Abstract
Aim
To examine the associations between variability in lipids and the risk of cardiovascular disease (CVD) and mortality in patients with type 2 diabetes based on low‐density lipoprotein‐cholesterol (LDL‐C), the total cholesterol (TC) to high‐density lipoprotein‐cholesterol (HDL‐C) ratio and triglycerides (TG).
Materials and methods
A retrospective cohort study included 125 047 primary care patients with type 2 diabetes aged 45‐84 years without CVD during 2008‐2012. The variability of LDL‐C, TC to HDL‐C and TG was determined using the standard deviation of variables in a mixed effects model to minimize regression dilution bias. The associations between variability in lipids and CVD and mortality risk were assessed by Cox regression. Subgroup analyses based on patients’ baseline characteristics were also conducted.
Results
A total of 19 913 CVD events and 15 329 mortalities were recorded after a median follow‐up period of 77.5 months (0.8 million person‐years), suggesting a positive linear relationship between variability in lipids and the risk of CVD and mortality. Each unit increase in the variability of LDL‐C (mmol/L), the TC to HDL‐C ratio and TG (mmol/L) was associated with a 27% (HR: 1.27 [95% CI: 1.20‐1.34]), 31% (HR:1.31 [95% CI: 1.25‐1.38]) and 9% (HR: 1.09 [95% CI: 1.04‐1.15]) increase in the risk of composite endpoint of CVD and mortality, respectively. Age‐specific effects were also found when comparing LDL‐C variability, with patients aged 45‐54 years (HR: 1.70 [95% CI: 1.42‐2.02]) exhibiting a 53% increased risk for the composite endpoints than those aged 75‐84 years (HR: 1.11 [95% CI: 1.01‐1.23]). Similar age effects were observed for both the TC to HDL‐C ratio and TG variability. Significant associations remained consistent among most of the subgroups.
Conclusions
Variability in respective lipids are significant factors in predicting CVD and mortality in primary care patients with type 2 diabetes, with the strongest effects related to LDL‐C and the TC to HDL‐C ratio and most significant in the younger age group of patients aged 45‐54 years. Further study is warranted to confirm these findings.
Keywords: CVD, HDL‐C, LDL‐C, lipids variability, mortality, TC, TG
1. INTRODUCTION
Cardiovascular disease (CVD) is the predominant cause of mortality, and its incidence has reached epidemic proportions globally. Patients with diabetes are predisposed to CVD with approximately a 2‐fold increased risk compared with those without diabetes. 1 Hence, several international guidelines for diabetes care have recommended optimal cholesterol level targets for the prevention of CVD and mortality, 2 , 3 thus generating renewed interest in the harmful effect of cholesterol variability on the risk of CVD and mortality. However, with clinicians and health professionals often focusing on target cholesterol levels, many remain unaware of the impact of intra‐individual cholesterol variability.
Alhough lipid reduction remains a major treatment goal for many clinicians and health professionals, the potentially harmful effects of intra‐individual cholesterol variability are rarely addressed in the literature. There have been eight studies evaluating the associations between cholesterol variability and the risk of CVD and mortality. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Of these, seven focused on either general 4 or CVD populations, 5 , 6 , 7 , 8 , 9 , 10 and thus may appear inapplicable to diabetes populations without CVD. Only one study focused on a diabetes population, thereby indicating the detrimental effects of low‐density lipoprotein‐cholesterol (LDL‐C) variability, although such impacts were not exhibited in triglycerides and high‐density lipoprotein‐cholesterol (HDL‐C). 11 This study also included a group of patients with CVD, therefore it was potentially susceptible to reverse causality. Furthermore, nearly all current studies have included a comparatively small number of patients and outcome events 7 , 8 , 9 , 10 , 11 or have introduced informative censoring and immortal time bias through the inclusion of postbaseline measurements when determining cholesterol variability. 5 , 6 , 7 , 8 Moreover, none of these studies accounted for measurement error in their measures of cholesterol variability, so the association between cholesterol variability between event outcomes may be biased because of regression dilution bias. All current evidence on cholesterol variability has focused primarily on LDL‐C, HDL‐C, total cholesterol (TC) and triglycerides; evidence regarding the TC to HDL‐C ratio is still lacking. Given that the TC to HDL‐C ratio is widely included in prediction models for CVD risk, evidence regarding the association of TC to HDL‐C variability on CVD and mortality could provide important clinical information for the treatment of different lipid traits. 12 , 13 In addition, despite the heterogeneity in diabetes populations, there is no available evidence about differences in the effect of cholesterol variability on CVD and mortality for different patient characteristics such as age and gender. This calls for further research into the relationship between cholesterol variability and the risk of CVD and mortality.
The aim of this population‐based cohort study was to evaluate the associations between variability in lipids, including LDL‐C, the TC to HDL‐C ratio and triglycerides, and incident CVD and mortality among patients with type 2 diabetes.
2. MATERIALS AND METHODS
2.1. Study design
This retrospective cohort study was carried out with data extracted from the national clinical database of the Hong Kong Hospital Authority (HA). The HA manages more than 90% of patients in Hong Kong with chronic disease 14 through its 43 public‐sector hospitals, 49 specialist outpatient clinics and 73 primary care clinics. Patients included in this study were aged 45‐84 years and diagnosed clinically with type 2 diabetes. The presence of diabetes was determined according to the International Classification of Primary Care‐2 (ICPC‐2) code of T90 and was routinely diagnosed by clinicians. A total of 125 047 patients, with no prior CVD diagnoses at baseline, were recruited from 1 January 2008 to 31 December 2012. All confidential data, such as patient profiles and event outcomes, are recorded in the HA clinical management system that has previously supported significant epidemiological studies, thus ensuring validity and coding accuracy. 15 , 16 , 17 All clinicians and healthcare professionals who are responsible for recording the related clinical information and patient demographics, such as patients' diagnoses, prescriptions, laboratory tests and results, emergency department visits, hospitalizations and specialist and primary care outpatient clinic visits during doctor consultations, are trained by the HA and deemed proficient in using the clinical management system. The follow‐up for lipid measurements and outcome determination in this study design is illustrated in Figure S1. Blood test for lipid measurements of patients with type 2 diabetes are performed annually in public primary care clinics to determine respective visit‐to‐visit cholesterol variability, giving annual readings after the first lipids measurement until the end of the 2‐year visit, at least three occasions in total, for estimating variability in lipids. Participants with less than three cholesterol records were excluded from the study. The baseline for each patient was determined by the first date of attendance of doctor consultation in the clinic or date of the third record of lipid measurements during the subject inclusion period. Each eligible patient was followed up from baseline to the incident date of outcome events, death or the final follow‐up visit before 31 December 2017, whichever occurred first.
2.2. Outcome measures
The primary outcome of the study was the composite outcome time to the first incidence CVD or all‐cause mortality, whichever occurred first; the secondary outcomes were the time to the first event of individual CVD, the subtype of coronary heart disease, stroke, heart failure, all‐cause mortality, CVD mortality and non‐CVD mortality. Clinical events were defined in accordance with ICPC‐2 or the International Classification of Diseases (ninth edition, Clinical Modification; ICD‐9‐CM). Respective mortality reports were provided by the internal population data of the Hong Kong Government Death Registry, in which CVD‐related mortality was classified as death with history of CVD or the main cause of death by I20‐I25, I50 and I60‐I69. Such codes have previously given positive predictive values of 85.4% (95% confidence interval [CI] 78.8%‐90.6%) and 91.1% (83.2%‐96.1%) in the diagnosis of myocardial infarction and stroke, respectively, 17 thus ensuring high coding accuracy in determination of CVD events. Further definitions for individual cardiovascular events are summarized in Table S1.
2.3. Ethical approval
Ethical approval for this study was obtained and reviewed by the Institutional Review Board of the Hong Kong Hospital Authority.
2.4. Lipid measurements
Lipid profiles were determined by analysis of 20 mL serum samples of venous blood drawn after an overnight fast in each subject. Blood‐taking is performed according to a standardized protocol across all clinics and hospitals in HA. Respective cholesterol measurements were obtained through Roche diagnostics using an automatic biochemical analyser (Cobas C6000 or equivalent). If triglycerides levels do not exceed 4.0 mmol/L, LDL‐C levels are routinely calculated via the Friedewald equation, 18 otherwise direct LDL‐C levels are measured.
2.5. Cholesterol variability measurements
Usual cholesterol level and cholesterol variability measures were obtained using a mixed effects location‐scale model, which treats the intra‐individual variability as an additional random effect. By using the mixed effects model, we used information across individuals to estimate the cholesterol variability more precisely, thus reducing regression dilution bias in the estimated association with the time‐to‐event outcome. The model was fitted in the Bayesian framework using Markov Chain Monte Carlo with JAGS version 4.3.0 and the R2jags package in R. 19 , 20 The usual cholesterol levels were estimated by the posterior mean of the random intercept, whereas the cholesterol variability measures were estimated by the posterior mean of the residual standard deviation, represented by the mean and standard deviation of lipid level corrected with regression dilution bias, respectively. Details of the statistical methods can be found in Appendix S1 and elsewhere in the literature. 21 , 22
2.6. Baseline characteristics
Baseline characteristics included gender, age, duration of diabetes, smoking status, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), HbA1c, estimated glomerular filtration rate (eGFR), 23 Charlson's co‐morbidity index, 24 , 25 the use of antidiabetic drugs (e.g. insulin, metformin, sulphonylurea), the use of antihypertensive drugs (e.g. angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers, calcium channel blockers, diuretics and others [hydralazine, methyldopa and prazosin]), statins and fibrates. All laboratory assays were performed in accredited laboratories by the College of American Pathologists, the Hong Kong Accreditation Service, or the National Association of Testing Authorities, Australia.
2.7. Data analysis
Multiple imputation was used to replace the missing data for all baseline characteristics except for lipid profile measures. Each missing value was imputed five times using the chained equation method adjusted for all baseline covariates and outcomes. The same analysis was conducted for all five imputed datasets, and the results were pooled using Rubin's rule. 26
Variability in lipids was categorized into quintiles. For each quintile group, descriptive statistics of patient characteristics were reported. The cumulative incidence and incidence rate of CVD, mortality and their composite events were calculated for each group, with CIs based on the Poisson distribution. To evaluate between variability in lipids and the risk of an event, multivariable Cox proportional hazards regression adjusted with each patient's characteristics and usual lipids level was applied in this study. The 95% CIs of the hazard ratios were estimated using the floating absolute risk, without the requirement of a reference group for reporting the standard error. 27 Proportional hazards assumptions were assessed using plots of the scaled Schoenfeld residuals against time for the covariates. Variance inflation factors were used to test for the existence of multi‐collinearity. The results suggested that the proportional hazards assumption holds for all models and that multi‐collinearity was not present. To ensure robustness, the analyses were repeated using two alternative measures of variability, the coefficient of variation (CV) and the variability independent of mean (VIM). Restricted cubic splines with three knots were used to investigate non‐linearity of the association between lipid variability as continuous variability and the time‐to‐event outcomes in the Cox models. Three sensitivity analyses of respective conditions were conducted. These included a complete case analysis, followed by an analysis excluding patients with a follow‐up period of less than 1 year, and finally an analysis with an extension of the patient inclusion period from 24 to 36 months.
To explore differences in the effect of cholesterol variability on the outcomes for different patient characteristics, subjects were classified into subgroups by gender (male, female), age group (45‐54, 55‐64, 65‐74 and 75‐84 years), smoking status (non‐smoker, smoker), duration of diabetes (<5, ≥5 years), BMI (<25, ≥25 kg/m2), usual cholesterol level (LDL‐C: <2.6, 2.6‐4.3, ≥4.3 mmol/L; TC‐HDL‐C ratio: <3.5, 3.5‐5, ≥5; triglycerides: <1.8, 1.8‐2.3, ≥2.3 mmol/L), baseline SBP (<130, ≥130 mmHg), HbA1c (<7%, ≥7% [53 mmoL/mol])), eGFR (<90, ≥90 mL/min/1.73m2), Charlson's Index (<4, ≥4), use of antihypertensive drugs (no, yes), use of antidiabetic drugs (no, yes), use of statins (no, yes), and use of fibrates (no, yes). P‐values were adjusted by Bonferroni correction to counteract the problem of multiple comparisons.
All significance tests were two‐tailed, and the statistical significance level was defined as P = .05. The statistical analysis was executed in Stata version 15.1.
3. RESULTS
A total of 125 047 patients fulfilled the inclusion and exclusion criteria. Data completion rates were 99% or higher for most baseline characteristics, except for the duration of diabetes (95.9%) and BMI (94.8%), as shown in Table S2. An average of 3.1 cholesterol measurements (SD: 0.5) were taken from each patient. The characteristics of each cholesterol variability group at baseline are summarized in Table 1. The mean age was 64.3 (SD: 9.7) years, with males accounting for 45.5% of patients overall. The mean values of LDL‐C, the TC to HDL‐C ratio and triglycerides variability were 0.49 (SD: 0.25) mmol/L, 0.57 (SD: 0.30) and 0.45 (SD: 0.43) mmol/L, respectively.
TABLE 1.
Descriptive statistics for baseline characteristics among patients stratified by LDL‐C, TC to HDL‐C ratio or triglycerides variability
| LDL‐C variability (mmol/L) | |||||
|---|---|---|---|---|---|
| <0.285 (N = 25 010) | 0.286‐0.375 (N = 25 009) | 0.376‐0.485 (N = 25 010) | 0.486‐0.680 (N = 25 009) | ≥0.681 (N = 25 009) | |
| Baseline characteristics | |||||
| Male, % | 49.3 | 47.9 | 46.1 | 45.2 | 38.9 |
| Age, y | 64.8 ± 9.9 | 64.3 ± 9.8 | 64.0 ± 9.8 | 63.8 ± 9.8 | 64.5 ± 9.5 |
| Duration of diabetes, y | 9.0 ± 6.9 | 8.8 ± 7.1 | 8.5 ± 7.0 | 8.2 ± 6.7 | 8.0 ± 6.8 |
| Current smoker, % | 10.0 | 9.8 | 9.7 | 9.9 | 9.1 |
| BMI, kg/m2 | 25.3 ± 4.0 | 25.4 ± 3.9 | 25.4 ± 4.0 | 25.5 ± 4.0 | 25.4 ± 3.9 |
| SBP, mmHg | 134.3 ± 16.6 | 134.5 ± 16.7 | 134.7 ± 17.1 | 134.7 ± 17.2 | 134.8 ± 17.4 |
| DBP, mmHg | 74.2 ± 9.9 | 74.5 ± 9.9 | 74.6 ± 10.0 | 74.7 ± 10.1 | 74.0 ± 10.1 |
| HbA1c, % | 7.3 ± 1.0 | 7.4 ± 1.0 | 7.4 ± 1.0 | 7.4 ± 1.1 | 7.4 ± 1.1 |
| eGFR, mL/min/1.73m2 | 102.5 ± 27.6 | 102.5 ± 29.2 | 101.8 ± 28.6 | 101.6 ± 29.3 | 99.0 ± 30.1 |
| Charlson Index | 3.2 ± 1.4 | 3.2 ± 1.4 | 3.2 ± 1.4 | 3.2 ± 1.5 | 3.3 ± 1.4 |
| Use of antidiabetic drugs, % | 85.5 | 84.1 | 83.5 | 82.7 | 82.5 |
| Use of antihypertensive drugs, % | 72.7 | 72.3 | 72.6 | 72.9 | 76.2 |
| Use of statins, % | 15.6 | 15.7 | 18.6 | 32.2 | 63.6 |
| Use of fibrates, % | 2.4 | 2.7 | 3.2 | 4.2 | 4.3 |
| Usual LDL‐C, mmol/L | 2.59 ± 0.53 | 2.89 ± 0.56 | 3.04 ± 0.58 | 3.16 ± 0.56 | 3.29 ± 0.49 |
| LDL‐C variability, mmol/L | 0.23 ± 0.03 | 0.33 ± 0.03 | 0.43 ± 0.03 | 0.57 ± 0.06 | 0.89 ± 0.21 |
| TC to HDL‐C ratio variability | |||||
|---|---|---|---|---|---|
| <0.339 (N = 25 010) | 0.34‐0.448 (N = 25 009) | 0.449‐0.577 (N = 25 010) | 0.578‐0.773 (N = 25 009) | ≥0.774 (N = 25 009) | |
| Baseline characteristics | |||||
| Male, % | 40.7 | 44.3 | 45.4 | 47.5 | 49.6 |
| Age, y | 64.9 ± 9.7 | 64.3 ± 9.7 | 64.1 ± 9.8 | 64.1 ± 9.8 | 63.9 ± 9.8 |
| Duration of diabetes, y | 9.3 ± 7.2 | 8.7 ± 6.8 | 8.4 ± 6.7 | 8.2 ± 6.8 | 7.7 ± 6.7 |
| Current smoker, % | 7.1 | 8.4 | 9.4 | 10.6 | 13.0 |
| BMI, kg/m2 | 24.6 ± 4.1 | 25.4 ± 4.1 | 25.6 ± 4.0 | 25.7 ± 3.9 | 25.8 ± 4.1 |
| SBP, mmHg | 133.8 ± 17.0 | 134.4 ± 16.7 | 134.8 ± 16.9 | 134.9 ± ± 16.9 | 135.2 ± 17.4 |
| DBP, mmHg | 73.3 ± 9.8 | 74.3 ± 9.9 | 74.7 ± 9.9 | 74.7 ± 10.0 | 74.9 ± 10.2 |
| HbA1c, % | 7.3 ± 1.0 | 7.3 ± 1.0 | 7.4 ± 1.0 | 7.4 ± 1.1 | 7.5 ± 1.1 |
| eGFR, mL/min/1.73m2 | 104.7 ± 27.3 | 103.0 ± 27.3 | 102.0 ± 30.1 | 100.3 ± 28.8 | 97.4 ± 30.7 |
| Charlson Index | 3.2 ± 1.4 | 3.2 ± 1.4 | 3.2 ± 1.4 | 3.2 ± 1.5 | 3.3 ± 1.5 |
| Use of antidiabetic drugs, % | 83.5 | 83.7 | 84.0 | 83.8 | 83.4 |
| Use of antihypertensive drugs, % | 68.6 | 72.4 | 73.6 | 75.7 | 76.4 |
| Use of statins, % | 18.6 | 20.0 | 24.7 | 34.7 | 47.7 |
| Use of fibrates, % | 1.1 | 1.9 | 2.8 | 3.9 | 7.1 |
| Usual TC to HDL‐C ratio | 3.18 ± 0.58 | 3.85 ± 0.65 | 4.20 ± 0.72 | 4.51 ± 0.74 | 5.02 ± 0.76 |
| TC to HDL‐C ratio variability | 0.27 ± 0.05 | 0.39 ± 0.03 | 0.51 ± 0.04 | 0.67 ± 0.06 | 1.03 ± 0.28 |
| Triglycerides variability (mmol/L) | |||||
|---|---|---|---|---|---|
| <0.187 (N = 25 010) | 0.188‐0.272 (N = 25 009) | 0.273‐0.391 (N = 25 010) | 0.392‐0.613 (N = 25 009) | ≥0.614 (N = 25 009) | |
| Baseline characteristics | |||||
| Male, % | 52.3 | 45.7 | 42.9 | 42.3 | 44.1 |
| Age, y | 64.9 ± 9.7 | 64.9 ± 9.7 | 64.7 ± 9.7 | 64.1 ± 9.8 | 62.8 ± 9.7 |
| Duration of diabetes, y | 9.7 ± 7.3 | 8.7 ± 7.0 | 8.3 ± 7.0 | 8.0 ± 6.7 | 7.7 ± 6.4 |
| Current smoker, % | 8.9 | 9.1 | 9.1 | 9.8 | 11.6 |
| BMI, kg/m2 | 23.9 ± 3.8 | 25.1 ± 4.0 | 25.7 ± 4.0 | 26.1 ± 3.8 | 26.2 ± 4.0 |
| SBP, mmHg | 133.0 ± 17.0 | 134.4 ± 16.9 | 134.9 ± 17.0 | 135.3 ± 16.9 | 135.4 ± 17.1 |
| DBP, mmHg | 72.8 ± 9.7 | 73.8 ± 9.9 | 74.4 ± 9.9 | 75.0 ± 10.0 | 75.9 ± 10.1 |
| HbA1c, % | 7.3 ± 1.1 | 7.3 ± 1.0 | 7.3 ± 1.0 | 7.4 ± 1.0 | 7.5 ± 1.1 |
| eGFR, mL/min/1.73m2 | 106.2 ± 27.0 | 102.3 ± 27.8 | 100.8 ± 28.2 | 99.9 ± 30.7 | 98.3 ± 30.4 |
| Charlson Index | 3.2 ± 1.4 | 3.3 ± 1.4 | 3.3 ± 1.4 | 3.2 ± 1.5 | 3.2 ± 1.5 |
| Use of antidiabetic drugs, % | 83.6 | 83.1 | 83.6 | 83.8 | 84.3 |
| Use of antihypertensive drugs, % | 65.5 | 72.2 | 75.1 | 76.7 | 77.3 |
| Use of statins, % | 23.5 | 29.1 | 31.1 | 32.3 | 29.8 |
| Use of fibrates, % | 1.0 | 1.4 | 1.8 | 2.4 | 10.1 |
| Usual triglycerides, mmol/L | 0.80 ± 0.17 | 1.16 ± 0.15 | 1.46 ± 0.17 | 1.81 ± 0.18 | 2.39 ± 0.31 |
| Triglycerides variability, mmol/L | 0.15 ± 0.03 | 0.23 ± 0.02 | 0.33 ± 0.03 | 0.49 ± 0.06 | 1.07 ± 0.62 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
All variables are expressed either in percentages or as means (standard deviation).
Over a median follow‐up of 77.5 months (0.8 million person‐years), 19 913 CVD and 15 329 mortalities were observed, a total of 28 841 incidents of any type of event. For the three cholesterol traits, a positive relationship was observed between increased cholesterol variability and both cumulative incidence and incident rates of CVD and mortality (Table 2 ). The positive linear association between LDL‐C, the TC to HDL ratio and triglycerides variability, and the risk of CVD and mortality, is presented in Figure 1. Other variability measurements, such as CV and VIM, were found to produce similar patterns with SD (Figure S2A,B). Figure S3A‐C also indicated a similar pattern for the risk of stroke, heart failure, CVD and non‐CVD mortality. In Figure S4A‐C, the results of the restricted cubic spline for lipid variability, as continuous variability in the Cox models, also suggested direct linear associations.
TABLE 2.
Number, incidence rate and hazard ratio of CVD and mortality, stratified by LDL‐C, TC to HDL‐C ratio or triglycerides variability
| LDL‐C variability (mmol/L) | |||||
|---|---|---|---|---|---|
| <0.285 (N = 25 010) | 0.286‐0.375 (N = 25 009) | 0.376‐0.485 (N = 25 010) | 0.486‐0.680 (N = 25 009) | ≥0.681 (N = 25 009) | |
| CVD | |||||
| Cumulative cases with event | 3724 | 3897 | 4011 | 4165 | 4116 |
| Incidence rate (95% CI) a | 24.4 (23.6, 25.2) | 25.3 (24.5, 26.1) | 25.9 (25.1, 26.7) | 27.3 (26.5, 28.1) | 27.5 (26.7, 28.4) |
| Hazard ratio b (95% CI) | 1.00 (0.97, 1.04) | 1.05 (1.01, 1.08)* | 1.06 (1.03, 1.09)* | 1.14 (1.10, 1.17)* | 1.11 (1.07, 1.15)* |
| All‐cause mortality | |||||
| Cumulative cases with event | 2995 | 2939 | 3036 | 3204 | 3155 |
| Incidence rate (95% CI) a | 18.3 (17.7, 19.0) | 17.7 (17.1, 18.4) | 18.2 (17.5, 18.8) | 19.4 (18.7, 20.1) | 19.5 (18.8, 20.2) |
| Hazard ratio b (95% CI) | 1.00 (0.96, 1.04) | 1.02 (0.98, 1.06)* | 1.05 (1.01, 1.08)* | 1.18 (1.14, 1.22)* | 1.22 (1.17, 1.27)* |
| All composite events | |||||
| Cumulative cases with event | 5606 | 5628 | 5808 | 5982 | 5817 |
| Incidence rate (95% CI) a | 36.7 (35.8, 37.7) | 36.5 (35.6, 37.5) | 37.5 (36.5, 38.5) | 39.2 (38.2, 40.2) | 38.9 (37.9, 39.9) |
| Hazard ratio b (95% CI) | 1.00 (0.97, 1.03) | 1.03 (1.00, 1.06)* | 1.06 (1.03, 1.09)* | 1.15 (1.12, 1.18)* | 1.13 (1.10, 1.16)* |
| TC to HDL‐C ratio variability | |||||
|---|---|---|---|---|---|
| <0.339 (N = 25 010) | 0.34‐0.448 (N = 25 009) | 0.449‐0.577 (N = 25 010) | 0.578‐0.773 (N = 25 009) | ≥0.774 (N = 25 009) | |
| CVD | |||||
| Cumulative cases with event | 3330 | 3736 | 3897 | 4218 | 4732 |
| Incidence rate (95% CI) a | 21.7 (21.0, 22.5) | 24.2 (23.5, 25.0) | 25.3 (24.5, 26.1) | 27.7 (26.8, 28.5) | 31.6 (30.7, 32.5) |
| Hazard ratio b (95% CI) | 1.00 (0.96, 1.04) | 1.04 (1.00, 1.07)* | 1.04 (1.00, 1.07)* | 1.07 (1.04, 1.10)* | 1.14 (1.10, 1.18)* |
| All‐cause mortality | |||||
| Cumulative cases with event | 2799 | 2843 | 2897 | 3087 | 3703 |
| Incidence rate (95% CI) a | 17.2 (16.6, 17.9) | 17.2 (16.6, 17.8) | 17.5 (16.8, 18.1) | 18.7 (18.0, 19.3) | 22.6 (21.9, 23.3) |
| Hazard ratio b (95% CI) | 1.00 (0.95, 1.05) | 1.04 (1.00, 1.08)* | 1.05 (1.02, 1.09)* | 1.13 (1.09, 1.17)* | 1.34 (1.29, 1.40)* |
| All composite events | |||||
| Cumulative cases with event | 5103 | 5504 | 5593 | 5966 | 6675 |
| Incidence rate (95% CI) a | 33.3 (32.4, 34.2) | 35.7 (34.8, 36.6) | 36.3 (35.4, 37.3) | 39.1 (38.1, 40.1) | 44.6 (43.5, 45.7) |
| Hazard ratio b (95% CI) | 1.00 (0.97, 1.04) | 1.05 (1.03, 1.08)* | 1.05 (1.02, 1.08)* | 1.10 (1.07, 1.12)* | 1.20 (1.17, 1.24)* |
| Triglycerides variability (mmol/L) | |||||
|---|---|---|---|---|---|
| <0.187 (N = 25 010) | 0.188‐0.272 (N = 25 009) | 0.273‐0.391 (N = 25 010) | 0.392‐0.613 (N = 25 009) | ≥0.614 (N = 25 009) | |
| CVD | |||||
| Cumulative cases with event | 3465 | 3802 | 4051 | 4114 | 4481 |
| Incidence rate (95% CI) a | 22.9 (22.1, 23.7) | 25.0 (24.2, 25.8) | 26.6 (25.8, 27.4) | 26.9 (26.1, 27.7) | 28.9 (28.1, 29.8) |
| Hazard ratio b (95% CI) | 1.00 (0.94, 1.06) | 1.03 (0.98, 1.07)* | 1.06 (1.03, 1.08)* | 1.05 (1.01, 1.10)* | 1.10 (1.02, 1.18)* |
| All‐cause mortality | |||||
| Cumulative cases with event | 3076 | 3072 | 2961 | 3071 | 3149 |
| Incidence rate (95% CI) a | 19.1 (18.4, 19.7) | 18.8 (18.2, 19.5) | 18.0 (17.4, 18.7) | 18.6 (17.9, 19.2) | 18.7 (18.0, 19.3) |
| Hazard ratio b (95% CI) | 1.00 (0.94, 1.07) | 1.00 (0.96, 1.05)* | 0.98 (0.95, 1.01)* | 1.05 (1.00, 1.10)* | 1.13 (1.03, 1.23)* |
| All composite events | |||||
| Cumulative cases with event | 5415 | 5671 | 5779 | 5817 | 6159 |
| Incidence rate (95% CI) a | 35.8 (34.8, 36.7) | 37.3 (36.3, 38.2) | 37.9 (37.0, 38.9) | 38.0 (37.1, 39.0) | 39.8 (38.8, 40.8) |
| Hazard ratio b (95% CI) | 1.00 (0.95, 1.05) | 1.02 (0.98, 1.05)* | 1.03 (1.01, 1.05)* | 1.04 (1.00, 1.08)* | 1.10 (1.03, 1.17)* |
Abbreviations: CI, confidence interval; CVD, cardiovasular disease; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; TC, total cholesterol.
Incidence rate (cases/1000 person‐years) with 95% CI based on Poisson distribution.
Hazard ratio was adjusted by age, gender, duration of diabetes, smoking status, body mass index, systolic blood pressure, diastolic blood pressure, HbA1c, estimated glomerular filtration rate, the use of antidiabetic drugs, antihypertensive drugs, statins and fibrates, Charlson's index and usual LDL‐C, TC to HDL‐C ratio or triglycerides (as appropriate). * Significance when confidence interval are wholly larger than 1.
FIGURE 1.
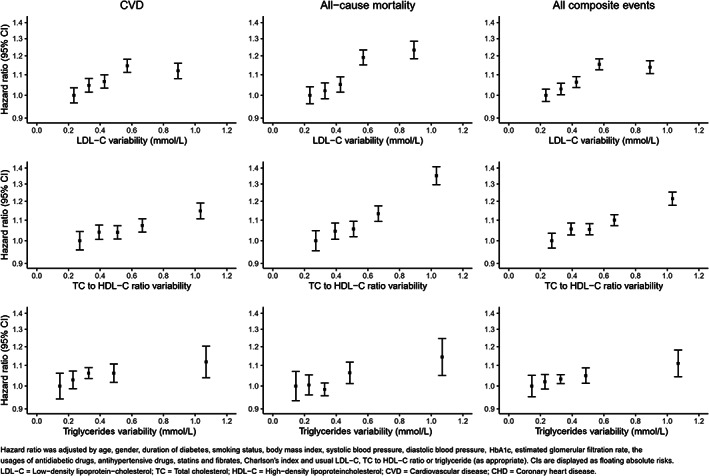
Hazard ratios for the association of a unit increase in LDL‐C, TC to HDL‐C ratio and triglycerides variability with CVD, all‐cause mortality and the composite outcome (CVD and mortality) from Cox regression models adjusted for baseline covariates
FIGURE 3.
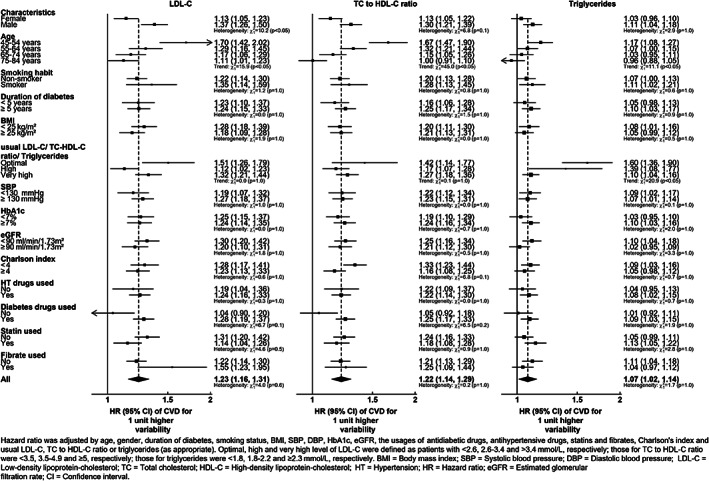
Hazard ratios for the association of a unit increase in LDL‐C, TC to HDL‐C ratio and triglyceridse variability with the composite outcome (CVD and mortality) from Cox regression models adjusted for baseline covariates. A separate model is fitted for each specified subgroup
Figure 2 further illustrates the associations between cholesterol variability and the risk of all outcomes, with cholesterol variability treated as a continuous outcome. Each unit increase in LDL‐C (mmol/L) variability was associated with a 21% (HR: 1.21 [95% CI 1.14‐1.30]), 49% (HR: 1.49 [95% CI 1.39‐1.60]) and 27% (HR: 1.27 [95% CI 1.20‐1.34]) higher risk of CVD, all‐cause mortality, and their composite events, respectively. Each unit increase in the TC to HDL‐C ratio variability was also associated with a 20% (HR: 1.20 [95% CI 1.13‐1.28]), 60% (HR: 1.60 [95% CI 1.50‐1.71]) and 31% (HR: 1.31 [95% CI 1.25‐1.38]) higher risk of respective CVD, all‐cause mortality, and their composite outcomes, respectively. Additionally, each unit increase in triglycerides (mmol/L) variability was also associated with a 7% (HR: 1.07 [95% CI 1.01‐1.13]), 14% (HR: 1.14 [95% CI 1.07‐1.22]) and 9% (HR: 1.09 [95% CI 1.04‐1.15]) higher risk of CVD, all‐cause mortality, and their composite outcomes, respectively. Although the strength of the effects of cholesterol variability on CVD, all‐cause mortality and their composite outcomes appeared to be attenuated when three lipid traits were applied in the multivariable model, the results largely remained significant, apart from the effect of triglycerides variability on mortality, as shown in Figure 2. This in turn reflected the weak correlation between LDL‐C, the TC to HDL‐C ratio and triglycerides variability. Three sensitivity analyses including (1) complete case analysis in Figure S5A, (2) exclusion of patients with less than 1‐year follow‐up in Figure S5B, and (3) extension of the patient inclusion period from 24 to 36 months in Figure S5C, revealed nearly identical results.
FIGURE 2.
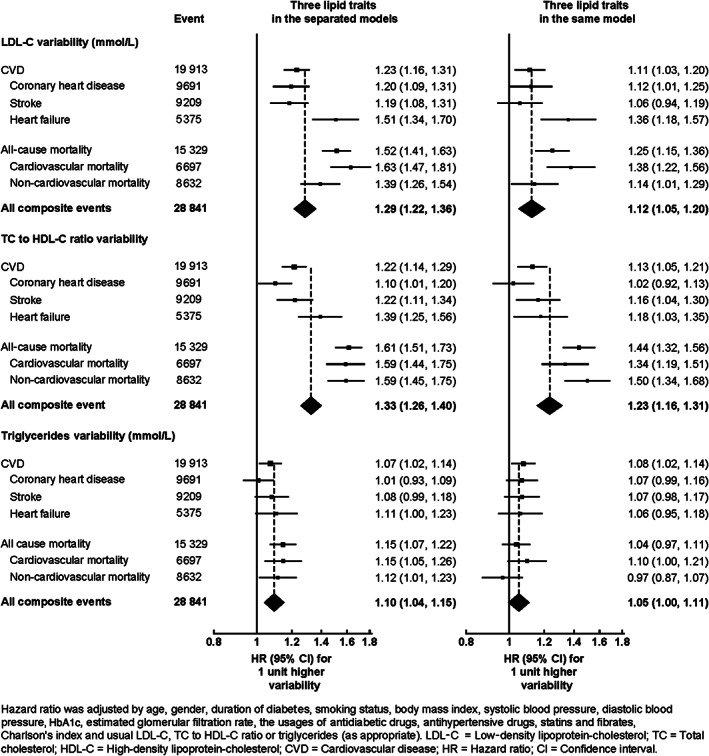
Hazard ratios for the risk of CVD, coronary heart disease, stroke, heart failure, all‐cause mortality, CVD mortality, non‐CVD mortality and their composite with each unit increasing LDL‐C, TC to HDL‐C ratio or triglycerides variability using Cox regressions adjusted for baseline
The results of the subgroup analyses are shown in the forest plots in Figure 3 and Figure S6A,B. In general, the hazard ratios (HRs) for all the outcomes suggest that age is inversely related to the cholesterol variability HR. In particular, the risk of composite CVD and mortality in the group aged 45‐54 years was ~ 53% larger than the group aged 75‐84 years, for both LDL‐C and the TC to HDL‐C ratio variabilities. Males had a higher LDL‐C variability HR then females for the composite event, with 39% higher CVD risk (HR: 1.37 [95% CI 1.26‐1.50]) per 1 mmol/L compared with an 18% increase in CVD risk for females (HR: 1.13 [95% CI 1.05‐1.23]) per 1 mmol/L. Other characteristics including usual cholesterol levels did not appear to affect the cholesterol variability associations.
4. DISCUSSION
This large‐scale cohort study suggested a positive linear association between the risk of CVD and mortality and variability in lipids that addresses LDL‐C and triglycerides as well as the TC to HDL‐C ratio in Chinese patients with type 2 diabetes. After adjusting for patient characteristics and absolute lipid levels, the effect of variability in lipids on all time‐to‐event outcomes remained significant. The effect of variability in lipids on adverse outcomes was greater in patients of a younger age, and was similar to other characteristics including absolute lipid level. These findings suggest that the lower the variability in lipids, the better the outcome, regardless of patient characteristics. Variability in lipids may potentially be useful for predicting the incidence of CVD events and mortality in patients with type 2 diabetes. Apart from optimizing lipids control, this study further shows the importance of lipids variability in younger age groups, suggesting this should be routinely monitored and evaluated in practice.
Although extant research on age‐specific differences and their associations with LDL‐C, the TC to HDL‐C ratio and triglycerides variability, as well as the risk of CVD and mortality, especially in a specific diabetes population, remain insufficiently explored, this study suggested that younger patients were more susceptible to adverse outcomes of cholesterol variability. Such differences could be explained by younger patients having a lower cardiovascular risk, thus exhibiting a heightened sensitivity to cholesterol variability. Furthermore, the effect of cholesterol variability in older patients could be masked by their co‐morbidities and frailty and thus could potentially explain the greater effect of lipid variability in lower lipid levels in our findings. Although the highest level of LDL‐C during therapy should typically be associated with the highest possible risk of variability in follow‐up, it is possible that lipid variability plays a more important factor in contributing to the CVD risk in patients with optimal LDL‐C levels. Those displaying higher levels of LDL‐C often suffer from other co‐morbidities, such that the effect, and in turn the risk of variability in these patients, could be masked, although further research is required to confirm such a hypothesis. Additionally, this study also revealed a possible gender effect on the relationship between LDL‐C variability and CVD risk and mortality, with a stronger association in males than in females. Although the exact mechanism requires further elucidation, it has been suggested that LDL‐C tends to be more variable in females than in males, 5 , 8 , 11 predominantly as a result of hormonal fluctuations in female menstrual cycles. 28 Consequently, females may be more adaptable to cholesterol variability, hence exhibiting a smaller effect in CVD risk regardless of the greater LDL‐C variability.
The variability of the three respective lipid traits, LDL‐C, the TC to HDL‐C ratio and triglycerides, were all found to be linearly associated with an increased risk of CVD in patients with type 2 diabetes. Only one study previously evaluated the relationship between LDL‐C/triglycerides variability and CVD risk in a specific diabetes population. This Taiwanese cohort study on 5354 patients with type 2 diabetes showed an association between CVD risk and LDL‐C variability, but not for triglycerides variability. 11 This could be explained by an ill‐defined cohort, with 10% of recruited patients having a diagnostic history of CVD. Furthermore, the effect of triglycerides variability could also be overshadowed in patients with severe health conditions. In comparison with the Taiwanese study, our investigation solely included patients without any previous history of CVD, and had a much larger sample size and number of outcome events, thereby providing more confidence in the observed associations between LDL‐C/triglycerides variability and adverse outcomes. Another post hoc analysis of the TNT trial in patients with coronary heart disease also supported the significant associations between LDL‐C/triglycerides variability and CVD risk. 5 , 6 Several other studies also showed that the TC to HDL‐C ratio was a stronger predictor of CVD risk, 29 and was included in the risk prediction model for CVD rather than individual TC and HDL‐C. 13 However, previous studies have focused on a single effect but not the joint effect of TC and HDL‐C variability on CVD risk 4 , 5 , 7 , 9 and therefore our findings help to extend the current evidence regarding the positive linear association between the TC to HDL‐C ratio variability and CVD risk. It should also be noted that the results showed a decreased magnitude of effect of LDL‐C variability on CVD risk after LDL‐C variability of 0.5 mmol/L (Figure S4A), although no literature to date has explored this observation. This may be related to resilience and adaptability to LDL‐C variability after this turning point. Moreover, patients with higher LDL‐C variability often display worse health conditions that could potentially overshadow the effect of LDL‐C variability, and further research is warranted to confirm such a hypothesis. In this study, the variability of LDL‐C and the TC to HDL‐C ratio were also found to be significantly associated with non‐CVD mortality. Recent studies have revealed that higher lipid variability may lead to an increased risk of renal function decline 30 , 31 and incidence of end‐stage renal disease. 32 With chronic kidney disease increasing all‐cause mortality, 25 this could possibly explain why lipid variability might contribute to non‐CVD mortality. Furthermore, higher lipid variability has also been suggested to be associated with new‐onset atrial fibrillation. 33 , 34 Previous studies reported that atrial fibrillation often co‐exists with a list of conditions, including (but not limited to) obstructive sleep apnoea and chronic obstructive pulmonary disease, 35 yet it is still hypothetical that the aforementioned causes led to increased non‐CVD mortality in this study. Because the mechanism of how lipid variability leads to non‐CVD mortality remains inadequately addressed, and with few studies focusing on a specific diabetes population, further studies are warranted to validate our findings.
Each lipid trait, LDL‐C, the TC to HDL‐C ratio and triglycerides variability, was independently associated with the risk of CVD in the multivariable model, indicating that each of these variabilities may provide independent predictive information about the risk of CVD. This implies that different pathophysiological mechanisms could be involved in the link between the different cholesterol variabilities and CVD risk. However, the mechanisms of these associations are complex and remain unclear. It has been widely postulated that LDL‐C variability is associated with plaque instability, often caused by enhanced recruitment of activated macrophages and impaired lipid efflux mechanism. 6 , 36 This in turn leads to repeated cholesterol crystallization and dissolution, thus resulting in plaque vulnerability and rupture, events inherently associated with CVD. 4 , 5 , 37 These effects could be further aggravated by endothelial dysfunction and inflammation, following the oxidation of endothelial walls of arteries with increased circulating LDL‐C 7 and carotid intima‐media thickness. A recent post hoc analysis study illustrated the associations between LDL‐C/TC to HDL‐C ratio variability and percentage of atheroma volume progression, 38 thereby reaffirming the pro‐atherosclerotic physiology observed in high lipoprotein variability and providing plausible explanations for a heightened susceptibility to CVD. In terms of triglycerides variability, the pathophysiological pathway to CVD is unknown. Potential mechanisms between hypertriglyceridaemia and atherosclerosis could also be associated with the accelerated formation of foam cells, lipid exchange, vascular endothelial dysfunction, inflammatory response, coagulation and inhibition of fibrinolysis. 39 Additional investigations should be considered to verify such mechanisms between triglycerides variability and CVD risk.
With the increasing popularity of injectable lipid‐lowering drugs, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, the effect of LDL‐C variability may be of greater interest as there might be a difference between weekly, bi‐weekly and monthly regimens. Lipid variability may also be useful in future clinical practice in predicting CV risk. The ultimate goal is to enable an earlier identification of those at higher risk so they can be directed to appropriate primary preventive measures. It has been proposed that medication adherence and statin intolerance might be associated with not only CVD but also cholesterol variability. 40 , 41 In the current study, there was a higher rate of statin use among the high variability group, but our subgroup analysis found no difference in the effect of cholesterol variability on CVD risk between patients with or without lipid‐lowering drugs. Although there were no data available on drug compliance, previous studies have reported that the effect of LDL variability on CVD risk remained unchanged after adjusting for statin non‐adherence. 6 , 7
Factors leading to higher LDL‐C variability have not been well studied, although previous studies reported that a younger age, female gender, smoking and a higher BMI were associated with larger LDL‐C variability. 5 , 8 , 11 Further studies should be conducted to look for other modifiable risk factors associated with lipid variability so as to identify those who are at higher risk in clinical practice.
4.1. Strengths and limitations
The major strengths of this cohort study were the large sample size and the use of an appropriate study design and advanced statistical methods. This effectively avoids bias because of informative observation of lipid measurements, immortal time bias and regression dilution bias, in turn ensuring confidence in the reliability of our results.
However, various limitations should be taken into consideration. First, the conclusion drawn is limited by the nature of this retrospective cohort study, only showing an associative but not a causative relationship between cholesterol variability and CVD risk. Multiple confounders were adjusted for in our analyses, and thus there remains a possibility of residual confounding, although the likelihood of reverse causation is minimal because patients with baseline CVD were excluded. This is further confirmed by the similar results generated from the sensitivity analysis that only included patients with a follow‐up period of more than 1 year. Second, lifestyle‐related factors, such as physical activity level, dietary intake and medication adherence, were not accounted for in this study. However, anthropometric and clinical variables, including BMI, HbA1c and blood pressure, were included to account for patients' disease severity and lifestyle habits. Additionally, HDL and non‐HDL variabilities were not addressed in this study. Future iterations of the study with HDL and non‐HDL variabilities should be conducted to examine the effect of variability of the entire lipid profile. Furthermore, the outcome events were evaluated based on diagnosis coding, hence underdiagnosis could be possible, revealing potential bias in the findings. Finally, the associations between cholesterol variability and elevated CVD risk shown in this study could be attributed to individual differences in our patients with type 2 diabetes, and thus the results may not extend to the population without diabetes.
In conclusion, this population‐based cohort study revealed a positive linear association between variability in lipids and CVD and mortality risks in Chinese patients with type 2 diabetes, shedding new light not only on LDL‐C and triglycerides lipid traits, but also on the variability in the TC to HDL‐C ratio, possibly a promising clinical indicator of CVD. These results support the hypothesis that increased lipids variability results in worse outcomes, although a greater impact was observed in younger age groups. Further studies are warranted to confirm our findings.
CONFLICT OF INTEREST
I.C.K.W. received funding from Pfizer, Bayer and Novartis to evaluate real‐world evidence on pharmacological treatments of cardiovascular diseases, but not related to the current study. E.W.Y.C. received research grants from Bayer, Bristol‐Myers Squibb, Janssen, a Division of Johnson and Johnson, Pfizer and Takeda to evaluate real‐world evidence on pharmacological treatments of cardiovascular diseases, but not related to the current study. The other authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
E.Y.F.W. and C.L.K.L. contributed to the study design and acquisition of data, researched the data, contributed to the statistical analysis and interpretation of the results, and wrote the manuscript. All of the authors contributed to the interpretation of the results, and reviewed and edited the manuscript. E.Y.F.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Appendix S1 S1. Supporting Information.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Hong Kong Hospital Authority for the contributions of data extraction. This study was funded by The Health Services Research Fund, Food and Health Bureau, HKSAR (ref. no. 14151181). The funding organization had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation of the manuscript.
Wan EYF, Yu EYT, Chin WY, et al. Greater variability in lipid measurements associated with cardiovascular disease and mortality: A 10‐year diabetes cohort study. Diabetes Obes Metab. 2020;22:1777–1788. 10.1111/dom.14093
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14093.
Funding information This study was funded by The Health Services Research Fund, Food and Health Bureau, HKSAR (ref. no. 14151181).
REFERENCES
- 1. Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S13‐S28. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation Working Group . IDF clinical practice recommendations for managing type 2 diabetes in primary care In: Aschner P. Adler A. Bailey C. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 4. Kim MK, Han K, Kim H‐S, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population‐based study. Eur Heart J. 2017;38(48):3560‐3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waters DD, Bangalore S, Fayyad R, et al. Visit‐to‐visit variability of lipid measurements as predictors of cardiovascular events. J Clin Lipidol. 2018;12(2):356‐366. [DOI] [PubMed] [Google Scholar]
- 6. Bangalore S, Breazna A, DeMicco DA, Wun C‐C, Messerli FH. Visit‐to‐visit low‐density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65(15):1539‐1548. [DOI] [PubMed] [Google Scholar]
- 7. Lee EY, Yang Y, Kim H‐S, et al. Effect of visit‐to‐visit LDL‐, HDL‐, and non‐HDL‐cholesterol variability on mortality and cardiovascular outcomes after percutaneous coronary intervention. Atherosclerosis. 2018;279:1‐9. [DOI] [PubMed] [Google Scholar]
- 8. Bangalore S, Fayyad R, Messerli FH, et al. Relation of variability of low‐density lipoprotein cholesterol and blood pressure to events in patients with previous myocardial infarction from the IDEAL trial. Am J Cardiol. 2017;119(3):379‐387. [DOI] [PubMed] [Google Scholar]
- 9. Boey E, Gay GMW, Poh K‐K, Yeo T‐C, Tan H‐C, Lee C‐H. Visit‐to‐visit variability in LDL‐and HDL‐cholesterol is associated with adverse events after ST‐segment elevation myocardial infarction: a 5‐year follow‐up study. Atherosclerosis. 2016;244:86‐92. [DOI] [PubMed] [Google Scholar]
- 10. Gu J, Yin Z‐F, Pan J‐A, Zhang J‐F, Wang C‐Q. Visit‐to‐visit variability in low‐density lipoprotein cholesterol is associated with adverse events in non‐obstructive coronary artery disease. Anatol J Cardiol. 2019;22(3):117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu W‐H, Lai C‐W, Lin K‐D, et al. Greater low‐density lipoprotein cholesterol variability increases the risk of cardiovascular events in patients with type 2 diabetes mellitus. Endocr Pract. 2019;25:918‐925. [DOI] [PubMed] [Google Scholar]
- 12. Wan EYF, Fong DYT, Fung CSC, et al. Development of a cardiovascular diseases risk prediction model and tools for Chinese patients with type 2 diabetes mellitus: a population‐based retrospective cohort study. Diabetes Obes Metab. 2018;20(2):309‐318. [DOI] [PubMed] [Google Scholar]
- 13. Hippisley‐Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau IT. A clinical practice guideline to guide a system approach to diabetes care in Hong Kong. Diabetes Metab J. 2017;41(2):81‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan EYF, Fung CSC, Yu EYT, Fong DYT, Chen JY, Lam CLK. Association of visit‐to‐visit variability of systolic blood pressure with cardiovascular disease and mortality in primary care Chinese patients with type 2 diabetes—a retrospective population‐based cohort study. Diabetes Care. 2017;40(2):270‐279. [DOI] [PubMed] [Google Scholar]
- 16. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran‐related gastrointestinal bleeding with gastroprotective agents: a population‐based study. Gastroenterology. 2015;149(3):586‐595. [DOI] [PubMed] [Google Scholar]
- 17. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 18. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499‐502. [PubMed] [Google Scholar]
- 19. Plummer M. JAGS version 4.3.0 user manual [computer software manual]. sourceforge net/projects/mcmc‐jags/files/Manuals/4 x. 2017;2.
- 20. Su Y‐S, Yajima M. R2jags: using R to run ‘JAGS’. R package version 05‐7. 2015; 34.
- 21. Barrett JK, Huille R, Parker R, Yano Y, Griswold M. Estimating the association between blood pressure variability and cardiovascular disease: an application using the ARIC study. Stat Med. 2019;38(10):1855‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hedeker D, Mermelstein RJ, Demirtas H. An application of a mixed‐effects location scale model for analysis of ecological momentary assessment (EMA) data. Biometrics. 2008;64(2):627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383(9932):1899‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 25. Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034‐2047. [DOI] [PubMed] [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Vol 81 Hoboken, New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 27. Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23(1):93‐104. [DOI] [PubMed] [Google Scholar]
- 28. Mumford SL, Dasharathy S, Pollack AZ. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin Lipidol. 2011;6(2):225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chi C, Teliewubai J, Lu Y‐Y, et al. Comparison of various lipid parameters in association of target organ damage: a cohort study. Lipids Health Dis. 2018;17(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ceriello A, De Cosmo S, Rossi MC, et al. Variability in HbA1c, blood pressure, lipid parameters and serum uric acid, and risk of development of chronic kidney disease in type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1570‐1578. [DOI] [PubMed] [Google Scholar]
- 31. Yan Y, Huang Y, Zhou D, Tang S, Feng Y‐Q, Research BP. Visit‐to‐visit variability in total cholesterol correlates with the progression of renal function decline in a Chinese community‐based hypertensive population. Kidney Blood Press Res. 2019;44(4):727‐742. [DOI] [PubMed] [Google Scholar]
- 32. Kim MK, Han K, Koh ES, et al. Variability in total cholesterol is associated with the risk of end‐stage renal disease: a nationwide population‐based study. Arterioscler Thromb Vasc Biol. 2017;37(10):1963‐1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee HJ, Lee SR, Choi EK, Han KD, Oh S. Low lipid levels and high variability are associated with the risk of new‐onset atrial fibrillation. J Am Heart Assoc. 2019;8(23):e012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roh E, Chung HS, Lee JS, et al. Total cholesterol variability and risk of atrial fibrillation: a nationwide population‐based cohort study. PLoS One. 2019;14(4):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferreira C, Providência R, Ferreira MJ, Gonçalves LM. Atrial fibrillation and non‐cardiovascular diseases: a systematic review. Arq Bras Cardiol. 2015;105(5):519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simpson WG. Biomarker variability and cardiovascular disease residual risk. Curr Opin Cardiol. 2019;34(4):413‐417. [DOI] [PubMed] [Google Scholar]
- 37. Alfonso F, Rivero F, Sánchez‐Madrid F. Variability in atherogenic lipoproteins and coronary artery disease progression. Eur Heart J. 2018;39:2559‐2561. [DOI] [PubMed] [Google Scholar]
- 38. Clark D III, Nicholls SJ, St. John J, et al. Visit‐to‐visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018;39(27):2551‐2558. [DOI] [PubMed] [Google Scholar]
- 39. Ji Y, Bai C. Research progress of hypertriglyceridemia and coronary heart disease. Heart Mind. 2018;2(2):40. [Google Scholar]
- 40. Serban M‐C, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all‐cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69(11):1386‐1395. [DOI] [PubMed] [Google Scholar]
- 41. Banach M, Stulc T, Dent R, Toth PP. Statin non‐adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016;225:184‐196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 S1. Supporting Information.


