Abstract
Introduction
It is pertinent to evaluate the impact of vaccination against human papillomavirus (HPV) in real life. The aim of the study was to evaluate the real‐life impact of HPV vaccination in the first birth cohort of Danish women offered free HPV vaccination as girls and invited to screening at the age of 23 years.
Material and methods
Women born in 1993 were offered free HPV vaccination at the age of 15 years but women born in 1983 have never been offered free HPV vaccination. We followed these two birth cohorts for 10 years from the age of 15 to after their first invitation to screening, and compared the risk of high‐grade cervical intraepithelial neoplasia (CIN). Data were obtained from Danish national health registers.
Results
Vaccination coverage was 91% in the 1993 birth cohort and <0.1% in the 1983 cohort. Screening coverage was close to 80% in both cohorts. CIN2+ was detected in 4% of the 15 748 screened women born in 1983 and in 3% of the 19 951 screened women born in 1993. The risk of high‐grade CIN was reduced by about 30% in the 1993 cohort compared with the 1983 cohort; for CIN2+ relative risk 0.74 (95% CI 0.66‐0.82) and for CIN3+ relative risk 0.68 (95% CI 0.58‐0.79).
Conclusions
This study investigated the real‐life impact of quadrivalent HPV vaccination by comparing a cohort of women offered HPV vaccination with a cohort of women not offered HPV vaccination. The observed decrease in the detection of high‐grade cervical lesions following HPV vaccination is in line with results from the randomized trials and has important implications for future cervical screening of HPV vaccinated cohorts.
Keywords: cervical intraepithelial neoplasia, early detection of cancer, human papillomavirus, prevention and control, vaccination
Abbreviations
- ASCUS
atypical squamous cells of undetermined significance
- CIN
cervical intraepithelial neoplasia
- HPV
human papillomavirus
- HSIL
high‐grade intraepithelial lesion
- LSIL
low‐grade intraepithelial lesion
- NILM
negative for intraepithelial lesion or malignancy
Key message.
This study examined the real‐life impact of HPV vaccination on cervical lesions by comparing entire populations from well‐defined areas. The risk of severe cervical lesions was reduced by 30% after HPV vaccination.
1. INTRODUCTION
Human papillomavirus (HPV) vaccination was introduced following randomized trials. 1 , 2 Young women recruited to the trials had no history of genital warts or abnormal cervical cytology, and a lifetime maximum of four sexual partners. 1 , 2 To compare, half of young Danish women reported a lifetime number of least five sexual partners. 3 As a result of these restrictions in the trial populations, and because HPV vaccination is now a public health initiative for the population in general, it is pertinent to determine the real‐life effectiveness of HPV vaccination. This poses two challenges. First, in a population‐based program uptake/not‐uptake of HPV vaccination is not randomly allocated. Second, high‐grade cervical intraepithelial neoplasia (CIN) does not cause symptoms, and is therefore, detected only in screened or otherwise examined women. We designed our study to take these factors into account.
In 2009, Denmark started recommending routine HPV vaccination for 12‐year‐old girls. From October 2008, girls aged 13‐15 years and born in 1993‐1995 were offered free vaccination with the quadrivalent HPV vaccine. 4 The quadrivalent HPV vaccine was used until February 2016, after which the bivalent HPV vaccine was used, until it was replaced by the nonavalent HPV vaccine in 2017. 5
We previously reported the impact of HPV vaccination on cytology outcome at first screening, and found a 40% reduction in the prevalence of high‐grade squamous intraepithelial lesions (HSIL). 6 Here we report on the impact of HPV vaccination on the prevalence of high‐grade CIN.
Previous observational studies on the effect of quadrivalent HPV vaccination compared vaccinated and unvaccinated women from the same birth cohorts. 7 To the best of our knowledge, ours is the first study to determine the real‐life impact of quadrivalent HPV vaccination by comparing entire populations from well‐defined areas. The objective of this population‐based cohort study was to evaluate the effect of HPV vaccination on prevalence of histologically confirmed high‐grade CIN.
2. MATERIAL AND METHODS
Women born in 1993 were defined as exposed to HPV vaccination, because they were offered free HPV vaccination at age 15 years in 2008. Women born in 1983 were defined as unexposed, because they had never been offered free HPV vaccination. Women born between 1985 and 1992 were targeted by a catch‐up program in 2012/13 and could consequently not be used as comparison cohort. 8
Women born in 1983 and 1993 were identified from the Danish Civil Registration System with their personal identification number allowing for linkage with other register data. A closed cohort was formed of women born in 1993 present in Denmark all the time from 1 January 2009 (time of offer of HPV vaccination) until end of follow up of screening outcomes in 31 December 2018. Similarly, a closed cohort was formed of women born in 1983 present from 1 January 1999 to 31 December 2008. The closed cohorts were followed for 10 years from age 15 to age 25 years (Figure 1). We excluded Copenhagen County before 2005 and 2015 because of incomplete pathology data. Design and analysis were in accordance with our previous study of screening cytology in the same populations. 6
FIGURE 1.
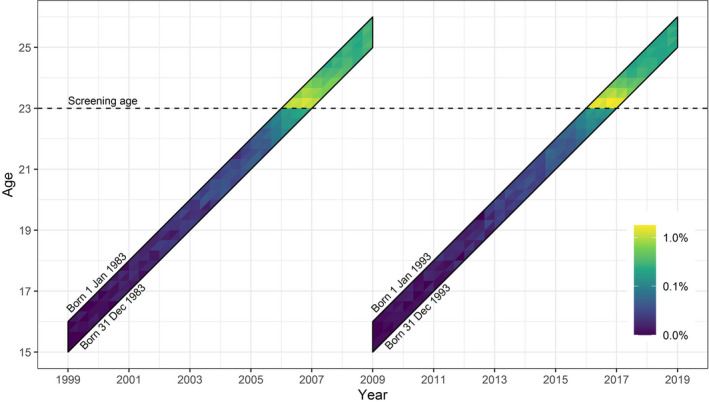
Lexis diagram of studied cohorts. Time of occurrence of worst diagnosis during follow up shown by colors
Primary outcome was CIN grade two or worse (CIN2+) during follow up. The National Register of Pathology contains all pathology diagnoses in Denmark, and uses the Danish Systematized Nomenclature of Medicine (SNOMED). The Bethesda classification was used from 2007, and the CIN nomenclature from 2012. 9 , 10 All M‐codes (morphology) were adapted to fit the Bethesda and CIN classifications. 11 Unclassifiable codes were reviewed to ensure that no relevant diagnosis was missed. T‐codes (topography) were used to distinguish between cervical histology and cytology (see Supplementary material, Table S1). For practical purposes, atypical glandular cells were included in the atypical squamous cells of undetermined significance (ASCUS) category. Atypical squamous cells cannot exclude HSIL and adenocarcinoma in situ were included in the HSIL category.
We acquired individual HPV vaccination data from the National Health Services Register for vaccines given free as part of the childhood immunization program, and from the Prescription Register for the limited number of self‐paid vaccines purchased before the start of the free program (see Supplementary material, Table S1). HPV vaccination status was defined as at least one dose, and age at vaccination was determined at first dose, regardless of register. We used population data on education as recorded in national statistics (statistikbanken.dk, HFUDD20, accessed 21 August 2019), data on smoking from school health surveys, 12 , 13 and data on sexual behavior from questionnaire‐based studies. 3 , 14 , 15 , 16
2.1. Cervical screening in Denmark
In Denmark, women are invited to cervical screening from age 23 years every third year, from age 50 to 59 years every fifth year, and at age 60‐64 to a check‐out test. In the birth cohorts studied here, women with HSIL or other severe diagnoses were referred to a gynecologist, but women with ASCUS or low‐grade squamous intraepithelial lesions (LSIL) were typically recommended repeated sampling after 3‐6 months and referred to a gynecologist if still abnormal. 17 HPV mRNA triage of ASCUS/LSIL was used for a subgroup of women born in 1993. 9 In one of the five Danish regions, conversion from conventional cytology to liquid‐based cytology started in 2002 and spread gradually to become nationwide in 2015, with most pathology departments using SurePath. 18 , 19 , 20
2.2. Statistical analyses
The closed cohorts were observed for 10 years from age 15 to 25 years. We tabulated regional distribution at study start, screening, and HPV‐vaccination coverage.
Screening outcome was defined as worst diagnosis. We applied one hierarchy of M codes in descending order. First came women with at least one histology diagnosis, the hierarchy was cervical intraepithelial neoplasia grade 3 and above (CIN3+), CIN2, CIN1, negative for intraepithelial lesion or malignancy (NILM) and unsatisfactory, or other. Then came women without a histology diagnosis but with at least one cytology diagnosis, the hierarchy was HSIL, LSIL, ASCUS, NILM, and unsatisfactory or other. The number of women without histology but with at least one abnormal cytology visualize women awaiting further examination. In a sub‐analysis, we stratified results for the 1993 birth cohort by vaccination status.
We calculated mean age at events and prevalence proportions with number of women screened, defined as women with a cervical histology and/or cytology registered in the National Register of Pathology, as denominator. As CIN does not give symptoms, only screened women can receive diagnosis. This means that screened women can only contribute to the numerator, therefore we used the same population as denominator. Relative risks (RR) and corresponding 95% CI were obtained from a multinomial logistic regression model (a generalized logit model; that is, a logit model with multiple responses). We compared the corresponding probabilities of each outcome in the two populations. RR here corresponded to prevalence ratios. Chi‐squared test for homogeneity was used to calculate P‐values. The significance level was set to 0.05. SAS statistical software v. 9.4 was used, together with the macros nlmeans v. 1.04 and nlestimate v. 1.51 to estimate the RR from the logistic regression. Plots were made in R v. 3.5.1, using the ggplot2 package.
2.3. Ethical approval
The study was approved by the Danish Data Inspection Agency 19 February 2016 (SUND‐2016‐22). According to Danish legislation, ethical approval is not required for register‐based research.
3. RESULTS
The closed cohorts included 19 629 women born in 1983 and 26 215 women born in 1993 (Table 1, see Supplementary material, Figure S1). The cohorts had similar regional distributions, but with the large numbers studied the difference was statistically significant (P = .001). The proportion of women who had at least a high school examination was slightly higher in the 1993 birth cohort—51% and 59%, respectively (statistikbanken.dk, HFUDD20, accessed 21 August 2019); median age of sexual debut was 16 years in both cohorts; 3 , 14 , 15 but the proportion of women with early sexual debut (≤14 years) seemed to have increased from 14.3% (95% CI 13.4%‐15.1%) to 18.4% (95% CI 17.1%‐19.7%). 16 The percentage of women aged 18‐24 years with >10 sexual partners increased from 14.3% (95% CI 13.4%‐15.2%) in a 2005 survey to 17.8% (95% CI 16.7%‐19.0%) in a 2012 survey. 16 The proportion of daily smokers at age 15 years decreased from 21% to 10%. 12 , 13 Cervical screening coverage was high; 80% for those born in 1983, and 76% for those born in 1993; a small difference, but statistically significant with the large numbers (P < .001).
TABLE 1.
Population characteristics; closed cohorts of Danish women born in 1983 and 1993
| Characteristics |
1983 cohort N (%) |
1993 cohort N (%) |
|---|---|---|
| Number of women included | 19 629 | 26 215 |
| Region of residence at start of follow up | ||
| Capital Region | 2968 (15.1) | 4342 (16.6) |
| Zealand | 3389 (17.3) | 4509 (17.2) |
| Southern Denmark | 5305 (27.0) | 6889 (26.3) |
| Central Denmark | 5336 (27.2) | 7061 (26.9) |
| Northern Denmark | 2631 (13.4) | 3414 (13.0) |
| High school examination a | 51% | 59% |
| Age of sexual debut b | 16 years | 16 years |
| Daily smoking at age 15 years 12 , 13 | 21% | 10% |
| Screening coverage at end of follow up | 15 748 (80.2) | 19 951 (76.1) |
| HPV‐vaccination coverage/cohort c | (<0.1%) | 23 968 (91.4) |
| HPV‐vaccination coverage/screened c | (<0.1%) | 18 612 (93.3) |
As expected, HPV‐vaccination coverage varied from <0.1% in the 1983 cohort to 91% in the 1993 cohort; and from <0.1% to 93% in the screened parts of these cohorts. In the 1993 cohort, 80% of women were vaccinated at the age of ≤15 years, and only 2.3% were vaccinated when older than 16 years; 84% were fully vaccinated with three doses, while 4% had one dose only; and only four women were vaccinated with the bivalent HPV vaccine.
In the 1983 cohort, 6.4% of all women had at least one histology diagnosis, but this was the case for 7.8% in the 1993 cohort (P < .001). Among screened women, the proportions were 8.0% and 10.2%, respectively (RR 1.28; 95% CI 1.19‐1.36) (Table 2). In screened women, the risk of CIN2+ in the 1993 cohort was 26% lower than that in the 1983 cohort (RR 0.74; 95% CI 0.66‐0.82). For CIN3+ a similar pattern was seen (RR 0.68; 95% CI 0.58‐0.79). The fact that slightly more screened women in the 1993 cohort than in the 1983 cohort had undergone biopsy, showed up in a larger risk of normal and low‐grade diagnoses; for CIN1 with an RR 1.23 (95% CI 1.05‐1.40), and for NILM with more than a doubled risk (RR 2.37; 95% CI 2.08‐2.67) (Figure 2).
TABLE 2.
Worst diagnosis during follow up out of women screened in closed 1983 and 1993 cohorts
|
1983 cohort N = 15 748 |
1993 cohort N = 19 951 |
1993 vs 1983 |
|
|---|---|---|---|
| N (%) | N (%) | RR (95% Cl) | |
| Any histology | 1262 (8.0) | 2042 (10.2) | 1.28 (1.19‐1.36) |
| Worst diagnosis | |||
| CIN2+ | 628 (4.0) | 590 (3.0) | 0.74 (0.66‐0.82) |
| CIN3+ | 361 (2.3) | 313 (1.6) | 0.68 (0.58‐0.79) |
| CIN2 | 267 (1.7) | 277 (1.4) | 0.82 (0.68‐0.96) |
| CIN1 | 296 (1.9) | 460 (2.3) | 1.23 (1.05‐1.40) |
| NILM histology | 320 (2.0) | 962 (4.8) | 2.37 (2.08‐2.67) |
| Unsatisfactory/other histology | 18 (0.1) | 30 (0.2) | 1.30 (0.55‐2.08) |
| Cytology only | 14 486 (92.0) | 17 909 (89.8) | 0.97 (0.97‐0.98) |
| Worst diagnosis | |||
| HSIL | 47 (0.3) | 11 (0.1) | 0.18 (0.06‐0.31) |
| LSIL | 566 (3.6) | 397 (2.0) | 0.55 (0.48‐0.62) |
| ASCUS | 437 (2.8) | 506 (2.5) | 0.91 (0.80‐1.03) |
| Any abnormal | 1050 (6.7) | 914 (4.6) | 0.69 (0.63‐0.75) |
| NILM cytology | 13 297 (84.4) | 16 965 (85.0) | 1.01 (1.00‐1.02) |
| Unsatisfactory/Other cytology | 139 (0.9) | 30 (0.2) | 0.17 (0.10‐0.24) |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance (incl. atypical glandular cells); CIN2+, cervical intraepithelial neoplasia grade 2 and above; CIN3+, cervical intraepithelial neoplasia grade 3 and above; CIN2, cervical intraepithelial neoplasia grade 2; CIN1, cervical intraepithelial neoplasia grade 1; HSIL, high‐grade squamous intraepithelial lesions (incl. atypical cells cannot exclude HSIL); LSIL, low‐grade squamous intraepithelial lesions; NILM, negative for intraepithelial lesion or malignancy.
FIGURE 2.
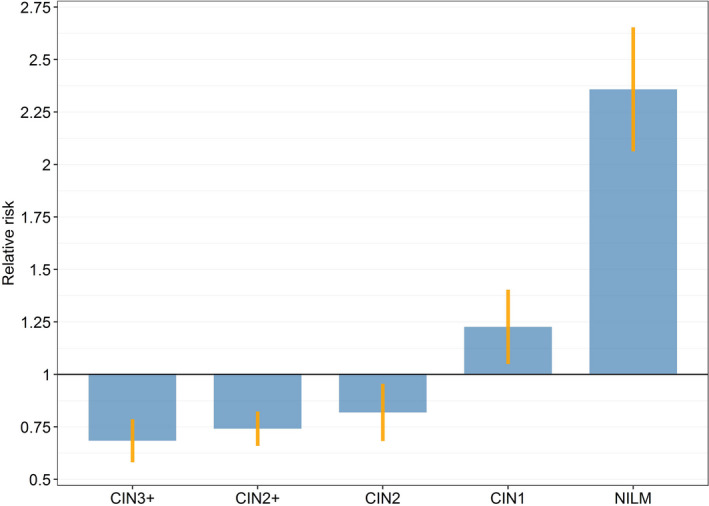
Relative risks for worst cervical histology in screened women from the 1993 cohort compared with the 1983 cohort. CIN, cervical intraepithelial neoplasia; NILM, negative for intraepithelial lesion or malignancy
In the 1983 cohort, 6.7% of screened women had abnormal cytology and awaited further diagnostics, but this proportion had decreased to 4.6% in the 1993 cohort (RR 0.69; 95% CI 0.63‐0.75). This decrease was seen primarily for HSIL with an RR of 0.18 (95% CI 0.06‐0.31) (Table 2). Age distribution at worst diagnosis was equal for the cohorts with mean age of 23.1 years.
In a sub‐analysis, we compared vaccinated and unvaccinated women from the 1993 birth cohort, where they had all been offered free HPV vaccination, the risk was 44% lower for CIN2+ and 52% lower for CIN3+ in vaccinated vs unvaccinated women (RR 0.56; 95% CI 0.42‐0.70 and RR 0.48; 95% CI 0.32‐0.63, respectively). The risks of CIN1 and NILM were the same in the two groups, reflecting that both groups had liquid‐based cytology.
4. DISCUSSION
We found a reduced risk of about 30% for CIN2+ and CIN3+ in the 1993 birth cohort offered free HPV vaccination as girls compared with the 1983 birth cohort not offered free vaccination. The observed decrease seems real, because it coincided with increased diagnostic activity. If anything, our data underestimated the effect of HPV vaccination. Fewer women in the 1993 cohort than in the 1983 cohort had HSIL without histology follow up, indicating that possible loss to follow up was lower among the 1993 cohort than among the 1983 cohort. On a national level, 1.7% of all high‐grade screening samples are lost to follow up. 21
It is important to evaluate if real‐life effectiveness of HPV vaccination is at the level expected from the randomized trials. In the FUTURE studies, 22 vaccination with at least one dose of the quadrivalent HPV vaccine reduced the risk of any CIN2+ by 43% in HPV‐naive women with normal cytology at the time of vaccination, and by 19% in the mixed group of both HPV‐naive and non‐naive women. In our study, 93% of screened women in the 1993 cohort had received at least one dose of HPV vaccination, and by far the majority were vaccinated at the age of 15 years. According to the Danish school health survey from 2010, 37% of 15‐year‐old girls reported being sexually active, 13 and our 1993 cohort, therefore, most likely included a mix of HPV‐naive and non‐naive girls at the time of vaccination. Bearing this in mind, the observed decrease of 30% in the risk of CIN2+ and CIN3+ was in concordance with the findings from the trials, indicating that with a high vaccination coverage, the real‐life effect of HPV vaccination can approach the effect found in the randomized trials.
Our findings are in line with a recent meta‐analysis of the real‐life impact of HPV vaccination showing a 31% reduction for CIN2+ among women aged 20‐24 years. 7 A Danish study comparing women vaccinated below age 16 years with unvaccinated women found a 43% reduction in CIN2+ among those who had reached screening age; 23 a finding similar to the 44% we found by comparing vaccinated with unvaccinated women born in 1993, but clearly higher than the 26% we found for all screened women. A Swedish study reported a 64% decrease in CIN2+ in women HPV‐vaccinated ≤16 years compared with unvaccinated women. However, this result was based entirely on tests taken before screening age, as only 93 person‐years and 0 CIN2+ cases were observed in screened women after the age of 23 years, when invitation to screening starts in Sweden. 24
Scotland started vaccination in 2008 with the bivalent HPV vaccine. A Scottish study reported a vaccine effectiveness of 65% against CIN2 and of 71% against CIN3+ in birth cohorts offered vaccination at age 15 years compared with birth cohorts not offered vaccination. 25 In the randomized trials, the efficacy for any CIN2+ in HPV‐naive girls was larger for the bivalent than for the quadrivalent HPV vaccine; a difference possibly explained by differences in inclusion criteria, HPV‐measurement techniques, and/or cross‐protection. 26 Notably, comparison of our quadrivalent vaccine data with the bivalent vaccine data published from Scotland indicated a similar pattern in real life.
It was a strength that we avoided selection bias by comparing two birth cohorts; one where HPV vaccination was offered and widely accepted, and one where HPV vaccination was not offered as part of the immunization program. Selection was limited further by the fact that cohort members had all reached screening age. The use of comprehensive, linkable data from high‐quality national registers was also a strength. By using closed cohorts we mimicked the randomized trial design.
Reasons for exclusions were residence in part of Copenhagen County, immigration, or emigration. Copenhagen County had a similar HPV‐vaccination coverage as the rest of Denmark, and this exclusion is unlikely to have affected the generalizability of the results. Women living in Denmark only during part of the observation time from age 15 to after first invitation to screening had to be excluded, because they were not under risk for both exposure/pseudo‐exposure and outcome. The proportions of excluded women were similar in the two cohorts, 25.5% and 23.2%, although there was a small difference in the proportion of women excluded because of emigration: 12% vs 9%, respectively (see Supplementary material, Figure S1). It should be noted though that as Denmark started HPV vaccination earlier than neighboring countries, we had vaccinated women emigrating and unvaccinated women immigrating. HPV‐vaccination coverage was therefore higher in our closed 1993 cohort than in women born in 1993 and living in Denmark in 2019, 91% vs 78% (statistik.ssi.dk, accessed 20 March 2019). The reduction in severe cervical lesions may be lower in the actual 2019 population than what we observed in the closed cohort because of this difference in vaccination coverage.
The study also had weaknesses. In young women, the shift from conventional cytology to mainly SurePath‐based liquid‐based cytology was associated with increased detection of ASCUS+, 19 , 20 with more diagnosed CIN cases, 27 and a slightly better protection against cervical cancer. 28 In Denmark, the shift in screening technology took place gradually between 2002 and 2015, and technology was not recorded in the National Register of Pathology. It was therefore not possible to estimate the exact impact of the shift on the effect of HPV vaccination, but if anything, the vaccination effect was underestimated. The transition may well explain the observed increase in normal and low‐grade diagnoses from the 1983 cohort to the 1993 cohort. Perhaps the introduction of HPV triage of ASCUS/LSIL could also have contributed to this increase if more women were referred to biopsy because of HPV positivity.
It was an underlying assumption that the two birth cohorts aside from HPV vaccination had comparable cervical cancer risk profiles; an assumption supported by the similarity between the two cohorts in geographical distribution, education, age of sexual debut, and screening coverage. Changes over time in sexual behavior have been reported from, for example, Britain. 29 In Denmark the median age of sexual debut has been stable at 16 years for the birth cohorts studied here, 14 , 15 but a recent survey reported a small increase in early sexual debut 16 and in proportion of women with a high number of sexual partners. 16 However, as the authors of the survey stated: “the relatively small recent changes in women's sexual behavior … are in themselves not likely to have strong implications for public health” 16 (p. 183). Daily smoking went down, and as smoking is a risk factor for cervical cancer, the effectiveness of HPV vaccination might be slightly overestimated. Nonetheless, based on a conservative assumption that the relative risk of CIN3+ for smokers vs non‐smokers is RR 2, 30 the expected relative risk, due to the smaller proportion of smokers, would be RR 0.9 (see Supplementary material, Table S2). In fact, our finding is still statistically significantly smaller than this value (ie, RR 0.68; 95% CI 0.58‐0.79) (Table 2).
5. CONCLUSION
To conclude, we found a c.30% reduction in CIN2+ and CIN3+ after introduction of HPV vaccination. Real‐life assessment of vaccine effectiveness poses problems; not all girls accept the offer of vaccination, and the transition to SurePath‐based liquid‐based cytology increased screening sensitivity. As this would tend to diminish a potential effect of HPV vaccination, the finding of a 30% reduction must be considered to be well in accordance with the 43% found in the quadrivalent HPV‐vaccination trials.
Many countries may face similar challenges in the study of vaccination effects, but real‐life knowledge on impact of HPV vaccination is necessary to optimize future screening of vaccinated birth cohorts. Furthermore, the evidence of real‐life effectiveness of HPV vaccination supports the existence of public HPV‐immunization programs.
CONFLICT OF INTEREST
LHT, LGL, and EL received test kits from Roche free of charge for a method study. GN has no conflicts of interests.
Supporting information
Fig S1
Table S1
Table S2
Thamsborg LH, Napolitano G, Larsen LG, Lynge E. High-grade cervical lesions after vaccination against human papillomavirus: A Danish cohort study. Acta Obstet Gynecol Scand. 2020;99:1290–1296. 10.1111/aogs.13935
Funding information
This work was supported by the Independent Research Fund Denmark under Grant (No. 4183‐00315, 2015); and Danish Health Foundation (grant no. 16‐8‐0227).
REFERENCES
- 1. Garland SM, Hernandez‐Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928‐1943. [DOI] [PubMed] [Google Scholar]
- 2. The FUTURE II study group . Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. N Engl J Med. 2007;356:1915‐1927. [DOI] [PubMed] [Google Scholar]
- 3. Olesen TB, Jensen KE, Munk C, Tolstrup S. Kjær SK. Liva – en befolkningsundersøgelse af kvinders seksualvaner [Liva – population survey of female sexual habits]. (In Danish). Ugeskr Laeger. 2010;172:3254‐3259. [PubMed] [Google Scholar]
- 4. Sander BB, Rebolj M, Valentiner‐Brandt P, Lynge E. Introduction of human papillomavirus vaccination in Nordic countries. Vaccine. 2012;30:1425‐1433. [DOI] [PubMed] [Google Scholar]
- 5. Danish Health Authority, Danish Medicine Agency, Statens Serum Institut . The childhood immunization program. Annual report 2017. (In Danish – summary in English). Available online at: https://www.ssi.dk//media/arkiv/dk/vaccination/boernevaccinationsprogrammet/boernevaccprogramaarsrap2017_23apr18.pdf?la=da (Accessed September 1, 2019)
- 6. Thamsborg LH, Napolitano G, Larsen LG, Lynge E. Impact of HPV vaccination on outcome of cervical cytology screening in Denmark – A register‐based cohort study. Int J Cancer. 2018;143:1662‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drolet M, Benard E, Perez N, Brisson M; HPV Vaccination Impact Study Group . Population‐level impact and herd effects following the introduction of human papillomavirus vaccination programs: updated systematic review and meta‐analysis. Lancet. 2019;394:497‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynge E, Rygaard C, Baillet MV. Cervical screening at crossroads. APMIS. 2014;122:667‐673. [DOI] [PubMed] [Google Scholar]
- 9. Danish Health Authority . National recommendations for cervical screening. 2012. (In Danish – summary in English). Available online at: https://www.sst.dk/~/media/B1211EAFEDFB47C5822E883205F99B79.ashx (Accessed January 5, 2019)
- 10. Danish Health Authority . National recommendations for cervical screening. 2007. (In Danish). Available online at: https://pure.vive.dk/ws/files/2193574/screening‐for‐livmoderhalskraeft‐oekonomi.pdf (Accessed January 5, 2019)
- 11. Beth Bjerregard. Patobank . Cervical cytology – distribution of diagnoses, Quality assurance and follow‐up. 2014. Available online at: http://www.patobank.dk/fundanemt/files//20150722_Cervical_cytology_Quality_assurance.pdf (Accessed January 30, 2019)
- 12. Rasmussen M, Due P, Holstein BE.Department of Public Health, University of Copenhagen. School survey 1998. 2000 (In Danish).
- 13. Rasmussen M, Due P;National Institute of Public Health, University of Southern Denmark . School Survey 2010. 2011. (In Danish). Available online at: http://www.hbsc.dk/downcount/HBSC‐Rapport‐2010.pdf (Accessed February 3, 2019)
- 14. Jensen KE, Munk C, Sparen P, et al. Women’s sexual behavior. Population‐based study among 65000 women from four Nordic countries before introduction of human papillomavirus vaccination. Acta Obstet Gynecol Scand. 2011;90:459‐467. [DOI] [PubMed] [Google Scholar]
- 15. Guleria S, Juul KE, Munk C, et al. Contraceptive non‐use and emergency contraceptive use at first sexual intercourse among nearly 12 000 Scandinavian women. Acta Obstet Gynecol Scand. 2017;96:286‐294. [DOI] [PubMed] [Google Scholar]
- 16. Hansen BT, Kjær SK, Arnheim‐Dahlström L, et al. Age at first intercourse, number of partners and sexually transmitted infection prevalence among Danish, Norwegian and Swedish women: estimates and trends from nationally representative cross‐sectional surveys of more than 100,000 women. Acta Obstet Gynecol Scand. 2019;00:1‐11. [DOI] [PubMed] [Google Scholar]
- 17. Bigaard J, Hariri J, Lynge E. Cervical cancer screening in Denmark. Eur J Cancer. 2000;36:2198‐2204. [DOI] [PubMed] [Google Scholar]
- 18. Danish Database for Quality Assurance of Cervical Cancer Screening . Annual report 2015 (In Danish). Available online at: https://www.sundhed.dk/content/cms/82/4682_dkls‐%C3%A5rsrapport‐2015.pdf (Accessed March 2, 2019)
- 19. Barken SS, Rebolj M, Lynge E, Junge J, Rygaard C. Outcomes in cervical screening using various cytology technologies: what’s age got to do with it? Eur J Cancer Prev. 2013;22:367‐373. [DOI] [PubMed] [Google Scholar]
- 20. Rask J, Lynge E, Franzmann M, et al. Impact of technology on cytology outcome in cervical cancer screening of young and older women. Int J Cancer. 2014;134:2168‐2179. [DOI] [PubMed] [Google Scholar]
- 21. Danish Database for Quality Assurance of Cervical Cancer Screening . Annual report 2018 (In Danish). Available online at: https://www.sundhed.dk/content/cms/82/4682_dkls_aarsrapport_2018_offentligversion.pdf (Accessed January 20, 20120)
- 22. Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)‐6/11/16/18 vaccine on HPV‐associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325‐329. [DOI] [PubMed] [Google Scholar]
- 23. Verdoodt F, Dehlendorff C, Kjær SK. Dose‐related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study. Clin Infect Dis. 2019;70:608‐614. [DOI] [PubMed] [Google Scholar]
- 24. Herweijer E, Sundström K, Ploner A, Uhnoo I, Sparén P, Arnheim‐Dahlström L. Quadrivalent HPV vaccine effectiveness against high‐grade cervical lesions by age at vaccination: a population‐based study. Int J Cancer. 2016;138(12):2867‐2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer T, Wallace L, Pollock KG, et al. Prevalence of cervical disease at age 20 after immunization with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ. 2019;365:l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arbyn M, Li XU, Simoens C. Martin‐Hirsch PLL. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursers. Cochrane Database Syst Rev. 2018;5:CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rebolj M, Rask J, van Ballegooijen M, et al. Cervical histology after routine ThinPrep or SurePath liquid‐based cytology and computer‐assisted reading in Denmark. Br J Cancer. 2015;113:1259‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rozemeijer K, Penning C, Siebers AG, et al. Comparing Sure Path, ThinPrep and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27:15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyle in Britain through the life course and over time: findings from the national surveys of sexual attitudes and lifestyle (Natsal). Lancet. 2013;382:1781‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. IARC Working Group . IARC Handbook of Cancer Prevention: Cervical Cancer Screening, Vol. 10. IARC Press Lyon. 2005. Available at: http://publications.iarc.fr/Book‐And‐Report‐Series/Iarc‐Handbooks‐Of‐Cancer‐Prevention/Cervix‐Cancer‐Screening‐2005 (Accessed April 10, 2019)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2


