Abstract
Receptor‐interacting protein kinase 1 (RIPK1), a regulator of inflammation and cell death, is a potential therapeutic target in immune‐mediated inflammatory diseases (IMIDs). The objective of this phase IIa multicenter, randomized, double‐blind, placebo‐controlled study was to evaluate safety, tolerability pharmacokinetics, pharmacodynamics, and preliminary efficacy of GSK2982772, a RIPK1 inhibitor, in plaque‐type psoriasis. Psoriasis patients (N = 65) were randomized to 60 mg twice daily (b.i.d.) or three times daily (t.i.d.), or placebo for 84 days. Most adverse events (AEs) were mild with no severe drug‐related AEs reported. Plaque Lesion Severity Sum improved with b.i.d. treatment compared with placebo; interpretation of t.i.d. treatment results was complicated by a high placebo response. Reductions in epidermal thickness and infiltration by CD3+ T cells in the epidermis and dermis were observed compared with placebo. Results support the rationale for additional studies on RIPK1 inhibition in IMIDs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Dysregulation of dendritic cell–mediated and T‐cell–mediated immunity has been shown to play a central role in the development of psoriasis. Increased release of proinflammatory cytokines (including tumor necrosis factor (TNF)) from immune cells is involved in the activation of a sustained inflammatory reaction, and receptor‐interacting protein kinase 1 (RIPK1) inhibitors have been shown to prevent TNF‐dependent inflammation in preclinical models. Thus, RIPK1 has emerged as a potential therapeutic target in immune‐mediated inflammatory diseases, including psoriasis.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Is GSK2982772, a first‐in‐class, RIPK1 small‐molecule inhibitor safe and effective in mild‐to‐moderate active plaque‐type psoriasis?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ GSK2982772 was safe and well tolerated dosed at 60‐mg t.i.d. Improvements in clinical efficacy measures and key biomarkers in this study indicate that RIPK1 inhibition by GSK2982772 may impact inflammation in plaque psoriasis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The data support future studies with GSK2982772 at higher dosages and in more active disease to assess the potential of this novel therapeutic approach.
Psoriatic skin disease is a chronic, immune‐mediated inflammatory disease (IMID) characterized by epidermal hyperproliferation and infiltration of mononuclear cells into the skin, that affects 2–3% of people worldwide. 1 , 2 The pathogenesis is complex, involving both innate and adaptive immunity mechanisms. Dysregulation of dendritic cell–mediated and T‐cell–mediated immunity has been shown to play a central role in its development, 3 and an increase in the release of proinflammatory cytokines (including tumor necrosis factor (TNF)) from immune cells is involved in the activation of a sustained inflammatory reaction. 4 Abnormalities have been observed involving antigen presentation, activation of nuclear factor–κB (NF‐κB) signaling pathways, and differentiation of helper T (TH)‐cell populations (especially TH17 cells associated with an enhanced interleukin 17 (IL‐17) response), promoting the host’s immune response and infiltration by immune cells. 3 Discrete differences in the activated inflammatory pathways account for a variety of clinical variants, including guttate, inverse, pustular, palmoplantar, erythrodermic, and the most common type, psoriasis vulgaris (plaque type). 3 Despite the availability of both topical and systemic treatments, there is still a significant unmet need for effective oral therapy. 5 , 6
Receptor‐interacting protein kinase 1 (RIPK1) is a critical kinase regulator of proinflammatory cytokine production, apoptosis, and necroptosis, notably in response to engagement of the TNF receptor 1. Cell death by necroptosis is highly inflammatory and the death of certain cell types can have profound effects on tissue homeostasis and disease pathogenesis of various IMIDs, including psoriasis. 4 RIPK1 inhibitors have been shown to prevent TNF‐dependent inflammation in multiple preclinical models (e.g., TNF shock, SHARPIN‐deficient murine models, and collagen antibody‐induced arthritis) and several rare human genetic mutations (NEMO, HOIL, and HOIP) that activate RIPK1 activity resulting in TNF‐dependent autoinflammation. 7 , 8 , 9 , 10 Thus, RIPK1 has emerged as a potential therapeutic target in a variety of IMIDs, including psoriasis, rheumatoid arthritis, and ulcerative colitis. 11
GSK2982772 is highly selective, binding to an allosteric pocket of the RIPK1 kinase domain to inhibit RIPK1‐mediated cell death and cytokine production. 11 The objective of the current study was to examine the safety, pharmacokinetic (PK), pharmacodynamic (PD), and preliminary efficacy of GSK2982772, a first‐in‐class, RIPK1 small‐molecule inhibitor in patients with active plaque‐type psoriasis, a prototypic IMID. Tissue analysis was included as previous work in psoriasis has shown that the number of T cells in the skin may correlate with measures of epidermal thickness and could be used to screen for efficacy of potentially active drugs in small experimental medicine studies 1 , 12 , 13 similar to findings for CD3+ T‐cell expression in synovial tissue in psoriatic arthritis. 14 , 15
Methods
Study design
This is a phase IIa multicenter, randomized, double‐blind, placebo‐controlled, repeat‐dose study (NCT02776033) to investigate the safety and tolerability, PK, PD, and preliminary efficacy of GSK2982772 in patients with mild‐to‐moderate active plaque‐type psoriasis (Figure 1 ). The GlaxoSmithKline (GSK) data review committee was the only group unblinded for the purpose of interim and ongoing reviews. The core GSK study team remained blinded to individual subject data throughout the study, apart from the study statistician. The study, initiated on August 30, 2016, was performed at four centers in Canada and consisted of a 30‐day screening period, an 84‐day treatment period, and a 28‐day follow‐up period. Patients were randomized in a 2:1 ratio to receive either GSK2982772 60 mg or placebo orally b.i.d. for 84 days. Approximately 1 year after the original protocol date, a second cohort of patients was randomized in a 3:1 ratio to receive either GSK2982772 60 mg or placebo orally t.i.d. for 84 days.
Figure 1.
Chemical structure of GSK2982772.
The completion date was January 4, 2018.
The study was approved by the ethics committee at every participating institution and was conducted according to the recommendations of Good Clinical Practice and the Declaration of Helsinki. The study protocol, amendments, informed consent, and other information requiring preapproval were reviewed and approved by an institutional review board. All patients provided written informed consent to participate in the study.
Patients
Eligible male and female patients were between 18–75 years of age, with a body mass index between 18.5–35.0 kg/m2 and had active plaque‐type psoriasis involving body surface area (BSA) ≥ 3% and Physician Global Assessment (PGA) ≥ 3, with at least two stable plaques on a site suitable for repeat biopsy at screening. Plaques were required to be ≥ 3 cm × 3 cm, have a Plaque Lesion Severity Sum (PLSS) score ≥ 2 (moderate or above) for the induration component, ≥ 1 for erythema, and scaling with a total score of ≥ 5 using Psoriasis Area Severity Index (PASI) severity assessment criteria.
Patients agreed to avoid prolonged exposure to natural sunlight for the duration of the study and were either naïve to biologic therapies for psoriasis or had previous exposure to a single anti‐TNF agent in a previous clinical trial, discontinued more than 8 weeks prior to their screening visit.
Some medications for the treatment of psoriasis such as emollients without salicylic acid and medicinal shampoos (but not corticosteroids) were allowed with specific restrictions. Prohibited medications included photochemical therapy with psoralens, psoralen and ultraviolet A therapy, ultraviolet B phototherapy, systemic immunomodulating agents, P‐glycoprotein inhibitors, narrow therapeutic index cytochrome P450 3A4 substrates, and live vaccinations.
Patients with tuberculosis, HIV, hepatitis B, or hepatitis C were excluded. Other exclusion criteria included any recurrent/chronic/active infection, a known history of significant neurologic disorders (e.g., multiple sclerosis or amyotrophic lateral sclerosis), or a history of suicidal ideation behavior.
Study assessments
Study visits occurred on days 1, 8, 15, 29, 43, 57, 71, and 85. Baseline clinical disease assessments included BSA, PLSS, PGA, and PASI. BSA was assessed on days 1 and 85. Other efficacy assessments were made on days 1, 15, 29, 43, 57, 71, and 85.
Skin biopsies were collected at baseline (predose day 1) and predose on day 43. The outer one third of one previously identified target lesion was used for assessment of histology, PK, and gene expression analysis. A second lesion was used for PLSS. A third skin biopsy from clear skin was performed at baseline only. All biopsy lesions were required to be on the trunk or extremities and to be shielded from natural light with clothing.
At the sites, 4 mm punch biopsies were obtained from lesional (two) and nonlesional (one) skin. Biopsies were divided (bisected) and processed as follows. For histological analyses, one bisected biopsy sample was placed in 20 mL of neutral‐buffered formalin and shipped to Q Squared Solutions (Valencia, CA) for paraffin embedding and storage. The remaining biopsy samples were either flash frozen for pharmacokinetic analyses or placed in 5 mL of RNAlater Thermo Fisher Scientific (Waltham, MA) for gene expression analyses. All samples were shipped to Q Squared Solutions for storage and at the end of the study, to GSK for sectioning and staining.
Formalin‐Fixed Paraffin‐Embedded (FFPE) skin biopsies were sectioned at 4µm. Staining was performed in the Ventana Discovery Ultra platform. CC1 was used for antigen retrieval followed by endogenous peroxidase blocking with inhibitor reagent. The HQ‐HRP system and purple chromogen was used for receptor‐interacting protein 1 (RIP1) detection. Stained slides were air dried at room temperature and coverslipped.
Whole Slide Images (20×) were manually annotated to analyze epidermis and dermis as separate layers and to exclude artifacts such as folds, tears, and chromogen precipitation areas. Epidermal skin thickness measurements were made with the ImageJ software program, National Institutes of Health, Bethesda, MD and University of Wisconsin, Madison, WI, using images under 10× magnification. 16
Predose plasma concentrations of GSK2982772 were measured on day 43 (for comparison with predose skin concentrations) and day 85. In addition, postdose (1, 2, 4, and 6 hours) plasma concentrations were assessed on days 1 and 43. All PK timepoints were used for the population PK analysis that was used to derive individual subject predicted trough concentrations for the PK/PD analysis of GSK2982772 plasma concentration tertiles vs. PLSS response. Patients recorded their daily study medication, concomitant medication administration, and any AEs while they were away from the clinical site. A 28‐day follow‐up period was entered after the initial 85‐day treatment period.
End points
Safety and tolerability were the primary end points in this study, including AEs, clinical laboratory values (clinical chemistry, hematology, and urinalysis), vital sign measurements (blood pressure, heart rate, respiratory rate, and body temperature), and 12‐lead electrocardiogram (ECG) monitoring.
Secondary end points included GSK2982772 plasma and skin trough concentrations, PD markers of change in histopathological scoring of psoriatic lesional biopsies from baseline, and messenger RNA (mRNA) expression of inflammatory genes. Efficacy end points included percent change from baseline and actual PLSS scores of the index lesion.
Exploratory efficacy end points included change from baseline in PASI, PGA, BSA, Itch Visual Analogue Scale (VAS), and Dermatology Life Quality Index (DLQI). Additionally, punch biopsies were taken for exploratory analysis of GSK2982772 concentrations in the skin.
Statistical analysis
The size of the study population was determined to support a 95% confidence interval around the probability of an observed safety event. Properties of the secondary end point PLSS were also considered. Assuming a standard deviation of 25%, it was estimated that the lower and upper bounds of the 95% confidence interval for the difference between GSK2982772 (n = 20) and placebo (n = 10) in percentage change in index lesion PLSS would be within approximately 20.3% of the point estimate.
Descriptive safety analyses, including review and display of AEs, clinical laboratory values, vital sign measurements, and 12‐lead ECG monitoring were based on the safety population, which included all patients who received at least one dose of the study treatment.
For PD analysis, the change observed from baseline on log‐transformed concentrations of inflammatory biomarkers from skin biopsy were statistically analyzed in the safety population using an analysis of covariance model. Log2 (intensity) mRNA expression of inflammatory gene transcripts were statistically analyzed using a linear repeated measures mixed effects model. For efficacy analysis (safety population), percentage change from baseline PLSS score was statistically analyzed using a mixed model repeated measures approach adjusted for baseline.
A population PK model was used to predict steady‐state GSK2982772 trough concentrations in plasma for each patient, and these values were used to evaluate the relationship between GSK2982772 systemic exposure and the percentage changes in PLSS score on day 43. The predicted trough plasma concentrations of GSK2982772 were divided into tertiles and were plotted against the corresponding percentage change in PLSS score within each tertile, together with the percentage change in PLSS score within the placebo.
For exploratory efficacy analyses (safety population), change from baseline in PASI, PGA, DLQI, and VAS score was analyzed using a mixed model repeated measures approach, and change from baseline in BSA was analyzed using an analysis of covariance model.
Results
A total of 65 patients were randomized, and 57 (88%) completed the study (Figure 2 ). In general, demographics and patient characteristics were balanced between treatment cohorts at baseline (Table 1 ). Patients in the placebo group had lower baseline scores for PASI and BSA compared with both GSK2982772 cohorts.
Figure 2.
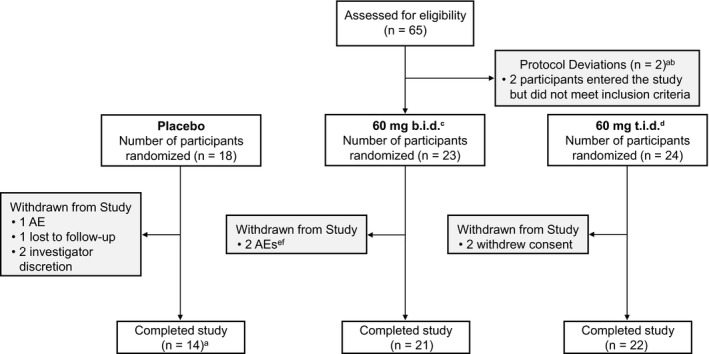
Patient disposition. aOne participant was found to have previously been exposed to 2 anti‐TNF biologic agents in the context of a previous clinical trial. The participant had already been dosed GSK2982772 and was therefore not withdrawn from the trial. bOne participant did not comply with contraception requirements. This was discovered on day 85, and the participant was therefore not withdrawn from the trial. cOne participant, randomized to the placebo group, erroneously received GSK2982772 on the day 29 visit. The participant was included in the 60‐mg b.i.d. treatment group. dOne participant was given captopril, a prohibited medication, for an SAE of hypertension on day 15 following a scheduled arthroscopic operation on the right knee. The participant was not withdrawn from the study as there were no safety concerns. eOne participant died following an accidental overdose of methylenedioxymethamphetamine 39 days after the start of the trial. fOne participant developed a herpes zoster infection on day 9 and was withdrawn from the study. AE, adverse event; b.i.d., twice daily; t.i.d., three times daily; SAE, serious adverse event.
Table 1.
Baseline patient demographics and clinical characteristics by treatment group
| Demographics |
GSK2982772 60 mg b.i.d. (N = 23) |
GSK2982772 60 mg t.i.d. (N = 24) |
Placebo b.i.d. (N = 10) |
Placebo t.i.d. (N = 8) | Total (N = 65) |
|---|---|---|---|---|---|
| Age in years (mean (SD)) a | 44.6 (12.52) | 47.5 (13.41) | 40.2 (15.98) | 55.6 (7.98) | 46.3 (13.40) |
| Sex (n (%)) | |||||
| Female | 6 (26) | 5 (21) | 3 (30) | 2 (25) | 16 (25) |
| Male | 17 (74) | 19 (79) | 7 (70) | 6 (75) | 49 (75) |
| BMI (kg/m2) (mean (SD)) | 28.22 (4.381) | 29.46 (3.110) | 28.52 (5.350) | 29.21 (3.293) | 28.85 (3.950) |
| Ethnicity (n (%)) | |||||
| Hispanic or Latino | 0 | 3 (13) | 0 | 0 | 3 (5) |
| Not Hispanic or Latino | 23 (100) | 21 (88) | 10 (100) | 8 (100) | 62 (95) |
| Race detail | |||||
| Asian/Native Hawaiian/other Pacific Islander | 3 (12) | 4 (16) | 2 (20) | 2 (13) | 11 (18) |
| White ‐ Arabic/North African heritage | 1 (4) | 1 (4) | 1 (10) | 0 | 3 (5) |
| White ‐ White/Caucasian/European heritage | 19 (83) | 19 (79) | 7 (70) | 6 (75) | 51 (78) |
| Body surface area (%) (mean (SD)) | 9.8 (9.94) | 10.3 (11.33) | 8.5 (9.10) | 5.1 (1.13) | 9.2 (9.73) |
| Duration in years (mean (SD)) | 16.9 (11.12) | 18.5 (12.58) | 14.3 (11.79) | 21.0 (14.89) | 17.6 (12.12) |
| Prior treatment (n (%)) | |||||
| Yes | 23 (100) | 24 (100) | 9 (90) | 8 (100) | 64 (98) |
| No | 0 | 0 | 1 (10) | 0 | 1 (2) |
| Naïve to phototherapy (n (%)) | |||||
| Yes | 6 (26) | 7 (29) | 4 (40) | 2 (25) | 19 (29) |
| No | 17 (74) | 17 (71) | 6 (60) | 6 (75) | 46 (71) |
| PASI (mean (SD)) | 10.3 (5.61) | 10.3 (6.11) | 8.4 (5.18) | 7.8 (2.87) | 9.7 (5.47) |
| Index lesion PLSS score (mean (SD)) | 8.1 (1.62) | 7.5 (1.44) | 6.9 (1.73) | 8.5 (1.51) | 7.7 (1.61) |
| Median (min‐max) | 8.0 (5–11) | 8.0 (5–11) | 6.0 (5–10) | 8.5 (7–11) | 7.0 (5–11) |
| Index lesion severity area (cm2) (mean (SD)) | 57.5 (50.01) | 68.9 (109.10) | 50.6 (35.41) | 67.3 (81.60) | 61.9 (78.04) |
| Biopsy lesion PLSS score (mean (SD)) | 7.5 (1.56) | 7.5 (1.41) | 6.7 (1.34) | 8.5 (1.51) | 7.5 (1.51) |
| Median (min‐max) | 7.0 (5–11) | 7.0 (5–11) | 6.5 (5–9) | 8.5 (7–11) | 7.0 (5–9) |
| Biopsy lesion severity area (cm2) (mean (SD)) | 84.1 (116.79) | 57.3 (61.93) | 54.9 (50.28) | 67.5 (120.80) | 67.7 (90.45) |
| PGA score (mean (SD)) | 3.4 (0.59) | 3.3 (0.48) | 3.3 (0.67) | 3.5 (0.53) | 3.4 (0.55) |
| History of tobacco use (n (%)) | |||||
| Never smoked | 8 (35) | 7 (29) | 4 (40) | 2 (25) | 21 (32) |
| Current smoker | 10 (43) | 8 (33) | 3 (30) | 2 (25) | 23 (35) |
| Former smoker | 5 (22) | 9 (38) | 3 (30) | 4 (50) | 21 (32) |
b.i.d., twice daily; BMI, body mass index; min, minimum; max, maximum; PASI, Psoriasis Area Severity Index; PGA, Physician Global Assessment; PLSS, Psoriatic Lesion Severity Sum; SD, standard deviation; t.i.d., three times daily.
Age is imputed when full date of birth is not provided.
Safety and tolerability
Based on the safety data collected, there was no clear‐cut difference in the rate of AEs between study treatment arms. The most frequently reported AEs are shown in Table 2 .
Table 2.
Adverse events
| n (%) | Placebo (N = 18) | 60 mg b.i.d. (N = 23) | 60 mg t.i.d. (N = 24) | Total (N = 65 a ) |
|---|---|---|---|---|
| Any event | 8 (44) | 21 (91) | 16 (67) | 45 (69) |
| AE by severity | ||||
| Serious AE | 0 | 2 (9) | 0 | 2 (3) |
| Moderate AE | 3 (17) | 10 (43) | 3 (13) | 16 (25) |
| Mild AE | 5 (28) | 9 (39) | 13 (54) | 27 (42) |
| Most frequent AE | ||||
| Nasopharyngitis | 4 (22) | 7 (30) | 7 (29) | 18 (28) |
| Headache | 4 (22) | 10 (43) | 3 (13) | 17 (26) |
| Arthralgia | 0 | 1 (4) | 2 (8) | 3 (5) |
| Back pain | 2 (11) | 0 | 1 (4) | 3 (5) |
| Cough | 0 | 2 (9) | 1 (4) | 3 (5) |
| Diarrhea | 2 (11) | 1 (4) | 0 | 3 (5) |
| Fatigue | 0 | 3 (13) | 0 | 3 (5) |
| Nausea | 1 (6) | 1 (4) | 1 (4) | 3 (5) |
| Upper respiratory tract infection | 0 | 2 (9) | 1 (4) | 3 (5) |
| Decreased appetite | 0 | 1 (4) | 1 (4) | 2 (3) |
| Dizziness | 0 | 2 (9) | 0 | 2 (3) |
| Gastroenteritis | 0 | 2 (9) | 0 | 2 (3) |
| Ligament sprain | 1 (6) | 0 | 1 (4) | 2 (3) |
| Pain in extremity | 1 (6) | 1 (4) | 0 | 2 (3) |
| Vomiting | 1 (6) | 1 (4) | 0 | 2 (3) |
| Most frequent drug‐related AE | 3 (17) | 7 (30) | 1 (4) | 11 (17) |
| Headache | 1 (6) | 2 (9) | 0 | 3 (5) |
| Diarrhea | 2 (11) | 0 | 0 | 2 (3) |
| Nausea | 1 (6) | 1 (4) | 0 | 2 (3) |
AE, adverse event; b.i.d., twice daily; t.i.d., three times daily.
Includes any patient given at least one dose of GSK2982772.
In the 60‐mg b.i.d. cohort, a study drug–related event of herpes zoster was reported as moderate in intensity and led to the withdrawal of study treatment. Two patients experienced severe AEs, which were determined not to be drug related by the investigator. One patient died after an accidental overdose with recreational use of methylenedioxymethamphetamine, and another reported abdominal distension. In the 60‐mg t.i.d. cohort, one serious AE, determined not to be drug related, was reported on day 14 due to hypertension following an arthroscopic operation on the right knee joint.
The incidence of drug‐related AEs was highest in the 60‐mg b.i.d. cohort (30%) with five patients reporting five AEs (four unique) of moderate intensity. These included headache (two patients), blurred vision (one patient), nasopharyngitis (one patient), and vitamin B12 deficiency (one patient). All other drug‐related AEs were mild in intensity. One patient in the 60‐mg t.i.d. cohort reported decreased appetite, which was considered a drug‐related AE of mild intensity.
Laboratory assessments, ECGs and vital signs of potential clinical importance were reported with no related AEs or obvious patterns detected across treatment groups or study visits. No suicidality was reported during any part of the study.
Clinical efficacy results
There was a decrease in PLSS compared with baseline in the 60‐mg b.i.d. cohort; the PLSS improved relative to placebo with a difference of −18% at day 85 (Figure 3 ). Similarly, there was a decrease in PLSS in the 60‐mg t.i.d. cohort; however, a strong response in the t.i.d. placebo group was observed, potentially due to an imbalance in baseline PLSS score.
Figure 3.
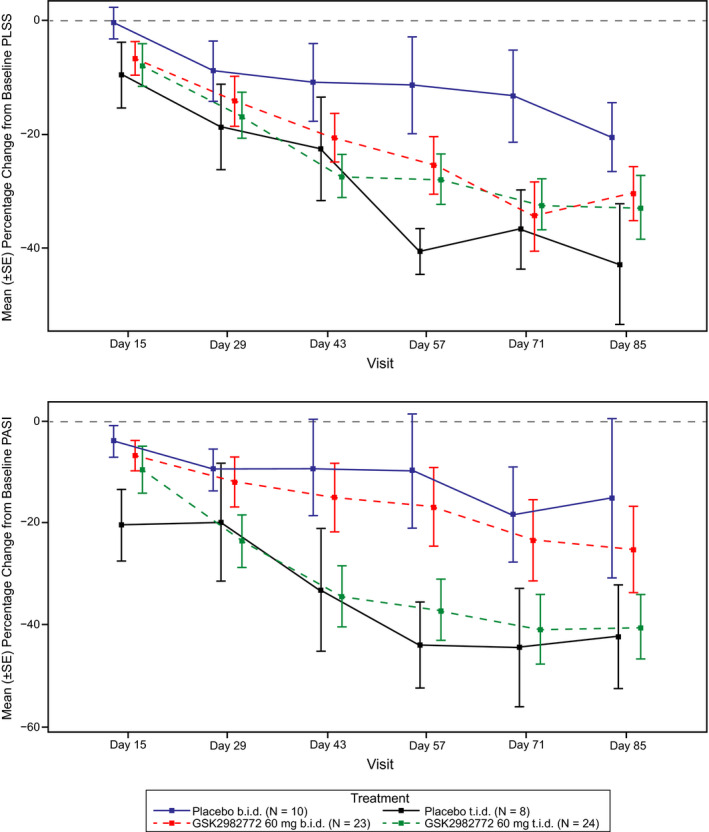
Plots represent mean (±SE) percentage change in (a) PLSS and (b) PASI scores over time by treatment group. The placebo t.i.d. group produced a higher‐than‐expected response to treatment. Representations did not adjust for baseline differences in PLSS and PASI. b.i.d., twice daily; PASI, Psoriasis Area Severity Index; PLSS, Plaque Lesion Severity Sum; SE, standard error; t.i.d., three times daily.
In the 60‐mg b.i.d. cohort, PASI score decreased from baseline, and improvements were seen relative to placebo at day 85 (final difference of −0.31). In the 60‐mg t.i.d. cohort, a decrease in baseline PASI was observed; however, similar to PLSS score, the observed decrease for GSK2982772 was lower than in the placebo group due to the strong response in the t.i.d. placebo arm. In both treatment arms, BSA decreased from baseline with a difference from placebo of −1.41 for 60 mg b.i.d. and −1.27 for 60 mg t.i.d. at day 85.
PGA scores decreased from baseline in both the 60‐mg b.i.d. and 60‐mg t.i.d. cohorts; however, there was no difference from placebo b.i.d. and a +0.50 (on a 0–5 scale) final difference from placebo t.i.d. at day 85. No patients achieved a PGA score of clear or almost clear on placebo b.i.d. Three patients (13%) treated in the GSK2982772 60‐mg t.i.d. cohort achieved a PGA score of clear or almost clear at day 85. The highest response was seen in the placebo t.i.d. cohort, where two patients (25%) had a PGA score of clear or almost clear.
In both the 60‐mg b.i.d. and 60‐mg t.i.d. cohorts at day 85, a decrease from baseline in DLQI and Itch VAS was observed. However, in the 60‐mg t.i.d. arm, DLQI and VAS increased relative to placebo.
There was a trend for a decrease in the PLSS score with increasing tertiles of predicted steady‐state trough concentrations of GSK2982772 (Figure 4 ).
Figure 4.
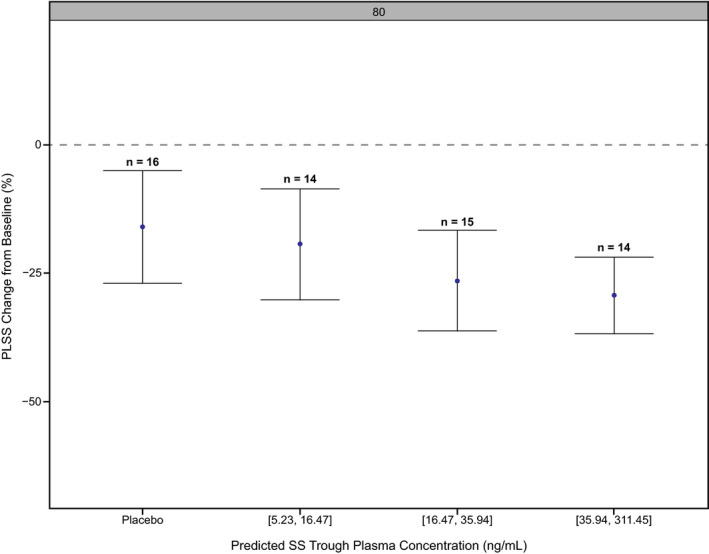
Tertiles of predicted steady‐state trough plasma concentrations of GSK2982772 vs. PLSS percentage change from baseline. Values of x‐axis represent the GSK2982772 plasma concentration range for each tertile in µg/mL. Negative values indicate a reduction of PLSS score from baseline. PLSS, Plaque Lesion Severity Sum; SS, steady state.
GSK2982772 levels in plasma and skin
On day 43, median total trough concentrations of GSK2982772 in skin were, on average, 1.35‐fold higher than the median total trough concentrations of GSK2982772 in plasma for both the 60‐mg b.i.d. and 60‐mg t.i.d. dose regimens. The increase in dose between 60 mg b.i.d. to 60 mg t.i.d. resulted in an approximately 1.6‐fold increase in skin and plasma trough concentrations.
The tissue response to GSK2982772 treatment
Histological measurement of epidermal thickness showed reductions of 21% and 17% in the 60‐mg b.i.d. treatment arm and 60‐mg t.i.d. treatment arm compared with placebo, respectively. There was a 28% reduction in CD3+ T cells in the dermis in the 60‐mg b.i.d. arm and a 29% reduction in the 60‐mg t.i.d. arm. In the epidermis, CD3+ T cells were reduced by 58% in both active treatment groups. For CD11+ myeloid dendritic cells in the dermis, reductions of 43% and 37% were observed in the 60‐mg b.i.d. and 60‐mg t.i.d. arms, respectively. For CD11+ myeloid dendritic cells in the epidermis, reductions of 47% and a 35% were observed in the 60‐mg b.i.d. arm and 60‐mg t.i.d. arm, respectively. All comparisons were made relative to placebo at day 43. (Table 3 ) There were no differences observed in other histopathological biomarkers. Additionally, no differences were observed in the mRNA expression of inflammatory gene transcripts (IL‐4, IL‐10, IL‐17, IL‐21, IL‐22, IL‐23, TNF, and interferon gamma) or the expression of the blood inflammatory biomarkers (C‐reactive protein, vascular endothelial growth factor, proteins S100A8 and S100A9, IL‐17A, IL‐17F, IL‐22, and TNF) for either treatment cohort compared with placebo.
Table 3.
Adjusted mean percentage change in histopathological scoring of psoriatic lesional biopsy
| Visit | N | Treatment | n | LS Mean (95% CI) | %CVb | Percent change of ratio to placebo (95% CI) |
|---|---|---|---|---|---|---|
| Parameter: CD11+ (cells/mm2) Skin: Dermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 8 | −16.08 (−49.68, 39.94) | 25.89 | |
| 8 | Placebo t.i.d. | 7 | −2.55 (−43.18, 67.14) | 27.36 | ||
| 23 | GSK2982772 60 mg b.i.d. | 18 | −52.35 (−66.17, −32.90) | 17.18 | −43.22 (−69.00, 4.01) | |
| 24 | GSK2982772 60 mg t.i.d. | 23 | −38.34 (−54.58, −16.29) | 15.32 | −36.73 (−65.81, 17.07) | |
| Parameter: CD11+ (cells/mm2) Skin: Epidermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 4 | −36.76 (−78.33, 84.53) | 56.64 | |
| 8 | Placebo t.i.d. | 6 | 4.86 (−56.19, 150.98) | 45.06 | ||
| 23 | GSK2982772 60 mg b.i.d. | 10 | −66.45 (−82.95, −33.99) | 34.29 | −46.95 (−85.08, 88.59) | |
| 24 | GSK2982772 60 mg t.i.d. | 20 | −31.91 (−57.78, 9.83) | 23.88 | −35.06 (−75.99, 75.65) | |
| Parameter: CD161+ (cells/mm2) Skin: Dermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 7 | −15.96 (−44.99, 28.40) | 21.28 | |
| 8 | Placebo t.i.d. | 7 | −2.61 (−35.63, 47.35) | 20.78 | ||
| 23 | GSK2982772 60 mg b.i.d. | 13 | −37.02 (−54.25, −13.29) | 15.97 | −25.06 (−54.44, 23.28) | |
| 24 | GSK2982772 60 mg t.i.d. | 23 | 6.87 (−15.57, 35.26) | 11.74 | 9.73 (−30.64, 73.59) | |
| Parameter: CD3+ (cells/mm2) Skin: Dermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 8 | −12.38 (−43.25, 35.28) | 21.91 | |
| 8 | Placebo t.i.d. | 7 | 6.76 (−32.68, 69.32) | 23.30 | ||
| 23 | GSK2982772 60 mg b.i.d. | 20 | −36.94 (−52.00, −17.15) | 13.67 | −28.02 (−56.87, 20.10) | |
| 24 | GSK2982772 60 mg t.i.d. | 23 | −23.87 (−41.02, −1.73) | 12.78 | −28.69 (−57.88, 20.73) | |
| Parameter: CD3+ (cells/mm2) Skin: Epidermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 8 | 40.51 (−17.29, 138.70) | 26.89 | |
| 8 | Placebo t.i.d. | 7 | 69.25 (−4.37, 199.53) | 29.05 | ||
| 23 | GSK2982772 60 mg b.i.d. | 20 | −40.97 (−57.64, −17.76) | 16.65 | −57.99 (−77.51, −21.52) | |
| 24 | GSK2982772 60 mg t.i.d. | 23 | −29.57 (−48.61, −3.47) | 15.81 | −58.39 (−78.53, −19.32) | |
| Parameter: Elastase (cells/mm2) Skin: Dermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 6 | 7.38 (−63.19, 213.26) | 57.01 | |
| 8 | Placebo t.i.d. | 5 | −33.29 (−79.30, 115.01) | 63.22 | ||
| 23 | GSK2982772 60 mg b.i.d. | 17 | −67.68 (−83.07, −38.30) | 32.88 | −69.90 (−91.49, 6.42) | |
| 24 | GSK2982772 60 mg t.i.d. | 19 | −61.18 (−78.72, −29.19) | 30.46 | −41.82 (−84.32, 115.91) | |
| Parameter: Elastase (cells/mm2) Skin: Epidermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 4 | −28.79 (−78.70, 138.04) | 61.87 | |
| 8 | Placebo t.i.d. | 4 | −64.02 (−89.53, 23.58) | 63.50 | ||
| 23 | GSK2982772 60 mg b.i.d. | 4 | −49.46 (−87.53, 104.85) | 73.91 | −29.03 (−88.53, 339.16) | |
| 24 | GSK2982772 60 mg t.i.d. | 9 | −57.23 (−83.08, 8.14) | 45.93 | 18.89 (−76.50, 501.58) | |
| Parameter: Epidermis Thickness (mm2) Skin: Epidermis | ||||||
| Day 43 | 10 | Placebo b.i.d. | 8 | −3.31 (−26.58, 27.34) | 13.79 | |
| 8 | Placebo t.i.d. | 7 | −12.24 (−34.86, 18.23) | 14.94 | ||
| 23 | GSK2982772 60 mg b.i.d. | 20 | −23.15 (−35.41, −8.57) | 8.68 | −20.52 (−42.60, 10.05) | |
| 24 | GSK2982772 60 mg t.i.d. | 23 | −27.53 (−38.48, −14.63) | 8.18 | −17.42 (−41.43, 16.45) | |
b.i.d., twice daily; CI, confidence interval; %CVb, coefficient of variation between subjects; LS, least squares; t.i.d., three times daily.
Discussion
In this first study exploring RIPK1 inhibition in human disease, repeat doses of GSK2982772 60 mg b.i.d. and 60 mg t.i.d. were well tolerated in patients with mild‐to‐moderate active plaque‐type psoriasis, with no apparent differences in terms of AEs between active drug and placebo over 12 weeks.
At day 43, data from the 60‐mg b.i.d. treatment cohort showed reductions in epidermal thickness, CD3+ T cells, CD11+ myeloid dendritic cells, and PLSS scores relative to baseline and placebo. Based on previous work, T‐cell infiltration of the psoriatic skin was used as a sensitive biomarker reflecting common final pathways associated with clinical improvement, which can be used to differentiate between active and ineffective treatment in small experimental medicine studies. 11 , 12 , 13 Differences in reduction in T‐cell infiltration between GSK2982772 treatment groups may be due, in part, to baseline differences in disease severity between the two groups. The decrease in T‐cell infiltration scores after GSK2982772 treatment compared with placebo support the notion that RIPK1 inhibition leads to inhibition of inflammation in psoriasis.
Clinical efficacy assessment was more difficult to interpret due to the unusually strong placebo response in the t.i.d. group. A baseline imbalance in the PLSS, PASI, BSA, DLQI, and VAS scores between the placebo b.i.d. and placebo t.i.d. cohorts was apparent, suggesting the possibility that a difference in the severity of disease between the two groups may have influenced these results. Of note, patients in the placebo t.i.d. cohort had higher baseline PLSS scores compared with other treatment groups. The difficulty in interpreting the clinical findings may also be explained, in part, by seasonal effects that were not considered in the analysis, as the b.i.d. and the t.i.d. arms were not recruited simultaneously. In contrast to clinical assessment, it is unlikely that results from tissue analysis were affected by seasonal differences in sun exposure, as all biopsy samples were obtained from tissue that was shielded from natural light with clothing.
The observed trend for a decrease in the PLSS score with increasing tertiles of GSK2982772 trough concentrations suggests that the efficacy may be further improved at higher systemic exposure to GSK2982772.
Because this study was conducted with mild‐to‐moderate disease activity, the ability to detect a difference in clinical improvement between active treatment vs. placebo may have been limited. The safety data combined with the improvement in T‐cell infiltration and the clinical improvement in the active b.i.d. and t.i.d. arms support future studies evaluating the effects of higher dosages in the future in more active psoriasis patient populations to assess the full potential of RIPK1 inhibition as a new therapy for psoriasis.
Conclusions
GSK2982772 treatment was generally well tolerated. No new safety issues were identified when dosed for 12 weeks in patients with mild‐to‐moderate active plaque‐type psoriasis. The PD marker results and relationship between PLSS and trough GSK2982772 concentrations indicate that inhibition of RIPK1 may have an impact on psoriasis, and possibly in other IMIDs. Future research will need to explore the full potential of GSK2982772 treatment when used at higher dosages and in patients with more active disease.
Funding
Funding for this study (NCT02776033 available from www.clinicaltrials.gov), editorial support, and graphic services was provided by GlaxoSmithKline.
Conflict of Interest
K.W., S.B., S.W., and P.P.T., are shareholders and were employees of GlaxoSmithKline (GSK) at the time of the study. K.P. reports grants and personal fees from AbbVie Inc., Akros Pharma Inc., Amgen, Boehringer Ingelheim, Celgene Corporation (Celgene), Coherus BioSciences, Eli Lilly and Company (Lilly), Galderma, Janssen Pharmaceutica (Janssen), Kyowa Hakko Kirin Co., Ltd., Merck (MSD), Merck‐Serono, Novartis Pharmaceuticals Corporation (Novartis), Pfizer Inc. (Pfizer), Sanofi Genzyme, Takeda Pharmaceutical Company, UCB, Inc. (UCB), Bausch Health Companies Inc. (Bausch), and PRCL Research Inc.; grants from Anacor Pharmaceuticals Inc., Arcutis Biotherapeutics, Astellas Pharma, Baxalta, Bristol‐Myers Squibb (BMS), Can‐Fite Biopharma Ltd., Dermira, Inc., Dow Pharmaceutical Sciences, Inc., Genentech, GSK, LEO Pharma, Inc., MedImmune, LLC, Regeneron Pharmaceuticals, Inc., F. Hoffmann‐La Roche Ltd (Roche), Gilead Sciences, Inc., InflaRx GmbH, Moberg Pharma AB, and Sun Pharmaceutical Industries Ltd. (Sun); and personal fees from Meiji Seika Pharma Co., Ltd., and Mitsubishi Tanabe Pharma. C.M. reports grants from GSK related to the submitted work and grants from Lilly, Asana, Bausch, AbbVie Inc., Dermavant Sciences Ltd., Janssen, Pfizer, and Boehringer Ingelheim. J.G.K., reports grants paid to his institution from Novartis, Pfizer, Amgen, Lilly, Boehringer Ingelheim, Innovaderm Research Inc., BMS, Janssen, AbbVie Inc., Parexel, LEO Pharma, Inc., Vitae Pharmaceuticals, Akros Pharma Inc., Regeneron Pharmaceuticals Inc., Allergan, Novan, Inc., Biogen MA Inc., Sienna Biopharmaceuticals, Inc. (Sienna), UCB, Celgene, Botanix Pharmaceuticals, Incyte Corporation, Avillion, LLP, and Exicure, Inc; he also reports personal fees from Novartis, Pfizer, Amgen, Lilly, Boehringer Ingelheim, BMS, Biogen Inc., Janssen, AbbVie Inc., LEO Pharma, Inc., Escalier Biosciences, Inc., Valeant Pharmaceuticals International, Inc., Aurigene Discovery Technologies Limited, Allergan plc, Sienna, UCB, Asana, Celgene, Nimbus Therapeutics, Menlo Therapeutics Inc., Aristea Therapeutics, Sanofi, Sun, Almirall SA, and Arena Pharmaceuticals, Inc. N.S., D.T., M.S., and J.B. are employees of and hold stock in GSK.
Author Contributions
S.W., S.B., K.W., C.M., J.G.K., N.S., D.T., M.S., J.B., and P.P.T. wrote the manuscript. K.W., J.G.K., N.S., D.T., J.B., and P.P.T. designed the research. K.P., C.M., N.S., D.T., and S.W. performed the research. K.W., S.B., K.P., C.M., J.G.K., N.S., D.T., S.W., M.S., and P.P.T. analyzed the data.
Data Sharing Statement
Anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com
Acknowledgments
Editorial support (Allyson Lehrman, DPM and Sarah Hummasti, PhD) and graphic services were provided by AOIC, LLC.
References
- 1. Goedkoop, A.Y. et al Early effects of tumour necrosis factor alpha blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 63, 769–773 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sawyer, L.M. , Cornic, L. , Levin, L.Å. , Gibbons, C. , Møller, A.H. & Jemec, G.B. Long‐term efficacy of novel therapies in moderate‐to‐severe plaque psoriasis: a systematic review and network meta‐analysis of PASI response. J. Eur. Acad. Dermatol. Venereol. 33, 355–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rendon, A. & Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 20, 1475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopalli, S.R. , Kang, T.‐B. & Koppula, S. Necroptosis inhibitors as therapeutic targets in inflammation mediated disorders – a review of the current literature and patents. Expert. Opin. Ther. Pat. 26, 1239–1256 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Schafer, P.H. et al Apremilast, a cAMP phosphodiesterase‐4 inhibitor, demonstrates anti‐inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 159, 842–855 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menter, A. et al Joint AAD‐NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 80, 1029–1072 (2019). [DOI] [PubMed] [Google Scholar]
- 7. Berger, S.B. et al Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN‐deficient mice. J. Immunol. 192, 5476–5480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger, S.B. et al Characterization of GSK'963: a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov. 1, 15009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Donnell, M.A. , Hase, H. , Legarda, D. & Ting, A.T. NEMO inhibits programmed necrosis in an NFkappaB‐independent manner by restraining RIP1. PLoS ONE 7, e41238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel, S. et al RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ 27, 161–175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisel, K. et al Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol. Res. Perspect. 5, e00365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Groot, M. et al A prospective, randomized, placebo‐controlled study to identify biomarkers associated with active treatment in psoriatic arthritis: effects of adalimumab treatment on lesional and nonlesional skin. Dermatology 225, 298–303 (2012). [DOI] [PubMed] [Google Scholar]
- 13. Goedkoop, A.Y. et al Alefacept therapy reduces the effector T‐cell population in lesional psoriatic epidermis. Arch. Dermatol. Res. 295, 465–473 (2004). [DOI] [PubMed] [Google Scholar]
- 14. van Kuijk, A.W. et al A prospective, randomised, placebo‐controlled study to identify biomarkers associated with active treatment in psoriatic arthritis: effects of adalimumab treatment on synovial tissue. Ann. Rheum. Dis. 68, 1303–1309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pontifex, E.K. et al Change in CD3 positive T‐cell expression in psoriatic arthritis synovium correlates with change in DAS28 and magnetic resonance imaging synovitis scores following initiation of biologic therapy–a single centre, open‐label study. Arthritis Res. Ther. 13, R7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim, J. et al Histological stratification of thick and thin plaque psoriasis explores molecular phenotypes with clinical implications. PLoS ONE 10, e0132454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


