SUMMARY
In recent years, research has increasingly focused on the key role of post‐transcriptional regulation of messenger ribonucleoprotein (mRNP) function and turnover. As a result of the complexity and dynamic nature of mRNPs, the full composition of a single mRNP complex remains unrevealed and mRNPs are poorly described in plants. Here we identify canonical Sm proteins as part of the cytoplasmic mRNP complex, indicating their function in the post‐transcriptional regulation of gene expression in plants. Sm proteins comprise an evolutionarily ancient family of small RNA‐binding proteins involved in pre‐mRNA splicing. The latest research indicates that Sm could also impact on mRNA at subsequent stages of its life cycle. In this work we show that in the microsporocyte cytoplasm of Larix decidua, the European larch, Sm proteins accumulate within distinct cytoplasmic bodies, also containing polyadenylated RNA. To date, several types of cytoplasmic bodies involved in the post‐transcriptional regulation of gene expression have been described, mainly in animal cells. Their role and molecular composition in plants remain less well established, however. A total of 222 mRNA transcripts have been identified as cytoplasmic partners for Sm proteins. The specific colocalization of these mRNAs with Sm proteins within cytoplasmic bodies has been confirmed via microscopic analysis. The results from this work support the hypothesis, that evolutionarily conserved Sm proteins have been adapted to perform a whole repertoire of functions related to the post‐transcriptional regulation of gene expression in Eukaryota. This adaptation presumably enabled them to coordinate the interdependent processes of splicing element assembly, mRNA maturation and processing, and mRNA translation regulation, and its degradation.
Keywords: cytoplasmic bodies, mRNP, cajal bodies, P‐bodies, stress granules, RIP‐seq
Significance Statement
The study reports the cyclic occurrence of cytoplasmic mRNP accumulations enriched in canonical Sm proteins but not in other spliceosomal components in Larix decidua microsporocytes. Based on transcriptomic analysis, 222 mRNAs were identified as cytoplasmic partners for Sm proteins, which were linked to many gene ontology terms. The results presented show that S‐bodies constitute newly described cytoplasmic domains involved in the post‐transcriptional regulation of highly expressed transcripts, particularly in cells in which mRNA synthesis occurs in transcriptional bursts.
INTRODUCTION
Post‐transcriptional regulation of gene expression plays an essential role in all aspects of the life cycle of a cell, including cell development, differentiation, survival, homeostasis, adaptation to stress and response to environmental signals.
The cytoplasm plays the key role in the regulation of gene expression by controlling the level of mRNA translation and decay. The transport, localization and degradation of mRNA within this compartment are highly ordered processes. This complexity is not surprising considering the high content of mRNA in the cytoplasm (up to 150 000 molecules in mammalian cells; Halbeisen et al., 2008), where two major counteracting processes of mRNA translation and decay must occur at the same time. As stated in the literature, the vast majority of mRNA post‐transcriptional regulation occurs within the specialized microdomains referred to as cytoplasmic bodies (Moser and Fritzler, 2010; Lavut and Raveh, 2012). These non‐membrane bound, highly dynamic structures enriched in ribonucleoproteins are evolutionarily conserved, as their occurrence has been confirmed in yeasts (Sheth and Parker, 2003; Lavut and Raveh, 2012), protozoans (López‐Rosas et al., 2012), nematodes (Barbee et al., 2002; Gallo et al., 2008), insects (Liu and Gall, 2007; Lee et al., 2009), amphibians (Bilinski et al., 2004), mammals (Chuma et al., 2003; Sen and Blau, 2005) and plants (Xu and Chua, 2009; Smoliński et al., 2011; Perea‐Resa et al., 2012). Several cytoplasmic bodies have been described to date, mainly in animal cells. These include P‐bodies (PBs), stress granules (SGs), neuronal granules, germinal granules and nuage, among others. The role and molecular composition of cytoplasmic bodies in plants remain less understood, however.
P‐bodies are sites of mRNA regulation, including 5′–3′ deadenylation‐dependent degradation (Sheth and Parker, 2003; Beggs, 2005), microRNA (miRNA)‐mediated decay (Sen and Blau, 2005), and mRNA stabilization and sequestration (Xu and Chua, 2009; Scarpin et al., 2017), as well as non‐sense‐mediated decay (NMD; Unterholzner and Izaurralde, 2004) and translational regulation (Andrei et al., 2005; Kedersha et al., 2005; Wilczynska et al., 2005; Chu and Rana, 2006). As a result of their composition, including proteins involved in both mRNA stabilization and turnover, P‐bodies constitute centers of mRNA sorting where transcripts are directed to either translation or degradation (Lavut and Raveh, 2012). It has been revealed that the components of P‐bodies accumulate transiently and can shuttle between the cytoplasm and other cytoplasmic granules (Kedersha et al., 2005; Wilczynska et al., 2005; Moser and Fritzler, 2010; Gutierrez‐Beltran et al., 2015). Despite relatively broad knowledge on P‐bodies in animal cells, their composition and function in plants is much less understood. Many components are shared between animal and plant P‐bodies: for example, LSm1‐7, DCP1/2, AGO1 and XRN exonuclease (Xu and Chua, 2009; Maldonado‐Bonilla, 2014). No homolog of GW182, which is a marker protein for animal P‐bodies, has been found in plants, however. Furthermore, plant P‐bodies accumulate proteins distinct from their animal counterparts: for example, AtTZF1 and hTTP (Pomeranz et al., 2010; Xu and Chua, 2011). Functionally, plant P‐bodies are involved in translational repression and mRNA decapping (Chantarachot and Bailey‐Serres, 2018). Mutational studies indicate that plant P‐bodies have a developmental rather than housekeeping function, and that their formation is triggered by the active repression of mRNA translation. Null mutants of DCP1 and DCP2 are lethal at the post‐embryonic stage in Arabidopsis thaliana, pointing to the role of P‐bodies and mRNA decapping in plant early development (Xu et al., 2006). Further studies identified DCP5 as another P‐body component in plants that is essential for the translational repression of seed storage protein mRNAs (e.g. OLEO1) and P‐body formation in germinating seedlings (Xu and Chua, 2009).
Stress granules (SGs) are evolutionarily conserved cytoplasmic microdomains representing aggregates of translationally stalled mRNPs that are formed upon environmental stress (Weber et al., 2008; Sorenson and Bailey‐Serres, 2014). Their formation is triggered by the inhibition of translation initiation of mRNAs (Protter and Parker, 2016). Importantly, upon stress recovery the transcripts stored in SGs might be targeted to PB‐mediated degradation or might be released to the cytoplasm for translation (Chantarachot and Bailey‐Serres, 2018). The proteomic analysis of isolated SGs from A. thaliana revealed 118 proteins present within these microdomains (Kosmacz et al., 2019). In addition to marker proteins related to SG formation and dynamics (e.g. eukaryotic translation initiation factors eIF4A, eIF4E and eIF3, poly‐A binding proteins PAB2, PAB8 and RBP47B, and oligouridilate‐binding proteins UBP1A, UBP1B and UBP1C), a number of enzymes (e.g. those involved in ethylene, glucosinolate and rhamnose metabolism), growth regulators (CDKA1) and other proteins related to plant stress responses (peroxidases, MKK5, MPK3 and SNRK kinases) were identified. It has been demonstrated that SGs are physically, compositionally and functionally linked to P‐bodies (Buchan and Parker, 2009; Chantarachot and Bailey‐Serres, 2018). A portion of the proteome of SGs overlaps with PBs, such as TUDOR‐SN proteins (TSN1 and TSN2) and tandem zinc‐finger proteins (AtTZF1, 4, 5, 6 and 9), which localize to SGs upon heat stress (Pomeranz et al., 2010; Gutierrez‐Beltran et al., 2015). Thus, the mRNA life cycle involves constant dynamic changes in mRNP composition and subcellular localization, where mRNPs can shuttle between polysomes, P‐bodies and stress granules, depending on external stimuli and the current metabolic needs of the cell. Despite growing research on the post‐transcriptional regulation of mRNA usage and its spatial organization within the cytoplasm, the full mechanisms and functional roles of mRNP assemblies into higher‐order microdomains must be elucidated.
In this study, we show that canonical Sm proteins are part of the cytoplasmic mRNP complex and thus function in the post‐transcriptional regulation of gene expression in plants. Sm proteins constitute an evolutionarily ancient family of small RNA‐binding proteins. In eukaryotic cells, these molecules are primarily involved in pre‐mRNA splicing. Only a few studies have indicated that, in addition to this well‐known function, Sm proteins could also impact mRNA at subsequent stages of its life cycle. In our research, using European larch (Larix decidua L.) microsporocytes as a model system, we show that there is a significant pool of Sm proteins that accumulate within distinct cytoplasmic bodies that also contain polyadenylated [poly(A)] RNA. A correlation between the cyclic occurrence of the cytoplasmic bodies and the metabolic activity of the cell was shown. The cytoplasmic bodies were localized after transcriptional bursts, indicating that these microdomains are formed as a result of pulsed, extensive mRNA synthesis. The European larch microsporocytes are male germline precursors that have been shown to be highly active periodically during diplotene (Kołowerzo‐Lubnau et al., 2015). The diplotene stage lasts approximately 5 months and it is possible to divide it into several substages and to observe each substage in detail. It has been demonstrated that in Larix species, the chromatin is subject to substantial rearrangements and the microsporocyte is highly metabolically active, increasing its volume sixfold during this stage (Zhang et al., 2008; Kołowerzo‐Lubnau et al., 2015). In L. decidua, metabolic activity is strictly driven by cyclic chromatin contraction and diffusion. Five periods of chromatin relaxation (diffuse stages) separated by four periods of genome contraction are distinguished during diplotene. In many animal and plant species, diplotene is characterized by significant chromatin relaxation, referred to as the diffuse stage of meiosis (Klasterska, 1976; Cenci et al., 1994; Sheehan and Pawlowski, 2009; Comizzoli et al., 2011; She et al., 2013; Colas et al., 2017). As a result, RNA synthesis is not a continuous process but occurs in transcriptional bursts: the diffuse stages are accompanied by large increases in renewed transcription of both mRNAs and rRNAs, as well as the expression and assembly of splicing subunits (Smoliński et al., 2007; Smoliński et al., 2011; Hyjek et al., 2015).
A total of 222 mRNAs were identified as cytoplasmic partners for Sm proteins, and they comprise three major categories of Sm protein‐associated mRNAs that code for proteins related to: (i) ribosomes/translation; (ii) mitochondria/energy metabolism; and (iii) chloroplasts/photosynthesis.
Based on the results obtained in this work and studies on other model eukaryotic cells, we propose that Sm protein–mRNA bodies constitute newly described cytoplasmic domains involved in the post‐transcriptional regulation of highly expressed transcripts, particularly in cells in which mRNA synthesis occurs in transcriptional bursts.
RESULTS
S‐bodies: cytoplasmic bodies rich in Sm proteins and polyadenylated mRNA
The specificity of Y12 antibodies against larch Sm proteins has been determined previously (Smoliński et al., 2011). In order to validate the specificity of the anti‐Sm antibodies used in these studies, immunoprecipitation and Western blot analyses were performed using cytoplasmic protein extract from larch anthers. All of the immunoglobulins tested specifically precipitated the SmB protein, with the Y12 antibody (not commercial) also effectively precipitating the low‐molecular SmD1–SmD3 proteins (Figure S1). In order to further confirm the specificity of the antibodies, the identification of proteins after immunoprecipitation (IP) was performed by mass spectrometry (using the IP‐SDS‐PAGE‐MS method). On the basis of the results obtained, in conjunction with the predicted mass of the studied polypeptides, seven of the eight core Sm proteins were identified: SmF (11 kDa), SmE (12 kDa), SmD1 (13 kDa), SmD2 (14 kDa), SmD3 (16 kDa), SmB (25 kDa) and SmB′ (26 kDa) (Figure S2).
A double labeling assay of the core spliceosomal Sm proteins and poly(A) RNA revealed a remarkable distribution of these molecules within the cytoplasm of microporocytes (Figures 1 and S3). In the larch anther, cytoplasmic bodies containing Sm proteins were limited to the microsporocyte cytoplasm. Other anther cells (epidermal cells, middle layer or tapetum cells) were devoid of Sm protein concentration in the cytoplasm (Figure S3).
Figure 1.

Cytoplasmic bodies rich in the Sm proteins and polyadenylated [poly(A)] RNA. (a–c) Localization of Sm proteins and poly(A) RNA in microsporocytes during diplotene using different types of anti‐Sm antibodies. Numerous cytoplasmic accumulations of Sm proteins in the colocalization with poly(A) RNA (arrowheads) are visible. The right‐hand panel represents the magnification of the fragments of the cytoplasm, which are marked with a square; CB, Cajal body; cyt, cytoplasm; n, nucleus; nu, nucleolus. Scale bars: 15 μm. (d, e) Ultrastructural analysis of microsporocytes. Non‐membrane‐bound cytoplasmic structures (arrowheads) are visible; cyt, cytoplasm; ER, endoplasmic reticulum; n, nucleus. Scale bars: 1 μm. (f, g) Localization of Sm proteins in the cytoplasm of the microsporocytes as determined by the immunogold method. Isolated accumulations of gold particles (20 nm) in distinctive cytoplasmic clusters (arrowheads) are clearly visible. Scale bars: 0.5 μm.
Distinct 0.5–1.0 µm cytoplasmic foci were observed, in which a significant quantity of polyadenylated transcripts had accumulated, in colocalization with Sm proteins. To verify the specificity of the observed labeling, three different antibodies were used for Sm protein localization, namely, commercially available Y12 MAbs (Figure 1a), Y12 MAbs from Matera’s laboratory (Figure 1b) and the ANA No. 5 human reference anti‐serum (Figure 1c). All of the antibodies used showed a similar pattern of labeling, with a well‐established and characteristic nuclear pool of Sm proteins present in the nucleoplasm and Cajal bodies (CBs), which are highly conserved nuclear domains involved in RNA metabolism, and with the maturation of spliceosomal units after being reimported from the cytoplasm (Darzacq et al., 2002; Staněk and Neugebauer, 2006; Bassett, 2012). Moreover, numerous discrete foci of Sm protein accumulation were observed in the cytoplasm and colocalized with poly(A) RNA (Figure S3). The ultrastructural analysis revealed spherical, non‐membrane‐bound microdomains with a diameter of 0.4–1.0 µm (Figure 1d,e). The immunogold assay confirmed that a noticeable portion of the Sm proteins accumulated within distinct cytoplasmic structures (Figure 1f,g).
The next aim was to examine whether the observed cytoplasmic accumulations are functionally related to other microdomains, such as P‐bodies or stress granules. The cellular localizations of well‐established markers for P‐bodies (DCP1 and DCP5) (Xu and Chua, 2009; Jang et al., 2019) and stress granules (PAB2) (Dufresne et al., 2008; Merret et al., 2013) were investigated. All of the antigens tested were specifically localized to the cytoplasm (Figure 2). In periods when the Sm‐rich cytoplasmic bodies were visible, DCP1 was distributed in a disperse manner throughout the cytoplasm and no distinct accumulations of P‐body markers could be observed (Figure 2a). Remarkably, P‐bodies were easily detected during periods of nuclear localization of the Sm proteins. Numerous cytoplasmic accumulations of DCP5 were observed at this stage, albeit showing no colocalization with Sm or poly(A) RNA (Figure 2b). Interestingly, the distribution of the polyadenylated RNA and the decapping machinery was clearly spatially separated. Although the cytoplasmic pool of poly(A) RNA was dispersed around the nucleus, the P‐bodies were located at the periphery of the cytoplasm (Figure 2b). For PAB2, only diffused fluorescence was visible in the cytoplasm (Figure 2c). The lack of distinct accumulations of PAB2 demonstrates that the microsporocytes do not form SGs during diplotene. This was expected, as the cells were not subject to stress conditions prior to analysis. Despite no significant accumulation of PAB2 within Sm‐rich cytoplasmic bodies, sometimes the colocalization of this protein with Sm accumulations could be observed, although the concentration of PAB2 was similar to that in the surrounding cytoplasm (Figure 2d). We attribute this to some residual pool of the poly(A) RNA that is still bound to PAB2 within the cytoplasmic bodies.
Figure 2.
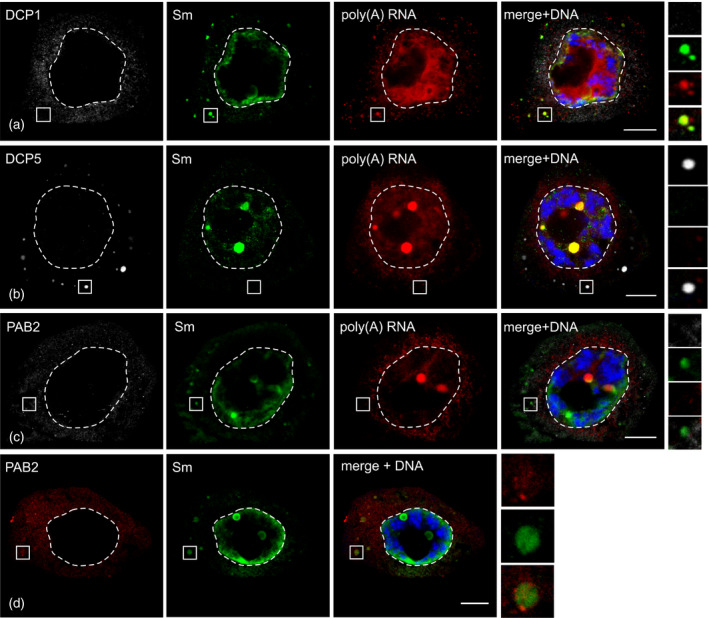
S‐bodies are not functionally related to P‐bodies or stress granules. Localization of proteins related to degradation (a, b) and translation regulation (c, d) in Larix decidua microsporocytes. The right‐hand panel represents the magnification of the fragments of the cytoplasm, which are marked with a square. Scale bars: 10 μm.
In summary, none of the protein markers investigated accumulated within the observed cytoplasmic bodies, indicating that they are not compositionally related to PBs or SGs. This conclusion is further confirmed by the fact that DCP1, DCP5 and PAB2 did not coprecipitate with Sm proteins from larch anthers (Table S2). Based on these observations and taking into consideration the general nomenclature used for this type of microdomain, we propose the name of S‐bodies for the structures observed in larch microsporocyte cytoplasm.
S‐bodies are formed during the cytoplasmic export of poly(A) RNA
Larch microsporocytes exhibit a rigorously regulated pattern of metabolic activity. During the diplotene stage of meiosis, five bursts of de novo synthesis of poly(A) RNA occur, separated by periods of transcriptional silencing (Kołowerzo‐Lubnau et al., 2015). Each of these cellular poly(A) RNA turnover cycles comprises subsequent stages of nuclear synthesis, cytoplasmic export and degradation of mRNA (Figure 3). A detailed microscopic analysis showed that the S‐bodies emerge periodically in each of the five cycles of poly(A) RNA turnover. Based on the transcription rate and localization of the poly(A) transcripts, the longest and most intensive fourth cycle could be divided into seven stages. Shortly after intensive synthesis in the nucleus (stages I and II; Figure 3a), poly(A) RNAs are transported to the cytoplasm, as indicated by an increasing level of labeling in this compartment (stages III–VII; Figure 3a). A remarkable ring‐like accumulation of poly(A) RNA was observed around the nucleus, reflecting the pool of newly exported transcripts. Additionally, starting at stage III of the cycle, distinct foci of the poly(A) RNA and Sm proteins were observed and represented the S‐bodies. At stage IV, the number of bodies was the highest observed in the whole cycle (380 ± 35 bodies per cell; Figure 3b). During the subsequent stages of cytoplasmic RNA localization, this number consequently decreased (stages V–VII; Figure 3b). It should be emphasized, however, that throughout the whole period when S‐bodies were observed, there was a significant colocalization of both poly(A) RNA and Sm proteins (Pearson correlation coefficient, PC = 0.69 ± 0.02; Figure 3d) in the cytoplasmic pool. Furthermore, the Manders’ overlap calculation demonstrated that a substantial fraction of Sm proteins in the cytoplasmic pool had colocalized with poly(A) RNA (67.43 ± 2.90%; Figure 3e).
Figure 3.

The spatial and temporal distribution of Sm proteins in the fourth cycle of poly(A) RNA turnover. (a) Localization of Sm proteins in the fourth cycle of poly(A) RNA turnover. I–VIII: successive stages of the cycle of poly(A) RNA turnover. A detailed description is provided in the text; CB, Cajal body. Scalr bars: 15 μm. (b) The mean number of S‐bodies per cell in subsequent stages of the cycle of poly(A) RNA turnover. Error bars indicate standard errors of the mean (SEMs). P < 0.05. (c) The mean volume of S‐bodies in subsequent stages of the cycle of poly(A) RNA turnover. (d, e) Analysis of the colocalization of poly(A) RNA and Sm proteins in the cytoplasm at subsequent stages of the poly(A) RNA cycle in the cell. The values are shown as means ± standard errors of the mean (SEMs); PC, Pearson colocalization coefficient. (f) The percentages were calculated on the basis of the Manders’ overlap coefficient, in which the value of 1 was converted to 100% colocalization. Error bars indicate the standard errors of the mean (SEMs); a–b, P < 0.01; a–c and b–c, P < 0.001.
The overlap coefficient gradually increased as the cytoplasmic export of mRNAs proceeded: from 35.76 ± 3.09% at stage III to 88.03 ± 2.13% of the total cytoplasmic Sm protein pool at stage VI (Figure 3e). We also estimated the percentage of the cytoplasmic poly(A) RNA pool present in S‐bodies during the fourth cycle of transcriptional activity at the diplotene stage. The analysis revealed that 3.08 ± 0.42% of the total cytoplasmic pool of poly(A) RNA had accumulated in cytoplasmic bodies, and this accumulation was consistent throughout the cytoplasmic phase of the RNA cell cycle (differences between stages were not statistically significant: analysis of variance, ANOVA, P > 0.05; Figure 3f).
To test the relationship between the occurrence of cytoplasmic bodies and the metabolic state of the cell, a quantitative analysis of the marker for transcriptional activity (active RNA pol II) was performed in the three cycles with the most intensive poly(A) RNA turnover (Figure 4). During the stages of RNA cytoplasmic export (stages III–VII; see Figure 3a), that is, when the Sm proteins and polyadenylated transcripts accumulate within cytoplasmic bodies, the transcriptional activity was considerably decreased compared with the transcriptional activity in the nuclear stages of mRNA synthesis (stages I and II; see Figures 3a and 4a). In contrast, the level of the marker for potential translational activity (5S rRNA) showed no correlation (Figure 4b). These results indicate that the formation of S‐bodies in the cytoplasm is slightly postponed with respect to bursts of nuclear mRNA synthesis, and that these microdomains appear in periods of decreased transcriptional activity.
Figure 4.
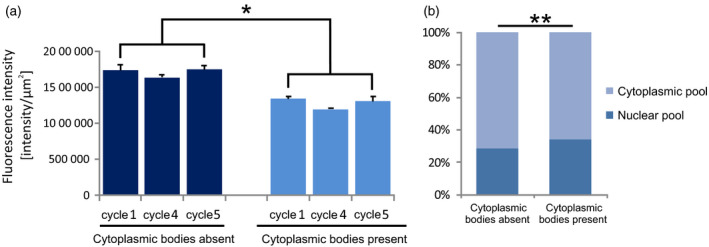
Quantitative analysis of the markers for transcriptional activity (RNA pol II and 5S rRNA). (a) Quantitative analysis of the active level of RNA polymerase II in the different cycles of poly(A) RNA synthesis in the presence and absence of S‐bodies in the cytoplasm of the cell. The error bars indicate the standard errors of the mean (SEMs); *P < 0.05. (b) Analysis of the nucleus:cytoplasm ratio of 5S rRNA in cells containing and not containing S‐bodies; **P < 0.01.
S‐bodies are not sites of spliceosomal particle accumulation
The well‐known and broadly documented role of Sm proteins in the nucleus is their participation in pre‐mRNA splicing. Sm proteins bind uridine‐rich small nuclear RNAs (U snRNAs) in the form of a heptameric ring around the transcript, creating the core spliceosomal ribonucleoparticles U1, U2, U4 and U5 snRNPs (Zieve et al., 1988; Baserga and Steitz, 1993; Raker et al., 1999; Shaw et al., 2008). The assembly of U snRNP involves a highly dynamic cytoplasmic stage at which the Sm proteins are loaded onto the U snRNA transcripts and the 5′ trimethyl‐guanosine (m3G) cap is formed (Mattaj, 1986; Urlaub et al., 2001; Yong et al., 2010). Moreover, our previous research (Hyjek et al., 2015) revealed that, during larch meiosis, the cytoplasmic stage of U snRNP assembly can occur in two spatially distinct manners: it may be diffused throughout the cytoplasm or localized within snRNP‐rich cytoplasmic bodies (CsBs). The assembly mode depends on the rate of de novo U snRNP formation and the level of U snRNP in the nucleus. Thus, it could not be precluded that the S‐bodies are related to the assembly and/or sequestration of spliceosomal elements. To test this possibility, the cellular distribution of both the nucleotide (U1, U2, U4 and U5 snRNA and m3G cap) and protein (U2B″) components of the U snRNPs were analyzed in relation to the S‐bodies (Figures 5 and S4). All the spliceosomal components were specifically localized within the nucleus, with significant accumulation in the CBs. In contrast, no signal in the form of distinct clusters of U snRNA, m3G cap or U2B″ protein was observed in the cytoplasm, indicating that the formation of S‐bodies does not coincide with the stage of U snRNP assembly in the cytoplasm. The lack of any U snRNP components and the presence Sm proteins in the S‐bodies demonstrates that the bodies represent the cytoplasmic pool of canonical Sm proteins acting outside of the spliceosome, and that this localization is presumably related to mRNA.
Figure 5.

S‐bodies are not sites of the U snRNP assembly. Labeling of Sm proteins in colocalization with selected splicing elements. There was no accumulation of U1 snRNA (a), m3G cap (b) or U2B″ proteins (c) in the S‐bodies (arrowheads). The right‐hand panel represents the magnification of the fragments of the cytoplasm, which are marked with a square; CB, Cajal body; T, anther tapetum cell. Scale bars: 10 μm.
Identification of cytoplasmic mRNA transcripts associated with Sm proteins
To identify the mRNA transcripts that accumulate within S‐bodies, we employed an RNA immunoprecipitation (RIP) approach against the Sm proteins from the larch microsporocyte cytoplasm, followed by high‐throughput sequencing of the immunopurified RNAs (RIP‐seq).
As all three anti‐Sm antibodies used for the assays in situ could label the cytoplasmic bodies with comparable specificity and strength (see Figure 1), an additional validation of the results from the RNA immunoprecipitation analyses was performed to select the most efficient antibody to use for further analysis (Figure 6a). The enrichment of U2 snRNA, a canonical cellular partner of the Sm proteins, was estimated and the results were normalized to 5S rRNA (which does not interact with Sm proteins). The experiment revealed that the Y12 MAbs obtained from Matera’s laboratory was the most effective for the RIP assay, precipitating over 1130 times more U2 snRNA than the control (mock IP, no antibody added). This was significantly different than the 84‐ and 12‐fold enrichment obtained using ANA No. 5 and the commercially available Y12, respectively.
Figure 6.
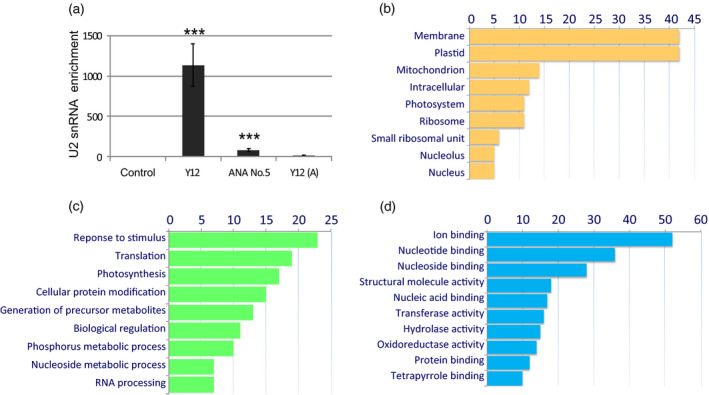
Identification of mRNA transcripts. (a) Determination of the efficiency of U2 snRNA immunoprecipitation using different anti‐Sm protein antibodies [Y12, Ana No. 5, and Y12 (A)] by real‐time (RT)‐PCR. The graph shows the enrichment values relative to the control (for the control, the value was 1), normalized to the level of 5S rRNA. The error bars indicate the standard errors of the mean (SEMs). ***P < 0.001. (b–d) The functional annotation of cytoplasmic mRNA precipitating with Sm proteins: biological process (b), molecular function (c) and cell compartment (d). The numbers along the horizontal axis indicate the number of genes with significant enrichment ratios, compared with the control, for all RIP transcripts.
The Sm protein‐associated transcripts are listed in Appendix S1. A total of 245 transcripts were identified as cytoplasmic partners of Sm proteins, 222 of which were classified as mRNAs. Most of the transcripts were homologous to Pinaceae. The gene ontology (GO) functional annotation revealed that the cytoplasmic Sm interactome consisted of mRNAs encoding a vast range of proteins, localizing to many cell compartments and linking a number of metabolic processes. A total of 141 sequences (58%) were annotated with 728 GO terms. This functional analysis revealed three major categories of Sm protein‐associated mRNAs, and they coded for proteins related to: (i) ribosomes/translation; (ii) mitochondria/energy metabolism; and (iii) chloroplasts/photosynthesis (Figure 6b–d).
To test whether the immunoprecipitated mRNAs are indeed accumulated within S‐bodies, the spatial distribution of the transcripts was analyzed in situ. From the 222 Sm protein‐associated mRNAs, 16 were selected representing different cellular functions and localizations. A double labeling assay of the larch microsporocytes was performed for the Sm proteins and each of the selected mRNAs (Figures 7 and S5).
Figure 7.
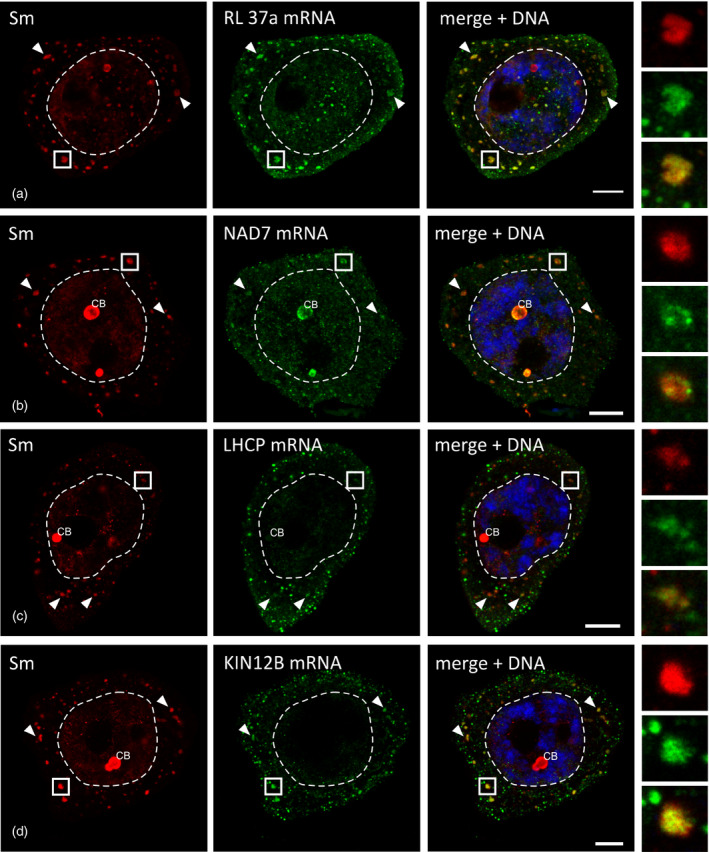
In situ localization of mRNA precipitating with Sm proteins. The panels represent stages IV–VI of poly(A) RNA turnover. Numerous clusters of mRNA that colocalized with the Sm proteins (arrowheads) are visible in the cytoplasm. The right‐hand panel represents the magnification of the fragments of the cytoplasm, which are marked with a square. (a) RL 37a, large ribosome subunit protein 37a; (b) NAD7, NADH 7 dehydrogenase subunit; (c) LHCP, protein binding chlorophyll a–b; (d) KIN12b, kinesin 12b‐like protein. CB, Cajal body. Scale bars: 10 μm.
The microscopic analysis confirmed the specific localizations of the 13 mRNAs within the cytoplasmic bodies. This specific distribution pattern was observed for mRNAs that encoded diverse proteins, including those involved in translation (Figures 7a and S5a,c,d), energy metabolism (Figures 7b and S5f) and photosynthesis (Figures 7c and S5i), as well as structural proteins of the cytoskeleton (Figures 7d and S5b). Additionally, S‐bodies were enriched in several mRNAs related to protein folding (Figure S5g,h), proteasomal degradation (Figure S5j) and miRNA biogenesis (Figure S5e).
A different pattern of localization was observed for only three mRNAs (Figure S5k–m). Transcripts encoding cellulase (Figure S5k) and tubulin (Figure S5l) showed a diffuse pattern of cytoplasmic distribution, with no distinct concentration in any particular area. Double labeling of these mRNAs with Sm proteins revealed that both of them were found in the cytoplasmic bodies, although without noticeable accumulation (i.e. there were no differences between the level of staining within the cytoplasmic bodies and in the surrounding cytoplasm). For the mRNA that encodes a light‐independent protochlorophyllide oxidoreductase (LI‐POR), the level of staining was below the limit of detection (Figure S5m).
In the next step, we performed an additional analysis of the SmD mRNAs that were identified in the cytoplasmic transcriptome of the larch microsporocytes, but not enriched in the Sm protein‐immunoprecipitated pool of transcripts obtained via RIP‐seq assay. The lack of cytoplasmic accumulation within these microdomains was observed in the case of mRNAs encoding SmD1, SmD2 and SmE proteins (Figure S6). This finding demonstrates that the S‐bodies are not sites of de novo Sm protein synthesis.
The cytoplasmic Sm proteins interactome
In order to explore the Sm proteic interactome, the cytoplasmic fraction of the larch anther cells was subjected to anti‐Sm immunoprecipitation, which was subsequently assessed by mass spectrometry. A total of 118 proteins were detected, of which five represented canonical Sm proteins (SmD1a, SmD1b, SmD3, SmD3b and SmB; Tables S2 and S3). Interestingly, there was a trend similar to the results obtained for mRNA identified by RIP‐seq. Similar to the Sm protein RNA interactome, a significant fraction of the proteic interactome included proteins related to: (i) ribosomes/translation (eIF2/IF5, RL10e, RS1, RS3, RS28 and OVA2); (ii) mitochondria/energy metabolism (SHM1 and SHM2 methyltransferases, IDH dehydrogenases and ATM1 ABC transporters); and (iii) plastids/photosynthesis (RCA RuBisCO activase and TKL1 transketolase) (Tables S2 and S3). Furthermore, two RNA‐binding protein families were identified as coprecipitating with Sm proteins: Alba (acetylation lowers binding affinity) and ECT (evolutionarily conserved C‐terminal region). Alba refers to a broad group of RNA‐ and DNA‐binding proteins implicated in chromatin organization, the regulation of transcription and translation, and RNA metabolism (Goyal et al., 2016). It has been shown that these proteins are involved in the regulation of plant cell development, where they perform essential roles in sperm cell specification in A. thaliana (Borg et al., 2011). Fewer ECT proteins have been investigated compared with mRNA‐binding proteins. The scarce ECT research indicates the role of ECT1 and ECT2 in calcium signal transduction from the cytoplasm to the nucleus that affects gene expression (Ok et al., 2005).
DISCUSSION
S‐bodies: novel cytoplasmic mRNA domains in plant cells
Thus far, no distinct Sm proteins and mRNA accumulation has been observed in the cytoplasm of plant cells; however, a growing body of evidence shows a specific localization of Sm proteins and particular mRNAs in animal germ granules, which has implications for transcript localization, translational control and mRNA turnover. It was demonstrated that SmE and SmG, localizing to P‐granules in Caenorhabditis elegans, are involved in transcriptional quiescence in germline precursors, and that this coordination of germline differentiation is splicing independent (Barbee et al., 2002; Barbee and Evans, 2006). P‐granules are structurally and functionally related to nuages in Xenopus oocytes, where the Sm proteins, but not the other splicing elements, are localized. It was proposed that Sm proteins facilitate the transport of the mRNAs specific to germ granules (e.g. Xcat2) from the nucleus to the cytoplasm (Bilinski et al., 2004). In mouse spermatocytes, Sm proteins accumulate in the RNP‐rich chromatoid body (Biggiogera et al., 1990; Moussa et al., 1994) and form a complex with a marker protein of these domains, MTR1 (Chuma et al., 2003). In Drosophila melanogaster, the accumulation of SmB and SmD3 at the posterior of the developing oocyte is directly linked to the unique localization and polarization of oskar mRNA. The proper localization of this transcript is crucial for germ‐cell specification during early embryogenesis (Gonsalvez et al., 2010; Jaglarz et al., 2011). Germline and early embryonic cells are highly metabolically active, with a spatiotemporally ordered pattern of expression for distinct groups of developmental genes. The European larch microsporocytes are male germline precursors that have been shown to be highly active periodically during diplotene (Kołowerzo‐Lubnau et al., 2015). In this context, the S‐bodies are similar to the germ granules observed in animals and might be considered, to some extent, as their plant counterparts.
Sm proteins as a part of the cytoplasmic mRNP complex
A growing body of evidence suggests additional roles for the canonical Sm proteins outside of the spliceosome in the processing, localization and translational control of mRNPs. Little is known about which mRNPs in the pool of all transcripts are regulated by Sm proteins, however, and what is the mechanism of their interaction. To determine which mRNAs are accumulated within the S‐bodies, we performed a transcriptomic analysis of cytoplasmic Sm protein‐mRNP complexes, followed by in situ detection analysis. The functional annotation showed that three major classes of Sm protein‐associated mRNAs could be distinguished, namely, those encoding proteins related to: (i) ribosomes/translation; (ii) mitochondria/energy metabolism; and (iii) chloroplasts/photosynthesis. This finding is in agreement with the results obtained for Drosophila ovaries and HeLa cells. Lu et al. (2014) demonstrated that among 72 and 30 Sm protein‐associated, fully spliced, polyadenylated mRNAs for Drosophila and HeLa cells, respectively, a significant portion encoded ribosomal and mitochondrial proteins. Some of the mRNAs show overlap with all of the Sm protein interactomes studied to date – plant (this work), insect and mammalian (Lu et al., 2014) – including those that encode small and large ribosomal subunit proteins, translation initiation factor eIF2Bα, NADH dehydrogenase and cytochrome oxidases. Furthermore, Sm proteins were also associated with the mRNA products of intronless genes (e.g. histone H2A mRNAs in human cells and 60S ribosomal L37 mRNA in larch cells), proving that this interaction is completely independent of pre‐mRNA splicing. The results from the animal cells do not clarify whether the Sm protein‐associated mRNAs are localized to the nucleus or to the cytoplasm, as the RIP‐seq was performed on whole‐cell extracts. Nevertheless, together with the aforementioned reports, our results indicate that the association between splicing‐independent Sm proteins and mRNA is conserved in invertebrates, vertebrates and plants.
Mutual post‐transcriptional coordination of mRNA subpopulations has been previously described by Keene and Tenenbaum (2002). The following hypothesis was proposed based on eukaryotic post‐transcriptional regulons (or operons): structurally and functionally related genes are regulated in groups by specific RNA‐binding proteins to facilitate the organization of gene expression during cell growth and development (Keene and Tenenbaum, 2002; Keene, 2007). The most acknowledged RBPs involved in this type of regulation are Pumilio proteins (PUF) that participate in the post‐transcriptional silencing of particular groups of genes in yeasts (Gerber et al., 2004), Drosophila (Gerber et al., 2006), mammals (Bohn et al., 2018; Goldstrohm et al., 2018) and plants (Tam et al., 2010). The post‐transcriptional regulons also operate in a number of developmental processes and stress responses (Gorospe, 2003; Mazan‐Mamczarz et al., 2003; Lü et al., 2006), including the immunological response in mammals (Lai et al., 1999; Ule et al., 2003; Cheadle et al., 2005; Lykke‐Andersen and Wagner, 2005). In HeLa cells, the spliceosomal proteins U2AF (U2 auxiliary factor) and PTB (polypyrimidine tract binding protein) bind distinct groups of mature mRNAs in both the nucleus and the cytoplasm (Gama‐Carvalho et al., 2006). Whereas U2AF interacts predominantly with transcriptional factors and cell cycle regulator mRNAs, PTB‐bound transcripts encode proteins related to intracellular and extracellular trafficking and apoptosis. Moreover, Ser–Arg‐rich (SR) spliceosomal proteins are linked to the cytoplasmic export of intronless histone mRNAs (Huang and Steitz, 2001; Änkö et al., 2012), the stimulation of translation (Sanford et al., 2004) and the cytoplasmic degradation of particular transcripts (Lemaire et al., 2002). Recently, the involvement of SmD1 in post‐transcriptional gene silencing (PTGS) was revealed in A. thaliana (Elvira‐Matelot et al., 2016). Based on the results from a genetic screening of PTGS‐defective mutants, it was proposed that, in addition to its role in pre‐mRNA splicing, SmD1 facilities cytoplasmic PTGS. By protecting transgene‐derived aberrant RNAs from degradation by the RNA quality control pathway (RQC) in the nucleus, SmD1 presumably promotes cytoplasmic export of transcripts to siRNA bodies, where PTGS occurs (Elvira‐Matelot et al., 2016). Thus, the involvement of the evolutionarily conserved canonical Sm proteins in the splicing‐independent post‐transcriptional control of mRNA metabolism is emerging as another regulatory component of gene expression. Taken together, these findings suggest that Sm proteins may represent a novel example of RNA‐binding proteins that regulate functionally related groups of mRNAs within the cytoplasm. In metabolically active microsporocytes (which enlarge sixfold during diplotene; Kołowerzo‐Lubnau et al., 2015), the most abundant groups of transcripts are those related to efficient translation, energy production and photosynthesis, which aligns with the results presented.
Therefore, it might be assumed that the Sm protein–mRNA interaction within a cell is a ubiquitous process, applying to a vast number of transcripts.
The mechanism of Sm protein–mRNA interaction
An open question concerns the mechanism governing the selectivity of Sm proteins towards certain types of mRNAs and the mechanisms for their interactions. The only Sm protein‐binding elements described to date are the Sm sites present in U snRNAs and yeast telomerase RNA (TLC1 and TER1) (Seto et al., 1999; Tang et al., 2012), and in the CAB box found in H/ACA CB‐specific RNAs (U85, U87 and U89 small cajal body‐specific RNAs [scaRNAs]) (Darzacq et al., 2002; Richard et al., 2003) and human telomerase RNA (hTR) (Fu and Collins, 2006). Sm sites or CAB box domains have not been found in mRNA to date. Furthermore, the specificity of scaRNA binding by Sm proteins is not fully understood. In both Drosophila and HeLa cells, Sm proteins coprecipitated only a fraction of the scaRNAs, with no apparent structural features that could explain this selectivity (Lu et al., 2014). Several studies have demonstrated the participation of spliceosomal U1 snRNPs in splicing‐independent post‐transcriptional regulation of gene expression, including nascent mRNA protection from premature cleavage and polyadenylation at cryptic sites (Kaida et al., 2010; Berg et al., 2012), regulation of polyadenylation of retroviral RNA (Ashe et al., 1997; Ashe et al., 2000; Schrom et al., 2013) as well as control of RNA pol II promoter directionality and promotion of sense mRNA transcription (Almada et al., 2013; Ntini et al., 2013). Recently, it was revealed that in plants U1 snRNP regulates miRNA biogenesis (Knop et al., 2017; Bhat et al., 2019) and mediates the interactions between the microprocessor, spliceosome and polyadenylation machinery (Szweykowska‐Kulinska et al., 2013; Stepien et al., 2016). Moreover, it was shown that in animal cells the interaction between Sm proteins and mRNAs is mediated by U1 and presumably other snRNPs. Twelve‐nucleotide putative binding sites for the 5′ end of the U1 snRNPs were identified in Sm protein‐associated mature mRNAs within the coding sequence, far from intron–exon boundaries. It was thus proposed that U1 snRNPs bind mature mRNAs to promote the recruitment of RNA processing factors, thereby affecting mRNA localization, translation and/or turnover (Lu et al., 2014). In the case of the larch microsporocytes, the Sm protein–mRNA interaction is apparently not mediated by U snRNPs, as there was no enrichment in U1 snRNA in the RIP‐seq analysis. Additionally, the in situ localization of splicing elements demonstrated that the U snRNAs and m3G caps did not colocalize within the S‐bodies, suggesting a different mechanism of Sm protein–mRNA interaction in these cells. Studies on Drosophila and C. elegans have revealed that only some Sm proteins associate with mature mRNAs (Barbee et al., 2002; Gonsalvez et al., 2010; Gonsalvez and Long, 2012). Sm proteins constitute an evolutionarily conserved family and are classified into canonical (spliceosomal) Sm and noncanonical LSm (Sm‐like) proteins (Hermann et al., 1995; Urlaub et al., 2001; Khusial et al., 2005; Scofield and Lynch, 2008). In Archaea, LSm proteins participate in tRNA maturation and ribosome synthesis (Mura et al., 2003). In Eubacteria, the Hfq homolog of the Sm protein regulates mRNA translation (Schumacher et al., 2002). The diversification of the ancestral Sm proteins in eukaryotes enabled their involvement in various processes. The LSm 2‐8 ring is found in U6 and U6 atac snRNPs (spliceosomal subunits), as well as in the U8 small nucleolar ribonucleoproteins (snoRNPs) involved in 5.8S and 28S rRNA processing (Peculis, 1997; Achsel et al., 1999). LSm 1‐7 forms a cytoplasmic complex that promotes mRNA decapping in P‐bodies (Bouveret et al., 2000; Decker and Parker, 2012). Another LSm ring variant, LSm 2‐7, binds snR5 snoRNA, which is essential for rRNA pseudouridylation (Fernandez et al., 2004). The unique LSm 10 and LSm 11 proteins are part of the U7 snRNP involved in the 3′ end maturation of histone mRNA in animal cells (Schümperli and Pillai, 2004). The Arabidopsis genome contains 42 Sm and LSm protein genes (Cao et al., 2011), many of which have not yet been characterized. Taking these findings into consideration, it could not be precluded that in larch microsporocytes the Sm protein–mRNA association is driven by another noncanonical Sm/LSm ring or is indirectly mediated by other RBPs/RNPs. This hypothesis is partially supported by an IP‐MS analysis that revealed a significant enrichment of the Alba proteins within the cytoplasmic Sm interactome. Alba proteins constitute an evolutionarily ancient family of dimeric nucleic acid‐binding proteins present in all domains of life (Goyal et al., 2016). With their broad functional plasticity, Alba proteins are implicated in genome organization, transcription regulation, RNA metabolism, cell differentiation and stress responses. Structurally, plant Alba paralogs display extensive diversity. Their association with target RNA is sequence independent, and the formation of the Alba–RNA complex facilitates the accessibility of the transcript for RNA processing factors (Goyal et al., 2016). It has been shown that the Alba interactome comprises a vast range of different RNAs and RBPs, including mRNA regulators such as poly(A)‐binding proteins (PABPs), ribosomal subunits and translational factors (Guo et al., 2003; Subota et al., 2011; Gissot et al., 2013). Furthermore, the formation of a particular complex within a cell depends on Alba protein abundance and local concentration, the composition of the dimer (homo or hetero) and post‐translational modifications, including arginine methylation (Lott et al., 2013; Ferreira et al., 2014). Interestingly, several studies point to a cytoplasmic fraction of the Alba protein within the cell. In Plasmodium falciparum, PfAlba 1, 2 and 4 are localized as distinct cytoplasmic foci during proliferation (Chêne et al., 2012). Cytoplasmic localization of OsAlba1 was confirmed in Oryza sativa (rice), and its level of expression increased in response to dehydration stress (Verma et al., 2014). PAlba 1–PAlba 3 from Plasmodium berghei coprecipitate with P‐granule components PABP and eIF4E (Mair et al., 2010). Additionally, the association of TbAlba 1–TbAlba 4 with cytoplasmic mRNAs was demonstrated in Trypanosoma brucei, in which they were localized to stress granules upon starvation, indicating their role in spatiotemporal translational regulation (Mani et al., 2011; Subota et al., 2011). Similarly, the larch microsporocyte cytoplasmic Sm protein interactome was enriched in both the Alba proteins and the ribosomal and translation‐related proteins.
Sm/poly(A) cytoplasmic bodies and the metabolic activity of the microsporocyte
The results presented unambiguously show that the S‐bodies accumulate a large pool of mRNAs encoding different proteins. What would be the functional role of this kind of microdomain? The cytoplasm is a highly dynamic cell compartment where the antagonistic processes of mRNA translation and decay are strictly regulated (Rissland, 2017). The spatial separation of the mRNA processing, translational and degradation machinery in distinct microdomains facilitates the efficiency of post‐transcriptional regulation, and protects the transcripts from uncontrolled translation or degradation, depending upon the recent needs of the cell (Parker and Sheth, 2007). In this study, we demonstrate that the S‐bodies do not accumulate proteins involved in mRNA degradation and therefore are not analogs of P‐bodies. We have also observed that large P‐bodies are distributed in a separate area of the cytoplasm poly(A) RNA, and that the occurrence of P‐bodies is most often separated temporally from periods of S‐body formation. This finding is in agreement with previous observations showing that the mRNAs that accumulate within P‐bodies are deadenylated (Sheth and Parker, 2003; Aizer et al., 2014), which is not the case with S‐bodies.
Our previous studies have shown that the diplotene stage is the most transcriptionally active stage of prophase I (Kołowerzo‐Lubnau et al., 2015). We comprehensively examined the transcriptional activity, levels and distribution of poly(A) RNA, RNA polymerase II (an enzyme responsible for mRNA synthesis) and snRNAs, which are responsible for mRNA maturation (Smoliński et al., 2007; Smoliński et al., 2011; Smoliński and Kołowerzo, 2012; Kołowerzo‐Lubnau et al., 2015; Hyjek et al., 2015). We also examined whether mRNA synthesis and maturation is accompanied by protein synthesis. The periods of intense transcription in larch microsporocytes positively correlated with periods of chromatin decondensation (the diffuse stages), whereas during the chromatin contraction stages, transcription levels decreased. As a result, RNA synthesis is not a continuous process but occurs in transcriptional bursts. The S‐bodies were localized after transcriptional bursts, indicating that these microdomains are formed as a result of pulsed, extensive mRNA synthesis in the diffuse stage.
The accumulation of a vast range of mRNAs in spatially separated cytoplasmic bodies is presumably a result of the effective organization of highly expressed transcripts during periods of greatly increased export of these molecules to the cytoplasm. As S‐bodies do not contain markers of degradation, the mRNAs are most likely sequestered to cope with the large number of molecules. This kind of regulation might act as a cellular strategy to control the accessibility of transcripts and their protein products during periods of genome contraction, at which time transcriptional regulation is not possible. This system enables the quick expression of proteins essential for normal cell growth and development (preparing the cell for division) and additionally provides the capability to respond to environmental changes and external stimuli. Recent studies have revealed that larch microsporocytes employ an unusual mechanism of nuclear mRNA retention, with a putative role for CBs. It was demonstrated that a significant pool of newly transcribed poly(A) RNAs is temporarily sequestered in the nucleoplasm and CBs before being exported to the cytoplasm (Kołowerzo et al., 2009; Smoliński and Kołowerzo, 2012). This functional connection between nuclear mRNA retention, accumulation in CBs and cytoplasmic localization is further supported by the observation that as many as 13 of the 16 mRNAs investigated as cytoplasmic partners for Sm proteins were also localized to the CBs (see Figures 6 and S5).
Conclusion
In summary, S‐bodies represent newly described intracellular microdomains that participate in mRNA post‐transcriptional regulation. Through the temporal storage of mRNAs and their spatial separation from degradation and translation factors, these domains presumably play a role in the coordination of the continuing, frequently overlapping and competing processes of transcript maturation, translation and degradation. This strict control of mRNA fate is particularly essential in cells such as microsporocytes, in which the chromatin is periodically switched on and off and gene expression occurs in bursts. We propose a model in which the accumulation of significant quantities of different types of mRNA in cytoplasmic bodies provides a mechanism for the temporal retention and storage of highly expressed transcripts until they are needed for further translation or turnover within the cytoplasm, according to the needs of the cell. The results obtained in this work support the hypothesis that evolutionarily conserved Sm proteins have been adapted to perform a plethora of functions related to the post‐transcriptional regulation of gene expression in Eukaryota. This multipurpose capacity presumably enabled them to coordinate the interdependent processes of splicing element assembly, mRNA maturation and processing, and mRNA translation regulation and degradation.
EXPERIMENTAL PROCEDURES
Plant material
For immunofluorescence/fluorescence in situ hybridization (FISH) assays, anthers of European larch (L. decidua Mill.) were collected between November and March at weekly intervals from the same tree in successive meiotic prophase (diplotene) stages to ensure consistent experimental conditions. The anthers were fixed in 4% paraformaldehyde in phosphate‐buffered saline (PBS), pH 7.2, for 12 h and squashed to obtain free meiocytes. Meiotic protoplasts were isolated from these cells according to the method of Kołowerzo et al. (2009). Isolated protoplasts were next subjected to immunostaining, FISH and immuno‐FISH assays.
For transmission electron microscopy (TEM) assays, the anthers were fixed in 4% paraformaldehyde and 0.25% glutaraldehyde (GA) in 0.1 m piperazine‐N,N′‐bis(2‐ethanesulfonic acid) (PIPES), pH 7.2, for 12 h. In addition, the samples for use in ultrastructural analysis were fixed with 2% OsO4 for 12 h at 4°C. Next, the samples were dehydrated in alcohol and embedded in LR Gold resin (Sigma‐Aldrich, https://www.sigmaaldrich.com). The material was sectioned using a Leica Ultracut UCT ultramicrotome (Leica Microsystems, https://www.leica‐microsystems.com), and the ultrathin sections were placed on nickel‐formvar‐coated grids. For observations of the ultrastructure, the samples were contrasted with 2.5% uranyl acetate for 30 min and then incubated with 2.5% lead citrate for 15 min.
For immunoprecipitation (IP) and RNA immunoprecipitation (RIP), the larch anthers were flash‐frozen in liquid nitrogen and stored at −80°C for further analysis.
Immunogold labeling of Sm proteins
Ultrathin sections were preincubated with 1% BSA in PBS, pH 7.2, for 30 min, followed by incubation with anti‐Sm antibody (ANA No. 5; Centers for Disease Control and Prevention, https://www.cdc.gov) at 1:800 dilution and 4°C overnight. After washing with PBS (5 × 10 min), the samples were incubated with secondary anti‐human antibody coupled to 20 nm colloidal gold particles (1:20 dilution in 1% BSA in PBS, pH 7.2; BBI Solutions, https://bbisolutions.com) for 1 h at 37°C, rinsed in PBS and water, and then contrasted with 1% phosphotungstic acid (PTA) and 2.5% uranyl acetate. The ultrastructural and immunogold analyses were performed with the use of a JEOL EM 1010 transmission electron microscope (JEOL, https://jeol.pl).
Design of the multi‐labeling reactions
Prior to the assay, the cells were treated with 0.1% Triton X‐100 in PBS for 25 min to induce cell membrane permeabilization. After labeling, the samples were stained for DNA detection with Hoechst (1:2000) and mounted in ProLong Gold Antifade reagent (Life Technologies, now ThermoFisher Scientific, https://www.thermofisher.com). The sequences for all the oligo probes used in this work are summarized in Table S1.
Double labeling of the poly(A) RNA/U snRNA/5S rRNA and Sm proteins
The samples were incubated with Cy3‐labeled probes: oligo(dT), U1 snRNA, U2 snRNA, U4 snRNA, U5 snRNA and 5S rRNA. The 100‐µm stock solutions with the probes were diluted 1:500 in hybridization buffer [30% v/v formamide, 4× saline sodium citrate (SSC), 5× Denhardt’s buffer, 1 mm ethylenediaminetetraacetic acid (EDTA) and 50 mm phosphate buffer] and incubated for 4 h [oligo(dT)] or overnight (other probes) at 27°C. After washing with 4× SSC (5 × 1 min), 2× SSC (5 × 1 min) and PBS (1 × 3 min), the samples were treated with the following antibodies for the detection of the Sm proteins: human anti‐Sm ANA No. 5 (1:300 in 0.2% bovine serum albumin, acetylated [acBSA] in PBS, pH 7.2), mouse anti‐Sm Y12 (1:100 in 0.01% acBSA in PBS, pH 7.2; Abcam, https://www.abcam.com), mouse anti‐Sm Y12 (1:500 in 0.01% acBSA in PBS, pH 7.2; a kind gift from Michael P. Terns, University of Georgia, Athens, GA, USA). The samples were then rinsed with PBS (5 × 3 min) and incubated for 1 h at 37°C with the following secondary antibodies: anti‐human Alexa 488 (1:750; Thermo‐Fisher Scientific) or anti‐mouse Alexa 488 (1:1000 in 0.01% acBSA in PBS, pH 7.2; Thermo‐Fisher Scientific).
Double labeling of the mRNA and Sm proteins
Immunodetection of Sm proteins with ANA No. 5 was performed first to detect the mRNA and Sm proteins according to the procedure described above. The secondary anti‐human tetramethylrhodamine (TRITC) antibody (1:200 in 0.01% acBSA in PBS, pH 7.2; Thermo‐Fisher Scientific) was used in this assay. Next, the samples were incubated overnight at 27°C with DIG‐labeled probes that are complementary to the distinct mRNAs (Table S1) diluted 1:200 in hybridization buffer. After washing with 4× SSC (5 × 1 min), 2× SSC (5 × 1 min), 1 × SSC (1 × 10 min) and PBS (1 × 3 min), the slides were incubated for 5 h at 8°C with mouse anti‐ digoxigenin (anti‐DIG) antibody (1:200 in 0.05% acBSA in PBS, pH 7.2; Roche, https://www.roche.com) and rinsed in PBS. The samples were then incubated for 1 h at 37°C with secondary anti‐mouse Alexa 488 at 1:1000 in 0.01% acBSA in PBS, pH 7.2.
Double localization of the proteins
Samples were incubated overnight at 4°C with the following primary antibodies: mouse anti‐m3G (1:50; Santa Cruz Biotechnology, https://www.scbt.com), mouse anti‐U2B″ (1:20; LS Bio, https://www.lsbio.com) and rat anti‐RNA pol II (elongated form, hyperphosphorylated Ser2 of the C‐terminal domain; 1:100; Chromotek, https://www.chromotek.com) in 0.01% acBSA in PBS, pH 7.2. After rinsing with PBS, the samples were incubated for 1 h at 37°C with the following secondary antibodies: anti‐mouse Alexa 546 (ThermoFisher Scientific), anti‐rat Alexa 488 (ThermoFisher Scientific) or anti‐rabbit Alexa 488 (ThermoFisher Scientific) 1:500 in 0.01% acBSA in PBS, pH 7.2. Next, the slides were rinsed with PBS, pre‐incubated with 2% BSA in PBS, pH 7.2, for 15 min, and incubated overnight at 4°C with the anti‐Sm ANA No. 5 antibody diluted 1:300 in 0.2% acBSA in PBS, pH 7.2. After rinsing with PBS, the samples were incubated for 1 h at 37°C with the appropriate respective anti‐human antibody: Alexa 488 at 1:750 or TRITC, at 1:200 in 0.01% acBSA in PBS, pH 7.2.
Triple labeling of the poly(A) RNA and proteins
For the triple‐labeling assays, the oligo(dT) probe was first hybridized according to the method described above (overnight incubation). Next, the samples were incubated overnight at 4°C with the following primary antibodies: mouse anti‐DCP1A (1:200; Sigma‐Aldrich), rabbit anti‐DCP5 (1:500; a gift from Prof. Shu‐Hsing Wu, Academia Sinica, Taipei, Taiwan) and rabbit anti‐PAB2 (1:200; a gift from Dr Cecile Bousquet‐Antonelli CNRS Perpignan Cedex, France) in 0.05% acBSA in PBS, pH 7.2. After washing with PBS, the slides were incubated for 1 h at 37°C with anti‐mouse Alexa 488 Plus (1:1000) or anti‐rabbit Alexa 488 Plus (1:500) in 0.01% acBSA in PBS, pH 7.2. The Sm proteins were labeled as described above with ANA No. 5 primary and anti‐human Alexa 633 (1:200; ThermoFisher Scientific) secondary antibodies.
Confocal microscopy and image analysis
The images were captured with a Leica TCS SP8 confocal microscope at wavelengths of 405, 488, 543, 561 and 633 nm. The optimized pinhole, long exposure time (400 kHz) and a 63× (numerical aperture 1.4) Plan Apochromat DIC H oil immersion lens were used. Images were acquired sequentially in the blue (4′,6‐diamidino‐2‐phenylindole, DAPI), green (Alexa 488), red (TRITC, Cy3, Alexa 546) and/or far red (Alexa 633) channels. The optical sections were collected at 0.5‐μm intervals. For the bleed‐through analysis and control experiments, las af software was used. For image processing and analysis, imagej software was used (Schneider et al., 2012).
For the quantitative measurements, each experiment was performed using consistent temperatures, incubation times, and concentrations of probes and antibodies. The images were collected under consistent conditions of acquisition (laser power, emission band, gain and resolution) to ensure comparable results. Before quantification of the fluorescence intensity, the background was eliminated by adjusting the threshold according to autofluorescence based on the negative control. Between 10 and 30 cells were analyzed for each stage (experimental variant), depending on the experiment. The level of fluorescence was expressed in arbitrary units (as the mean intensity per μm2).
The statistical analysis was performed using sigmaplot 11.0. To compare two groups, Student’s t‐tests were used. To compare between several groups, statistical significance was determined by the Kruskal–Wallis test. The significance level was set at P < 0.05 for all statistical tests.
Colocalization analysis was performed with the imagej plugin jacop (Cordelières and Bolte, 2008). Between 10 and 25 cells were analyzed for each stage (experimental variant). Only the cytoplasmic area of the cells was subject to all colocalization analyses. The colocalization coefficients were calculated (Pearson's correlation coefficient, PC; M1 and M2, Manders’ overlap coefficients) for each stage and evaluated for statistical significance with Costes’ randomization test (P < 0.05).
The number and volume of the cytoplasmic bodies per cell were measured with imagej plugin 3d objects counter (Bolte and Cordelières, 2006). Between 10 and 25 cells for each stage (experimental variant) were analyzed. The statistical significance was determined by one‐way analysis of variance (ANOVA) followed by multiple comparisons using the Holm–Šídák post hoc test.
Immunoprecipitation assays
For proteomic analysis of immunoprecipitates (IP‐LC‐MS/MS), contents from the cytoplasmic fraction of the cells were immunoprecipitated according to a modified protocol based on that used by Oliva et al. (2016). Briefly, larch anthers were ground in liquid nitrogen and homogenized in protein extraction buffer at 3 ml per 1 g of plant material. The homogenates were then centrifuged twice at 14 000 g, followed by centrifugation at 16 000 g for 30 min at 4°C, to obtain native cytosolic protein extract. The supernatant (cytosolic fraction) was pre‐cleared with MagnaChiP A/G magnetic beads (Merck, https://www.merckmillipore.com) for 1 h at 4°C and incubated with anti‐Sm Y12 antibody (a kind gift from Michael P. Terns, University of Georgia, Athens, GA, USA) overnight at 4°C. Next, a fresh set of magnetic beads was added, and the mixture was incubated for 1 h at 4°C. The beads were precipitated by immobilization on a magnetic separator (ThermoFisher Scientific), washed four times with PBST (0.1% Tween 20) and resuspended in water. The experiment was performed in quadruplicate for the IP assay and triplicate for the mock IP assay (no antibody added). The LC‐MS/MS analysis was performed by the Mass Spectrometry Laboratory (IBB PAS, Warsaw, Poland) with the use of an Orbitrap mass spectrometer (ThermoFisher Scientific). The proteins were identified with mascot (Matrix Science, http://www.matrixscience.com) and the TAIR10 database (The Arabidopsis Information Resource, TAIR, https://www.arabidopsis.org). Next, the samples were analyzed with mscan softare (http://proteom.ibb.waw.pl/mscan/index.html). The list of Sm protein‐immunoprecipitated proteins was corrected with the results from the mock IP experiments. The protein was considered Sm protein‐immunoprecipitated when it was present in at least three IP samples and not present in at least two control (mock IP) samples.
For the RNA immunoprecipitation (RIP) from the cytoplasmic fraction, the assays were performed according to a modified protocol from Sorenson and Bailey‐Serres (2015). Briefly, larch anthers were ground in liquid nitrogen and RNP extraction buffer [200 mm Tris‐HCl, pH 9.0, 110 mm potassium acetate, 0.5% (v/v) Triton X‐100, 0.1% (v/v) Tween 20, 2.5 mm dithiothreitol (DTT), complete protease inhibitor (Roche) and 0.04 U μl–1 RNase inhibitor] was added at 10 ml per 1.5 g of tissue. After thawing on ice, the material was filtered through Miracloth (∅ 22–25 μm; Merck) and centrifuged at 1500 g for 2 min at 4°C. The supernatant (10 ml per sample) containing the cytoplasmic fraction (Figure S7) of the anther cells was pre‐cleared with MagnaChiP A/G magnetic beads for 1 h at 4°C and incubated with anti‐Sm antibodies [ANA No. 5, 15 μl per sample; Y12 (Abcam), 1 μg per sample; Y12 (Terns), 10 μl per sample; mock RIP, no antibody] for 2 h at 4°C. Next, a fresh set of magnetic beads was added and incubated for 1 h at 4°C. The beads were precipitated on a magnetic separator (ThermoFisher Scientific), washed four times with wash buffer [200 mm Tris‐HCl, pH 9.0, 110 mm potassium acetate, 0.5% (v/v) Triton X‐100, 0.1% (v/v) Tween 20 and 2.5 mm DTT], resuspended in 100 μl of cold wash buffer and subjected to RNA purification and cDNA library preparation.
cDNA library preparation and sequencing
RNA was purified with a TRIzol:chloroform extraction and incubated with TURBO DNA‐free DNase (ThermoFisher Scientifc), according to the manufacturer’s protocol. Next, the RNA samples were further purified by phenol:chloroform (25:24; Sigma‐Aldrich) extraction. The RNA pellet was resuspended in 20 μl of RNase‐free water.
For qPCR, the RNA was reverse transcribed with Superscript III (Invitrogen, now ThermoFisher Scientific) enzyme using random hexamers according to the manufacturer’s protocol. The qPCR reaction mix contained 1 µl of template cDNA, 5 µl of 2× concentrated PowerSYBR Green PCR MasterMix (ThermoFisher Scientific), 2 pmoles of each primer and water up to 10 µl. The primers specific for U2 snRNA or 5S rRNA (Supl. 1) were used. The assays were carried out in a 7900HT Fast Real‐Time PCR system (Applied Biosystems, now ThernmoFisher Scientific), and the cycling conditions were as follows: 95°C for 10 min; 95°C for 15 sec, and 40 cycles of 60°C for 1 min. The relative expression level of the U2 snRNA was calculated via the ΔΔC t method. The results were normalized to the 5S rRNA expression level and compared with the control (RNA isolated from the mock‐RIP experiment), for which a value of 1 was assigned.
After qualitative (Bioanalyzer 2100; Agilent, https://www.agilent.com) and quantitative (NanoDrop 2000, Qubit; ThermoFisher Scientific) evaluation, the RNA was used for cDNA library preparation with TruSeq Stranded Total RNA with a Ribo‐Zero Plant kit (Illumina, https://www.illumina.com) according to the manufacturer’s protocol. As a result of the extremely low quantity of RNA available, 30 cycles of library amplification were performed. The libraries were run on 1.5% agarose gels, and the 250–350 bp fragments were excised, eluted from the gel (GelElute gel extraction kit; Sigma‐Aldrich) and quantified via qPCR with a KAPA Library Quantification Kit for Illumina (KAPA Biosystems, now Roche) according to the manufacturer's protocol. The libraries were next sequenced on MiSeq (Illumina) with MiSeq Reagent Kit v3 (150 cycles). The reads were filtered in terms of quality via prinseq‐lite (Schmieder and Edwards, 2011). The sequence stretches with an average quality of <20 over a window of 20 nt were removed, and the reads were cut immediately before the first incidence of a degenerated base. Furthermore, trimmed reads shorter than 36 nt were removed. The reads from all libraries were pooled and assembled de novo using trinity 2.8.2 with default settings (Grabherr et al., 2011). The transcript sequences were next analyzed with blastx (for putative mRNAs) and blastn (for other RNAs) with the use of the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov) nr and nt databases, respectively. Consensus functions and ontologies were found by LCA (least common ancestor) methodologies implemented in blast2go 4.1 (Götz et al., 2008).
AUTHOR CONTRIBUTIONS
MSH participated in experimental design, performed the majority of experiments and participated in writing the article. MB and PN performed immunoprecipitation assays and cDNA library preparation. MG performed bioinformatical analysis and participated in the discussion, analysis of results and writing of the article. AKL performed confocal microscopy and image analysis and participated in discussion. AJ designed experiments and participated in the discussion and analyses of the results. DJS performed immunogold labeling, double localization of the proteins, triple labeling of the RNA and proteins, confocal microscopy and image analysis, designed experiments, participated in the discussion and analyses of the results, and in the writing of the article.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Figure S1. Western blot analysis of protein extracts obtained after immunoprecipitation using different types of anti‐Sm antibodies: Y12, ANA No. 5 and Y12 (Abcam).
Figure S2. Identification of Sm Larix decidua proteins by IP‐SDS‐PAGE‐MS.
Figure S3. Localization of the Sm proteins and poly(A) RNA on semi‐thin sections of Larix decidua Mill. anther.
Figure S4. Labeling of Sm proteins and selected splicing U snRNAs in larch microsporocytes.
Figure S5. Localization of mRNA precipitating with Sm proteins in situ.
Figure S6. Localization of the Sm mRNAs and Sm proteins in situ.
Figure S7. Validation of the Larix decidua anther cell fractionation method.
Table S1. Real‐time RT‐PCR primers and probe sequences used for in situ hybridization.
Table S2. Identification of the proteins that form a complex with Sm proteins in the cytoplasm determined by IP‐LC‐MS/MS.
Table S3. The cytoplasmic Sm protein interactome.
Appendix S1. List of Sm protein‐associated transcripts and sequences determined by RIP‐seq.
ACKNOWLEDGEMENT
This work was supported by Polish National Science Center NCN grant nos N 303 799640 and 2014/15/N/NZ3/04525.
Contributor Information
Malwina Hyjek‐Składanowska, Email: mhyjek@iimcb.gov.pl.
Dariusz Jan Smoliński, Email: darsmol@umk.pl.
DATA AVAILABILITY STATEMENT
Data supporting the findings of this work are provided in the main text and the supporting information. Raw reads generated during this project were deposited in the NCBI SRA database and are accessible through BioProject no. PRJNA624007. The full data set for the transcriptomic analysis is available at https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA624007. All other data and materials used in this study will be available at request from the corresponding authors.
REFERENCES
- Achsel, T. , Brahms, H. , Kastner, B. , Bachi, A. , Wilm, M. and Lührmann, R. (1999) A doughnut‐shaped heteromer of human Sm‐like proteins binds to the 3’‐end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 18, 5789–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer, A. , Kalo, A. , Kafri, P. , Shraga, A. , Ben‐yishay, R. , Jacob, A. and Kinor, N. (2014) Quantifying mRNA targeting to P bodies in living human cells reveals a dual role in mRNA decay and storage. J. Cell Sci. 127, 4443–4456. [DOI] [PubMed] [Google Scholar]
- Almada, A.E. , Wu, X. , Kriz, A.J. , Burge, C.B. and Sharp, P.A. (2013) Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature, 499, 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei, M.A. , Ingelfinger, D. , Heintzmann, R. , Achsel, T. , Rivera‐Pomar, R. and Lührmann, R. (2005) A role for eIF4E and eIF4E‐transporter in targeting mRNPs to mammalian processing bodies. RNA, 11, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Änkö, M.‐L. , Müller‐McNicoll, M. , Brandl, H. , Curk, T. , Gorup, C. , Henry, I. , Ule, J. and Neugebauer, K.M. (2012) The RNA‐binding landscapes of two SR proteins reveal unique functions and binding to diverse RNA classes. Genome Biol. 13, R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe, M.P. , Furger, A. and Proudfoot, N.J. (2000) Stem‐loop 1 of the U1 snRNP plays a critical role in the suppression of HIV‐1 polyadenylation. RNA, 6, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe, M.P. , Pearson, L.H. and Proudfoot, N.J. (1997) The HIV‐1 5’ LTR poly (A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J. 16, 5752–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee, S.A. and Evans, T.C. (2006) The Sm proteins regulate germ cell specification during early C. elegans embryogenesis. Dev. Biol. 291, 132–143. [DOI] [PubMed] [Google Scholar]
- Barbee, S.A. , Lublin, A.L. and Evans, T.C. (2002) A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr. Biol. 12, 1502–1506. [DOI] [PubMed] [Google Scholar]
- Baserga, S.J. and Steitz, J.A. (1993) The diverse world of small ribonucleoproteins. RNA World, 24, 359–381. [Google Scholar]
- Bassett, C.L. (2012) Cajal bodies and plant RNA metabolism. CRC Crit. Rev. Plant Sci. 31, 258–270. [Google Scholar]
- Beggs, J.D. (2005) Lsm proteins and RNA processing. Biochem. Soc. Trans. 33, 433–438. [DOI] [PubMed] [Google Scholar]
- Berg, M.G. , Singh, L.N. , Younis, I. et al . (2012) U1 snRNP determines mRNA length and regulates isoform expression. Cell, 150, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, S.S. , Bielewicz, D. , Grzelak, N. et al . (2019) mRNA adenosine methylase (MTA) deposits m6A on pri‐miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana. bioRxiv. 10.1101/557900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggiogera, M. , Fakan, S. , Leser, G. , Martin, T.E. and Gordon, J. (1990) Immunoelectron microscopical visualization of ribonucleoproteins in the chromatoid body of mouse spermatids. Mol. Reprod. Dev. 26, 150–158. [DOI] [PubMed] [Google Scholar]
- Bilinski, S.M. , Jaglarz, M.K. , Szymanska, B. , Etkin, L.D. and Kloc, M. (2004) Sm proteins, the constituents of the spliceosome, are components of nuage and mitochondrial cement in Xenopus oocytes. Exp. Cell Res. 299, 171–178. [DOI] [PubMed] [Google Scholar]
- Bohn, J.A. , Etten, J.L.V. , Schagat, T.L. , Bowman, B.M. , McEachin, R.C. , Freddolino, P.L. and Goldstrohm, A.C. (2018) Identification of diverse target RNAs that are functionally regulated by human pumilio proteins. Nucleic Acids Res. 46, 362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte, S. and Cordelières, F.P. (2006) A guided tour into subcellular colocalisation analysis in light microscopy. J. Microsc. 224, 213–232. [DOI] [PubMed] [Google Scholar]
- Borg, M. , Brownfield, L. , Khatab, H. , Sidorova, A. , Lingaya, M. and Twell, D. (2011) The R2R3 MYB transcription factor DUO1 activates a male germline‐specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell, 23, 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouveret, E. , Rigaut, G. , Shevchenko, A. , Wilm, M. and Séraphin, B. (2000) A Sm‐like protein complex that participates in mRNA degradation. EMBO J. 19, 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan, J.R. and Parker, R. (2009) Eukaryotic stress granules : the ins and out of translation. Mol. Cell, 36, 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. , Shi, F. , Liu, X. , Jia, J. , Zeng, J. and Huang, G. (2011) Genome‐wide identification and evolutionary analysis of Arabidopsis sm genes family. J. Biomol. Struct. Dyn. 28, 535–544. [DOI] [PubMed] [Google Scholar]
- Cenci, G. , Bonaccorsi, S. , Pisano, C. , Verni, F. and Gatti, M. (1994) Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 107, 3521–3534. [DOI] [PubMed] [Google Scholar]
- Chantarachot, T. and Bailey‐Serres, J. (2018) Polysomes, stress granules, and processing bodies: a dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiol. 176, 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle, C. , Fan, J. , Cho‐Chung, Y.S. , Werner, T. , Ray, J. , Do, L. , Gorospe, M. and Becker, K.G. (2005) Control of gene expression during T cell activation: alternate regulation of mRNA transcription and mRNA stability. BMC Genom. 6, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chêne, A. , Vembar, S.S. , Rivière, L. et al . (2012) PfAlbas constitute a new eukaryotic DNA/RNA‐binding protein family in malaria parasites. Nucleic Acids Res. 40, 3066–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C.Y. and Rana, T.M. (2006) Translation repression in human cells by microRNA‐induced gene silencing requires RCK/p54. PLoS Biol. 4, 1122–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma, S. , Hiyoshi, M. , Yamamoto, A. , Hosokawa, M. , Takamune, K. and Nakatsuji, N. (2003) Mouse Tudor Repeat‐1 (MTR‐1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech. Dev. 120, 979–990. [DOI] [PubMed] [Google Scholar]
- Colas, I. , Darrier, B. , Arrieta, M. , Mittmann, S.U. , Ramsay, L. , Sourdille, P. and Waugh, R. (2017) Observation of extensive chromosome axis remodeling during the “diffuse‐phase” of meiosis in large genome cereals. Front. Plant Sci. Sci. 8, 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli, P. , Pukazhenthi, B.S. and Wildt, D.E. (2011) The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum. Reprod. 26, 2165–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelières, F.P. and Bolte, S. (2008) JACoP v2. 0: improving the user experience with co‐localization studies In ImageJ User & Developer Conference. pp. 174–181. [Google Scholar]
- Darzacq, X. , Jády, B.E. , Verheggen, C.C. et al . (2002) Cajal body‐specific small nuclear RNAs: a novels class of 2′‐O‐methylation and pseudouridylation guide RNAs. Embo J. 21, 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, C.J. and Parker, R. (2012) P‐bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 4, a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne, P.J. , Ubalijoro, E. , Fortin, M.G. and Laliberte, J.F. (2008) Arabidopsis thaliana class II poly(A)‐binding proteins are required for efficient multiplication of turnip mosaic virus. J. Gen. Virol. 89, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Elvira‐Matelot, E. , Bardou, F. , Ariel, F. , Jauvion, V. , Bouteiller, N. , Masson, I.L. , Cao, J. , Crespi, M.D. and Vaucheret, H. (2016) The nuclear ribonucleoprotein SmD1 interplays with splicing, RNA quality control and post‐transcriptional gene silencing in Arabidopsis. Plant Cell, 28, 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, C.F. , Pannone, B.K. , Xinguo, C. , Fuchs, G. and Wolin, S.L. (2004) An Lsm2–Lsm7 Complex in Saccharomyces cerevisiae Associates with the Small Nucleolar RNA snR5. Mol. Biol. Cell, 15, 2842–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, T.R. , Alves‐Ferreira, E.V.C. , Defina, T.P.A. , Walrad, P. , Papadopoulou, B. and Cruz, A.K. (2014) Altered expression of an RBP‐associated arginine methyltransferase 7 in Leishmania major affects parasite infection. Mol. Microbiol. 94, 1085–1102. [DOI] [PubMed] [Google Scholar]
- Fu, D. and Collins, K. (2006) Human telomerase and Cajal body ribonucleoproteins share a unique specificity of Sm protein association. Genes Dev. 20, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, C.M. , Munro, E. , Rasoloson, D. , Merritt, C. and Seydoux, G. (2008) Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev. Biol. 323, 76–87. [DOI] [PubMed] [Google Scholar]
- Gama‐Carvalho, M. , Barbosa‐Morais, N.L. , Brodsky, A.S. , Silver, P.A. and Carmo‐Fonseca, M. (2006) Genome‐wide identification of functionally distinct subsets of cellular mRNAs associated with two nucleocytoplasmic‐shuttling mammalian splicing factors. Genome Biol. 7, R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, A.P. , Herschlag, D. and Brown, P.O. (2004) Extensive association of functionally and cytotopically related mRNAs with Puf family RNA‐binding proteins in yeast. PLoS Biol. 2, 0342–0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, A.P. , Luschnig, S. , Krasnow, M.A. , Brown, P.O. and Herschlag, D. (2006) Genome‐wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA, 103, 4487–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissot, M. , Walker, R. , Delhaye, S. et al . (2013) Toxoplasma gondii Alba proteins are involved in translational control of gene expression. J. Mol. Biol. 425, 1287–1301. [DOI] [PubMed] [Google Scholar]
- Goldstrohm, A.C. , Hall, T.M.T. and McKenney, K.M. (2018) Post‐transcriptional regulatory functions of mammalian Pumilio proteins. Trends Genet. 34, 972–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez, G.B. and Long, R.M. (2012) Spatial regulation of translation through RNA localization. F1000 Biol. Rep. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez, G.B. , Rajendra, T.K. , Wen, Y. , Praveen, K. and Matera, A.G. (2010) Sm proteins specify germ cell fate by facilitating oskar mRNA localization. Development, 137, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe, M. (2003) HuR in the mammalian genotoxic response: post‐transcriptional multitasking. Cell Cycle, 2, 412–414. [PubMed] [Google Scholar]
- Götz, S. , García‐Gómez, J.M. , Terol, J. et al . (2008) High‐throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36, 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, M. , Banerjee, C. , Nag, S. and Bandyopadhyay, U. (2016) The Alba protein family: structure and function. BBA Proteins Proteom. 1864, 570–583. [DOI] [PubMed] [Google Scholar]
- Grabherr, M.G. , Haas, B.J. , Yassour, M. et al . (2011) Full‐length transcriptome assembly from RNA‐Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, R. , Xue, H. and Huang, L. (2003) Ssh10b, a conserved thermophilic archaeal protein, binds RNA in vivo. Mol. Microbiol. 50, 1605–1615. [DOI] [PubMed] [Google Scholar]
- Gutierrez‐Beltran, E. , Moschou, P.N. , Smertenko, A.P. and Bozhkov, P.V. (2015) Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell, 27, 926–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbeisen, R.E. , Galgano, A. , Scherrer, T. and Gerber, A.P. (2008) Post‐transcriptional gene regulation: From genome‐wide studies to principles. Cell. Mol. Life Sci. 65, 798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, H. , Fabrizio, P. , Raker, V.A. , Foulaki, K. , Hornig, H. , Brahms, H. and Lührmann, R. (1995) snRNP Sm proteins share two evolutionarily conserved sequence motifs which are involved in Sm protein‐protein interactions. EMBO J. 14, 2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. and Steitz, J.A. (2001) Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell, 7, 899–905. [DOI] [PubMed] [Google Scholar]
- Hyjek, M. , Wojciechowska, N. , Rudzka, M. , Kołowerzo‐Lubnau, A. and Smoliński, D.J. (2015) Spatial regulation of cytoplasmic snRNP assembly at the cellular level. J. Exp. Bot. 66, 7019–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglarz, M.K. , Kloc, M. , Jankowska, W. , Szymanska, B. and Bilinski, S.M. (2011) Nuage morphogenesis becomes more complex: two translocation pathways and two forms of nuage coexist in Drosophila germline syncytia. Cell Tissue Res. 344, 169–181. [DOI] [PubMed] [Google Scholar]
- Jang, G.J. , Yang, J.Y. , Hsieh, H.L. and Wu, S.H. (2019) Processing bodies control the selective translation for optimal development of Arabidopsis young seedlings. Proc. Natl. Acad. Sci. USA, 116, 6451–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida, D. , Berg, M.G. , Younis, I. , Kasim, M. , Singh, L.N. , Wan, L. and Dreyfuss, G. (2010) U1 snRNP protects pre‐mRNAs from premature cleavage and polyadenylation. Nature, 468, 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha, N. , Stoecklin, G. , Ayodele, M. et al . (2005) Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene, J.D. (2007) RNA regulons: coordination of post‐transcriptional events. Nat. Rev. Genet. 8, 533–543. [DOI] [PubMed] [Google Scholar]
- Keene, J.D. and Tenenbaum, S.A. (2002) Eukaryotic mRNPs may represent posttranscriptional operons. Mol. Cell, 9, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Khusial, P. , Plaag, R. and Zieve, G.W. (2005) LSm proteins form heptameric rings that bind to RNA via repeating motifs. Trends Biochem. Sci. 30, 522–528. [DOI] [PubMed] [Google Scholar]
- Klasterska, I. (1976) A new look on the role of the diffuse stage in problems of plant and animal meiosis. Hereditas, 82, 193–204. [Google Scholar]
- Knop, K. , Stepien, A. , Barciszewska‐pacak, M. , Taube, M. , Bielewicz, D. , Michalak, M. , Borst, J.W. , Jarmolowski, A. and Szweykowska‐Kulinska, Z. (2017) Active 5 splice sites regulate the biogenesis efficiency of Arabidopsis microRNAs derived from intron‐containing genes. Nucleic Acids Res. 45, 2757–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołowerzo‐Lubnau, A. , Niedojadło, J. , Świdziński, M. , Bednarska‐Kozakiewicz, E. and Smoliński, D.J. (2015) Transcriptional activity in diplotene larch microsporocytes, with emphasis on the diffuse stage. PLoS ONE, 10, e0117337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołowerzo, A. , Smoliński, D.J. and Bednarska, E. (2009) Poly(A) RNA a new component of Cajal bodies. Protoplasma, 236, 13–19. [DOI] [PubMed] [Google Scholar]
- Kosmacz, M. , Gorka, M. , Schmidt, S. et al . (2019) Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 222, 1420–1433. [DOI] [PubMed] [Google Scholar]
- Lai, W.S. , Carballo, E. , Strum, J.R. , Kennington, E.A. , Phillips, R.S. and Blackshear, P.J. (1999) Evidence that tristetraprolin binds to AU‐rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell. Biol. 19, 4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavut, A. and Raveh, D. (2012) Sequestration of highly expressed mrRNAs in cytoplasmic granules, P‐bodies, and stress granules enhances cell viability. PLoS Genet. 8, e1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L. , Davies, S.E. and Liu, J.L. (2009) The spinal muscular atrophy protein SMN affects Drosophila germline nuclear organization through the U body‐P body pathway. Dev. Biol. 332, 142–155. [DOI] [PubMed] [Google Scholar]
- Lemaire, R. , Prasad, J. , Kashima, T. , Gustafson, J. , Manley, J.L. and Lafyatis, R. (2002) Stability of a PKCI‐1 ‐related mRNA is controlled by the splicing factor ASF/SF2: a novel function for SR proteins. Genes Dev. 16, 594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.‐L. and Gall, J.G. (2007) U bodies are cytoplasmic structures that contain uridine‐rich small nuclear ribonucleoproteins and associate with P bodies. Proc. Natl. Acad. Sci. USA, 104, 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Rosas, I. , Orozco, E. , Marchat, L. et al . (2012) mRNA decay proteins are targeted to poly(A)+ RNA and dsRNA‐containing cytoplasmic foci that resemble P‐bodies in Entamoeba histolytica. PLoS ONE, 7, e45966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott, K. , Li, J. , Fisk, J.C. , Wang, H. , Aletta, J.M. , Qu, J. and Read, L.K. (2013) Global proteomic analysis in trypanosomes reveals unique proteins and conserved cellular processes impacted by arginine methylation. J. Proteomics, 91, 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü, X. , De La Peña, L. , Barker, C. , Camphausen, K. and Tofilon, P.J. (2006) Radiation‐induced changes in gene expression involve recruitment of existing messenger RNAs to and away from polysomes. Cancer Res. 66, 1052–1061. [DOI] [PubMed] [Google Scholar]
- Lu, Z. , Guan, X. , Schmidt, C.A. and Matera, A.G. (2014) RIP‐seq analysis of eukaryotic Sm proteins identifies three major categories of Sm‐containing ribonucleoproteins. Genome Biol. 15, R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke‐Andersen, J. and Wagner, E. (2005) Recruitment and activation of mRNA decay enzymes by two ARE‐mediated decay activation domains in the proteins TTP and BRF‐1. Genes Dev. 19, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair, G.R. , Lasonder, E. , Garver, L.S. et al . (2010) Universal features of post‐transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 6, e1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado‐Bonilla, L.D. (2014) Composition and function of P bodies in Arabidopsis thaliana. Front. Plant Sci. 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, J. , Güttinger, A. , Schimanski, B. , Heller, M. , Acosta‐Serrano, A. , Pescher, P. , Späth, G. and Roditi, I. (2011) Alba‐domain proteins of trypanosoma brucei are cytoplasmic RNA‐Binding proteins that interact with the translation machinery. PLoS ONE, 6, e22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj, I.W. (1986) Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell, 46, 905–911. [DOI] [PubMed] [Google Scholar]
- Mazan‐Mamczarz, K. , Galban, S. , de Silanes, I.L. , Martindale, J.L. , Atasoy, U. , Keene, J.D. and Gorospe, M. (2003) RNA‐binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. USA, 100, 8354–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merret, R. , Martino, L. , Bousquet‐Antonelli, C. , Fneich, S. , Descombin, J. , Billey, É. , Conte, M.R. and Deragon, J.M. (2013) The association of a La module with the PABP‐interacting motif PAM2 is a recurrent evolutionary process that led to the neofunctionalization of La‐related proteins. RNA, 19, 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, J.J. and Fritzler, M.J. (2010) Cytoplasmic ribonucleoprotein (RNP) bodies and their relationship to GW/P bodies. Int. J. Biochem. Cell Biol. 42, 828–843. [DOI] [PubMed] [Google Scholar]
- Moussa, F. , Oko, R. and Hermo, L. (1994) The immunolocalization of small nuclear ribonucleoprotein particles in testicular cells during the cycle of the seminiferous epithelium of the adult rat. Cell. Tissue. Res. 278, 363–378. [DOI] [PubMed] [Google Scholar]
- Mura, C. , Kozhukhovsky, A. , Gingery, M. , Phillips, M. and Eisenberg, D. (2003) The oligomerization and ligand‐binding properties of Sm‐like archaeal proteins (SmAPs). Protein Sci. 12, 832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntini, E. , Järvelin, A.I. , Bornholdt, J. et al. (2013) Polyadenylation site‐induced decay of upstream transcripts enforces promoter directionality. Nat. Struct. Mol. Biol. 20, 923–928. [DOI] [PubMed] [Google Scholar]
- Ok, S.H. , Jeong, H.J. , Bae, J.M. , Shin, J.‐S. , Luan, S. and Kim, K.‐N. (2005) Novel CIPK1‐associated proteins in Arabidopsis contain an evolutionarily conserved C‐terminal region that mediates nuclear localization. Plant Physiol. 139, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, M. , Butenko, Y. , Hsieh, T.‐F. , Hakim, O. , Katz, A. , Smorodinsky, N.I. , Michaeli, D. , Fischer, R.L. and Ohad, N. (2016) FIE, a nuclear PRC2 protein, forms cytoplasmic complexes in Arabidopsis thaliana . J. Exp. Bot. 67, 6111–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, R. and Sheth, U. (2007) P bodies and the control of mRNA translation and degradation. Mol. Cell, 25, 635–646. [DOI] [PubMed] [Google Scholar]
- Peculis, B.A. (1997) The sequence of the 5′ end of the U8 small nucleolar RNA is critical for 5.8S and 28S rRNA maturation. Mol. Cell. Biol. 17, 3702–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea‐Resa, C. , Hernández‐Verdeja, T. , López‐Cobollo, R. , del Mar Castellano, M. and Salinas, J. (2012) LSM proteins provide accurate splicing and decay of selected transcripts to ensure normal Arabidopsis development. Plant Cell, 24, 4930–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz, M. , Lin, P.C. , Finer, J. and Jang, J.C. (2010) AtTZF gene family localizes to cytoplasmic foci. Plant Signal. Behav. 5, 190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter, D.S.W. and Parker, R. (2016) Principles and properties of stress granules. Trends Cell Biol. 26, 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raker, V.A. , Hartmuth, K. , Kastner, B. and Lührmann, R. (1999) Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol. Cell. Biol. 19, 6554–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, P. , Darzacq, X. , Bertrand, E. , Jády, B.E. , Verheggen, C. and Kiss, T. (2003) A common sequence motif determines the Cajal body‐specific localization of box H/ACA scaRNAs. EMBO J. 22, 4283–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissland, O.S. (2017) The organization and regulation of mRNA‐protein complexes. Wiley Interdiscip. Rev. RNA, 8, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, J.R. , Gray, N.K. , Beckmann, K. and Cáceres, J.F. (2004) A novel role for shuttling SR proteins in mRNA translation A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 18, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpin, M.R. , Sigaut, L. , Temprana, S.G. , Boccaccio, G.L. , Pietrasanta, L.I. and Muschietti, J.P. (2017) Two Arabidopsis late pollen transcripts are detected in cytoplasmic granules. Plant Direct, 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder, R. and Edwards, R. (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics, 27, 863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. and Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrom, E.‐M. , Moschall, R. , Hartl, M.J. , Weitner, H. , Fecher, D. , Langemeier, J. , Bohne, J. , Wöhrl, B.M. and Bodem, J. (2013) U1snRNP‐mediated suppression of polyadenylation in conjunction with the RNA structure controls poly (A) site selection in foamy viruses. Retrovirology, 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M.A. , Pearson, R.F. , Moller, T. , Valentin‐Hansen, P. and Brennan, R.G. (2002) Structures of the pleiotropic translational regulator Hfq and an Hfq‐RNA complex: A bacterial Sm‐like protein. EMBO J. 21, 3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli, D. and Pillai, R.S. (2004) The special Sm core structure of the U7 snRNP: far‐reaching significance of a small nuclear ribonucleoprotein. Cell. Mol. Life Sci. 61, 2560–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, D.G. and Lynch, M. (2008) Evolutionary diversification of the Sm family of RNA‐associated proteins. Mol. Biol. Evol. 25, 2255–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, G.L. and Blau, H.M. (2005) Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–636. [DOI] [PubMed] [Google Scholar]
- Seto, A.G. , Zaug, A.J. , Sobel, S.G. , Wolin, S.L. and Cech, T.R. (1999) Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature, 401, 177–180. [DOI] [PubMed] [Google Scholar]
- Shaw, D.J. , Eggleton, P. and Young, P.J. (2008) Joining the dots: production, processing and targeting of U snRNP to nuclear bodies. Biochim. Biophys. Acta, 1783, 2137–2144. [DOI] [PubMed] [Google Scholar]
- She, W. , Grimanelli, D. , Rutowicz, K. , Whitehead, M.W.J. , Puzio, M. , Kotliński, M. , Jerzmanowski, A. and Baroux, C. (2013) Chromatin reprogramming during the somatic‐to‐reproductive cell fate transition in plants. Development, 140, 4008–4019. [DOI] [PubMed] [Google Scholar]
- Sheehan, M.J. and Pawlowski, W.P. (2009) Live imaging of rapid chromosome movements in meiotic prophase I in maize. Proc. Natl. Acad. Sci. USA, 106, 20989–20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, U. and Parker, R. (2003) Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science, 300, 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliński, D.J. and Kołowerzo, A. (2012) mRNA accumulation in the Cajal bodies of the diplotene larch microsporocyte. Chromosoma, 121, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliński, D.J. , Niedojadło, J. , Noble, A. and Górska‐Brylass, A. (2007) Additional nucleoli and NOR activity during meiotic prophase I in larch (Larix decidua Mill.). Protoplasma, 232, 109–120. [DOI] [PubMed] [Google Scholar]
- Smoliński, D.J. , Wróbel, B. , Noble, A. , Zienkiewicz, A. and Górska‐Brylass, A. (2011) Periodic expression of Sm proteins parallels formation of nuclear cajal bodies and cytoplasmic snRNP‐rich bodies. Histochem. Cell Biol. 136, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson, R. and Bailey‐Serres, J. (2014) Selective mRNA sequestration by OLIGOURIDYLATE‐BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA, 111, 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson, R. and Bailey‐Serres, J. (2015) Rapid immunopurification of ribonucleoprotein complexes of plants. Methods Mol. Biol. 1284, 209–219. [DOI] [PubMed] [Google Scholar]
- Staněk, D. and Neugebauer, K.M. (2006) The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma, 115, 343–354. [DOI] [PubMed] [Google Scholar]
- Stepien, A. , Knop, K. , Dolata, J. , Taube, M. , Bajczyk, M. , Barciszewska‐pacak, M. , Pacak, A. , Jarmolowski, A. and Szweykowska‐Kulinska, Z. (2016) Posttranscriptional coordination of splicing and miRNA biogenesis in plants. Wiley Interdiscip. Rev. RNA, 8, e1403 10.1002/wrna.1403 [DOI] [PubMed] [Google Scholar]
- Subota, I. , Rotureau, B. , Blisnick, T. , Ngwabyt, S. , Durand‐Dubief, M. , Engstler, M. and Bastin, P. (2011) ALBA proteins are stage regulated during trypanosome development in the tsetse fly and participate in differentiation. Mol. Biol. Cell, 22, 4205–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szweykowska‐Kulinska, Z. , Jarmolowski, A. and Vazquez, F. (2013) The crosstalk between plant microRNA biogenesis factors and the spliceosome. Plant Signal. Behav. 8, e26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, P.P. , Barrette‐Ng, I.H. , Simon, D.M. , Tam, M.W. , Ang, A.L. and Muench, D.G. (2010) The Puf family of RNA‐binding proteins in plants: phylogeny, structural modeling, activity and subcellular localization. BMC Plant Biol. 10, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. , Kannan, R. , Blanchette, M. and Baumann, P. (2012) Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature, 484, 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule, J. , Jensen, K. , Roggiu, M. , Mele, A. , Ule, A. and Darnell, R.B. (2003) CLIP identifies Nova‐regulated RNA networks in the brain. Science, 302, 1212–1215. [DOI] [PubMed] [Google Scholar]
- Unterholzner, L. and Izaurralde, E. (2004) SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell, 16, 587–596. [DOI] [PubMed] [Google Scholar]
- Urlaub, H. , Raker, V.A. , Kostka, S. and Lührmann, R. (2001) Sm protein‐Sm site RNA interactions within the inner ring of the spliceosomal snRNP core structure. EMBO J. 20, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, J.K. , Gayali, S. , Dass, S. , Kumar, A. , Parveen, S. , Chakraborty, S. and Chakraborty, N. (2014) OsAlba1, a dehydration‐responsive nuclear protein of rice (Oryza sativa L. ssp. indica), participates in stress adaptation. Phytochemistry, 100, 16–25. [DOI] [PubMed] [Google Scholar]
- Weber, C. , Nover, L. and Fauth, M. (2008) Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 56, 517–530. [DOI] [PubMed] [Google Scholar]
- Wilczynska, A. , Aigueperse, C. , Kress, M. , Dautry, F. and Weil, D. (2005) The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118, 981–992. [DOI] [PubMed] [Google Scholar]
- Xu, J. and Chua, N.‐H. (2009) Arabidopsis decapping 5 is required for mRNA decapping, P‐body formation, and translational repression during postembryonic development. Plant Cell, 21, 3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. and Chua, N.‐H. (2011) Processing bodies and plant development. Curr. Opin. Plant Biol. 14, 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Yang, J.‐Y. , Niu, Q.‐W. and Chua, N.‐H. (2006) Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell, 18, 3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong, J. , Kasim, M. , Bachorik, J.L. , Wan, L. and Dreyfuss, G. (2010) Gemin5 delivers snRNA precursors to the SMN complex for snRNP biogenesis. Mol. Cell, 38, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S.G. , Yang, W.H. , Qi, Y.C. , Li, M.X. , Wang, J.H. , Sun, X.M. , Wang, X.S. and Qi, L.W. (2008) Development of male gametophyte of Larix leptolepis Gord. with emphasis on diffuse stage of meiosis. Plant Cell Rep. 27, 1687–1696. [DOI] [PubMed] [Google Scholar]
- Zieve, G.W. , Sauterer, R.A. and Feeney, R.J. (1988) Newly synthesized small nuclear RNAs appear transiently in the cytoplasm. J. Mol. Biol. 199, 259–267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Western blot analysis of protein extracts obtained after immunoprecipitation using different types of anti‐Sm antibodies: Y12, ANA No. 5 and Y12 (Abcam).
Figure S2. Identification of Sm Larix decidua proteins by IP‐SDS‐PAGE‐MS.
Figure S3. Localization of the Sm proteins and poly(A) RNA on semi‐thin sections of Larix decidua Mill. anther.
Figure S4. Labeling of Sm proteins and selected splicing U snRNAs in larch microsporocytes.
Figure S5. Localization of mRNA precipitating with Sm proteins in situ.
Figure S6. Localization of the Sm mRNAs and Sm proteins in situ.
Figure S7. Validation of the Larix decidua anther cell fractionation method.
Table S1. Real‐time RT‐PCR primers and probe sequences used for in situ hybridization.
Table S2. Identification of the proteins that form a complex with Sm proteins in the cytoplasm determined by IP‐LC‐MS/MS.
Table S3. The cytoplasmic Sm protein interactome.
Appendix S1. List of Sm protein‐associated transcripts and sequences determined by RIP‐seq.
Data Availability Statement
Data supporting the findings of this work are provided in the main text and the supporting information. Raw reads generated during this project were deposited in the NCBI SRA database and are accessible through BioProject no. PRJNA624007. The full data set for the transcriptomic analysis is available at https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA624007. All other data and materials used in this study will be available at request from the corresponding authors.


