Abstract
Background
Insulinomas are found in 10–15 per cent of patients with multiple endocrine neoplasia type 1 (MEN1) and lead to life‐threatening hypoglycaemia. Surgical outcome and the optimal surgical strategy for MEN1‐related insulinoma are unknown.
Methods
Patients with MEN1‐related insulinomas were identified in 46 centres in Europe and North America between 1990 and 2016. Insulinomas were considered localized if the lesion was in the pancreatic head or body/tail. Patients with pancreatic neuroendocrine tumours throughout the pancreas were suspected of having multifocal insulinoma. The primary outcome was postoperative hypoglycaemia, defined as persistent hypoglycaemia, or recurrent hypoglycaemia caused by a new insulinoma or insulin‐producing liver metastases. Hypoglycaemia‐free survival was estimated by the Kaplan–Meier method.
Results
Ninety‐six patients underwent resection for MEN1‐related insulinoma. Sixty‐three and 33 patients had localized and multifocal insulinomas respectively. After a median follow‐up of 8 (range 1–22) years, one patient (1 per cent) had persistent disease and six (6 per cent) had developed recurrent disease, of whom four had a new insulinoma. The 10‐year hypoglycaemia‐free survival rate was 91 (95 per cent c.i. 80 to 96) per cent. Of those with localized disease, 46 patients underwent pancreatic resection and 17 enucleation. One of these patients had persistent disease and one developed recurrent insulinoma. Among patients with multifocal disease, three developed new insulinomas and two developed insulin‐producing liver metastases.
Conclusion
Surgery for MEN1‐related insulinoma is more successful than previously thought.
In this cohort of 96 patients with resected multiple endocrine neoplasia 1 (MEN1)‐related insulinomas, seven patients (7 per cent) developed postoperative persistent or recurrent hypoglycaemia after a median follow‐up of 8 years. The 10‐year hypoglycaemia‐free survival rate was 91 (95 per cent c.i. 80 to 96) per cent. For patients with localized insulinoma, enucleation seems the preferred procedure. PPPD, pylorus‐preserving pancreatoduodenectomy.
Outcomes good
Antecedentes
Del 10% al 15% de los pacientes con MEN1 presentan insulinomas que pueden desencadenar una hipoglucemia potencialmente mortal. Se desconoce el resultado de la cirugía y la estrategia quirúrgica óptima para el tratamiento del insulinoma relacionado con el MEN1.
Métodos
Se identificaron los pacientes con insulinomas relacionados con el MEN1 en 46 centros de Europa y América del Norte entre 1990 y 2016. Los insulinomas se consideraron localizados si el tumor se localizaba en la cabeza o en el cuerpo/cola del páncreas. Se sospechó la existencia de un insulinoma multifocal en los pacientes con tumores neuroendocrinos pancreáticos (pNETs). El objetivo primario de este estudio fue evaluar la hipoglucemia postoperatoria, definida como hipoglucemia persistente, hipoglucemia recidivante causada por un nuevo insulinoma o debida a metástasis hepáticas productoras de insulina. La supervivencia libre de hipoglucemia se estimó mediante el método de Kaplan‐Meier.
Resultados
A 96 se les realizó una resección por insulinoma en el contexto del MEN1. Un total de 63 y 33 pacientes presentaron insulinomas localizados y multifocales, respectivamente. Después de una mediana de seguimiento de 7,8 años (rango 1‐22), un paciente (1%) tenía enfermedad persistente y seis pacientes (6%) presentaron enfermedad recidivante, de los cuales cuatro desarrollaron un nuevo insulinoma. La supervivencia libre de hipoglucemia fue del 91% a los 10 años (i.c. del 95%, 80%‐96%). De los pacientes con enfermedad localizada, 46 fueron sometidos a resección pancreática y 17 pacientes a enucleación. Entre éstos, un paciente tenía enfermedad persistente y uno desarrolló insulinoma recidivante, respectivamente. De los pacientes con enfermedad multifocal, tres desarrollaron nuevos insulinomas y dos desarrollaron metástasis hepáticas productoras de insulina.
Conclusión
La cirugía para el insulinoma en el contexto del MEN1 es más exitosa de lo que parecía en principio.
Introduction
Insulinoma is a pancreatic neuroendocrine tumour (pNET) that produces insulin and leads to symptomatic and life‐threatening hypoglycaemia1. Currently, surgical resection is the only curative treatment2, 3. Some 4–8 per cent of insulinomas are associated with multiple endocrine neoplasia type 1 (MEN1), a rare hereditary disorder occurring in two to three per 100 000 people4, 5. Patients with MEN1 develop pNETs with a very high and age‐related penetrance. These pNETs are insulin‐producing in 10–15 per cent of patients6, 7, 8. Patients with MEN1‐related insulinoma are often young and have multiple pNETs, making the decision regarding the extent of surgery complicated9.
Localization of the insulin‐producing pNET is a major challenge in the presence of a diffuse background of non‐functioning pNETs (NF‐pNETs) in MEN1. Extensive resections were initially proposed, such as 80 per cent resection of the pancreas left of the superior mesenteric/portal vein, with subsequent enucleations of pNETs in the pancreatic head8, 10. Although persistent and recurrent hypoglycaemia seem uncommon after this aggressive approach, the procedure is associated with pancreatic insufficiency8, 10, 11. The sensitivity of CT and MRI has improved, and the availability of endoscopic ultrasonography (EUS) has increased. In addition, glucagon‐like peptide‐1 receptor (GLP‐1R) imaging using 68Ga‐DOTA‐exendin‐4 ([Nle14, Lys40(Ahx‐DOTA‐68Ga)NH2] exendin‐4) PET/CT, an emerging localization technique, might overcome the difficulties of localizing MEN1‐related insulinomas12. These advances in preoperative localization raise the question of the optimal surgical procedure for MEN1‐related insulinomas, taking both short‐term cure of hypoglycaemia and long‐term risk of recurrence into account.
The European Neuroendocrine Tumour Society (ENETS) and the MEN1 clinical practice guidelines2, 3 lack well grounded recommendations regarding the optimal surgical strategy because there is limited evidence. In the case of a single pNET on CT, MRI or EUS, the ENETS guidelines3 recommend pancreas‐sparing surgery, based on two single‐centre studies13, 14 that included only 13 and eight patients with MEN1 respectively. Persistence or recurrence of hypoglycaemia has been reported in 25–50 per cent after enucleation and 2·6–20 per cent after extensive resection11, 13, 15. Most data are, however, based on old and small single‐centre series9, 10, 11, 13, 15, 16, hampering comparisons between surgical strategies. In the only population‐based cohort study17, a higher risk of recurrent hypoglycaemia was observed after enucleation (33·3 per cent) than after distal pancreatectomy (8·7 per cent), but 42 per cent of the patients had surgery before 1990. Studies often also failed to differentiate between recurrent hypoglycaemia because of insulin‐producing liver metastases and new insulin‐producing pNETs.
The aim of this study was to provide evidence for surgical decision‐making in patients with MEN1‐related insulinoma. The risk of recurrence after pancreatic surgery was investigated in a comprehensive international cohort of patients with MEN1‐related insulinoma.
Methods
This study was an international collaboration between 40 hospitals in Europe and six in North America. Patients with MEN1 were identified in the hospital databases using ICD‐9/10 codes for MEN1 and insulinoma. Eligible patients underwent surgery for insulinoma between 1990 and 2016, had a pNET tumour confirmed histologically, and follow‐up for at least 1 year after surgery. The MEN1 diagnosis was established either by genetic testing, family history or clinically, according to most recent practice guidelines2. Patients who underwent total pancreatectomy and those with distant metastases at diagnosis were excluded. Clinical and demographic data were collected by review of medical records in a standardized manner using predefined definitions. The medical records were examined by an investigator at the collaborating institution and discussed with the coordinating investigators. The study protocol was approved by the medical ethics committees or institutional review boards of all participating centres.
Insulinoma diagnosis
The presence of an insulinoma was based on a positive 72‐h supervised fasting test18, 19. If no test was performed, the diagnosis was based on symptoms or signs of hypoglycaemia with concomitant biochemical endogenous hyperinsulinaemic hypoglycaemia, according to clinical practice guidelines18, 19. The date of diagnosis was based on the date of the supervised 72‐h fasting test or the date of symptoms accompanied by endogenous hyperinsulinaemic hypoglycaemia.
Insulinoma localization
The evaluation for MEN1‐related insulinoma was dependent on the surgeon's preference and availability of localization techniques in each centre at the time of surgery. Generally, conventional imaging (CT, MRI, EUS) was undertaken before surgery, eventually followed by more invasive techniques, such as arterial (calcium) stimulation venous sampling or GLP‐1R receptor imaging. Most importantly, intraoperative findings, based on intraoperative ultrasonography and/or bimanual palpation, were used to localize the insulinoma and subsequently guide intraoperative surgical decision‐making.
Based on all preoperative and intraoperative findings, the insulinoma was localized to the pancreatic head or body/tail, or the surgeon suspected multifocal insulinomas in both the head and body/tail. Subsequent surgical decisions were based on whether during surgery the surgeon considered the insulinoma to be localized (suspected to be located in pancreatic head or body/tail) or possibly multifocal (insulinomas in both pancreatic head and body/tail).
Surgical strategy
Patients with localized insulinoma underwent enucleation, Whipple/pylorus‐preserving pancreatoduodenectomy (PPPD) or distal pancreatectomy. In these patients, enucleation was compared with resection (distal pancreatectomy or Whipple/PPPD).
Patients with multifocal insulinomas underwent combined procedures involving the pancreatic head and body/tail, including multiple enucleations, distal pancreatectomy plus enucleation of a pNET in the pancreatic head, Whipple/PPPD plus enucleation and Whipple/PPPD plus distal pancreatectomy. In such patients, distal pancreatectomy combined with enucleation of tumour in the head of the pancreas was compared with multiple enucleations, Whipple/PPPD plus enucleation or Whipple/PPPD plus distal pancreatectomy. In addition, patients who underwent one or multiple enucleations were compared with those who had other resections.
Pathology
The total number of pNETs, number of immunohistochemically insulin‐positive pNETs in the head and body/tail, size of the largest insulin‐positive pNET of the head and body/tail, and number of tumour‐positive locoregional lymph nodes were registered.
Postoperative and long‐term outcomes
The primary outcome was hypoglycaemia at 3 months after surgery. Patients with hypoglycaemia within 3 months of surgery were considered to have persistent disease. Insulinoma recurrence was defined as recurrence of hypoglycaemia more than 3 months after surgery owing to a new insulinoma in the remaining pancreas. Patients with recurrence of hypoglycaemia and newly diagnosed liver metastases were considered to have insulin‐producing liver metastases.
Secondary outcomes were early and late complications after surgery. Postoperative pancreatic fistula (POPF), postpancreatectomy haemorrhage (PPH), bile leak and delayed gastric emptying (DGE) were graded according to the International Study Group of Pancreatic Surgery (ISGPS) classification20, 21, 22, 23. Clavien–Dindo grade III–IV postoperative complications within 30 days after surgery, or during the hospital stay, were recorded24. Postoperative mortality was defined as death within 30 days of surgery. Exocrine pancreatic insufficiency was defined by use of pancreatic enzyme supplementation for at least 6 months. New‐onset diabetes was defined as the use of antidiabetic medication for 6 months or more after surgery.
Statistical analysis
Data are presented as median (range) or count (percentage). The time to recurrence of hypoglycaemia and insulinoma was assessed using Kaplan–Meier analysis25, and hypoglycaemia‐ and insulinoma‐free survival probabilities were estimated. Follow‐up started on the date of insulinoma surgery and ended on the date of hypoglycaemia or insulinoma recurrence, last follow‐up or death. Analyses were done separately for patients with localized versus multifocal disease, and for patients with one or more enucleation(s) and those who had other resections (all resections other than one or more enucleation(s)). Statistical analyses were undertaken using SPSS® version 25.0 (IBM, Armonk, New York, USA).
Results
A total of 159 patients with MEN1 were identified, of whom 96 met the inclusion criteria. Sixty‐three patients did not meet the inclusion criteria for the following reasons: no surgery 15, liver metastases at diagnosis 6, no proper diagnosis 3, follow‐up less than 1 year 34, total pancreatectomy 4 or no details on surgical procedure 1. Demographics and clinical characteristics of the cohort are shown in Table 1. Median age at diagnosis was 30 (range 5–81) years and 15 of the 96 patients (16 per cent) were younger than 21 years at diagnosis. There were 58 female patients (60 per cent). The insulinoma diagnosis was confirmed following a 72‐h fasting test in 64 patients (67 per cent).
Table 1.
Baseline characteristics of multiple endocrine neoplasia type 1 study cohort undergoing insulinoma surgery
| Overall (n = 96) | Localized insulinoma (n = 63) | Multifocal insulinoma (n = 33) | |
|---|---|---|---|
| Age at insulinoma diagnosis (years)* | 30 (5–81) | 31 (5–81) | 30 (14–61) |
| Age at surgery (years)* | 32 (6–82) | 32 (6–82) | 31 (13–62) |
| Aged less than 21 years at insulinoma surgery | |||
| Yes | 15 (16) | 8 (13) | 7 (21) |
| No | 81 (84) | 55 (87) | 26 (79) |
| Sex ratio (M : F) | 38 : 58 | 24 : 39 | 14 : 19 |
| Diagnosis | |||
| Fasting test | 64 (67) | 43 (68) | 21 (64) |
| Clinical and biochemical | 32 (33) | 20 (32) | 12 (36) |
| No. of pNETs on conventional imaging (n = 91) | |||
| 0 | 4 (4) | 3 (5) | 1 (3) |
| 1 | 45 (49) | 31 (52) | 14 (45) |
| 2 | 19 (21) | 12 (20) | 7 (23) |
| ≥3 | 23 (25) | 14 (23) | 9 (29) |
| Distribution of pNETs on conventional imaging (n = 92) | |||
| None | 4 (4) | 3 (5) | 1 (3) |
| Head only | 11 (12) | 6 (10) | 5 (16) |
| Body/tail only | 51 (55) | 39 (65) | 12 (38) |
| Multifocal (head and body/tail) | 26 (28) | 12 (20) | 14 (44) |
| Size of largest pNET on preoperative imaging (mm)* (n = 79) | 20 (4–90) | 20 (4–60) | 22·5 (10–90) |
| pNET ≥ 2 cm on preoperative imaging (n = 81) | |||
| None | 36 (44) | 26 (50) | 10 (34) |
| Head | 13 (16) | 6 (12) | 7 (24) |
| Body/tail | 29 (36) | 19 (37) | 10 (34) |
| Head and body/tail | 3 (4) | 1 (2) | 2 (7) |
| Suspected lymph node metastases on preoperative imaging (n = 92) | 1 (1) | 1 (2) | 0 (0) |
| Time interval of surgery | |||
| 1990–2006 | 48 (50) | 29 (46) | 19 (58) |
| 2006–2016 | 48 (50) | 34 (54) | 14 (42) |
| Type of resection | |||
| Enucleation | 17 (18) | 17 (27) | 0 (0) |
| Multiple enucleations | 3 (3) | 0 (0) | 3 (9) |
| Distal pancreatectomy | 41 (43) | 41 (65) | 0 (0) |
| Distal pancreatectomy and enucleation | 26 (27) | 0 (0) | 26 (79) |
| Whipple/PPPD | 5 (5) | 5 (8) | 0 (0) |
| Whipple/PPPD and enucleation | 2 (2) | 0 (0) | 2 (6) |
| Whipple/PPPD and distal pancreatectomy | 2 (2) | 0 (0) | 2 (6) |
Values in parentheses are percentages unless indicated otherwise; *values are median (range). pNET, pancreatic neuroendocrine tumour; PPPD, pylorus‐preserving pancreatoduodenectomy.
Preoperative imaging and type of surgery
Preoperative imaging results and surgical strategies are shown in Table 1 and Fig. 1. The insulinoma was localized in 63 patients (66 per cent), and these patients underwent typical resections: single enucleation 17, distal pancreatectomy 41 or Whipple/PPPD 5. The disease was considered multifocal in 33 patients (34 per cent), leading to combined pancreatic resections, of which the majority (26 of 33) were distal pancreatectomies with enucleation of tumour in the pancreatic head. Clinical characteristics of patients undergoing one or multiple enucleations and other resections are shown in Table S1 (supporting information). Twenty‐nine of 48 patients operated before 2006 were considered to have localized insulinoma compared with 34 of 48 who had surgery from 2006 onwards (Table S2, supporting information).
Figure 1.
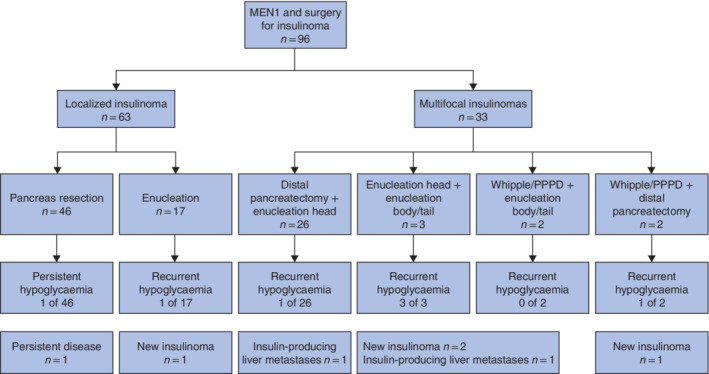
Study flow chart showing insulinoma location, surgical procedures and postoperative hypoglycaemia MEN1, multiple endocrine neoplasia type 1; PPPD, pylorus‐preserving pancreatoduodenectomy.
Of 92 patients with preoperative imaging, 45 (49 per cent) had a solitary pNET on imaging. Despite having a solitary pNET on imaging, 14 of these patients underwent resection for multifocal disease, based on invasive imaging or intraoperative findings. Ten had distal pancreatectomy with enucleation, one had Whipple/PPPD with enucleation and three had multiple enucleations.
Pathology
The median number of pNETs resected was 3 (range 1–74), of which a median of 1 (0–4) was insulin‐positive. Histopathological reports for four patients could not be retrieved, and detailed information regarding the exact number of insulin‐positive pNETs was not available for another 17 patients. Three patients had no insulin‐positive pNETs, all of whom were cured by surgery. A single insulin‐positive pNET was observed in 40 of 50 patients with a localized insulinoma. Preoperative conventional imaging and outcomes of histology in patients with localized disease are summarized in Table S3 (supporting information).
Among 25 patients with multifocal insulinomas, one, two and at least three insulin‐positive pNETs were observed in 12, eight and four respectively, and one patient had no insulin‐positive pNET. Eight of these patients had an insulin‐positive pNET in the pancreatic head and in the body/tail (Table S4, supporting information). In the remaining eight patients with multifocal disease, no detailed information regarding insulin staining was available. Among the patients with a solitary pNET on imaging but the finding of multifocal disease during surgery, five of 11 had multifocal insulin‐positive pNETs.
Lymph nodes were resected in 45 patients and were tumour‐positive in seven, but as insulin immunohistochemistry of metastatic lymph nodes was not undertaken routinely, the exact source could not be assessed.
Intraoperative data, postoperative complications and hospital stay
Median duration of operation was 225 (range 43–440) min, and median blood loss was 200 (0–4150) ml. Median operating time was shorter (133 versus 244 min) and there was less blood loss (50 versus 250 ml) in enucleations compared with other procedures. Postoperative outcomes are shown in Table 2. There was no postoperative death. ISGPS grade B/C POPF, DGE, PPH and bile leak developed in 16 of 87 (18 per cent), five of 87 (6 per cent), none of 87 and none of nine patients respectively. No relevant differences were observed between the procedures. Median hospital stay was 10 (range 3–53) days, and ten of 85 patients (12 per cent) were readmitted. Patients who underwent Whipple/PPPD alone 4 or distal pancreatectomy with enucleation 15 had a relatively long hospital stay: median 19 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 and 15 (7–53) days respectively.
Table 2.
Procedure‐specific outcomes after insulinoma surgery in patients with multiple endocrine neoplasia type 1
| Localized | Multifocal | ||||||
|---|---|---|---|---|---|---|---|
| Enucleation (n = 17) | Whipple/PPPD (n = 5) | Distal pancreatectomy (n = 41) | Distal pancreatectomy + enucleation head (n = 26) | Enucleation head + enucleation body/tail (n = 3) | Whipple/PPPD + enucleation body/tail (n = 2) | Whipple/PPPD + distal pancreatectomy (n = 2) | |
| Early postoperative outcomes | |||||||
| ISGPS grade B/C complications | |||||||
|
POPF B C |
3 of 16 3 0 |
0 of 4 – – |
6 of 36 4 2 |
5 of 24 2 3 |
1 of 3 1 0 |
0 of 2 – – |
1 of 2 1 0 |
| DGE | 0 of 16 | 0 of 4 | 3 of 36 | 1 of 24 | 1 of 3 | 0 of 2 | 0 of 2 |
| PPH | 0 of 16 | 0 of 4 | 0 of 36 | 0 of 24 | 0 of 3 | 0 of 2 | 0 of 2 |
| Bile leak | – | 0 of 4 | – | – | – | 0 of 2 | 0 of 2 |
| Other Clavien–Dindo grade III–IV complication | 0 of 13 | 1 of 4 | 2 of 27 | 1 of 24 | 0 of 2 | 0 of 2 | 0 of 2 |
| Death | 0 of 17 | 0 of 5 | 0 of 41 | 0 of 26 | 0 of 3 | 0 of 2 | 0 of 2 |
| Duration of hospital stay (days)* (n = 57) | 8·5 (4–12) | 19 (14–23) | 9 (3–25) | 15 (7–53) | – | 15 (15–15) | – |
| Readmission | 3 of 14 | 0 of 4 | 3 of 36 | 2 of 25 | 1 of 3 | 1 of 1 | 0 of 2 |
| Long‐term outcomes | |||||||
| Endocrine or exocrine insufficiency | 0 of 17 | 1 of 5 | 14 of 41 | 7 of 26 | 0 of 3 | 1 of 2 | 2 of 2 |
| Exocrine insufficiency | 0 of 17 | 1 of 5 | 1 of 41 | 2 of 26 | 0 of 3 | 1 of 2 | 2 of 2 |
| New‐onset diabetes | 0 of 17 | 0 of 5 | 13 of 41 | 5 of 26 | 0 of 3 | 0 of 2 | 0 of 2 |
| Development of liver metastases | 0 of 17 | 0 of 5 | 3 of 41 | 2 of 26 | 1 of 3 | 0 of 2 | 0 of 2 |
| Death | 0 of 17 | 1 of 5 | 3 of 41 | 4 of 26 | 1 of 3 | 0 of 2 | 0 of 2 |
| Follow‐up (years)* | 5 (1–22) | 9 (7–13) | 8 (1–22) | 8 (1–21) | 18 (10–20) | 3 (1–5) | 11 (8–14) |
Values are median (range). PPPD, pylorus‐preserving pancreatoduodenectomy; ISGPS, International Study Group of Pancreatic Surgery; POPF, postoperative pancreatic fistula; DGE, delayed gastric emptying; PPH, postpancreatectomy haemorrhage.
Recurrence of hypoglycaemia and insulinoma
The distribution of patients with postoperative hypoglycaemia is shown in Fig. 1. After a median follow‐up of 8 (range 1–22) years, seven patients (7 per cent) had hypoglycaemia. One patient (1 per cent) had persistent hypoglycaemia and six (6 per cent) had recurrent hypoglycaemia. Of those with recurrent hypoglycaemia, four had a new insulinoma and two developed insulin‐producing liver metastases. The patient with persistent disease underwent distal pancreatectomy to remove a 5‐mm insulinoma with immunohistochemistry positive for insulin. Postoperative EUS showed two lesions in the pancreatic body that were resected 10 months later, resulting in biochemical cure.
Estimated 10‐year hypoglycaemia‐free and insulinoma‐free survival rates were 91 (95 per cent c.i. 80 to 96) and 93 (83 to 97) per cent respectively (Fig. 2). The estimated 10‐year hypoglycaemia‐free survival rate was 96 (84 to 98) per cent for patients with localized insulinoma and 81 (58 to 92) per cent among those with multifocal insulinomas (Fig. 3). Ten patients (10 per cent) had follow‐up of less than 2 years, 34 (35 per cent) less than 5 years, 63 (66 per cent) less than 10 years, 81 (84 per cent) less than 15 years, and the remaining 15 patients (16 per cent) had follow‐up of 15 years or more. Five of 64 patients (8 per cent) who were diagnosed according to a 72‐h fasting test developed recurrent hypoglycaemia and two of 32 who were diagnosed based on clinical criteria. Outcomes for patients who had one or more enucleations versus those with other resections are summarized in Table S1 and Fig. S1 (supporting information). Of the 15 patients aged less than 21 years at the time of surgery, two developed recurrent insulinoma.
Figure 2.

Persistent or recurrent hypoglycaemia‐ and recurrent insulinoma‐free survival after surgery for multiple endocrine neoplasia type 1‐related insulinoma a Persistent or recurrent hypoglycaemia and b recurrent insulinoma. Dotted lines indicate 95 per cent confidence interval.
Figure 3.
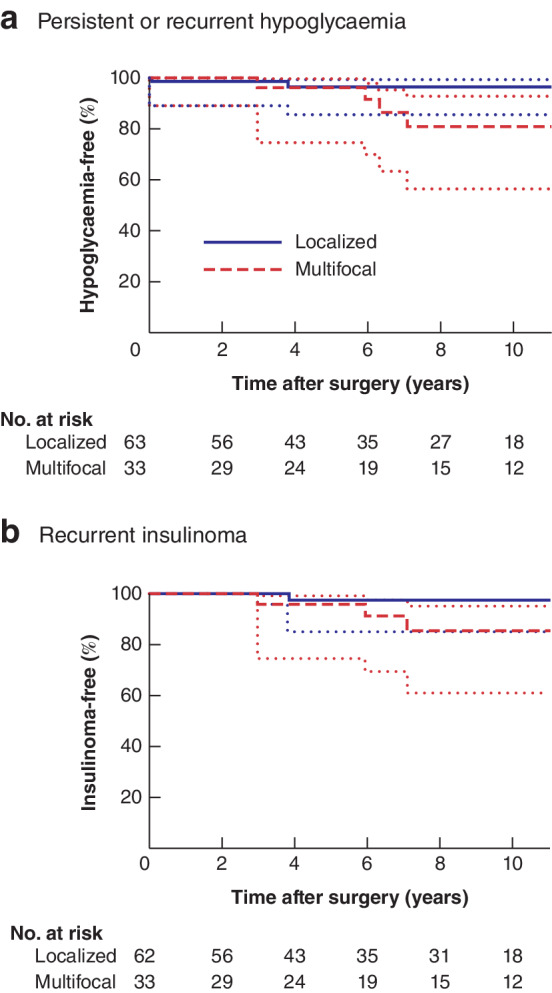
Persistent or recurrent hypoglycaemia‐ and recurrent insulinoma‐free survival after surgery for multiple endocrine neoplasia type 1‐related insulinoma, stratified by location of insulinoma a Persistent or recurrent hypoglycaemia and b recurrent insulinoma. Dotted lines indicate 95 per cent confidence interval.
Pancreatic insufficiency, liver metastases and death
Twenty‐five patients (26 per cent) developed pancreatic insufficiency (Table 2). Seven patients developed exocrine pancreatic insufficiency, and 18 had new‐onset diabetes. None of the patients who underwent enucleation developed exocrine pancreatic insufficiency or new‐onset diabetes. New‐onset diabetes was only observed after distal pancreatectomy with or without enucleation, and occurred in 27 per cent of patients. Six patients developed liver metastases, of whom two had insulin‐producing liver metastases. Nine patients died during follow‐up but no deaths were insulinoma‐related.
Discussion
These data from a large international cohort showed that surgery for MEN1‐related insulinoma was effective as only one patient (1 per cent) had persistent disease. In addition, only four patients (4 per cent) developed a new insulinoma after a median follow‐up of 8 years, leading to an estimated 10‐year insulinoma‐free survival rate of 93 per cent. Enucleation appeared to be the favourable surgical strategy for treatment of localized MEN1‐related insulinoma, owing to the absence of pancreatic insufficiency and high rate of symptom resolution. In patients with multifocal disease, distal pancreatectomy combined with enucleation of tumours in the head led to cure of hypoglycaemia, but less extensive resections were also effective in some patients.
In a series17 from the French Endocrine Tumour Study Group (GTE), 73 patients with MEN1‐associated insulinomas were analysed, including a large group of patients who underwent surgery before 1990. After a median follow‐up of 9 years, the rate of persistent postoperative hypoglycaemia was 4 per cent and the rate of late recurrence of hypoglycaemia was 14 per cent. Interestingly, the rate of overall persistent or recurrent hypoglycaemia (14 per cent) was much higher than that in the present study (7 per cent). This is likely explained by an improvement in perioperative imaging and operative techniques over the past three decades. More than 40 per cent of patients in the GTE cohort were treated before 1990, whereas the present study only included patients who had surgery from 1990 onwards. A more recent German publication13 supports this as late recurrent hypoglycaemia was shown in only one of 13 patients with MEN1 who underwent surgery between 1997 and 2013, and the authors concluded that enucleation and limited resection can provide long‐term cure in patients with solitary or dominant tumours.
Current ENETS guidelines advise using pancreas‐sparing surgery (enucleation or limited resection) in patients with a solitary insulinoma on MRI, CT or EUS. Although this is reasonable for sporadically occurring insulinomas, patients with MEN1 are often affected by multiple concurrent NF‐pNETs which might be missed on conventional imaging26. A solitary pNET was observed on imaging in 45 of 92 patients in the present cohort, but 14 of these patients underwent combined resections. In only five of these patients were insulin‐positive pNETs found throughout the pancreas. In addition, the only patient with persistent disease underwent resection of an insulin‐positive pNET, but postoperative EUS revealed multiple pNETs, which were not observed on preoperative CT. This underscores that the decision to resect a single lesion after findings on conventional imaging should be made with caution, as limited resections are performed using minimally invasive techniques without the opportunity for intraoperative bimanual palpation27, 28. In addition, patients with a solitary lesion on conventional imaging should be counselled about the substantial risk of a complex procedure.
For patients with equivocal preoperative imaging, 68Ga‐DOTA–exendin‐4 PET/CT could improve preoperative insulinoma localization, facilitate minimally invasive surgery and offer better surgical outcomes12, 29. Outcomes of arterial stimulation venous sampling, an invasive localization technique, have been reported for only a small number of patients with MEN1; this technique could not adequately localize the insulinoma in all of the patients, questioning its value in the evaluation of MEN1‐related insulinoma13, 29. Almost all insulinomas express GLP‐1R, offering the opportunity to target these receptors and visualize insulinomas using PET/CT with 68Ga‐labelled tracer and exendin‐4.
In the present study, 33 patients were deemed to have multifocal insulinomas by the operating surgeons, leading to combined resections of which the majority were distal pancreatectomy with enucleation. Patients undergoing this classical approach for MEN1‐related insulinomas had a median hospital stay of 15 days. Although all patients who had combined resections were cured of hypoglycaemia, 17 of 25 patients only had an insulin‐positive pNET in the head or body/tail. Some of these patients had a pNET of 2 cm or larger, which is nowadays considered as an indication for surgery, but at least a subgroup of the patients with multifocal disease might have undergone resections that were too extensive3, 30, 31. Prevention of long‐term complications is important, because pancreatic insufficiency decreases quality of life in this young and otherwise unaffected population. Insulinoma is often the first manifestation of MEN1 and a common surgical indication in children and adolescents with MEN1, which underscores the importance of long‐term pancreatic function32, 33, 34, 35. None of the patients in this cohort who underwent enucleation developed endocrine or exocrine insufficiency, which is in line with other studies36, 37. New‐onset diabetes, on the contrary, was observed after distal pancreatectomies with or without enucleations in 26 per cent of patients. Adequate preoperative localization of the insulinoma can lead to preservation of pancreatic tissue and function.
Enucleation for patients with MEN1 and a localized insulinoma seems preferable if surgically feasible, as it is associated with a high rate of cure of hypoglycaemia, low risk of recurrent disease and absence of long‐term complications. The feasibility of enucleation depends on the insulinoma size, location and relation to the main pancreatic duct. In patients with multifocal disease, a more aggressive approach seems advisable based on the present findings, but localization of the insulinoma(s) in these patients is particularly important. Surgical decision‐making in patients with multiple pNETs on preoperative imaging should be tailored to the individual patient's needs and guided by the location of the insulinoma. Concurrent large NF‐pNETs carry a substantial risk of malignancy and ultimately determine the life expectancy of patients with MEN138, 39, 40. Furthermore, surgeons must be aware of the risk of new‐onset diabetes or exocrine pancreatic insufficiency after distal pancreatectomy with or without enucleations of tumours in the head, which was 31 per cent in the present series.
Although curative resection is the recommended therapy for MEN1‐related insulinoma, radiofrequency ablation (RFA) has been reported as a successful treatment2, 3. The feasibility, efficacy and safety of percutaneous, intraoperative and EUS‐guided RFA for pNETs was described in ten patients in 201441. Although all patients had a complete ablation, severe complications requiring reintervention were observed in three. More recently, a retrospective study42 from two tertiary referral centres showed that EUS‐guided RFA led to complete relief of the symptoms of hypoglycaemia in all of seven patients with insulinomas, of whom one had a MEN1‐related insulinoma. Another nine patients with insulinomas and symptom improvement or resolution after EUS‐guided RFA were described in the literature review43. Complications after EUS‐guided RFA have been reported in two of 18 and two of 12 patients with pNETs42, 43. Considering the short follow‐up (less than 1 year), relatively small pNET size (under 30 mm), selection of patients (those who had either refused surgery or were ineligible for surgery), and the limitations of RFA for pNETs close to surrounding structures or to the pancreatic duct, further comparative studies, ideally RCTs, are needed to clarify the role of EUS‐guided RFA for MEN1‐related insulinomas. For patients ineligible for surgery, EUS‐guided RFA seems a viable alternative.
There are limitations to this study. The retrospective design has known disadvantages, and it was not possible to correct for possible confounding factors influencing surgical strategy, such as age, tumour size, treatment period, centre and localization of the insulinoma. A large prospective observational study or RCT comparing different surgical strategies could overcome this issue, but would be unrealistic owing to the rarity of the disease. Furthermore, identification of insulinoma on pathology is challenging because NF‐pNETs might also express insulin and some insulinomas might not express insulin44, as also observed here. Future use of enhancer signatures might differentiate more accurately between pNET subtypes in MEN145.
Surgery for MEN1 insulinoma is associated with higher cure rates than previously reported. Enucleation is recommended for MEN1 with a suspected solitary insulinoma if feasible surgically. Distal pancreatectomy combined with enucleation of pancreatic head lesions seems favourable for patients with MEN1 and multiple insulinomas, but this type of resection is probably too extensive for some patients.
Collaborators
Members of the International MEN1 Insulinoma Study Group: P. Goudet (Centre Hospitalier Universitaire de Dijon, Dijon, France), on behalf of the Groupe d'étude des Tumeurs Endocrines (GTE); A. Vella, D. Donegan (Mayo Clinic, Rochester, Minnesota, USA); D. K. Bartsch, J. Manoharan (Philipps University Marburg, Marburg, Germany); N. D. Perrier, I. Christakis (University of Texas MD Anderson Cancer Center, Houston, Texas, USA); M. L. Brandi (University of Florence, Florence, Italy); R. Zarnegar, E. L. Postma (New York Presbyterian Hospital, Weill Cornell Medical Centre, New York, USA); E. Kebebew, P. Nockel (National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA); L. Brunaud (Université de Lorraine, Hôpital Brabois Adultes, Centre Hospitalier Universitaire, Nancy, France); J. D. Pasternak, W. P. Kluijfhout (University Health Network, Toronto, Ontario, Canada); C. Sturgeon, S. Giri (Northwestern University, Chicago, Illinois, USA); B. A. Bonsing (Leiden University Medical Centre, Leiden, the Netherlands); C. H. van Eijck (Erasmus Medical Centre, Rotterdam, the Netherlands); H. van Goor (Radboud University Medical Centre, Nijmegen, the Netherlands); R. H. J. de Kleine (University of Groningen, University Medical Centre Groningen, Groningen, the Netherlands); E. J. Nieveen van Dijkum (Cancer Centre Amsterdam, Amsterdam UMC location, Academic Medical Centre, Amsterdam, the Netherlands); C. H. C. Dejong (Maastricht University Medical Centre, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht, the Netherlands, and Universitätsklinikum Aachen, Aachen, Germany).
Supporting information
Table S1 Patients undergoing enucleation(s) versus other resections
Table S2 Difference between patients operated on before and after 2006
Table S3 Preoperative imaging and histopathological outcomes of patients with localized insulinomas
Table S4 Immunohistochemistry outcomes in patients with multifocal insulinomas.
Fig. S1 Freedom from persistent or recurrent hypoglycaemia after enucleations versus other resections for multiple endocrine neoplasia type 1‐related insulinoma
Acknowledgements
D.J.v.B. and S.N. contributed equally to this article. No preregistration exists for the study reported in this article. Because of the sensitive nature of the data collected for this study, the authors do not wish to make the data publicly available. This work was supported by a Dutch Cancer Society research grant (UU 2014‐7102). The DutchMEN study group was supported by an unrestricted grant from Ipsen Pharmaceutical and a start‐up grant from the Comprehensive Cancer Centre of the Netherlands (IKNL). The funding sources had no influence on the study question, design, data acquisition, statistical analysis, and interpretation of data.
Disclosure: The authors declare no conflict of interest.
Presented to the Annual Meeting of the American Association of Endocrine Surgeons, Orlando, Florida, USA, April 2017, and to the Annual Meeting of the European Society of Endocrine Surgeons, Oxford, UK, April 2017
Contributor Information
M. R. Vriens, Email: mvriens@umcutrecht.nl.
the International MEN1 Insulinoma Study Group:
P. Goudet, A. Vella, D. Donegan, D. K. Bartsch, J. Manoharan, N. D. Perrier, I. Christakis, M. L. Brandi, R. Zarnegar, E. L. Postma, E. Kebebew, P. Nockel, L. Brunaud, J. D. Pasternak, W. P. Kluijfhout, C. Sturgeon, S. Giri, B. A. Bonsing, C. H. van Eijck, H. van Goor, R. H. J. de Kleine, E. J. Nieveen van Dijkum, and C. H. C. Dejong
References
- 1. Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935; 101: 1299–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR et al; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012; 97: 2990–3011. [DOI] [PubMed] [Google Scholar]
- 3. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M et al; Vienna Consensus Conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non‐functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016; 103: 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008; 15: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert‐Buck MR et al Positional cloning of the gene for multiple endocrine neoplasia‐type 1. Science 1997; 276: 404–407. [DOI] [PubMed] [Google Scholar]
- 6. de Laat JM, van der Luijt RB, Pieterman CR, Oostveen MP, Hermus AR, Dekkers OM et al MEN1 redefined, a clinical comparison of mutation‐positive and mutation‐negative patients. BMC Med 2016; 14: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pieterman CR, Conemans EB, Dreijerink KM, De Laat JM, Timmers HTM, Vriens MR et al Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr Relat Cancer 2014; 21: R121–R142. [DOI] [PubMed] [Google Scholar]
- 8. Norton JA, Fang TD, Jensen RT. Surgery for gastrinoma and insulinoma in multiple endocrine neoplasia type 1. J Natl Compr Canc Netw 2006; 4: 148–153. [DOI] [PubMed] [Google Scholar]
- 9. Crippa S, Zerbi A, Boninsegna L, Capitanio V, Partelli S, Balzano G et al Surgical management of insulinomas: short‐ and long‐term outcomes after enucleations and pancreatic resections. Arch Surg 2012; 147: 261–266. [DOI] [PubMed] [Google Scholar]
- 10. O'Riordain DS, O'Brien T, van Heerden JA, Service FJ , Grant CS. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg 1994; 18: 488–493. [DOI] [PubMed] [Google Scholar]
- 11. Tonelli F, Giudici F, Nesi G, Batignani G, Brandi ML. Operation for insulinomas in multiple endocrine neoplasia type 1: when pancreatoduodenectomy is appropriate. Surgery 2017; 161: 727–734. [DOI] [PubMed] [Google Scholar]
- 12. Antwi K, Nicolas G, Fani M, Heye T, Pattou F, Grossman A et al 68Ga‐exendin‐4 PET/CT detects insulinomas in patients with endogenous hyperinsulinemic hypoglycemia in MEN‐1. J Clin Endocrinol Metab 2019; 104: 5843–5852. [DOI] [PubMed] [Google Scholar]
- 13. Bartsch DK, Albers M, Knoop R, Kann PH, Fendrich V, Waldmann J. Enucleation and limited pancreatic resection provide long‐term cure for insulinoma in multiple endocrine neoplasia type 1. Neuroendocrinology 2014; 98: 290–298. [DOI] [PubMed] [Google Scholar]
- 14. Giudici F, Nesi G, Brandi ML, Tonelli F. Surgical management of insulinomas in multiple endocrine neoplasia type 1. Pancreas 2012; 41: 547–553. [DOI] [PubMed] [Google Scholar]
- 15. Tonelli F, Fratini G, Falchetti A, Nesi G, Brandi ML. Surgery for gastroenteropancreatic tumours in multiple endocrine neoplasia type 1: review and personal experience. J Intern Med 2005; 257: 38–49. [DOI] [PubMed] [Google Scholar]
- 16. Anlauf M, Bauersfeld J, Raffel A, Koch CA, Henopp T, Alkatout I et al Insulinomatosis: a multicentric insulinoma disease that frequently causes early recurrent hyperinsulinemic hypoglycemia. Am J Surg Pathol 2009; 33: 339–346. [DOI] [PubMed] [Google Scholar]
- 17. Vezzosi D, Cardot‐Bauters C, Bouscaren N, Lebras M, Bertholon‐Grégoire M, Niccoli P et al Long‐term results of the surgical management of insulinoma patients with MEN1: a Groupe d'Etude des Tumeurs Endocrines (GTE) retrospective study. Eur J Endocrinol 2015; 172: 309–319. [DOI] [PubMed] [Google Scholar]
- 18. Service FJ . Hypoglycemic disorders. N Engl J Med 1995; 332: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 19. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94: 709–728. [DOI] [PubMed] [Google Scholar]
- 20. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007; 142: 761–768. [DOI] [PubMed] [Google Scholar]
- 21. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138: 8–13. [DOI] [PubMed] [Google Scholar]
- 22. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al Postpancreatectomy hemorrhage (PPH) – an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007; 142: 20–25. [DOI] [PubMed] [Google Scholar]
- 23. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011; 149: 680–688. [DOI] [PubMed] [Google Scholar]
- 24. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 26. van Treijen MJC, van Beek D‐J, van Leeuwaarde RS, Vriens MR, Valk GD. Diagnosing nonfunctional pancreatic NETs in MEN1: the evidence base. J Endocr Soc 2018; 2: 1067–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nell S, Brunaud L, Ayav A, Bonsing BA, Groot Koerkamp B, Nieveen van Dijkum EJ et al Robot‐assisted spleen preserving pancreatic surgery in MEN1 patients. J Surg Oncol 2016; 114: 456–461. [DOI] [PubMed] [Google Scholar]
- 28. Lopez CL, Albers MB, Bollmann C, Manoharan J, Waldmann J, Fendrich V et al Minimally invasive versus open pancreatic surgery in patients with multiple endocrine neoplasia type 1. World J Surg 2016; 40: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 29. Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR et al Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 2009; 94: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nell S, Verkooijen HM, Pieterman CRC, De Herder WW, Hermus AR, Dekkers OM et al Management of MEN1 related nonfunctioning pancreatic NETs: a shifting paradigm: results from the DutchMEN1 Study Group. Ann Surg 2018; 267: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 31. Triponez F, Sadowski SM, Pattou F, Cardot‐Bauters C, Mirallié E, Le Bras M et al Long‐term follow‐up of MEN1 patients who do not have initial surgery for small ≤ 2 cm nonfunctioning pancreatic neuroendocrine tumors, an AFCE and GTE study. Ann Surg 2018; 268: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goudet P, Dalac A, Le Bras M, Cardot‐Bauters C, Niccoli P, Lévy‐Bohbot N et al MEN1 disease occurring before 21 years old: a 160‐patient cohort study from the Groupe d'étude des Tumeurs Endocrines. J Clin Endocrinol Metab 2015; 100: 1568–1577. [DOI] [PubMed] [Google Scholar]
- 33. Gonçalves TD, Toledo RA, Sekiya T, Matuguma SE, Maluf Filho F, Rocha MS et al Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. J Clin Endocrinol Metab 2014; 99: E89–E96. [DOI] [PubMed] [Google Scholar]
- 34. Manoharan J, Raue F, Lopez CL, Albers MB, Bollmann C, Fendrich V et al Is routine screening of young asymptomatic MEN1 patients necessary? World J Surg 2017; 41: 2026–2032. [DOI] [PubMed] [Google Scholar]
- 35. Herath M, Parameswaran V, Thompson M, Williams M, Burgess J. Paediatric and young adult manifestations and outcomes of multiple endocrine neoplasia type 1. Clin Endocrinol 2019; 91: 633–638. [DOI] [PubMed] [Google Scholar]
- 36. Nell S, Borel Rinkes IHM, Verkooijen HM, Bonsing BA, van Eijck CH, van Goor H et al; DMSG. Early and late complications after surgery for MEN1‐related nonfunctioning pancreatic neuroendocrine tumors. Ann Surg 2018; 267: 352–356. [DOI] [PubMed] [Google Scholar]
- 37. Ratnayake CBB, Loveday BP, Windsor JA, Lawrence B, Pandanaboyana S. Patient characteristics and clinical outcomes following initial surgical intervention for MEN1 associated pancreatic neuroendocrine tumours: a systematic review and exploratory meta‐analysis of the literature. Pancreatology 2019; 19: 462–471. [DOI] [PubMed] [Google Scholar]
- 38. Goudet P, Murat A, Binquet C, Cardot‐Bauters C, Costa A, Ruszniewski P et al Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg 2010; 34: 249–255. [DOI] [PubMed] [Google Scholar]
- 39. Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger–Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 2013; 92: 135–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vinault S, Mariet A‐S, Le Bras M, Mirallié E, Cardot‐Bauters C, Pattou F et al Metastatic potential and survival of duodenal and pancreatic tumors in multiple endocrine neoplasia type 1. Ann Surg 2018; 10.1097/SLA.0000000000003162 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41. Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L et al Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas 2014; 43: 938–945. [DOI] [PubMed] [Google Scholar]
- 42. Oleinikov K, Dancour A, Epshtein J, Benson A, Mazeh H, Tal I et al Endoscopic ultrasound‐guided radiofrequency ablation: a new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2019; 104: 2637–2647. [DOI] [PubMed] [Google Scholar]
- 43. Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S et al Endoscopic ultrasound‐guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy 2019; 51: 836–842. [DOI] [PubMed] [Google Scholar]
- 44. Andreassen M, Ilett E, Wiese D, Slater EP, Klose M, Hansen CP et al Surgical management, pre‐operative tumor localization and histopathology of 80 patients operated for insulinoma. J Clin Endocrinol Metab 2019; 104: 6129–6138. [DOI] [PubMed] [Google Scholar]
- 45. Cejas P, Drier Y, Dreijerink KMA, Brosens LAA, Deshpande V, Epstein CB et al Enhancer signatures stratify and predict outcomes of non‐functional pancreatic neuroendocrine tumors. Nat Med 2019; 25: 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Patients undergoing enucleation(s) versus other resections
Table S2 Difference between patients operated on before and after 2006
Table S3 Preoperative imaging and histopathological outcomes of patients with localized insulinomas
Table S4 Immunohistochemistry outcomes in patients with multifocal insulinomas.
Fig. S1 Freedom from persistent or recurrent hypoglycaemia after enucleations versus other resections for multiple endocrine neoplasia type 1‐related insulinoma


