Abstract
Pigmentary uveitis (PU), also known as Golden Retriever Pigmentary Uveitis (GRPU), is a common ocular condition of Golden Retrievers that has severe, vision‐threatening ocular complications and can require surgical intervention. In order to ensure consistency in the diagnosis of GRPU between examiners, a specified set of diagnostic criteria must be applied. This is critical to ensure owners, breeders, and veterinary ophthalmologists maintain confidence in the ocular certification process. Therefore, current and former members of the American College of Veterinary Ophthalmologists’ Genetics Committee came together to draft this Viewpoint Article on the challenges of diagnosis and treatment of Golden Retriever Pigmentary Uveitis for veterinary ophthalmologists, Golden Retriever owners, and Golden Retriever breeders.
Keywords: cyst, dog, Golden Retriever, pigment, pigmentary uveitis, uveitis
1. INTRODUCTION
Pigmentary uveitis (PU), also known as Golden Retriever Pigmentary Uveitis (GRPU), is a common, inherited ocular condition of Golden Retrievers that has severe, vision‐threatening ocular complications and can require surgical intervention. Pigmentary uveitis is a significant health issue for Golden Retriever breeders and owners. As awareness of the condition has grown within the breed, so has the need for earlier identification of affected dogs to alter breeding and reduce incidence of the disease, and improved treatment protocols to either prevent or delay the progression of the disease. As such, the Golden Retriever Foundation has supported several research projects through the American Kennel Club Canine Health Foundation.
Golden Retriever breeders and owners have also expressed concern regarding perceived inconsistencies in the diagnosis of GRPU by veterinary ophthalmologists. This Viewpoint Article provides a review of the current literature regarding GRPU. In addition, challenges regarding both the diagnosis and treatment of GRPU are discussed. Finally, an overview is given of the American College of Veterinary Ophthalmologists’ Genetics Committee and the OFA Companion Animal Eye Registry (CAER) examination review process. We hope that this information will be helpful to Golden Retriever breeders, owners, and veterinary ophthalmologists.
The diagnosis and treatment of PU have been evaluated and reported in 6 peer‐reviewed publications over the past 17 years. 1 , 2 , 3 , 4 , 5 , 6 The mean age at presentation is 8.12 to 8.6 years, but the age of onset ranges from 4.5 to 14.5 years of age. 1 No sex predilection has been detected. 1 , 4 The condition is frequently, but not always bilateral (59%‐89% of dogs). 1 , 3 The condition was first described in 1996 by the American College of Veterinary Ophthalmologists’ Genetics Committee based on examination findings from Canine Eye Registry Foundation (CERF) examinations of Golden Retrievers. 7 In 1996, GRPU was defined in The Blue Book: Ocular Disorders Presumed to be Inherited in Purebred Dog as, “A unique uveitis observed in the Golden Retriever that is not associated with other ocular or systemic disorders. Adhesions develop between iris and lens and the peripheral iris and cornea. Pigment dispersion (exfoliation) occurs across the anterior lens capsule from the pigmented cells of the posterior iris. Other complications include secondary cataract and obstructive glaucoma. Onset is usually between 5 and 10 years of age.”
The first manuscript describing clinical cases of GRPU was focused on Golden Retrievers in the Northeastern United States. 1 A pedigree review at the time, two decades ago, showed a common ancestry and suggested a founder effect. 1 However, the inability to identify dogs who would later become affected by GRPU allowed the condition to become widespread. The reported overall prevalence is 5.5% for PU in GR within the Midwestern United States 4 and 5.9% in Western Canada. 5 This prevalence is much higher than the 1.3% reported by the Orthopedic Foundation for Animals (OFA), 8 likely because the dogs presenting for Companion Animal Eye Registry examinations tend to be younger animals and animals without overt abnormalities that are excluded by breeders. 5 The published prevalence of PU is 9.9% for GR in the Midwest greater than 8 years of age. 4 However, based on current examination data, the national prevalence for dogs greater than 8 years of age is now 23.9%, with no significant regional variations (unpublished data, WT). Confirming the widespread nature of the condition, no significant regional variation is seen in current OFA data (Eddie Dziuk personal communication). GRPU is now recognized in Golden Retrievers throughout the United States and Canada.
The late onset of the condition 1 , 4 , 5 has stymied the efforts of conscientious breeders as a dog may be bred multiple times or produce multiple generations before being diagnosed with GRPU. The Golden Retriever Club of America in the GRCA Code of Ethics requires continued certifying eye examinations throughout the dog's lifetime so that their status with regards to GRPU will be known. 9 Identification of affected animals can increase awareness of potentially affected offspring and help avoid further breeding of affected lineage. Because of the potential for severe visual impairment and ocular pain, the ACVO Genetics Committee recommends that dogs affected with GRPU should not be bred.
Pigmentary uveitis is strongly suspected to be inherited, as it has been diagnosed exclusively in GR, or offspring of GR/Labrador Retriever or GR/Poodle crosses. Holly et al 5 suggested that GRPU was autosomal dominant with incomplete penetrance after creating a pedigree of Golden Retrievers in Western Canada. Similarly, a polygenic mode of inheritance could also explain this pedigree. Determining the mode of inheritance for an older‐onset disease such as PU can be quite difficult because many presumably affected individuals may not live long enough to show the disease phenotype if they succumb to other conditions first.
Uveal cysts have been noted as a significant risk factor for the development of GRPU. 5 , 10 In one study, eyes with cysts on the first examination had a higher percentage of PU on the second examination (36%) than those with no cysts (1%). 10 Eyes with multiple cysts on the first examination had a significantly higher percentage of PU (58%) than those with solitary cysts (10%) or without cysts (1%). 10 In another study, 56.5% of dogs diagnosed with thin‐walled, attached iridociliary cysts had a high risk of being diagnosed with PU at future examinations. 5 In the Midwestern United States, uveal cysts were seen in 35% of Golden Retrievers. 4 Uveal cysts were seen in 7.9% of Golden Retrievers presented for OFA Companion Animal Eye Registry (CAER) examinations. 8 However, not all Golden Retrievers with uveal cysts go on to develop PU. Therefore, dogs with uveal cysts are currently considered to be breeder option with regards to breeding recommendations from the ACVO Genetics Committee. 8
Histologically, the PU phenotype is characterized by cysts within the posterior chamber even though the uveal cysts are often not visualized during the clinical examination of a dog affected by GRPU. 3 The immunohistochemical staining pattern of the cysts is consistent with a cellular origin from the ciliary body epithelium. 3 The cysts contain an alcian blue‐positive material that is consistent with hyaluronic acid. 2 Additional histologic findings may include: (a) free pigment within the trabecular meshwork, (b) cysts stretched across the anterior face of the vitreous, (c) cysts attached to the lens capsule, (d) posterior synechia, (e) peripheral anterior synechia, (f) a pre‐iridal fibrovascular membrane, (g) a mild‐lymphoplasmacytic uveitis, and (h) deposition of a hyaluronic acid and collagen‐rich material on the equatorial and anterior lens capsule that corresponds with the fibrinous material seen clinically. 1 , 2 , 3 The exact role of uveal cysts in GRPU and the GRPU phenotype has not yet been determined.
2. DIAGNOSIS
The challenge of diagnosing GRPU lies not in identifying severely affected eyes, but instead with determining the minimum threshold of clinical findings to make a diagnosis of GRPU. Once the underlying genetic mutations and thereby the underlying pathophysiology have been identified, the various expressions of the phenotype will be better understood and the point at which the individual becomes “affected” by GRPU (manifests the ocular changes associated with the genetic mutations) will be easier to define.
Eyes with the most severe manifestations of GRPU present with pigment deposited in a radial fashion on the anterior lens capsule, varying degrees of iridal hyperpigmentation, fibrinous material within the anterior chamber, and in the most severe cases, glaucoma (Figures 1, 2, 3). The fibrinous material has been seen in anterior chamber of 18% to 37% of eyes affected by GRPU and examined by veterinary ophthalmologists in the non‐breeding screening setting. 1 , 4 Glaucoma has occurred in 30% to 46% of eyes affected by GRPU. 1 , 3 At times the web of fibrinous material within the anterior chamber and/or significant corneal edema may hinder visualization of the anterior lens capsule (Figure 4). As such, during a Companion Animal Eye Registry (CAER) examination the examiner can still diagnose the dog as GRPU affected based on the other clinical findings. The clinical findings leading to the diagnosis (web of fibrinous material obscuring visualization of the entire anterior lens capsule) should be recorded in detail within the comments section. The description of the clinical findings used to make the diagnosis of GRPU is of critical importance to the ACVO Genetics Committee and researchers investigating GRPU so that accurate and detailed phenotypic information can be collected.
Figure 1.
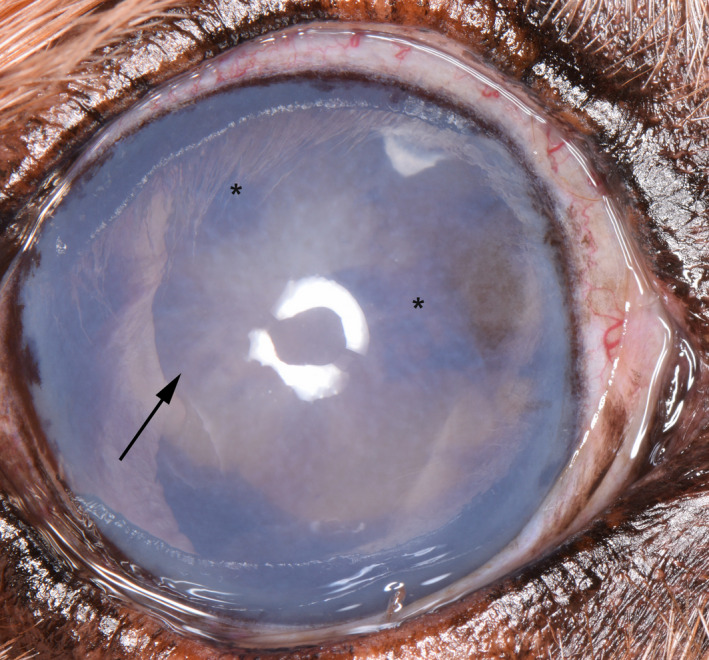
Right eye of a 12‐y‐old, male, neutered Golden Retriever severely affected by GRPU. He is blind in the eye due to secondary glaucoma. He has diffuse, dense corneal edema. Radial pigment can be seen on the anterior lens capsule (arrow) as well as dense clumps of pigment on the anterior lens capsule (*)
Figure 2.
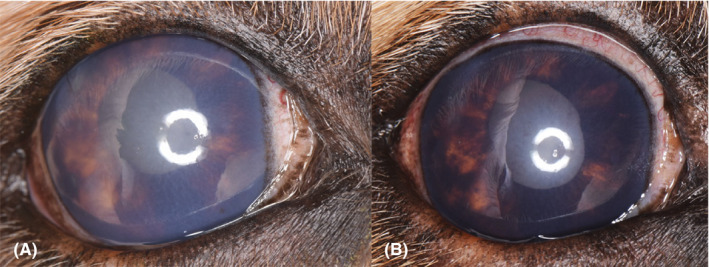
(A) Right eye of an 11‐y‐old, intact, male Golden Retriever severely affected by GRPU. Note the moderate, diffuse corneal edema. The radial pigmentation on the anterior lens capsule is difficult to discern because of the corneal edema. A web of fibrinous material is present across the anterior lens capsule and within the anterior chamber, but is difficult to visualize in the image due to the corneal edema. Iris bombé is present. The globe is buphthalmic and has an intraocular pressure of 31 mm Hg. (B) The same eye pictured in (A), this image was taken 1 y prior. The radial pigmentation on the lens is more easily discerned as the corneal edema is less severe. The pupil does not dilate completely due to multiple areas of posterior synechia and thickening of the iris and pupillary margin due to pigment deposition within the iridal stroma
Figure 3.

Right eye of a 9‐y‐old, male, neutered Golden Retriever blind due to glaucoma secondary to GRPU. He has mild corneal edema, a darkly pigmented iris, and dense deposition of pigment along the corneal endothelium near the limbus extending further than the irregular border of endothelial pigment seen in many unaffected Golden Retrievers. Radial and scattered pigment is present along the anterior lens capsule. The pupil is mid‐range due to extensive posterior synechia. Iris bombé is present
Figure 4.

Left eye of an 8‐year‐old, intact, female Golden Retriever severely affected by GRPU. Note the severe, diffuse corneal edema. The radial pigmentation on the anterior lens capsule is difficult to discern because of the corneal edema, but clumps of pigment can be seen (*). A web of fibrinous material is present in the anterior chamber and strands can be seen contacting the corneal endothelium (arrows). Pigment is also present on the corneal surface of this individual which is not a characteristic feature of GRPU
Eyes with more moderate manifestations of GRPU present with radial pigmentation on the anterior lens capsule, varying degrees of iris hyperpigmentation, and/or posterior synechia (adhesions between the iris and lens) (Figures 5 and 6). Posterior synechia has been noted in 50% of eyes affected by GRPU and examined by veterinary ophthalmologists in the non‐breeding screening setting. 1 Cataracts may or may not be present as well. Cataracts have been described in 37% to 43% of eyes affected by GRPU and examined by veterinary ophthalmologists in the non‐breeding screening setting. 1 , 3
Figure 5.

Left eye of the Golden Retriever whose right eye is shown in Figure 1. This eye is moderately affected by GRPU and retains vision. There is diffuse radial pigmentation and pigment clumping along the anterior lens capsule. The iris is darkly pigmented and the pupillary margin is thickened due to pigment deposition. The arrow denotes dense pigment deposited along the corneal endothelium at the limbus, again extending farther axially then the typical endothelial pigment seen in many Golden Retrievers near the temporal limbus
Figure 6.

(A) Left eye of an 8‐y‐old, intact, male, Golden Retriever moderately affected by GRPU. Note the area of posterior synechia at the 9'clock position and 12 clock hours of radial pigmentation. (B) The same eye 3 y prior. Notice the well‐dilated pupil and the presence of radial pigmentation through only 7 clock hours
Uveal cysts do not have to be visualized in order to make a diagnosis of GRPU. Visible uveal cysts have been noted in only 13.3% to 42% of eyes affected by GRPU. 1 , 3 , 4 , 5 As GRPU progresses, the thickening of the iris due to pigment deposition and areas of posterior synechia often preclude complete pupillary dilation, thereby preventing visualization of the uveal cysts. When examined using ultrasound biomicroscopy (UBM), all eyes affected by GRPU have multiple uveal cysts detected (Figure 7). 11
Figure 7.

This is a series of ultrasound biomicroscopy (UBM) images from the right (A and B) and left (C and D) eyes. The clinical images are seen in Figures 1 and 5. In the clinical images, uveal cysts cannot be visualized. In Figure 7A, the yellow line denotes the diameter (1.2 mm) of the uveal cyst. In Figure 7B, each asterisk is within a uveal cyst along the ciliary processes. In Figure 7C, the arrow points to a well‐delineated uveal cyst posterior to the iris. In Figure 7D, the asterisk denotes a cluster of uveal cysts within the posterior chamber
Eyes with mild manifestations of GRPU may present with only radial pigment on the anterior lens capsule which has been described as the hallmark of the GRPU phenotype (Figures 8, 9, 10, 11, 12). 1 , 3 , 4 , 5 , 8 Anecdotally, some ophthalmologists have elected to make a diagnosis of GRPU only if iridal hyperpigmentation, aqueous flare, free floating pigmented cells, hypotony, and/or conjunctival hyperemia have also been noted. In order to ensure consistency in the diagnosis of GRPU between all examiners, the authors recommend the following criteria: if radial pigment is present on the anterior lens capsule, the eye should be marked as affected by GRPU even if aqueous flare, free floating pigmented cells, hypotony, and/or conjunctival hyperemia are not present. The reason for this recommendation is that when followed over years, dogs with radial pigment do progress to moderate to severe GRPU (Figures 13, 14, 15, 16, 17). Twenty six dogs with radial pigment alone were followed for 1 to 5 years (unpublished data). Of those, 14/26 (54%) developed moderate to severe manifestations of GRPU. Eight dogs were followed for 3 to 5 years, and 7/8 (87.5%) developed moderate to severe manifestations of GRPU. The progression can be slow and very subtle and difficult to quantify if photographs are not taken. For the same reason, Golden Retrievers with even faint radial lines of pigmentation on the anterior lens capsule should be classified as being affected with GRPU (Figures 18 and 19); other breeds with these faint radial lines of pigmentation on the anterior lens capsule should be marked “other suspect inherited “ or “other suspect not inherited” as interpreted by the examiner. Regardless of breed, examiners should draw the pigment present on the anterior lens capsule in the comments section of the Companion Animal Eye Registry form. The drawings and any comments will allow progression of clinical lesions, particularly in cases with radial pigment alone, to be tracked within the CAER database, and thus further define the relationship of this lesion with the overall GRPU disease. For Golden Retrievers, while the PU affected / do‐not‐breed criteria of radial pigment alone may seem stringent, the precedent for this is appropriate and common in the breeding screening setting where erring on the side of caution/exclusion, especially with a late onset, potentially blinding and painful condition is warranted. This is quite similar to the history of the decision to fail / do‐not‐breed recommendation for Labrador Retrievers and Samoyeds with retinal dysplasia – folds alone, due to the potential association with oculoskeletal dysplasia in these breeds, until the underlying genetic mutation was identified and allows affected dogs to be screened and potentially cleared to passing with breeder option for the folds if negative. If only a few specks of pigment are present or pigment is present only adjacent to a collapsed cyst and not radial, this does not constitute a diagnosis of GRPU in the Golden Retriever or any other breed (Figures 20 and 21). However, the findings should be recorded in the comments section, and for Golden Retrievers, with concerns that the dog is at increased risk for GRPU and a recommendation for a follow‐up examination in 6‐12 months.
Figure 8.

Right eye of an 8‐y‐old, female, spayed Golden Retriever. Radial pigment is present on the anterior lens capsule for 12 clock hours. An incipient cataract is present ventronasally. This dog should be diagnosed as affected with pigmentary uveitis
Figure 9.

Right eye of a 10‐y‐old, intact, female Golden Retriever. Two collapsed uveal cysts are present, one axially and one nasally. Radial pigment is present through 6 clock hours on the anterior lens capsule temporally with other randomly scattered pigment present on the remainder of the lens capsule. A mature cataract is present. This dog should be diagnosed as affected with pigmentary uveitis
Figure 10.
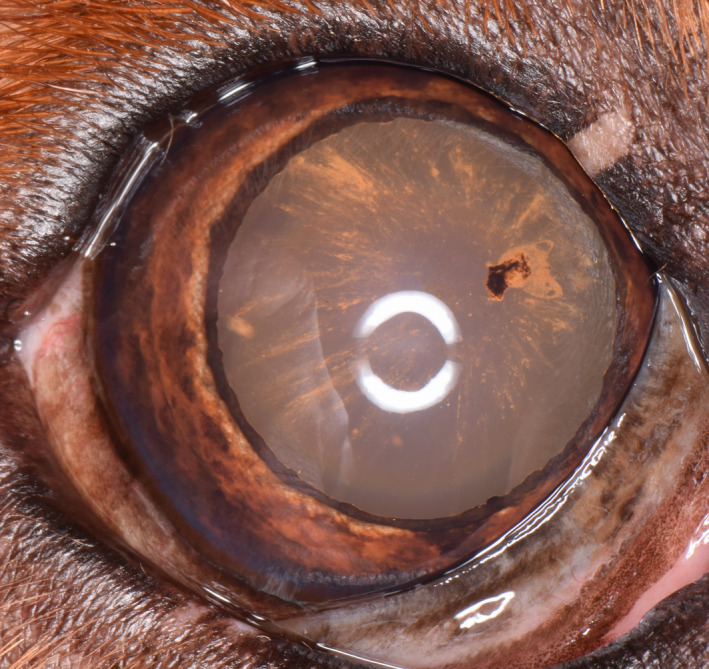
Right eye of an 8‐y‐old, male, neutered Golden Retriever. Note the 12 clock hours of radial pigmentation and clump of pigment at the 2 o'clock position. Even though the iris is not darkly pigmented, this dog should be diagnosed as affected with pigmentary uveitis
Figure 11.

Right eye of the dog in Figure 6. Radial pigment is present on the anterior lens capsule over 6 clock hours. This eye should be diagnosed as affected with pigmentary uveitis and demonstrates the asymmetry that can occur between eyes
Figure 12.

Both eyes of a 9‐y‐old, intact, male Golden Retriever. (A) The right eye with 12 clock hours of radial pigmentation and areas of pigment clumping. (B) The left eye with 12 clock hours of radial pigmentation and larger areas of pigment clumping and slightly darker coloration of the iris. Both eyes are affected with GRPU
Figure 13.

(A) Right eye of a 6‐y‐old, male, neutered Golden Retriever. Four uveal cysts are visible at the nasal pupillary margin. The white arrow denotes 3 lines of radial pigmentation with associated underlying cataract. The iris does not have increased pigmentation. (B) The same eye two years later. The arrows denote the extension of the pigmentation which now spans the majority of the anterior lens capsule. The iris has become more darkly pigmented and slightly thickened although the pupil still dilates completely. The cataract is more extensive. Uveal cysts are still visible along the pupillary margin. Darker, finely scattered, stippled pigment is also present across the anterior lens capsule
Figure 14.
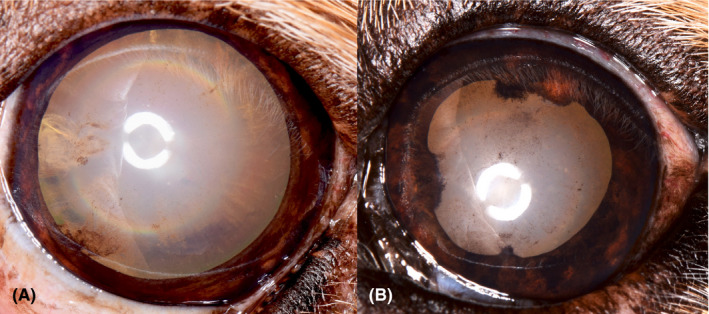
Left eye of the dog in Figure 2. (A) This image was taken at 11 y of age. Fine radial pigment is dispersed for 12 clock hours across the anterior lens capsule. A collapsed cyst is seen at the 8 o'clock position. An incipient cataract is present at the 9 o'clock position. The iris is orange brown in color. (B) The same eye 2 years later. The iris has areas of increased pigmentation. More pigment is scattered across the anterior lens capsule. Areas of posterior synechia are present at the 6, 9, and 12 o'clock positions
Figure 15.

Both eyes of a 13‐y‐old, female, spayed Golden Retriever. (A) The right eye has faint areas of radial pigmentation (black arrows) present on the anterior lens capsule. This eye is affected by GRPU. (B) The left eye was blind secondary to glaucoma. Iris bombé is present. Radial and clumped areas of pigment are present on the anterior lens capsule. Posterior synechia is present (*)
Figure 16.

(A) Right and (B) left eyes of a 7‐y‐old, male, neutered Golden Retriever. The white arrows denote the fine areas of radial pigmentation. Collapsed cysts are seen in both eyes. Small, radial areas of pigment are also noted in the left eye. (C and D) are the same eyes 5 y later. Increased areas of pigmentation are present. A broad area of posterior synechia has developed in both eyes at the four o'clock position. Fine areas of posterior synechia are present at the 10, 11, and 12 o'clock positions in the left eye. The amount of pigmentation has increased both in density and surface area of the lens affected
Figure 17.

(A) Left eye of a 5‐y‐old, male, neutered Golden Retriever. The bright white circular region near 6 o'clock is corneal lipid. Uveal cysts are present at 3 and 7 o'clock. Faint areas of radial pigment are present from 7‐8 and 10‐12 o'clock. (B) The same eye 2 y later. The radial pigment now involves all 12 clock hours. A cataract is present at the 12 o'clock position. The uveal cysts are still present at the 3 o'clock position. The iris has become more darkly pigmented
Figure 18.
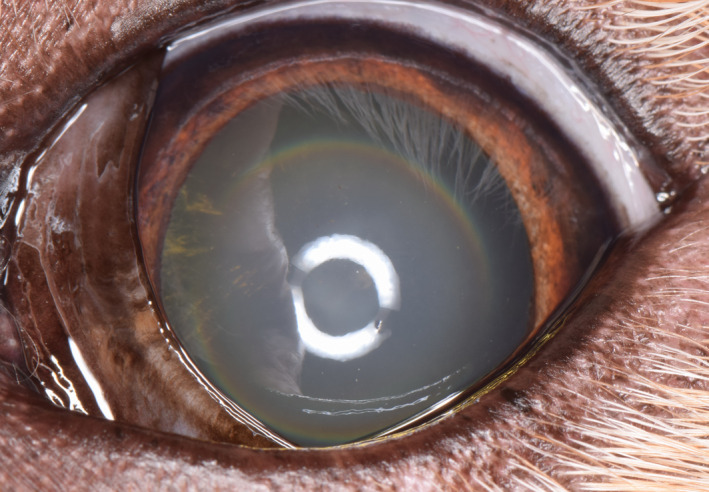
Left eye of a 10‐y‐old, female, spayed Golden Retriever. Fine radial pigment is present on the anterior lens capsule from 8 to 10 o'clock. This dog is affected with GRPU
Figure 19.

Left eye of a 6‐y‐old, female, spayed Golden Retriever. A collapsed uveal cysts are present at the 9 o'clock position with pigment scattered adjacent to the collapsed cyst. In addition through, radial pigment is present from 7 to 8 and 10‐11 o'clock. Iris to iris persistent pupillary membranes are present temporally. This dog is affected with GRPU
Figure 20.
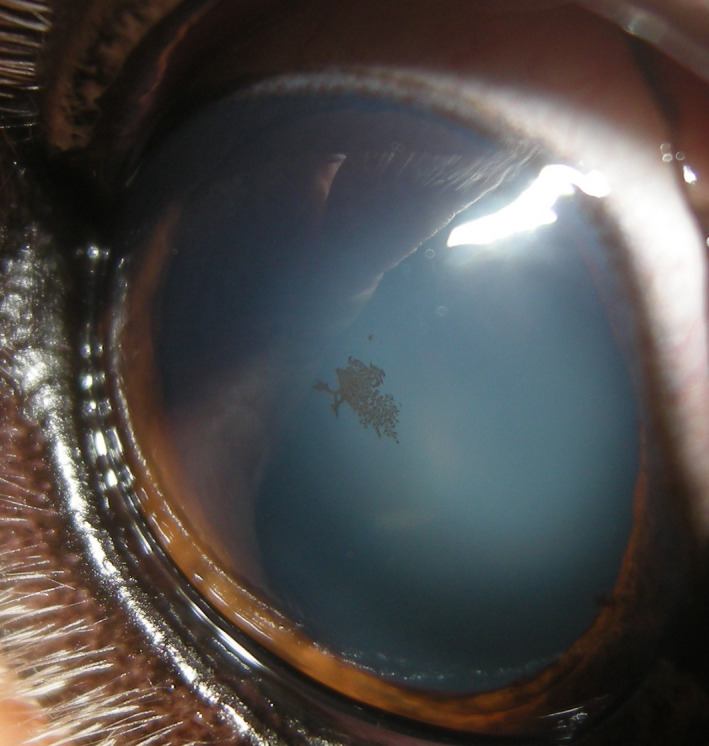
Right eye of an 11‐y‐old, female, spayed Golden Retriever. Stippled pigment is present on the central aspect of the anterior lens capsule. This pigment had not been noted at multiple previous examinations and thus does not represent the lens pigment foci/no strands presentation of persistent pupillary membranes, which are congenital and unchanging. As the pigment is not deposited in a radial fashion, these findings should be recorded in the comments section with concern that the dog may be at increased risk for GRPU with a recommendation for a follow‐up examination in 6‐12 mo
Figure 21.

Left eye of a 7‐y‐old, intact, male Golden Retriever. Multiple uveal cysts are present. Several have collapsed on the anterior lens capsule. Radial pigment deposition is not present. This dog should be diagnosed as having multiple uveal cysts only
While pigment dispersion and deposition on the iridal and corneal endothelial surfaces may be present, these factors without radial pigment on the anterior lens capsule do not constitute a diagnosis of GRPU. These findings should be recorded in the comments section. Some lines of Golden Retrievers have very dark iridal coloration from a very young age, particularly some of those with lighter coat colors (Figure 22). Some Golden Retrievers also have irregular endothelial pigmentation and/or a rim of bluish “haze” at the limbus (usually temporal) from a very young age. Again without radial pigment on the anterior lens capsule, this finding would not constitute a diagnosis of GRPU. If the examiner feels the dog is at high risk for developing GRPU because of these findings, the examiner should write suspect GRPU but lacks radial pigment within the comments section and list recommendations for follow‐up examinations. These animals will pass their CAER examination without notation unless other breeder option (including cysts) or failing conditions are also present and marked.
Figure 22.

Both eyes of a 2‐y‐old, intact, female Golden Retriever. Very darkly pigmented irides in otherwise normal eyes. This dog should not be diagnosed as affected with GRPU. This individual is now 10 y old and has had yearly ophthalmic examinations. She remains free of any clinical signs of GRPU
Hypotony does not have to be present for a diagnosis of GRPU. The presence of hypotony also should not trigger a diagnosis of GRPU. In one abstract, the intraocular pressure was not significantly different for GR without visible uveal cysts, with visible uveal cysts, or affected by GRPU. 12 There was also no significant difference in mean intraocular pressure between dogs without cysts detectable on ultrasound biomicroscopy (UBM), with solitary cysts on UBM, 2‐4 cysts on UBM, greater than 4 cysts on UBM, or affected by GRPU. 12 The mean IOP for all eyes, measured using applanation tonometry, was 7 ± 3 mm Hg which was lower than is typical for most breeds. 12
3. TREATMENT AND PROGNOSIS
Treatment of GRPU remains challenging because the underlying pathophysiology of the disease has not yet been elucidated. Clinically GRPU appears to have an inflammatory component, although histologically, minimal inflammation has been noted in eyes affected by GRPU. 1 , 3 Whether this is a reflection of the lack of inflammation in the disease process or control of the inflammation by topical medications is difficult to discern as most patients do receive topical anti‐inflammatory medications. At this time, treatment with topical or systemic anti‐inflammatory medications is the standard of care. Medications to control ocular hypertension and glaucoma are added as required.
Golden Retriever Pigmentary Uveitis causes secondary glaucoma and vision loss in 45%‐46% of eyes. 1 , 5 Per Sapienza, the prognosis for vision for dogs affected with this condition is guarded, particularly those with extensive posterior synechia and fibrinous material. 1 In that set of GR, the average time to development of glaucoma was 9 months. 1 Treatment with contact diode trans‐scleral cyclophotocoagulation successfully controlled intraocular pressure in 91% (10/11) cases. 1 However, all patients were blind before treatment and remained blind after treatment. 1 In the paper by Esson et al, 3 the time from initial presentation to the development of glaucoma was even shorter at 4.8 months.
In patients with fibrinous material present within the anterior chamber, the injection of tissue plasminogen activator (TPA) has been shown to lyse the fibrinous material. 1 However, 1 of 3 patients treated with intracameral TPA developed ocular hypertension. 1 Therefore, the author suggested TPA injections should be performed with caution. 1 Anecdotally, veterinary ophthalmologists report a failure of TPA to lyse the fibrinous material within the anterior chamber or frequent recurrence of the fibrinous material.
A recent abstract reported vision loss due to glaucoma in only 21% of GRPU‐affected dogs. 11 The dogs in the study were followed for at least 10 months with an average time between examinations of 35 months. The lower rate of progression to glaucoma may have been due to earlier detection of PU as these dogs were diagnosed during screening examinations as opposed to after the development of overt clinical signs. All dogs in the study received some form of topical anti‐inflammatory treatment. There was no difference in rate of progression between dogs treated with topical ophthalmic corticosteroids and topical ophthalmic non‐steroidal anti‐inflammatory drugs. 11 There was also no difference in the rate of progression between dogs receiving OcuGloTM in addition to topical anti‐inflammatory therapy. Similar to previous reports, 1 the presence of synechia and fibrinous material were each noted as significant risk factors for the development of glaucoma. 11
4. ACVO GENETICS COMMITTEE AND OCULAR CERTIFYING EXAMINATIONS
The American College of Veterinary Ophthalmologists (ACVO) Genetics Committee is a scientific advisory committee composed of 8 Diplomates of the ACVO, two advisors (one from ACVO and one from European College of Veterinary Ophthalmologists ‐ ECVO), and the Diplomate serving as the Orthopedic Foundation for Animals (OFA) liaison. The committee members serve a 4‐year term, and two new members are elected to rotate onto the committee each year. New members are chosen from a pool of ACVO members that have shown an interest in the Genetics Committee through self‐nomination. An effort is made to maintain a balance between members practicing ophthalmology in an academic setting vs in private practice.
The genetics committee's main goal is to furnish guidelines regarding ocular disorders proven or suspected to be inherited or of major concern in pure bred dogs. The committee members are charged with review of literature published each year that may pertain to advances in canine ocular genetics, genetic testing, and inherited ocular diseases. Pertinent references and information are added yearly to the newest edition of The Blue Book: Ocular Disorders Presumed to Be Inherited in Purebred Dog written by the Genetics Committee of the American College of Veterinary Ophthalmologists, often known simply as The Blue Book. The genetics committee is responsible for designing and editing ophthalmic examination forms such as the OFA Companion Animal Eye Registry (CAER) examination form and the gonioscopy examination form. Breed clubs direct concerns to the committee for consideration and guidance. Committee members also update data in The Blue Book by reviewing frequency of ophthalmic conditions submitted to OFA for each breed. At each annual conference meeting of the ACVO, the committee members meet to make final adjustments to the most current edition of The Blue Book. This allows the college to monitor for disease trends and document changes in significance of ophthalmic conditions over time. The Blue Book, therefore, maintains up to date information regarding prevalence of ophthalmic diseases for each breed and current recommendations for breeding.
The OFA liaison is the Diplomate responsible for relaying any problems or information that needs to be communicated from the OFA office to the genetics committee members or Diplomates of ACVO and vice versa. This member also reviews all OFA ophthalmic examination forms with any cataract lesion marked, any "other" lesion marked, any comments made, unusual findings (eg, a condition noted in an atypical breed), and or conflicting results with another form for the same dog. These forms are also cross‐referenced with any previous examinations of the same dog, and frequently reviewed and discussed further with the actual examiners, before an OFA diagnosis/code(s) and associated ACVO breeding recommendations are determined for the breeder/owner/dog certificate, as well as for the ACVO breed statistics data code. As consistent internal* and external** standards are applied on a case‐by‐case basis, examiner findings and interpretations may be recoded to a different diagnosis, a hold of the application pending follow‐up may be recommended, and or an additional opinion(s) may be recommended or required as a "tie‐breaker" in the case of conflicting findings.
*(the OFA and the OFA liaison apply the ACVO genetics committee and blue book, and OFA, protocol and "rules" in the same manner to all cases).
**(reconciling different ways of reporting the same lesion across different examiners).
5. CONCLUSION
Golden Retriever Pigmentary Uveitis remains a challenging disease for Golden Retriever breeders and owners. The late age of onset makes breeding choices very challenging. In order to maintain consistency within the OFA CAER examination process, all dogs with radial pigment on the anterior lens capsule should be diagnosed as affected with GRPU. When examiners make a diagnosis of GRPU, the specific examination findings which led to the diagnosis should be recorded and drawn in the comments section of the Companion Animal Eye Registry form. A diagnosis of GRPU should only be rendered if radial pigment is present on the anterior lens capsule or the above described other classic severe or moderate case findings are present and obscure visualization of such pigment.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
We would like to thank Dr Gustavo Aguirre for reviewing this manuscript and providing critical insight into structure and content.
Townsend WM, Huey JA, McCool E, King A, Diehl KA. Golden retriever pigmentary uveitis: Challenges of diagnosis and treatment. Vet Ophthalmol. 2020;23:774–784. 10.1111/vop.12796
Funding information
Open Access Publication of this manuscript has been funded by the American Kennel Club Canine Health Foundation.
REFERENCES
- 1. Sapienza JS, Simo FJ, Prades‐Sapienza A. Golden Retriever uveitis: 75 cases (1994–1999). Vet Ophthalmol. 2000;3:241‐246. [DOI] [PubMed] [Google Scholar]
- 2. Deehr AJ, Dubielzig RR. A histopathological study of iridociliary cysts and glaucoma in Golden Retrievers. Vet Ophthalmol. 1998;1:153‐158. [DOI] [PubMed] [Google Scholar]
- 3. Esson D, Armour M, Mundy P, et al. The histopathological and immunohistochemical characteristics of pigmentary and cystic glaucoma in the Golden Retriever. Vet Ophthalmol. 2009;12:361‐368. [DOI] [PubMed] [Google Scholar]
- 4. Townsend W, Gornik K. Prevalence of uveal cysts and pigmentary uveitis in Golden Retrievers in three Midwestern states. J Am Vet Med Assoc. 2013;243:1298‐1301. [DOI] [PubMed] [Google Scholar]
- 5. Holly VL, Sandmeyer LS, Bauer BS, et al. Golden retriever cystic uveal disease: a longitudinal study of iridociliary cysts, pigmentary uveitis, and pigmentary/cystic glaucoma over a decade in western Canada. Vet Ophthalmol. 2016;19:237‐244. [DOI] [PubMed] [Google Scholar]
- 6. Tofflemire K, Whitley R, Ben‐Shlomo G, et al. Uveal hematocysts in a golden retriever dog. Case Rep Vet Med. 2013;2013:1‐3. [Google Scholar]
- 7. Genetics Committee of the American College of Veterinary Ophthalmologists . Ocular Disorders Presumed to be Inherited in Purebred Dogs, 2nd ed. Phoenix, AZ: Genetics Committee of the American College of Veterinary Ophthalmologists, 1996. [Google Scholar]
- 8. Genetics Committee of the American College of Veterinary Ophthalmologists . Presumed To Be Inherited in Purebred Dogs. Phoenix, AZ: Genetics Committee of the American College of Veterinary Ophthalmologists, 2018; https://www.ofa.org/diseases/eye‐certification/blue‐book. Accessed March 19, 2020. [Google Scholar]
- 9. America Golden Retriever Club of America . GRCA Code of Ethics, 2020. https://www.grca.org/about‐grca/grca‐code‐of‐ethics/. Accessed March 19, 2020.
- 10. Townsend W, Ashmore J, Moore G. Association of uveal cysts with development of golden retriever pigmentary uveitis (abstract #137). Vet Ophthalmol. 2013;16:E48. [Google Scholar]
- 11. Jost H, Townsend W, Moore G. Vision loss and glaucoma in Golden Retrievers with pigmentary uveitis, risk factors for glaucoma, and effect of treatment on disease progression. Vet Ophthalmol. 2019;22:E46. [DOI] [PubMed] [Google Scholar]
- 12. Townsend W. Intraocular pressures in eyes of golden retrievers without uveal cysts, with uveal cysts, and with pigmentary uveitis (abstract #101). Vet Ophthalmol. 2015;18:E33. [Google Scholar]


