is present as in cancer; (b) Inactivity—sedentary lifestyle or disease‐related immobility or disability; or (c) Malnutrition—as a result of inadequate energy or protein caused by anorexia, malabsorption, food insecurity or limited ability to eat. 13
There is no global consensus on the diagnostic criteria for sarcopenia. Commonly used definitions include the European Working Group on Sarcopenia in Older People (EWGSOP) 1 12 and EWGSOP 2, 13 and the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Sarcopenia Project 37 (Table 4). However, these definitions and cut‐points have been developed based on research in older adults and have not been extensively researched in cancer populations. Most research in cancer has assessed sarcopenia based on low muscle mass alone from computed tomography images using cut‐points prognostic for poor survival. 40 , 44 The various methods used for diagnosing sarcopenia are presented in Table 4. Selection of assessment method may depend on patient factors, access to the required technical resources and the purpose of testing. Clinicians and researchers should understand the strengths and limitations of the assessment method being applied and understand these are not interchangeable. 12 , 13 Ongoing research will contribute to identifying cut‐points relative to patient population and ethnicity.
TABLE 4.
| Definition | Low muscle strength | Low muscle mass | Poor muscle function |
|---|---|---|---|
| EWGSOP 1 (2010)—Low muscle mass + low muscle strength or low physical performance | Grip strength: <30 kg men; <20 kg women | ALM a /height (m)2: <7.26 kg/m2 men; <5.50 kg/m2 women | Gait speed:≤0.8 m/second (4 m walk) or SPPB Score ≤8 points |
| FNIH (2014)—Low muscle mass + low muscle strength | Grip strength: <26 kg men; <16 kg women | ALM/BMI: <0.789 kg/BMI men; <0.512 kg/BMI women | NA |
| EWGSOP 2 (2019)—Low muscle strength + low muscle mass or low physical performance | Grip strength: <27 kg men; <16 kg women or Chair Stands: >15 seconds five rises | ALM/height (m)2: <7.00 kg/m2 men; <5.50 kg/m2 women or ALM: <20 kg men; <15 kg women | Gait speed: ≤0.8 m/second (4 m walk) or SPPB Score ≤8 points or TUG ≥20 seconds or 400 m walk ≥6 minutes or non‐completion. Only used to classify severity of sarcopenia. |
| Cancer‐specific CT image analysis research b —Low muscle mass | NA | SMI (SMA/height [m]2) at L3: <52.4 cm2/m2 (men) 18 ; <38.5 cm2/m2 (women) 18 ; or <43 cm2/m2 (men with BMI < 24.9) 40 ; <53 cm2/m2 (men with BMI ≥ 25) 40 ; <41 cm2/m2 (women of any BMI) 40 | NA |
Notes: Muscle mass outputs produced automatically by BIA and BIS devices may be less accurate due potential to be affected by fluid shifts and a lack of transparency regarding the population‐specific regression equations underpinning their outputs owing to their proprietary nature 39 , 41 , 42 , 43 ; however, some guidelines support use in clinical practice.
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; CT, computed tomography; EWGSOP 1, European Working Group on Sarcopenia in Older People; EWGSOP 2, European Working Group on Sarcopenia in Older People updated definition; FNIH, Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project; SMA, skeletal muscle area determined from cross‐sectional CT image at the third lumbar vertebrae; SMI, Skeletal Muscle Index; SPPB, short physical performance battery; TUG, timed up and go. 13
ALM can be assessed using dual energy X‐ray absorptiometry (DXA), magnetic resonance imaging (MRI), CT, raw bioelectrical impedance analysis (BIA) or bioelectrical impedance spectroscopy (BIS).
Multiple cut‐points for SMI at the third lumbar vertebrae are used in the literature, depending on population and ethnicity. These are suggested cut‐points; however, clinicians are advised to research the most appropriate cut‐point for their population.
3.3. Treating cancer‐related malnutrition and sarcopenia
All people with cancer‐related malnutrition and sarcopenia should have access to the core components of treatment including individualised medical nutrition therapy, targeted exercise prescription and physical activity advice and physical and psychological symptom management.
Treatment for cancer‐related malnutrition and sarcopenia should be individualised, in collaboration with the multidisciplinary team (MDT), and tailored to consider multi‐morbidities and meet needs at each stage of cancer treatment.
Treatment of cancer‐related malnutrition and sarcopenia aims to provide adequate nutritional intake and participation in exercise in order to optimise body composition with a focus on preserving or improving lean mass and physical function, as well as assisting patients to complete cancer treatment. 6 , 25 , 29 However, people with cancer commonly report unmet needs in regards to access and provision of nutrition and exercise advice for the management of their malnutrition or sarcopenia, and are seeking consistent care from health professionals across their treating team to address these needs. 45 , 46 , 47 , 48
Medical nutrition therapy that considers symptoms, social situation and treatment plan, has been found to improve nutritional intake, weight and patient‐reported physical function and reduce treatment breaks and unplanned hospital admissions. 3 , 6 , 25 Sufficient nutritional intake of energy and protein is required to treat malnutrition, with evidence‐based guidelines recommending between 105 and 125 kJ/kg/day and between 1.0 and 1.5 g/kg/day in protein. 3 Preliminary data suggest that in fact a higher protein intake of 2.0 g/kg/day may be required to combat muscle loss; however, this is not yet supported by sufficient research. 17 Specific recommendations regarding the use and timing of initiation of medical nutrition therapy, including enteral and parenteral nutrition, and Enhanced Recovery After Surgery protocols are available for some cancer types including head and neck and upper and lower gastrointestinal cancers. 3 , 6 , 24 , 25
For medical nutrition therapy to be optimally effective, it needs to occur alongside exercise intervention. 49 Exercise training provides the necessary support for nutrition intervention to improve (or prevent worsening) in body composition, specifically, preservation or increases in lean tissue, with or without changes in body weight. 3 , 50 , 51 Resistance exercise is more effective than aerobic exercise at improving muscle mass and strength. 52 However, aerobic exercise remains relevant as it also modulates muscle metabolism, insulin sensitivity and levels of inflammation in a way that could potentially aid in preservation of muscle mass and function. 52 As such, in line with the most up to date Australian exercise prescription guidelines for people with cancer, individually prescribed multimodal exercise training (including targeted aerobic and resistance exercise) at moderate to high intensity, with emphasis on resistance exercise incorporating exercises for major muscle groups, is recommended. 52
Although promising, evidence is variable for the use of anabolic or appetite stimulating agents, such as progestins, or non‐steroidal anti‐inflammatory agents, and their use should be evaluated on an individual basis by the MDT or disease protocol. 3 Supportive care screening to identify unmet needs, including symptom management and psychosocial needs, is important for a holistic approach.
Interventions are most effective when commenced early, with a focus on prevention or the treatment of mild to moderate malnutrition and/or sarcopenia. 22 However, despite the evidence for treatment of cancer‐related malnutrition and sarcopenia, consistent and equitable access to care is lacking. 3 , 47 , 48 The potential benefit to the health system through implementing evidence‐based treatment of malnutrition and sarcopenia is significant, estimated to be the equivalent of AU$800 000 per 100 000 population. 27 , 53 Detailed recommendations regarding evidence‐based treatment for people undergoing different treatment modalities and for specific cancer diagnoses can be found in the relevant evidence‐based guidelines. 3 , 6 , 24 , 25 , 52
3.4. Role of the multidisciplinary team
Health services should ensure a broad range of health care professionals across the MDT have the skills and confidence to recognise malnutrition and sarcopenia to facilitate timely referrals and treatment.
MDTs should work towards an individualised and coordinated approach to treating cancer‐related malnutrition and sarcopenia.
A multidisciplinary approach to identifying and treating cancer‐related malnutrition and sarcopenia is essential. Alongside medical nutrition therapy, interventions that have been shown to contribute to optimising nutritional status and body composition include exercise, management of treatment side effects through optimising medications and the management of anorexia and dysphagia. 3 , 25 Frameworks, such as the team mental model, have been found to optimise team processes and functioning. 54 Multidisciplinary cancer prehabilitation and rehabilitation programs providing individualised care demonstrate capacity to improve nutritional status, muscle mass and strength, fatigue and performance status. 55 , 56 , 57 , 58 These interventions require the specialist skills of various MDT members as part of a coordinated approach to individualised treatment of cancer‐related malnutrition and sarcopenia and can be accessed through acute hospital services, community‐based services or general practices.
Guidance regarding optimal practice at a national level is expected to improve the management of cancer‐related malnutrition and sarcopenia across health sectors. Implementation of the position statement recommendations within health services will require the support and training of multidisciplinary health professionals, health service managers and policy makers. The position statement recommendations and pathway provide a framework for achieving optimal care.
ENDORSING AND SUPPORTING ORGANISATIONS
The COSA Position Statement on Cancer‐Related Malnutrition and Sarcopenia is a stand‐alone document, the content of which is not influenced by any other authority. The position statement is endorsed by Cancer Council Australia, Dietitians Australia, Cancer Nurses Society of Australia and the Australia New Zealand Society for Sarcopenia and Frailty Research. Supporting organisations include the Multinational Association of Supportive Care in Cancer, Beyond Five, Australian Physiotherapy Association, Australia New Zealand Head and Neck Society, Australasian Lung Cancer Trials Group, Pancare Foundation, Lung Foundation Australia, Australia New Zealand Society of Palliative Medicine, Exercise and Sports Science Australia, Australian Gastro‐intestinal Trials Group, Australasian Leukaemia and Lymphoma Group and Australia New Zealand Children's Haematology and Oncology Group, Psycho‐oncology Cooperative Research Group.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
N. K., M. F., J. B., J. L., E. I. developed the structure for the position statement. All authors contributed to the literature review and drafting content, recommendations and implementation tips. N. K. drafted the manuscript and chaired the working group. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere. N. K. is supported by a fellowship from the Victorian Cancer Agency. Cancer Council Queensland is to be acknowledged for S. H. fellowship support for contributions made during the fellowship funding period.
Kiss N, Loeliger J, Findlay M, et al. Clinical Oncology Society of Australia: Position statement on cancer‐related malnutrition and sarcopenia. Nutr Diet. 2020;77:416–425. 10.1111/1747-0080.12631
[Correction added on 24 August 2020, after first online publication: affiliation 3 has been amended and funding information section has been removed.]
REFERENCES
- 1. Allison SP. Malnutrition, disease, and outcome. Nutrition. 2000;16:590‐593. [DOI] [PubMed] [Google Scholar]
- 2. Baracos VE. Cancer‐associated malnutrition. Eur J Clin Nutr. 2018;72:1255‐1259. [DOI] [PubMed] [Google Scholar]
- 3. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11‐48. [DOI] [PubMed] [Google Scholar]
- 4. Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M. The health economics burden of sarcopenia: a systematic review. Maturitas. 2019;119:61‐69. [DOI] [PubMed] [Google Scholar]
- 5. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enteral Nutr. 2014;38:196‐204. [DOI] [PubMed] [Google Scholar]
- 6. Isenring E, Zabel R, Bannister M, et al. Updated evidence‐based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutr Diet. 2013;70:312‐324. [Google Scholar]
- 7. Marshall KM, Loeliger J, Nolte L, Kelaart A, Kiss NK. Prevalence of malnutrition and impact on clinical outcomes in cancer services: a comparison of two time points. Clin Nutr. 2019;38:644‐651. [DOI] [PubMed] [Google Scholar]
- 8. Loeliger J, Kiss N. Phase II Malnutrition in Victorian Cancer Services: Summary Report. Melbourne, Australia: Department of Health and Human Services, State Governerment of Victoria; 2015. [Google Scholar]
- 9. Pressoir M, Desné S, Berchery D, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102:966‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agarwal E, Ferguson M, Banks M, Bauer J, Capra S, Isenring E. Nutritional status and dietary intake of acute care patients: results from the nutrition care day survey 2010. Clin Nutr. 2012;31:41‐47. [DOI] [PubMed] [Google Scholar]
- 11. Ramos Chaves M, Boleo‐Tome C, Monteiro‐Grillo I, et al. The diversity of nutritional status in cancer: new insights. Oncologist. 2010;15:523‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;48:16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition—an ESPEN consensus statement. Clin Nutr. 2015;34:335‐340. [DOI] [PubMed] [Google Scholar]
- 15. Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1‐9. [DOI] [PubMed] [Google Scholar]
- 16. Baracos V, Reiman T, Mourtzakis M, et al. Body composition in patients with non‐small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S‐1137S. [DOI] [PubMed] [Google Scholar]
- 17. Bauer J, Morley JE, Schols AMWJ, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10:956‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629‐635. [DOI] [PubMed] [Google Scholar]
- 19. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1339‐1344. [DOI] [PubMed] [Google Scholar]
- 20. Prado CMM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920‐2926. [DOI] [PubMed] [Google Scholar]
- 21. Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29:ii1‐ii9. [DOI] [PubMed] [Google Scholar]
- 22. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489‐495. [DOI] [PubMed] [Google Scholar]
- 23. Fearon KCH. The 2011 ESPEN Arvid Wretlind lecture: cancer cachexia: the potential impact of translational research on patient‐focused outcomes. Clin Nutr. 2012;31:577‐582. [DOI] [PubMed] [Google Scholar]
- 24. Academy of Nutrition and Dietetics . Oncology evidence‐based nutrition practice guideline. 2013. http://andevidenceanalysislibrary.com
- 25. Findlay M, Bauer J, Brown T, et al. Evidence based practice guidelines for the nutritional managment of adult patients with head and neck cancer. 2011 [updated April 2011]. http://wiki.cancer.org.au/australia/COSA:Head_and_neck_cancer_nutrition_guidelines
- 26. Kiss N, Bauer J, Boltong A, et al. Awareness, perceptions and practices regarding cancer‐related malnutrition and sarcopenia: a survey of cancer clinicians. Support Care Cancer. 2020. 10.1007/s00520-020-05371-7. [DOI] [PubMed] [Google Scholar]
- 27. Elia M, British Association of Parenteral and Enteral Nutriton (BAPEN), National Institute for Health Research Southhampton Biomedical Research Centre . The Cost of Malnutrition in England and Potential Cost Savings from Nutritional Interventions (Short Version). Southhampton, England: National Institute for Health Research Southhampton Biomedical Research Centre; 2015. [Google Scholar]
- 28. SEO Economic Research . Malnutrition Underestimated: The Costs of Malnutrition and the Return on Medical Nutrition. Amsterdam, The Netherlands: SEO Economic Research; 2014. [Google Scholar]
- 29. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr. 2017;36:1187‐1196. [DOI] [PubMed] [Google Scholar]
- 30. Barazzoni R, Bischoff SC, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37:1787‐1793. [DOI] [PubMed] [Google Scholar]
- 31. Cawood AL, Elia M, Sharp SK, et al. Malnutrition self‐screening by using MUST in hospital outpatients: validity, reliability, and ease of use. Am J Clin Nutr. 2012;96:1000‐1007. [DOI] [PubMed] [Google Scholar]
- 32. Di Bella A, Blake C, Young A, et al. Reliability of patient‐led screening with the malnutrition screening tool: agreement between patient and health care professional scores in the cancer care ambulatory setting. J Acad Nutr Diet. 2018;118:1065‐1071. [DOI] [PubMed] [Google Scholar]
- 33. Malmstrom TK, Morley JE. SARC‐F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531‐532. [DOI] [PubMed] [Google Scholar]
- 34. Fu X, Tian Z, Thapa S, et al. Comparing SARC‐F with SARC‐CalF for screening sarcopenia in advanced cancer patients. Clin Nutr. 2020. 10.1016/j.clnu.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 35. Ottery F. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15‐S19. [DOI] [PubMed] [Google Scholar]
- 36. Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr. 1987;11:8‐13. [DOI] [PubMed] [Google Scholar]
- 37. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheean P, Gonzalez MC, Prado CM, McKeever L, Hall AM, Braunschweig CA. American Society for Parenteral and Enteral Nutrition clinical guidelines: the validity of body composition assessment in clinical populations. J Parenter Enteral Nutr. 2019;44:12‐43. 10.1002/jpen.1669. [DOI] [PubMed] [Google Scholar]
- 40. Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539‐1547. [DOI] [PubMed] [Google Scholar]
- 41. Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr. 2014;38:940‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Price KL, Earthman CP. Update on body composition tools in clinical settings: computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur J Clin Nutr. 2019;73:187‐193. [DOI] [PubMed] [Google Scholar]
- 43. Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019;73:194‐199. [DOI] [PubMed] [Google Scholar]
- 44. Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amano K, Maeda I, Morita T, et al. Need for nutritional support, eating‐related distress and experience of terminally ill patients with cancer: a survey in an inpatient hospice. BMJ Support Palliat Care. 2016;6:373‐376. [DOI] [PubMed] [Google Scholar]
- 46. Choi K‐H, Park J‐H, Park S‐M. Cancer patients' informational needs on health promotion and related factors: a multi‐institutional, cross‐sectional study in Korea. Support Care Cancer. 2011;19:1495‐1504. [DOI] [PubMed] [Google Scholar]
- 47. Hwang SS, Chang VT, Cogswell J, et al. Study of unmet needs in symptomatic veterans with advanced cancer: incidence, independent predictors and unmet needs outcome model. J Pain Symptom Manage. 2004;28:421‐432. [DOI] [PubMed] [Google Scholar]
- 48. Isenring E, Cross G, Kellett E, Koczwara B, Daniels L. Nutritional status and information needs of medical oncology patients receiving treatment at an Australian public hospital. Nutr Cancer. 2010;62:220‐228. [DOI] [PubMed] [Google Scholar]
- 49. Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance‐type exercise training: a meta‐analysis. Am J Clin Nutr. 2012;96:1454‐1464. [DOI] [PubMed] [Google Scholar]
- 50. Aversa Z, Costelli P, Muscaritoli M. Cancer‐induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9:369‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sadeghi M, Keshavarz‐Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91‐104. [DOI] [PubMed] [Google Scholar]
- 52. Hayes SC, Newton RU, Spence RR, Galvão DA. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22:1175‐1199. [DOI] [PubMed] [Google Scholar]
- 53. SEO Economic Research . The Social Costs and Benefits of Dietetics for Malnourished Patients in Hospital. Amsterdam, The Netherlands: SEO Economic Research; 2015. [Google Scholar]
- 54. Portman DG, Thirlwell S, Donovan KA, et al. Leveraging a team mental model to develop a cancer anorexia‐cachexia syndrome team. J Oncol Pract. 2016;12:1046‐1052. [DOI] [PubMed] [Google Scholar]
- 55. Chasen M, Bhargave R. A rehabilitation program for patients with gastrointestinal cancer: a pilot study. Support Care Cancer. 2010;18:S35‐S40. [DOI] [PubMed] [Google Scholar]
- 56. Gagnon B, Murphy J, Eades M, et al. A prospective evaluation of an interdiscipinary nutrition‐rehabilitation program for patients with advanced cancer. Curr Oncol. 2013;20:310‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parmar M, Swanson T, Jagoe RT. Weight changes correlate with alterations in subjective physical function in advanced cancer patients referred to a specialized nutrition and rehabilitation team. Support Care Cancer. 2013;21:2049‐2057. [DOI] [PubMed] [Google Scholar]
- 58. Schmidt H, Boese S, Bauer A, et al. Interdisciplinary care programme to improve self‐management for cancer patients undergoing stem cell transplantation: a prospective non‐randomised intervention study. Eur J Cancer Care. 2017;26:e12458. [DOI] [PubMed] [Google Scholar]


