Abstract
The impact of primary cytomegalovirus infection (pCMV) on renal allograft function and histology is controversial. We evaluated the influence on incidence of acute rejection, allograft loss, allograft function and interstitial fibrosis/tubular atrophy (IF/TA). Retrospective case–control study, recipients transplanted between 2000 and 2014. Risk of acute rejection and allograft loss for those who experienced pCMV infection compared with those who did not, within an exposure period of two months after transplantation. Besides, its influence on allograft function and histology at one to three years after transplantation. Of 113 recipients experienced pCMV infection, 306 remained CMV seronegative. pCMV infection in the exposure period could not be proven as increasing the risk for acute rejection [HR = 2.18 (95% CI 0.80–5.97) P = 0.13] or allograft loss [HR = 1.11 (95%CI 0.33–3.72) P = 0.87]. Combination of pCMV infection and acute rejection posed higher hazard for allograft loss than acute rejection alone [HR = 3.69 (95% CI 1.21–11.29) P = 0.02]. eGFR(MDRD) values did not significantly differ at years one [46 vs. 50], two [46 vs. 51] and three [46 vs. 52]. No association between pCMV infection and IF/TA could be demonstrated [OR = 2.15 (95%CI 0.73–6.29) P = 0.16]. pCMV infection was not proven to increase the risk for acute rejection or allograft loss. However, it increased the risk for rejection‐associated allograft loss. In remaining functioning allografts, it was not significantly associated with decline in function nor with presence of IF/TA.
Keywords: primary CMV infection, renal transplantation, renal allograft outcome
Introduction
Cytomegalovirus (CMV) infection is the most common viral infectious complication following renal transplantation. CMV IgG seronegative recipients may experience primary CMV (pCMV) infection after transplantation with a renal allograft obtained from a CMV IgG seropositive donor. This pCMV infection often manifests as CMV disease with symptoms as fever, bone marrow suppression, pneumonitis, hepatitis and/or colitis. However, the primary infection may also run an asymptomatic course [1]. Besides, in renal allograft recipients who already have encountered CMV prior to renal allograft transplantation, CMV reactivation frequently occurs. Although the implementation of CMV prophylaxis has been shown to reduce CMV‐associated morbidity and mortality, pCMV infection may still occur after discontinuation of CMV prophylaxis [2].
A causal relationship between CMV infection and allograft injury has been observed in experimental studies [3, 4], but definitive proof in clinical transplantation is still lacking [1, 5]. Furthermore, data about histological changes after CMV infection in humans are relatively scarce [6]. Previous studies showed an association between CMV disease and occurrence of acute rejection [7, 8, 9, 10, 11], while others did not [12, 13]. Moreover, the association between CMV infection and chronic allograft dysfunction remains controversial [6, 12, 14, 15, 16, 17].
Hence, the aim of this study was to evaluate the effect of pCMV infection on incidence of acute rejection and rejection‐associated allograft loss, on renal allograft function, as well as on the presence of interstitial fibrosis/tubular atrophy (IF/TA) in renal allograft biopsies taken between six and 24 months after transplantation.
Patients and methods
Hospital setting
This study was conducted in our University Medical Center that serves as a tertiary referral centre for patients with end‐stage renal disease. Patients from the North and the Middle of the Netherlands are referred for renal transplantation. One year after transplantation, they return back for follow up to their referring medical centre. Patient approval according to the guidelines of the Medical Ethical Committee of our University Medical Center was obtained from every renal allograft recipient included in this study.
Study setup
We have performed a retrospective case–control study on renal allograft recipients transplanted for the first time between 2000 and 2014, who had been screened for CMV infection by measurement of specific IgM and IgG antibodies as well as PCR. Heparinized peripheral blood samples had been collected before transplantation and weekly during the first 16 weeks after transplantation. Next, every month during the first year and every three months thereafter.
Among a total of 1028 patients, we identified 113 who experienced pCMV infection and 306 who never developed CMV infection, defined as persistent CMV seronegativity (IgM and IgG) and negative CMV‐PCR during the entire study follow‐up of three years post‐transplantation. These two groups were compared for the incidence of acute rejection, renal allograft loss, and renal allograft function as measured by eGFR in ml/min per 1.73 m2 with MDRD formula [18] at one, two and three years after transplantation. Moreover, presence of IF/TA was determined in renal allograft biopsies taken between 6 and 24 months after transplantation. In the group of recipients who had experienced pCMV infection, these outcome parameters were evaluated at time points following the infection.
Definitions
Primary CMV infection was defined as CMV viremia demonstrated by PCR in an initially IgM and IgG seronegative renal allograft recipient. The diagnosis was confirmed by seroconversion. In case of CMV‐associated symptoms (fever, pneumonitis, hepatitis and/or colitis and/or cytopenia), the infection was classified as pCMV disease, conform published guidelines [19, 20]. CMV seronegative renal allograft recipients were defined as those who were IgM and IgG negative at time of transplantation and remained so during entire follow‐up. These patients did also not experience CMV replication at any time point during follow‐up, as measured by PCR. Delayed graft function was defined as the requirement of dialysis within the first week after transplantation because of acute tubular necrosis. Primary nonfunction was defined as post‐transplant acute tubular necrosis without recovery of function even after several weeks [21]. Acute rejection was defined as a decline in renal allograft function along with renal allograft biopsy confirmation.
Immunosuppressive therapy and co‐medication
The patients were treated according to national guidelines and from 2010 according to the international KDIGO guidelines [22]. Immunosuppressive maintenance therapy consisted of steroids, mycophenolate mofetil and a calcineurin inhibitor, mostly tacrolimus but alternatively cyclosporin. CD25 monoclonal antibody (basiliximab) was administered as induction therapy to patients receiving a renal allograft obtained from a living donor, non‐Caucasians and those with panel reactive antibodies> 30%. Acute T‐cell‐mediated rejection (TCMR) was treated with intravenous steroid pulse therapy. Antibody‐mediated rejection (ABMR), combined (cellular and antibody‐mediated) and steroid‐resistant acute TCMR episodes were treated with plasma exchange and anti‐thymocyte globulin (ATG). From one year after transplantation, immunosuppressive treatment generally consisted of double therapy, that is prednisolone and mycophenolate mofetil. Those patients who had experienced acute rejection episode(s) remained on triple therapy as described above.
From 2007, valganciclovir prophylaxis was routinely provided to recipients who had an increased risk for developing CMV replication (transplantation of a renal allograft from a CMV seropositive donor to a CMV seronegative recipient, or in case of ATG treatment) for a period of three months following renal transplantation. From 2010, the time period of prophylaxis with valganciclovir was extended to a period of six months.
When pCMV infection was diagnosed, the dosage of mycophenolate mofetil was gradually decreased in each patient. In addition, CMV disease was treated with intravenous ganciclovir for at least 14 days.
Renal allograft biopsy protocols
We intended to take renal allograft biopsies according to protocol in all patients. Selected time points were perioperatively, at six months, and at one and two years after transplantation. Indication biopsies were taken to diagnose underlying medical conditions, which resulted in deterioration of the renal allograft function and/or unexplainable proteinuria. All renal biopsies were reviewed by one renal pathologist (SF) for the purpose of this study. C4d‐staining results were available for the indication biopsies. T‐cell‐mediated rejection (TCMR), antibody‐mediated rejection (ABMR) and interstitial fibrosis and tubular atrophy (IF/TA) were defined according to the 2018 Reference Guide to the Banff Classification of renal allograft pathology [23]. For the analysis of IF/TA, a binary score was used. We considered IFTA grade 0 and I not significant (no inflammation present; score 0) and IF/TA grade II and III as significant (score 1).
Statistical analyses
Normal distributed continuous variables were expressed as the mean with standard deviation, and non‐normal distributed data were expressed as the median and 25–75% interquartile range (IQR). Student’s t‐test was used to compare continuous variables in case of normal distribution. In case of non‐normal distribution, Mann–Whitney U‐test was used. The distribution of nominal variables between groups was statistically analysed with the chi‐square test.
The outcomes of patient and renal allograft survival are presented by Kaplan–Meier curves and analysed according to the landmark method as described by Gleiss et al. [25]. In a landmark analysis, a period of time between a baseline date (cohort entry) and a study start date (the landmark date) is designated the exposure period and chosen a priori. Only outcome variables that occur after the landmark date are counted in the analysis. Hence, participants who experience the outcome of interest during the exposure window are excluded from subsequent analyses to avoid reverse causality and immortal time bias. Landmark was set at two months after transplantation because the median time between transplantation and occurrence of pCMV infection was 60 days. In the assessment of patient and allograft survival, pCMV infection and/or acute rejection occurring within the exposure window of two months after transplantation were used as exposure variables.
Landmark analysis was also performed to determine pCMV infection as a risk factor for subsequent acute rejection. Because pCMV was the exposure variable, the landmark was also set at two months after transplantation. Consequently, early acute rejection episodes, occurring mostly within two months after transplantation were excluded [26, 27, 28]. Additionally, allograft loss as result of surgical complications was excluded, as well as primary nonfunction occurring within the first two months. In the multivariable model, outcome was adjusted for CMV prophylaxis, donor age, allograft type (deceased vs. living donation).
To determine the independent influence of pCMV infection on renal allograft function, uni‐ and multivariable linear regression analysis were performed. Only pCMV infection events occurring before the measured endpoint of eGFR (MDRD) were included in this analysis. Because of variation between pCMV infection and the fixed eGFR (MDRD) endpoints, we additionally investigated association of ‘time gap’ and eGFR (MDRD). Time gap was defined as the period of time (in months) between pCMV infection and subsequent measurement of eGFR (MDRD) at one, two and three years after transplantation. Time gap and eGFR (MDRD) were assessed with Pearson correlation tests. The eGFR values were logarithmically transformed to fulfil the normality criteria. To enhance interpretability, regression coefficients were transformed backwards by the inverse of the log function, now corresponding to the ratio of the geometric mean of eGFR (MDRD) between pCMV infection and CMV seronegative groups. A 10B value greater than 1 indicated an increase in eGFR (MDRD) after experiencing pCMV infection whereas a 10B value smaller than 1 indicated a decline in eGFR (MDRD) after experiencing pCMV infection. In multivariable linear regression analysis, the influence of pCMV infection on eGFR (MDRD) was adjusted for body mass index (BMI) of recipient, diabetes mellitus, donor age, type of allograft (living vs. obtained from a deceased donor), administration of CMV prophylaxis, delayed graft function and acute rejection.
To determine the influence of pCMV infection on the presence of IF/TA in the renal allograft biopsies, binary logistic regression analysis was performed in which the presence of IF/TA was the dependent variable and pCMV infection the independent variable. Only biopsies obtained after the event of pCMV infection were included. Biopsies taken from patients in the CMV seronegative group served as control. Since the renal allograft biopsies were obtained on both indication and protocol, they were categorized in two periods: biopsies obtained between six to 12 months and between 13 and 24 months after transplantation. In multivariable analysis, outcome IF/TA was adjusted for group differences: age of the donor, acute rejection and administration of CMV prophylaxis. In line with the eGFR (MDRD) analysis, here is also a ‘time gap’ present, which is defined as the period of time (in months) between pCMV infection and subsequent allograft biopsy procedure. Time gap and presence of IF/TA were assessed with Spearman’s rs correlation tests.
It should be emphasized that both outcome parameters, that is graft function and presence of IF/TA are conditional on both graft and patient survival.
Statistical analyses were performed with SPSS software (IBM Corp. Released 2017. SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Landmark analyses were analysed using R studio built under R version 3.5.3 (R Core Team 2013, Vienna, Austria) with package survival [24].
Results
Study groups: pCMV infection versus CMV seronegative
We analysed 419 renal allograft recipients of whom 113 had experienced pCMV infection and 306 remained CMV seronegative and CMV‐PCR negative during entire three‐year follow‐up. 36 Per cent of the seronegative recipients had been transplanted with a kidney from a CMV seropositive donor. These were post‐traumatic donors and polytransfusees, whereby they tested falsely positive in the CMV serology. In view of the persistently negative CMV‐PCR test in their recipients over a time course of 3 years, we consider them as truly CMV negative. The median age of the donor was moderately but significantly higher in the pCMV infection group (P = 0.03), and 97% of the patients in this group received a renal allograft obtained from a CMV IgG‐positive donor (Table 1). Antiviral prophylaxis was implemented in 2007; 131 patients in our total cohort received this prophylactic treatment (Table 1). Table S1 presents a comparison of patients with and without prophylaxis within the pCMV infection group. The median time interval between transplantation and pCMV infection was 60 days (interquartile range (IQR) of 41–215 days) and was significantly longer among those who received prophylaxis compared to those who did not (median 225 days (IQR 97–299) vs. 46 days, IQR 32–60; P < 0.01). The incidence of acute rejection was significantly lower in the prophylaxis group (P = 0.02). The majority of the recipients (101/113; 89%) experiencing pCMV infection were symptomatic, hence experienced pCMV disease.
Table 1.
Comparison of primary CMV infection and CMV seronegative group
| Primary CMV infection | CMV seronegative | OR (95% CI) | P value | |
|---|---|---|---|---|
| n = 113 | n = 306 | |||
| Variables recipient | ||||
| Age (years) | 51 (34–62) | 51 (37–61) | 1.00 (0.98–1.01) | 0.56 |
| Female gender | 40 (35%) | 99 (32%) | 1.15 (0.73–1.80) | 0.56 |
| BMI (m/kg2) | 24.4 ± 3.8 | 25.0 ± 4.4 | 0.97 (0.91–1.02) | 0.22 |
| Diabetes mellitus | 22 (20%) | 59 (19%) | 1.01 (0.59–1.75) | 0.97 |
| Variables donor | ||||
| Deceased donor | 63 (56%) | 144 (47%) | 1.42 (0.92–2.19) | 0.14 |
| Age (years) | 55 (47–63) | 51 (43–61) | 1.02 (1.01–1.03) | 0.03 |
| Donor CMV IgG+ | 109 (97%) | 111 (36%) | 47.87 (17.18–133.37) | <0.01 |
| HLA mismatches | ||||
| Locus A | 71 (63%) | 209 (68%) | 0.79 (0.50–1.25) | 0.31 |
| Locus B | 79 (70%) | 234 (76%) | 0.72 (0.44–1.17) | 0.18 |
| Locus DR | 77 (68%) | 212 (69%) | 0.95 (0.59–1.53) | 0.83 |
| Post‐transplantation | ||||
| CMV prophylaxis | 48 (43%) | 83 (27%) | 1.98 (1.27–3.11) | <0.01 |
| Delayed graft function | 27 (24%) | 86 (28%) | 0.81 (0.49–1.33) | 0.4 |
| Acute rejection | 35/113 (31%) | 50 (16%) | 2.30 (1.39–3.79) | <0.01 |
| Borderline TCMR | 5/35 | 10/50 | ||
| TCMR type I | 23/35 | 23/50 | ||
| TCMR type II | 3/35 | 8/50 | ||
| BMR | 3/35 | 3/50 | ||
| combined | 3/35 | 6/50 | ||
| Renal allograft outcome | ||||
| Allograft loss | 17/113 (15%) | 16/306 (5%) | 3.21 (2.56–6.60) | <0.01 |
| due to rejection | 10/113 (9%) | 7/306 (2%) | 4.32 (1.60–11.70) | <0.01 |
| other causes | 7/113 (6%) | 9/306 (3%) | 2.35 (0.85–6.49) | 0.13 |
| Allograft function in eGFR † | ||||
| 1 year | 46 (34–60) | 50 (42–62) | ** | ** |
| 2 year | 46 (34–58) | 51 (39–62) | ** | ** |
| 3 year | 46 (32–58) | 52 (39–61) | ** | ** |
| Renal allograft biopsies | ||||
| Presence of IF/TA ‡ | ||||
| At reperfusion (n = 81) | 0/26 | 1/55 (2%) | ||
| 6 to 12 months after Tx (n = 106) | 10/35 (29%) | 8/71 (11%) | *** | *** |
| 13 to 24 months after Tx (n = 58) | 7/17 (41%) | 12/41 (29%) | *** | *** |
Continuous variables are depicted as mean ± standard deviation or as median with (25–75%) interquartile range. Nominal variables are depicted as the total number (percentages). ABMR, antibody‐mediated rejection; BMI, body mass index; CMV, cytomegalovirus; Combined, mixed cellular – humoral rejection; TCMR, T‐cell mediated rejection
eGFR (MDRD) in ml/min/1.73 m2.
Interstitial fibrosis and tubular atrophy.
for statistical analyses: see Table 4.
for statistical analyses: see Table 5.
The influence of primary CMV infection on patient survival
Figure 1 displays Kaplan–Meier patient survival curves stratified according to pCMV infection and/or acute rejection. Landmark was set at two months after transplantation. Here, no significant differences in three‐year patient survival (log‐rank P = 0.12) were observed.
Figure 1.
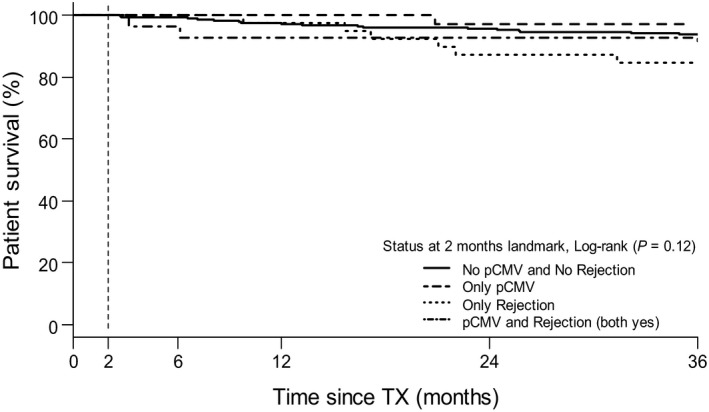
Landmark analysis for patient survival according to having experienced primary CMV infection (pCMV) and/or rejection
Association between primary CMV infection and acute renal allograft rejection
In both the pCMV and the CMV seronegative group, the majority of rejection episodes concerned TCMR (Table 1). Of 35 renal allograft recipients had experienced both pCMV infection and acute rejection during follow‐up. Considering all acute rejection episodes within the pCMV group in more detail, it appeared that 14 of 35 patients (40%) had experienced pCMV infection preceding an acute rejection episode, whereas in 21 of 35 patients (60%), pCMV infection occurred after acute rejection episode (Fig. 2). To determine pCMV infection as a risk factor for subsequent acute rejection, landmark analysis was performed with landmark set at two months after transplantation, being the median time between transplantation and occurrence of pCMV infection. Therefore, in both groups only acute rejection episodes occurring after this landmark date were analysed. Multivariable analysis indicated that pCMV infection could not be proven as increasing the risk for occurrence of subsequent acute rejection [HR = 2.18 (95% CI 0.80 ‐ 5.97), P = 0.13] (Table 2).
Figure 2.
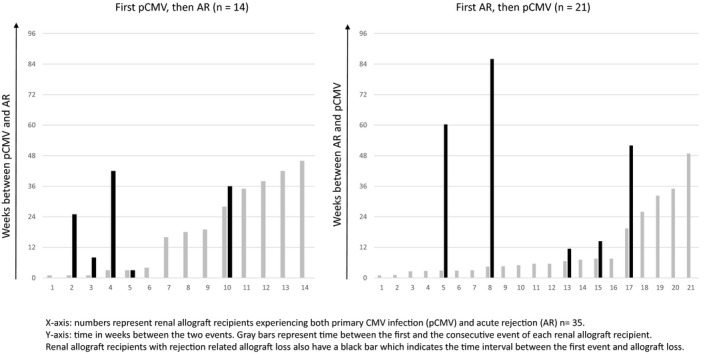
Time between the events primary CMV infection (pCMV)–acute rejection (AR) and occurrence of rejection‐related allograft loss. X‐axis: numbers represent renal allograft recipients experiencing both primary CMV infection (pCMV) and acute rejection (AR) n = 35. Y‐axis: time in weeks between the two events. Grey bars represent time between the first and the consecutive event of each renal allograft recipient. Renal allograft recipients with rejection‐related allograft loss also have a black bar which indicates the time interval between the first event and allograft loss
Table 2.
(A) Landmark analysis for primary CMV infection as a risk factor for subsequent occurrence of acute rejection. (B) Landmark analysis for primary CMV infection as an independent risk factor for renal allograft loss from 2 months to 3 years following transplantation
| Univariable | Multivariable* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| (A) | ||||
| Status at landmark at 60 days after Tx | ||||
| pCMV | 2.67 (0.99–7.18) | 0.05 | 2.18 (0.80–5.97) | 0.13 |
| (B) | ||||
| Status at landmark 60 days after Tx | ||||
| No pCMV and No AR | 1.00 [reference] | – | 1.00 [reference] | – |
| Only pCMV | 1.08 (0.33–3.58) | 0.89 | 1.11 (0.33–3.72) | 0.87 |
| Only AR | 3.51 (1.69–7.29) | <0.01 | 3.54 (1.69–7.41) | <0.01 |
| Both pCMV and AR | 7.53 (3.52–16.08) | <0.01 | 6.40 (2.83–14.47) | <0.01 |
Adjusted for CMV prophylaxis, donor age, allograft type (deceased vs. living donation).
The influence of primary CMV infection on allograft survival
Figure 3 displays Kaplan–Meier curves for allograft survival. Landmark was set at two months after transplantation, using pCMV infection and/or acute rejection as exposure variables. Stratified according to these two parameters, there was a significant difference in allograft survival (log‐rank P < 0.01). Compared to the reference group that did not experience pCMV infection nor acute rejection (no pCMV no AR), having experienced only pCMV infection within the exposed period could not be proven to result in a higher hazard for allograft loss [HR = 1.11 (95% CI 0.33–3.72), P = 0.87 (Table 2). Furthermore, patients who experienced both pCMV infection and acute rejection (both pCMV and AR) within the exposure period appeared to pose a significantly higher hazard for allograft loss if compared to the group that only experienced acute rejection. [HR = 3.69 (95% CI 1.21 – 11.29), P = 0.02). Table 3 displays the histology data of the failed allografts. Within the pCMV infection group, 59% of allograft loss resulted from acute rejection. Cases of nonrejection‐related allograft loss in the pCMV group were not related to CMV infection.
Figure 3.
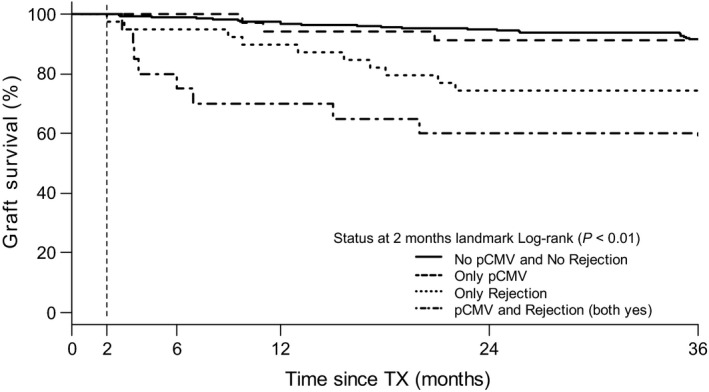
Landmark analysis for allograft survival according to having experienced primary CMV infection (pCMV) and/or rejection
Table 3.
Causes of renal allograft loss according to histological examination of the transplantectomy specimen
| Primary CMV infection | CMV seronegative | |
|---|---|---|
| n = 17 | n = 16 | |
| Allograft loss by acute rejection | ||
| TCMR type 1 | 4 | 1 |
| TCMR type 2 | 1 | 3 |
| ABMR | 3 | 2 |
| Combined rejection | 2 | 1 |
| Allograft loss by other causes | ||
| Primary nonfunction | 1 | 2 |
| Surgical complications | 3 | 2 |
| Recurrence of primary renal disease | 2 | 2 |
| Thrombotic micro‐angiopathy | 1 | 1 |
| Others (deceased/multiple myeloma) | 0 | 2 |
ABMR, antibody‐mediated rejection; TCMR, T‐cell‐mediated rejection.
No influence of pCMV infection on renal allograft function measured by eGFR (MDRD)
In the pCMV group, the eGFR (MDRD) of the remaining functioning allografts at one, two and three years after transplantation was slightly lower than in the 290 patients from the CMV seronegative group (Table 1). Compared to the CMV seronegative group, having experienced primary CMV infection resulted in a 10% lower eGFR (MDRD) at one year after transplantation (univariable linear regression analysis, P = 0.02) as shown in Table 4. However, multivariable linear regression analysis, adjusted for the variables known to influence allograft function, showed that this decline in eGFR (MDRD) was not significant at year 1 (P = 0.12), nor at year 2 (P = 0.21) or year 3 (P = 0.55) after transplantation (Table 4). Of note, ‘time gap’ (i.e. duration between pCMV infection and eGFR (MDRD) measurement) was also not significantly associated with variation of eGFR (MDRD) at year one (r = 0.05, P = 0.63), year two (r = 0.08, P = 0.47) and year three (r = 0.08, P = 0.46) after transplantation.
Table 4.
Linear regression analysis for the influence of primary CMV infection on renal allograft function measured by eGFR (MDRD)
| Unadjusted* | Adjusted † | |||
|---|---|---|---|---|
| 10B (95% CI) | P value | 10B (95% CI) | P value | |
| Year 1 after Tx | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 0.90 (0.82–0.98) | 0.02 | 0.93 (0.84–1.02) | 0.12 |
| Year 2 after Tx | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 0.90 (0.82–0.99) | 0.04 | 0.93 (0.84–1.04) | 0.21 |
| Year 3 after Tx | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 0.87 (0.77–0.98) | 0.02 | 0.96 (0.83–1.10) | 0.55 |
B is the regression coefficient of log‐transformed eGFR (MDRD)
Unadjusted model: association between primary CMV infection (pCMV) and eGFR (MDRD)
Adjusted for BMI of recipient, diabetes mellitus, age of the donor, type of allograft (living vs. deceased donor), delayed graft function, acute rejection and CMV prophylaxis
No association between CMV infection and the presence of IF/TA in renal allograft biopsies taken between six and 24 months after transplantation
Table 1 displays the number of renal allograft biopsies taken in each patient group. Only one of the biopsies taken at moment of reperfusion showed signs of IF/TA. Hereafter, biopsies were obtained within a time period of six to 24 months after transplantation. Renal allograft biopsies obtained after the event of pCMV infection were analysed. Among biopsies taken between six to 12 months after transplantation, having experienced pCMV infection was significantly associated with presence of IF/TA [odds ratio (OR) 3.08 (95% CI 1.06–8.94) P = 0.04] (Table 5). However, after adjustment for group differences (donor age, acute rejection and CMV prophylaxis) in multivariable analysis, it could not be proven that pCMV infection was significantly associated with the presence of IF/TA [OR 1.93 (95% CI = 0.50–7.46) P = 0.34]. Also, among biopsies taken between 13 and 24 months after transplantation, no significant association between pCMV infection and the presence of IF/TA was observed by multivariable analysis [OR 1.78 (95% CI 0.30–10.68), P = 0.53]. Comparable outcome was observed after multivariable analysis of all biopsies taken between six and 24 months after transplantation [OR 2.15 (95% CI 0.73–6.29) P = 0.16]. Of note, ‘time gap’ (time span between pCMV infection and subsequently allograft biopsy procedure) was not associated with the presence of IF/TA (rs 0.21, P = 0.17). Unfortunately, the number of biopsies according to protocol was lower than expected, mainly because lack of permission by patients who were afraid for damage to their good functioning kidney transplant. Therefore, we again analysed the 154 patients from whom biopsies were available as a separate group. As shown in Table S2 and Table S3, the baseline characteristics and eGFR (MDRD) outcome in this biopsy subgroup were largely similar to those in the original group.
Table 5.
Logistic regression analysis for the influence of primary CMV infection on the presence of IF/TA in renal allograft biopsies
| Unadjusted* | Adjusted † | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Biopsies taken between 6 and 12 months | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 3.08 (1.06–8.94) | 0.04 | 1.93 (0.50–7.46) | 0.34 |
| Biopsies taken between 13 and 24 months | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 1.69 (0.52–5.49) | 0.38 | 1.78 (0.30–10.68) | 0.53 |
| All biopsies (between 6 and 24 months) | ||||
| No pCMV | 1.00 [reference] | 1.00 [reference] | ||
| Yes pCMV | 2.30 (1.06–4.97) | 0.03 | 2.15 (0.73–6.29) | 0.16 |
Unadjusted model: association between experiencing primary CMV infection (pCMV) and the presence of IF/TA in renal biopsy specimen.
Adjusted for age of the donor, acute rejection and CMV prophylaxis.
Discussion
In this large case–control study, we studied the influence of primary CMV infection acquired after renal transplantation on the incidence of acute rejection, allograft loss, renal function and the presence of IF/TA in the first 3 years after transplantation.
The association between CMV infection and acute rejection is controversial, since the interpretation of studies on a possible relationship between CMV infection following renal transplantation and allograft dysfunction is hampered by a lack of uniform definitions regarding CMV infection, such as primary infection versus CMV reactivation, asymptomatic versus symptomatic infection and whether or not prophylactic antiviral therapy has been administered after transplantation. Many research groups have observed an association between CMV infection and occurrence of acute rejection [7, 8, 9, 11], whereas others did not [12, 13, 14]. Patients who acquire pCMV infection following transplantation are reported to have an increased risk of rejection‐associated allograft loss [11, 29, 30]. Although we observed a higher incidence of both acute rejection and allograft loss in the total group of patients who experienced pCMV infection, in landmark analysis pCMV infection could not be proven as increasing the risk for acute rejection or graft loss in patients who first experienced pCMV infection, followed by either an acute rejection episode or allograft loss. Interestingly, nonrejection‐related causes of allograft loss were noninfectious complications which were not related to pCMV infection, supporting the idea that in absence of acute rejection, pCMV infection itself does not result in allograft loss within three years after transplantation.
In two studies, an association between CMV infection and allograft loss of any cause was reported. Luan et al reported that late‐onset CMV disease was associated with an increased risk for graft loss during a 5‐year study period [31]. Reischig et al observed that CMV DNAemia of more than 2000 copies/ml was independently associated with renal allograft loss [32]. However, both studies did not report histology data on the aetiology of allograft loss. A unique aspect of our current study is the analysis of renal allograft outcome stratified according to the occurrence of acute rejection. Thereby, the independent influence of pCMV infection on allograft outcome could be determined.
Both sequences in time have been described: CMV infection may occur prior to acute rejection and vice versa [29]. Indeed, we observed both sequences of these two events. pCMV infection may be triggered by intensifying immunosuppressive therapy as treatment for acute rejection. Conversely, a decrease in intensity of immunosuppressive drug medication because of occurrence of pCMV infection may very well be causative in triggering acute rejection. Another explanation for acute rejection following pCMV infection may be found in cross‐reactivity of CMV‐specific T cells to donor alloantigens, as demonstrated previously [33]. Interestingly, we observed that the sequence of pCMV infection and acute rejection did not influence the incidence of rejection‐related allograft loss.
To determine the influence of pCMV infection on the remaining functioning renal allografts, we measured eGFR (MDRD) and observed that pCMV infection did not independently contribute to impairment of renal allograft function up to three years after transplantation. In view of the variable time gap, defined as time (in months) between the moment of pCMV infection and eGFR (MDRD) measurements, we tested its possible influence on eGFR (MDRD) variation and observed that a longer time gap did not significantly influence the variation in the eGFR (MDRD). Regarding allograft functional outcome, comparable observations have been reported by McLaughlin et al, who concluded from a retrospective study that CMV seronegative recipients receiving a renal allograft from a CMV seropositive donor were at risk for renal allograft loss resulting from acute rejection, but that it was not associated with poorer renal allograft function measured at three years after transplantation [30].
Another aim of this study was to determine the influence of pCMV infection on the presence of IF/TA in renal allografts biopsies. We did not observe an association between pCMV infection and the occurrence of IF/TA for up to 2 years following transplantation. It should be noticed that in our study population none of the available perioperative biopsies showed IF/TA, except for one specimen, excluding that observed histological outcomes were influenced by pre‐existing differences. Since the time span between the event of pCMV infection and the moment of biopsy was variable, we additionally determined whether this influenced the presence of IF/TA, which was not the case.
Until now, only few studies have evaluated the influence of CMV infection on renal allograft histology among humans [6, 12, 14, 15]. Moreover, histology data on this topic are mostly available until six months after transplantation. In agreement with our data, Helantera et al demonstrated that symptomatic CMV infection was not associated with increased vascular or other histopathological changes in six‐month protocol biopsy specimens [15]. Erdbrugger et.al. observed that IF/TA was not increased prevalent in patients with CMV infection, defined as detectable viremia irrespective of clinical symptoms. [6]. In contrast, in two other studies, an association between CMV infection and increased IF/TA was observed. The first study showed that CMV viremia (≥2000 copies/ml) was associated with increased risk of IF/TA in protocol biopsies at three months after transplantation [14]. However, these same authors did not observe an impact of CMV DNAemia on the incidence of moderate‐to‐severe IF/TA in protocol biopsies at 36 months [32]. In another study, increased fibrosis was observed in 6‐month protocol biopsies from patients with previous CMV infection [16]. Interpretation of an association between CMV infection and an increase in histological abnormalities in previously published studies is complicated by the absence of reference biopsies taken at the moment of transplantation. Moreover, comparison of these previously published histological studies is hampered by differences in definitions of CMV infection.
Hence, the strength of our study is its setup. By comparing the pCMV infection group to the CMV seronegative group, bias caused by the influence of CMV latency and/or asymptomatic CMV reactivation on renal allograft outcome was excluded. Moreover, the time point of pCMV infection after transplantation was accurately determined by frequent CMV‐PCR monitoring.
A noteworthy limitation of our study is that we cannot make any statement on the influence of asymptomatic viremia following pCMV infection, because nearly all our patients were symptomatic. The same holds true for the influence of chronic asymptomatic CMV reactivation on the functional and histological outcome of the renal allograft since this research question did not fit within the scope of our study. Reischig et al. [32] showed that a low viral load (<2000 copies/ml) may not be harmful for transplant outcomes, but that renal transplant recipients with a load of ≥ 2000 copies/ml in the first year after transplantation, irrespective of the time of onset, were at increased risk for graft loss at 4 years after transplantation. Next, our study was not designed to examine the relationship between antiviral prophylaxis and graft outcome. Other studies have been published on that subject: several meta‐analyses concluded that CMV prophylaxis prevents CMV infection, symptomatic CMV disease and CMV‐associated mortality. However, it did not reduce the risk for developing acute rejection or graft loss [2, 34, 35]. We observed a slightly lower incidence of acute rejection in the prophylaxis group (P = 0.02) as shown in Table S1.
Another limitation of our study is the limited number of available biopsies. However, re‐analysis of the data from the separate group of patients from whom biopsies was available showed comparable baseline characteristics and eGFR (MDRD) outcome.
In conclusion, our study demonstrates that pCMV infection occurring after renal transplantation could not be proven to increase the risk for subsequent acute rejection episodes or allograft loss. However, having experienced both pCMV infection and acute rejection increased the risk of allograft loss considerably as compared to the risk exerted by acute rejection only. pCMV infection did not seem to contribute to functional impairment of the remaining functioning grafts in the first three years after transplantation. Finally, no association was observed between pCMV infection and the presence of chronic allograft damage, as measured by IF/TA up to 24 months after transplantation.
Authorship
RS: involved in study setup, data collection and data analysis, wrote and prepared the manuscript. HP‐S: involved in statistical analyses and data interpretation and prepared the manuscript. EBMR and RAWvL: collected and interpreted the data and prepared the manuscript. UY: involved in study setup, collected and interpreted the data and prepared the manuscript. KAMIvdP, FJB and NCvdW: involved in patient follow‐up, interpreted the data and prepared the manuscript. JJTHR and SF: involved in interpretation of biopsy data, histology analysis and prepared the manuscript. IJMtB: involved in study setup, data collection, and analysis wrote and prepared the manuscript.
Funding
This study was in part funded by the Dutch Kidney Foundation; CP09.04, Consortium Grant (‘ALLOVIR’).
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Table S1 Comparison of primary CMV infection patients (n = 113) with and without antiviral prophylaxis.
Table S2 Comparison of primary CMV infection and CMV seronegative group in biopsy subgroup (n = 154).
Table S3 Uni‐ and multivariable analysis of the renal allograft function measured by eGFR (MDRD) in the biopsy subgroup (n = 154).
Acknowledgements
This study was in part funded by the Dutch Kidney Foundation; CP09.04, Consortium Grant ‘ALLOVIR’. We also greatly acknowledge our colleagues in both the AMC and our affiliated clinical centers for their help in collecting all patient data.
References
- 1. Baron C, Forconi C, Lebranchu Y. Revisiting the effects of CMV on long‐term transplant outcome. Curr Opin Organ Transplant 2010; 15: 492. [DOI] [PubMed] [Google Scholar]
- 2. Hodson EM, Ladhani, M , Webster, AC , Strippoli, GF , Craig, JC . Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev 2013; 2: Cd003774. [DOI] [PubMed] [Google Scholar]
- 3. Inkinen K, Soots A, Krogerus L, Bruggeman C, Ahonen J, Lautenschlager I. Cytomegalovirus increases collagen synthesis in chronic rejection in the rat. Nephrol Dial Transplant 2002; 17: 772. [DOI] [PubMed] [Google Scholar]
- 4. Lautenschlager I, Soots A, Krogerus L, et al Time‐related effects of cytomegalovirus infection on the development of chronic renal allograft rejection in a rat model. Intervirology 1999; 42: 279. [DOI] [PubMed] [Google Scholar]
- 5. Kaminski H, Fishman JA. The cell biology of cytomegalovirus: implications for transplantation. Am J Transplant 2016; 16: 2254. [DOI] [PubMed] [Google Scholar]
- 6. Erdbrugger U, Scheffner I, Mengel M, Schwarz A, Haller H, Gwinner W. Long‐term impact of CMV infection on allografts and on patient survival in renal transplant patients with protocol biopsies. Am J Physiol Renal Physiol 2015; 309: F925. [DOI] [PubMed] [Google Scholar]
- 7. Reischig T, Jindra P, Svecova M, Kormunda S, Opatrny K Jr, Treska V. The impact of cytomegalovirus disease and asymptomatic infection on acute renal allograft rejection. J Clin Virol 2006; 36: 146. [DOI] [PubMed] [Google Scholar]
- 8. Toupance O, Bouedjoro‐Camus MC, Carquin J, et al Cytomegalovirus‐related disease and risk of acute rejection in renal transplant recipients: a cohort study with case‐control analyses. Transpl Int 2000; 13: 413. [DOI] [PubMed] [Google Scholar]
- 9. Sagedal S, Nordal KP, Hartmann A, et al The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2002; 2: 850. [DOI] [PubMed] [Google Scholar]
- 10. Hakimi Z, Aballea S, Ferchichi S, et al Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transpl Infect Dis 2017; 19: e12732. [DOI] [PubMed] [Google Scholar]
- 11. McLaughlin K, Wu C, Fick G, Muirhead N, Hollomby D, Jevnikar A. Cytomegalovirus seromismatching increases the risk of acute renal allograft rejection. Transplantation 2002; 74: 813. [DOI] [PubMed] [Google Scholar]
- 12. Erdbruegger U, Scheffner I, Mengel M, et al Impact of CMV infection on acute rejection and long‐term renal allograft function: a systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol Dial Transplant 2012; 27: 435. [DOI] [PubMed] [Google Scholar]
- 13. Dickenmann MJ, Cathomas G, Steiger J, Mihatsch MJ, Thiel G, Tamm M. Cytomegalovirus infection and graft rejection in renal transplantation. Transplantation 2001; 71: 764. [DOI] [PubMed] [Google Scholar]
- 14. Reischig T, Jindra P, Hes O, Bouda M, Kormunda S, Treska V. Effect of cytomegalovirus viremia on subclinical rejection or interstitial fibrosis and tubular atrophy in protocol biopsy at 3 months in renal allograft recipients managed by preemptive therapy or antiviral prophylaxis. Transplantation 2009; 87: 436. [DOI] [PubMed] [Google Scholar]
- 15. Helantera I, Koskinen P, Tornroth T, Loginov R, Gronhagen‐Riska C, Lautenschlager I. The impact of cytomegalovirus infections and acute rejection episodes on the development of vascular changes in 6‐month protocol biopsy specimens of cadaveric kidney allograft recipients. Transplantation 2003; 75: 1858. [DOI] [PubMed] [Google Scholar]
- 16. Helantera I, Teppo AM, Koskinen P, Tornroth T, Gronhagen‐Riska C, Lautenschlager I. Increased urinary excretion of transforming growth factor‐beta(1) in renal transplant recipients during cytomegalovirus infection. Transpl Immunol 2006; 15: 217. [DOI] [PubMed] [Google Scholar]
- 17. Martin‐Gandul C, Mueller NJ, Pascual M, Manuel O. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant 2015; 15: 3024. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461. [DOI] [PubMed] [Google Scholar]
- 19. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094. [DOI] [PubMed] [Google Scholar]
- 20. Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant 2005; 5: 218. [DOI] [PubMed] [Google Scholar]
- 21. Halloran PF, Hunsicker LG. Delayed graft function: state of the art, November 10‐11, 2000. Summit meeting, Scottsdale, Arizona, USA. Am J Transplant 2000; 2001: 115. [PubMed] [Google Scholar]
- 22. Kasiske BL, Zeier MG, Chapman JR, et al KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 2010; 77: 299. [DOI] [PubMed] [Google Scholar]
- 23. Roufosse C, Simmonds N, Clahsen‐van Groningen M, et al A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation 2018; 102: 1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Therneau T. (2020). A Package for Survival Analysis in R. R package version 3.1‐11, https://CRAN.R‐project.org/package=survival2020.
- 25. Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time‐dependent events. Transpl Int 2018; 31: 125. [DOI] [PubMed] [Google Scholar]
- 26. Nasr M, Sigdel T, Sarwal M. Advances in diagnostics for transplant rejection. Expert Rev Mol Diagn 2016; 16: 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol 2010; 125(Suppl. 2): S324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basadonna GP, Matas AJ, Gillingham KJ, et al Early versus late acute renal allograft rejection: impact on chronic rejection. Transplantation 1993; 55: 993. [DOI] [PubMed] [Google Scholar]
- 29. Nett PC, Heisey DM, Fernandez LA, Sollinger HW, Pirsch JD. Association of cytomegalovirus disease and acute rejection with graft loss in kidney transplantation. Transplantation 2004; 78: 1036. [DOI] [PubMed] [Google Scholar]
- 30. McLaughlin K, Sandhu S, Wu C, Muirhead N, Hollomby D, Jevnikar A. Transplanting kidneys from CMV‐seropositive donors to CMV‐seronegative recipients is not associated with poorer renal allograft function or survival. Nephrol Dial Transplant 2005; 20: 176. [DOI] [PubMed] [Google Scholar]
- 31. Luan FL, Kommareddi M, Ojo AO. Impact of cytomegalovirus disease in D+/R‐ kidney transplant patients receiving 6 months low‐dose valganciclovir prophylaxis. Am J Transplant 2011; 11: 1936. [DOI] [PubMed] [Google Scholar]
- 32. Reischig T, Kacer M, Hruba P, et al The impact of viral load and time to onset of cytomegalovirus replication on long‐term graft survival after kidney transplantation. Antivir Ther 2017; 22: 503. [DOI] [PubMed] [Google Scholar]
- 33. Heutinck KM, Yong SL, Tonneijck L, et al Virus‐specific CD8(+) T cells cross‐reactive to donor‐alloantigen are transiently present in the circulation of kidney transplant recipients infected with CMV and/or EBV. Am J Transplant 2016; 16: 1480. [DOI] [PubMed] [Google Scholar]
- 34. Couchoud C, Cucherat M, Haugh M, Pouteil‐Noble C. Cytomegalovirus prophylaxis with antiviral agents in solid organ transplantation: a meta‐analysis. Transplantation 1998; 65: 641. [DOI] [PubMed] [Google Scholar]
- 35. Stern M, Hirsch H, Cusini A, et al Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation 2014; 98: 1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of primary CMV infection patients (n = 113) with and without antiviral prophylaxis.
Table S2 Comparison of primary CMV infection and CMV seronegative group in biopsy subgroup (n = 154).
Table S3 Uni‐ and multivariable analysis of the renal allograft function measured by eGFR (MDRD) in the biopsy subgroup (n = 154).


