Abstract
Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 inhibitor for the treatment of mild to moderate atopic dermatitis. Results from 2 randomized, double‐blind, vehicle‐controlled phase 3 studies showed that twice‐daily crisaborole in children and adults with mild to moderate atopic dermatitis was efficacious and well tolerated. Initial pharmacokinetics (PK) studies of crisaborole indicated absorption with measurable systemic levels of crisaborole. The current analysis was conducted to correlate steady‐state systemic exposure parameters with ointment dose and identify covariates impacting PK parameters in healthy participants and patients with atopic dermatitis or psoriasis. A nonlinear regression analysis was conducted using ointment dose and noncompartmental PK parameters at steady state (area under the curve [AUCss] and maximum concentration [Cmax,ss]). PK data were available from 244 participants across 6 clinical studies (AUCss, N = 239; Cmax,ss, N = 241). Disease condition had the greatest impact on slope in both models, corresponding to 2.5‐fold higher AUCss and Cmax,ss values at a given ointment dose in patients with atopic dermatitis or psoriasis relative to healthy participants. Disease severity, race/ethnicity, and sex had marginal effects on AUCss and Cmax,ss. Systemic exposures were similar across age groups ≥2 years of age when the same percentage of body surface area (%BSA) was treated. Predictive performance plots for AUCss and Cmax,ss for different age groups demonstrated that the models adequately describe the observed data. Model predictions indicated that systemic exposure to crisaborole in pediatric patients (2‐17 years) is unlikely to exceed systemic exposure in adults (≥18 years), even at the highest possible ointment dose corresponding to a %BSA of 90.
Keywords: crisaborole, atopic dermatitis, pharmacokinetics, steady state, area under the curve, maximum concentration
The intracellular enzyme phosphodiesterase 4 (PDE4) plays a key role in regulating inflammatory processes, such as cytokine production, through the degradation of cyclic adenosine monophosphate (cAMP). 1 PDE4 is overexpressed or overactivated in immune cells in patients with inflammatory conditions such as psoriasis and atopic dermatitis (AD), 2 , 3 , 4 which led to the clinical development of PDE4 inhibitors for the treatment of several inflammatory disorders. PDE4 inhibition allows the accumulation of intracellular cAMP, which activates protein kinase A, leading to suppression of proinflammatory cytokine synthesis. 1 The orally administered PDE4 inhibitor apremilast is approved for patients with psoriasis; however, this requires dose titration to reduce the risk of gastrointestinal side effects due to PDE4 inhibition in nontarget tissues, which prompted interest in topically delivered alternatives for inflammatory skin diseases. 5 , 6
Crisaborole ointment, 2%, is a nonsteroidal PDE4 inhibitor for the treatment of mild to moderate AD. 7 The boron moiety in crisaborole plays a central role in its PDE4 inhibitory activity and allows for a low molecular weight (251 kDa), thus enabling effective skin penetration and topical administration of the drug. 8 , 9 PDE4 inhibition by crisaborole leads to increased intracellular cAMP levels and modulation of pathways, such as nuclear factor‐κB‐mediated inflammatory cytokine synthesis, resulting in suppression of the production of proinflammatory cytokines (eg, interferon‐γ, tumor necrosis factor‐α, interleukin [IL]‐2, IL‐5, IL‐10). 10 Additionally, crisaborole significantly modulates key molecular pathways, including type 1 helper T cell (Th1), Th2, and Th17/Th22 axes. 11
The efficacy and safety of twice‐daily topical crisaborole in adults and children (aged ≥2 years) with mild to moderate AD was demonstrated in 2 identically designed, randomized, double‐blind, vehicle‐controlled phase 3 studies. 12 Crisaborole improved the severity of AD skin manifestations in both trials. A significantly greater proportion of crisaborole‐ versus vehicle‐treated patients achieved the primary end point of Investigator's Static Global Assessment (ISGA) success (clear [0] or almost clear [1] with ≥2‐grade improvement from baseline), with improvements also observed in percentage of body surface area (%BSA) affected, in pruritus, and in other signs and symptoms of AD. 12
Small early‐phase pharmacokinetic (PK) studies of crisaborole in children and adolescents with AD indicated low systemic exposure (mean maximum concentration [Cmax] 105‐111 ng/mL on day 1) and rapid absorption (median time to Cmax [Tmax] 2.4‐3.0 hours on day 1) of crisaborole. 13 , 14 Although PK data from individual studies of crisaborole are available, a population PK analysis using structural compartmental models has not been possible because of variable absorption profiles following topical administration and limited data from the absorption phase in patients with AD. Therefore, a nonlinear regression analysis was performed using noncompartmental PK parameters at steady state (area under the curve [AUCss] and Cmax,ss) to describe the relationship between ointment dose and systemic exposure of crisaborole in healthy participants and patients with AD or psoriasis. The dose of a topical agent used to treat AD or psoriasis is dictated by the %BSA affected by the disease and body size of the patient. The ointment amount is unlikely to be fixed between patients of different ages because body size increases with age until adulthood and with body weight gain in adults.
The objectives of the current analysis are to correlate systemic exposure parameters of crisaborole with ointment dose and identify covariates that impact the PK parameters of crisaborole in healthy participants and in patients with AD or psoriasis. The described methodology is a comprehensive approach to analyze PK of topical agents with assessed steady‐state PK parameters while enabling the approximation and prediction of systemic exposure to treatment across age groups.
Methods
Participants and Samples
Data from 6 clinical studies of crisaborole were included in the analysis: 3 phase 1 studies in healthy adults (C3291010, AN2728‐PSR‐104, NCT01258088; C3291019, AN2728‐TQT‐108; and C3291009, AN2728‐PK‐101), 1 phase 1b study in patients aged 12‐17 years with mild to moderate AD (C3291007, AN2728‐AD‐203, NCT01652885), 13 1 phase 1b maximal‐use systemic exposure (MUSE) study in patients aged 2‐17 years with mild to moderate AD (C3291006, AN2728‐AD‐102), 14 and 1 phase 1b MUSE study in adult patients with mild to very severe psoriasis (C3291012, AN2728‐PSR‐106) (Supplemental Table S1). The timing of pre‐ and postdose blood sampling for determination of plasma crisaborole concentrations varied across studies, as did the days on which noncompartmental PK parameters were calculated (Supplemental Table S1), but in general, noncompartmental PK parameters were calculated for 2 time points (day 1 and days 7, 8, or 9) in each study.
The characteristics of the 244 patients included in the noncompartmental analysis of PK parameters are shown in Table 1.
Table 1.
Demographics and Baseline Characteristics
| N = 244 | |
|---|---|
| Age, mean (SD), y | 30.4 (14.0) |
| Male, n (%) | 139 (57) |
| Body weight, mean (SD), kg | 69.6 (21.6) |
| Ointment dose, median (range), mg | 16 500 (4800‐47 100) |
| Disease condition, n (%) | |
| Healthy | 154 (63) |
| Atopic dermatitis | 57 (23) |
| Mild (ISGA 2) | 25 (44) |
| Moderate (ISGA 3) | 32 (56) |
| Psoriasis | 33 (14) |
| Moderate (PGA 2 or 3) a | 22 (67) |
| Severe (PGA 4 or 5)b | 11 (33) |
| Race/ethnicity, n (%) | |
| White | 184 (75) |
| Black | 30 (12) |
| Latino | 17 (7) |
| Other | 13 (5) |
ISGA indicates Investigator's Static Global Assessment; PGA, Physician's Global Assessment.
A PGA score of 2 is characterized as mild but for this analysis was combined with a score of 3 and categorized as moderate. Overall, there were 3 patients with a PGA score of 2 and 19 patients with a PGA score of 3.
bA PGA score of 5 is characterized as very severe but for this analysis was combined with a score of 4 and categorized as severe. Overall, there were 9 patients with a PGA score of 4 and 2 patients with a PGA score of 5.
Pharmacokinetic Assessments and Analysis
Plasma crisaborole and 2 major oxidative metabolite concentrations were measured using a validated high‐performance liquid chromatography/tandem mass spectrometry assay. Blood samples with K2EDTA anticoagulant were collected at the times specified in the individual studies (Supplemental Table S1), processed to plasma, and acidified with phosphoric acid, 1% (v/v). The acidified plasma sample was placed in an ice bath, fortified with isotope‐labeled crisaborole‐d4 as an internal standard, and extracted by liquid‐liquid or supported liquid extraction using hexane, methyl tert‐butyl ether, and/or dichloromethane. The extract was dried under a stream of nitrogen, and the reconstituted residue was injected onto a reverse‐phase C18 high‐performance liquid chromatography column. Chromatographic separation was achieved by isocratic elution using a mixture of aqueous and methanol‐ or acetonitrile‐based mobile phases containing oxalic acid. Crisaborole and its internal standard were detected as oxalic acid adducts by tandem mass spectrometry using negative ion electrospray. The monitored ion transitions were m/z 322→250 for crisaborole and m/z 326→254 for the internal standard. The validated crisaborole concentration calibration range was 0.200 to 100 ng/mL. Interrun accuracy (percentage relative error) across the studies ranged from −12.0% to 7.0%, and interrun precision (percentage coefficient of variation) was ≤8.3%. Nonlinear regression analysis was performed using R (version 3.2.2; R Foundation, Vienna, Austria). Goodness‐of‐fit assessments and nonparametric bootstrap procedures were conducted using the library nlstools (version 1.0‐2); plotting was performed using lattice (version 0.2‐33) and ggplot2 (version 1.0.1). Patients with missing steady‐state PK parameters or ointment dose and PK parameters collected before day 5 after twice‐daily dosing were excluded from the analysis, as it was restricted to steady‐state PK parameters.
Nonlinear Regression Model Development
Nonlinear regression analysis correlating steady‐state PK parameters to ointment dose was conducted using data from each of the 6 clinical studies included in this analysis. The base model for describing the relationship between systemic exposure (AUCss or Cmax,ss) and ointment dose was defined by the following, with body weight included as covariate of slope as a multiplicative term:
where Oint dosei is the ointment dose in milligrams for the ith patient, α0 is the intercept fixed at 0 based on the expectation of no drug concentration without treatment, β0 is the slope, Wti is the baseline body weight for the ith patient, and γ is the exponent. Both estimated and fixed values based on allometric principles were evaluated for γ.
The slope parameter β0 translates to PK parameters based on PK first principles. For AUC:
where F is bioavailability and CL is clearance. Therefore,
Hence, β0 for AUC is defined as:
For Cmax, assuming a first‐order absorption:
where ka is first‐order absorption rate constant, ke is elimination rate constant, Vd is volume of distribution, t is the time to reach maximum concentration, and τ is interdose interval. Hence, β0 for Cmax is defined as:
where Tmax is the time to Cmax.
Outliers were identified by visual assessment of observed data and standardized residual plots of base models. Outliers were considered influential if the parameter estimates of slope and γ differed by >10% with versus without outliers.
Race/ethnicity, sex, disease condition (ie, healthy volunteer, AD, or psoriasis), baseline disease severity (moderate or severe, as assessed by ISGA for patients with AD or Physician's Global Assessment [PGA] for those with psoriasis), and age were added as covariates to the base model to estimate their influence on the slope parameter. Multicategory covariates, such as race/ethnicity, were converted to binary covariates (eg, white versus nonwhite) because of insufficient numbers of participants in each category. Disease severity had 2 categories in the data set for patients with AD (mild and moderate). For patients with psoriasis, the severity ranged from mild to very severe, but for the purposes of this analysis patients with PGA scores of 2 (mild) or 3 (moderate) were designated as moderate, and those with scores of 4 (severe) or 5 (very severe) were designated as severe. Covariates with high correlation/collinearity or that caused ill‐conditioning of the model were eliminated. In addition, the potential for additional nonlinearity was evaluated by estimating an exponent on ointment dose. Nonparametric bootstrap procedures were used to derive 95%CIs for final model parameters.
Model Assessment
Goodness of fit during model development was assessed based on successful convergence of the model, magnitude, and precision of parameter estimates and on visual examination of diagnostic plots. Models were compared using Akaike information criteria and analysis of variance. The predictive performance of the final model was assessed using nonparametric bootstrap methods to obtain a set of 1000‐parameter vectors and calculate the median and 95%CI for AUCss or Cmax,ss stratified by each respective clinical study with %BSA capped at 90 to account for hair‐bearing regions (eg, scalp), where the use of ointment has not been studied and is not recommended. Predictions were generated for ointment doses 1000‐55 000 mg, corresponding to a BSA of ∼350‐18 000 cm2 at an application rate of 3 mg/cm2.
Results
Participants and Samples
Among the 310 participants in the 6 included clinical studies, measured steady‐state AUCss or Cmax,ss values were available for 244 participants (AUCss, N = 239; Cmax,ss, N = 241). The mean age of participants was 30.4 years and ranged from 2.1‐70.0 years. The median ointment dose was 16 500 mg (range, 4800‐47 100 mg) (Table 1). Among the 57 (23%) participants with AD, 25 (44%) had mild and 32 (56%) had moderate disease per ISGA at baseline; of the 33 (14%) participants with psoriasis, 22 (67%) had moderate and 11 (33%) had severe disease per PGA at baseline (Table 1).
Development of Nonlinear Regression Model
Base Model
The equations for the base models describing the relationship between AUCss or Cmax,ss and ointment dose and results of base model testing and parameter estimates are shown in Table 2 and Table 3. For AUCss, body weight was included as a body size metric with a fixed exponent of −0.75 to account for changes in clearance with age. 15 Based on analysis of variance and Akaike information criteria of base models with fixed and estimated γ, with prior knowledge of accepted allometric scaling of clearance using the exponent 0.75, 15 the value of γ was fixed at −0.75 in quantifying the relationship between AUCss and ointment dose. Body weight was included in the Cmax,ss base model with an estimated exponent γ. A fixed exponent for γ was not considered in the Cmax,ss model due to a lack of precedence and the relationship between β0 and PK parameters as described in the Methods section (Nonlinear Regression Model Development). Participants identified as outliers (1 in the AUCss base model and 2 in the Cmax,ss base model) were excluded during development of the final models; their influence on parameter estimates is shown in Supplemental Table S2.
Table 2.
Testing, Selection, and Parameter Estimates for AUCss Base Model
| Model No. | Model | AIC | Comparison | P Valuea | |
|---|---|---|---|---|---|
| 1 |
|
3718.9 | ‐ | ‐ | |
| 2 |
|
3699.6 | Model 1 versus 2 | <0.001 | |
| 3 |
|
3700.2 | Model 2 versus 3 | 0.103 | |
| Selected Base Model Parameter | Estimate | Lower 95%CI | Upper 95%CI | ||
| β0 (ng·h/mL/mg) | 0.0196 | 0.0173 | 0.0219 |
AIC indicates Akaike information criteria; AUCss, area under concentration‐time curve at steady state; β0, slope; γ, exponent; Oint Dosei, ointment dose for ith participant in milligrams; Wti, body weight for ith participant in kilograms.
Table 3.
Testing, Selection, and Parameter Estimates for Cmax,ss Base Model
| Model No. | Model | AIC | Comparison | P Value | |
|---|---|---|---|---|---|
| 1 |
|
2928.9 | ‐ | ‐ | |
| 2 |
|
2919.4 | Model 1 versus 2 | <0.001 | |
| Selected Base Model Parameter | Estimate | Lower 95%CI | Upper 95%CI | ||
| β0 (ng·h/mL/mg) | 0.0025 | 0.0021 | 0.0029 | ||
| γ | −0.9885 | −1.2880 | −0.5561 |
AIC indicates Akaike information criteria; β0, slope; Cmax,ss, mean maximum concentration at steady state; γ, exponent; Oint Dosei, ointment dose for ith participant in milligrams; Wti, body weight for ith participant in kilograms.
Final Model
The final models describing the relationship between AUCss or Cmax,ss and ointment dose are described by:
and
where GE, RC, DISad, ADms, DISps, and PSOse represent parameters for sex, race/ethnicity, patients with AD, patients with moderate AD, patients with psoriasis, and patients with severe psoriasis, respectively. The AD and psoriasis severity parameters are included as nested parameters with AD and psoriasis disease parameters, respectively. POPad, POPps, and PSOSEse are dichotomized indicator variables that take a value of 1 if the relevant condition is true and zero otherwise. RACE1 is an indicator variable for race/ethnicity and takes a value of zero for white and 1 for other (black+Asian+other). SEX is an indicator variable for sex and takes a value of 1 for male and 2 for female.
The effects of covariates on slope are shown in Table 4. Disease condition had the greatest impact on slope for both AUCss and Cmax,ss (Figure 1A and Figure 2). Slope was ∼2.5‐fold higher for patients with AD or psoriasis relative to healthy participants. Hence, for every milligram increase in ointment dose, patients with AD or psoriasis had ∼2.5‐fold greater unit increase in AUCss and Cmax,ss compared with healthy participants. Disease severity had a marginal impact on slope for AUCss in patients with moderate AD (∼25% lower) and in patients with severe psoriasis (∼1.3‐fold higher) (Figure 1B). No significant impact was found for disease severity on slope for Cmax,ss in patients with AD, whereas patients with severe psoriasis had a marginally higher slope (∼1.3‐fold). Race/ethnicity and sex also had marginal effects on AUCss and Cmax,ss (∼20‐30% lower for categories of black+Latino+other and females in both models; Figure 1A and Figure 2).
Table 4.
Parameter Estimates for AUCss and Cmax,ss Final Models
| Parameter | Estimate | RSE, % | Median (95%CI) a |
|---|---|---|---|
| AUCss final model | |||
| β0 (ng·h/mL/mg) | 0.0178 | 4.26 | 0.0177 (0.0164‐0.0192) |
| γ (effect of body weight) | −0.75 (fixed) | – | – |
| GE (effect of sex, female) | 0.7536 | 6.16 | 0.7528 (0.6607‐0.8593) |
| RC (effect of race/ethnicity, other) | 0.7046 | 7.63 | 0.7057 (0.6168‐0.8234) |
| DISad (effect of disease, atopic dermatitis) | 2.5451 | 8.91 | 2.5329 (2.1369‐3.0034) |
| ADms (effect of atopic dermatitis severity, moderate) | 0.7542 | 10.5 | 0.7610 (0.6237‐0.9161) |
| DISps (effect of disease, psoriasis) | 2.5051 | 10.7 | 2.5105 (2.0758‐3.0894) |
| PSOse (effect of psoriasis disease severity, severe) | 1.2800 | 11.2 | 1.2862 (1.0516‐1.5913) |
| Cmax,ss final model | |||
| β0 (ng·h/mL/m) | 0.0022 | 4.63 | 0.0022 (0.0020‐0.0024) |
| γ (effect of body weight) | 0.4387 | 21.6 | 0.4436 (0.2544‐0.6203) |
| GE (effect of sex, female) | 0.7716 | 6.79 | 0.7742 (0.6686‐0.8870) |
| RC (effect of race/ethnicity, other) | 0.7681 | 7.42 | 0.7673 (0.6595‐0.8839) |
| DISad (effect of disease, atopic dermatitis) | 2.6459 | 9.33 | 2.6401 (2.1968‐3.1571) |
| DISps (effect of disease, psoriasis) | 2.4732 | 10.8 | 2.4801 (2.0004‐3.0592) |
| PSOse (effect of psoriasis disease severity, severe) | 1.2842 | 10.7 | 1.2838 (1.0276‐1.5993) |
AUCss indicates area under concentration‐time curve at steady state; Cmax,ss, mean maximum concentration at steady state; RSE, relative standard error.
Median and 95%CIs were obtained from a nonparametric bootstrap procedure for the final model.
Figure 1.
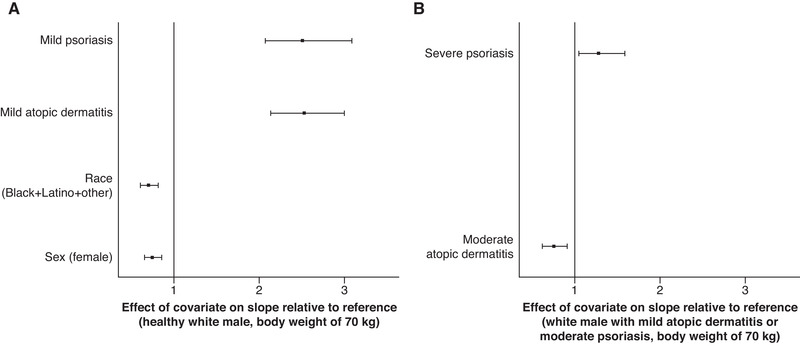
Effect of covariates on slope (β0) for AUCss final model: disease condition, race/ethnicity, and sex relative to a healthy 70‐kg white man (A) and moderate atopic dermatitis (AD) or severe psoriasis relative to a 70‐kg white man with mild AD or moderate psoriasis (B). Solid squares represent medians, and error bars represent 95%CIs obtained by a nonparametric bootstrap procedure for the final model. AUCss indicates area under concentration‐time curve at steady state.
Figure 2.
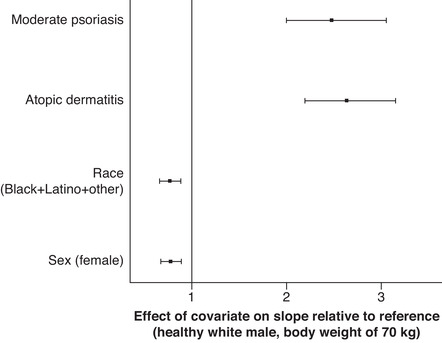
Effect of covariates on slope (β0) for Cmax,ss final model: disease condition, race/ethnicity, and sex relative to a healthy 70‐kg white man. Solid squares represent medians, and error bars represent 95%CIs obtained by nonparametric bootstrap procedure for the final model. Cmax,ss indicates mean maximum concentration at steady state.
Inclusion of an exponent for ointment dose to explain additional nonlinearity was not necessary in the final AUCss model, as the 95%CI from the nonparametric bootstrap procedure for the exponent estimate (0.7441‐1.0167) included 1. For Cmax,ss, inclusion of an exponent for ointment dose highly correlated with slope (correlation = –0.998), making it unidentifiable; therefore, it was not retained in the final model.
Model Assessment
The predictive performance of the final models was evaluated by plotting median predictions and 95%CIs overlaid with observed data from each clinical study included in the analysis. Predictive performance plots for AUCss (Figure 3) and Cmax,ss (Figure 4) for different age cohorts in the MUSE study of crisaborole in pediatric and adolescent patients (2‐5 years, 6‐11 years, and 12‐17 years) 14 demonstrated that the models adequately describe the observed data. To determine whether systemic exposure of crisaborole varied by age, final models were used to predict mean AUCss and Cmax,ss in white male patients aged 2‐20 years with moderate AD at the maximum possible ointment dose (%BSA = 90) (Supplemental Figure S1). This resulted in similar predictions for AUCss across age ranges and marginally lower Cmax,ss in younger patients compared with older patients. The observed data from studies conducted in patients with AD subcategorized by treated %BSA confirms that there is no trend of higher exposure in children as young as 2 years of age (Supplemental Figure S1).
Figure 3.
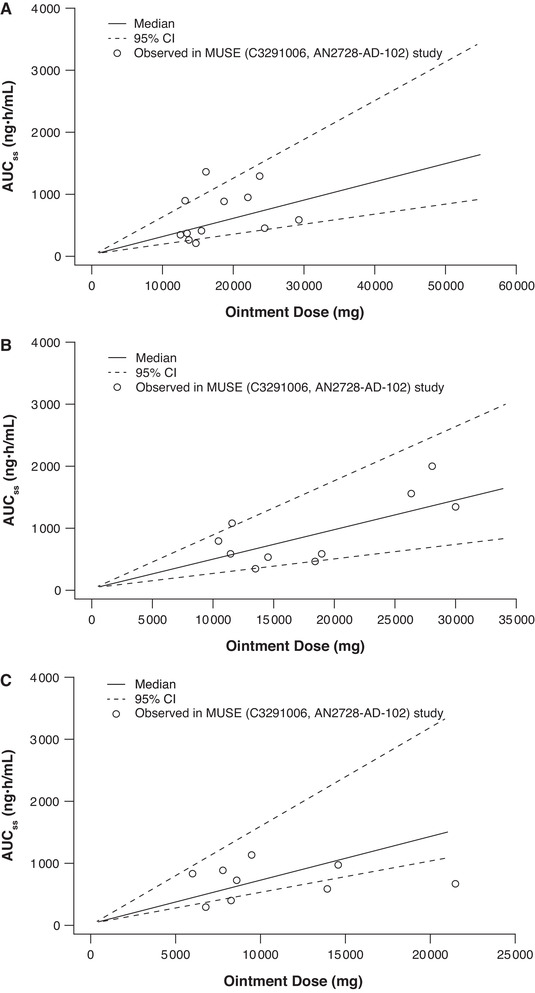
Predictive performance of AUCss final model across age cohorts in the maximal‐use systemic exposure (MUSE) study. 14 Cohort 1 (12‐17 years) (A), cohort 2 (6‐11 years) (B), and cohort 3 (2‐5 years) (C). Ointment dose was limited to the maximum possible BSA for cohorts 2 and 3 (12 901 cm 2 and 8113 cm2, respectively) due to smaller patient size. AUCss indicates area under concentration‐time curve at steady state; BSA, body surface area.
Figure 4.
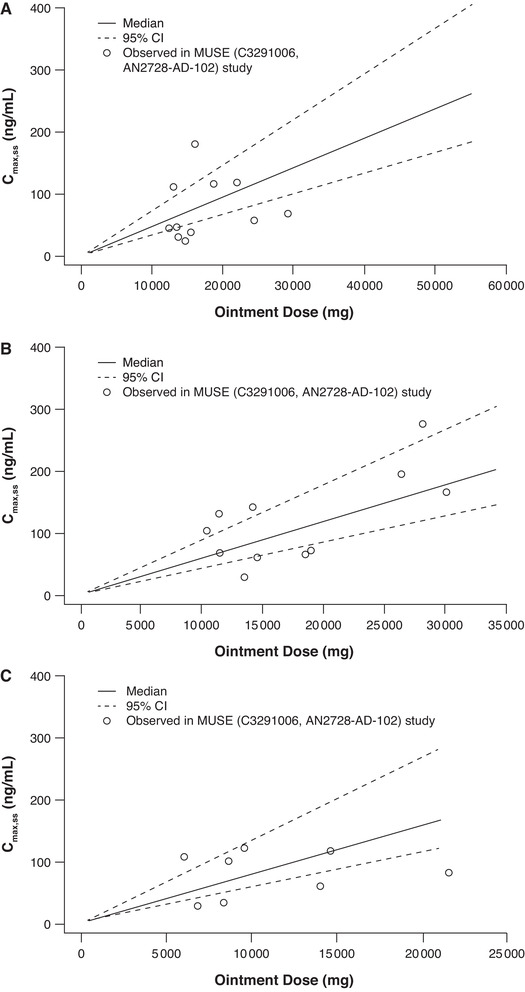
Predictive performance of Cmax,ss final model across age cohorts in the maximal‐use study. 14 Cohort 1 (12‐17 years) (A), cohort 2 (6‐11 years) (B), and cohort 3 (2‐5 years) (C). Ointment dose was limited to the maximum possible BSA for cohorts 2 and 3 (12 901 cm 2 and 8113 cm2, respectively) due to smaller patient size. BSA indicates body surface area; Cmax,ss, maximum concentration at steady state; MUSE, maximal‐use systemic exposure.
The predictive performance plots for the other clinical studies included in this analysis demonstrate that the AUCss (Supplemental Figure S2) and Cmax,ss (Supplemental Figure S3) models adequately described the data.
Discussion
Treatment with topical corticosteroids or topical calcineurin inhibitors can result in systemic exposure, the extent of which depends on factors that include patient age, treated %BSA, and the presence of skin inflammation. 16 This study demonstrates that similar factors affect the systemic exposure of crisaborole in patients with AD or psoriasis. To our knowledge, this is the first comprehensive cross‐study analysis that correlates PK exposure parameters to applied dose of a topical drug. These models can be utilized to predict systemic exposures over a range of ointment doses in patients aged ≥2 years.
This study used a nonlinear regression model, with weight as an allometric power function included as a covariate on slope. The model was developed using steady‐state PK parameters from 6 clinical studies in healthy participants and patients with AD or psoriasis, and it adequately described the observed relationship between crisaborole systemic exposure (AUCss and Cmax,ss) and ointment dose. Ointment dose of crisaborole ointment 2% in milligrams was selected as the independent variable versus crisaborole dose (ointment dose in milligrams × 0.02) for ease of future communication and to avoid the need to convert crisaborole dose back to ointment dose when providing usage instructions. Covariates, such as disease status, disease severity, sex, and race/ethnicity, were identified and included in the final model.
Disease condition had the largest impact on slope in the final crisaborole nonlinear regression models for both AUCss and Cmax,ss, corresponding to ∼2.5‐fold higher AUCss and Cmax,ss values at a given ointment dose in patients with AD or psoriasis relative to healthy participants. This is consistent with the pathology of impaired epidermal barrier function observed in both AD and psoriasis, 17 , 18 which allows for greater drug penetration to the dermis and subsequently into systemic circulation. The current analysis suggests that the epidermal barrier function of the patients in these crisaborole studies may have been compromised to a similar degree in patients with AD and psoriasis. Hence, compared with healthy participants, patients with AD or psoriasis can have lower apparent clearance due to higher bioavailability as a result of compromised skin barrier function. For patients with moderate AD the slope for AUCss was ∼25% lower than the slope in patients with mild AD, while no significant difference was identified between AD severity groups for Cmax,ss. Patients with moderate AD could have more severe lichenification compared with patients with mild AD, which may hinder the permeation of crisaborole from the applied ointment through the epidermis. For patients with psoriasis, the slope for patients with severe disease was marginally higher (∼1.3‐fold) for both AUCss and Cmax,ss, relative to patients with moderate psoriasis. The marginal differences due to disease severity in patients with AD or psoriasis are unlikely to result in clinically meaningful differences in crisaborole systemic exposure.
Both race/ethnicity and sex had a marginal impact on slope and are unlikely to result in clinically relevant differences in systemic exposure. For race/ethnicity, the categories of black (n = 30), Latino (n = 17), and other (n = 13 patients with varying race/ethnicities: American Indian [n = 2], Pacific Islander [n = 4], Asian [n = 4], Hispanic [n = 2], and Middle Eastern [n = 1]) were combined because of limited representation of each of these categories. These marginal differences observed for race/ethnicity are possibly due to slight differences in skin properties, structure, and/or physiology of white versus nonwhite patients. 19
The systemic exposure of absorbed drugs depends on clearance, which has a nonlinear relationship with body size. Because the analysis data set included patients with ages ranging from 2.1 to 70.0 years, weight (body size metric) was included as a covariate on slope as an allometric power function for both AUCss and Cmax,ss models. The inclusion of weight accounts for the difference in clearance across the age range. A commonly reported concern with topical agents in pediatric dermatology is that absorption, and thus systemic exposure, is disproportionately higher in children due to their greater ratio of BSA to body weight. 20 The final models for AUC and Cmax had similar predictions for AUCss across age ranges and marginally lower Cmax,ss in younger patients compared with older patients. These results suggest that systemic exposure to crisaborole in children as young as 2 years of age is unlikely to exceed systemic exposure in adults, even at the highest possible ointment dose. This result can be explained by the relationship of ointment dose and clearance to body size. Considering maximum possible ointment dose, the ointment dose for treating a %BSA of 90 in a 2‐year‐old patient is 14 900 mg (calculated using actual BSA [cm2] for the 50th percentile height and body weight, and ointment application rate of 3 mg/cm2), which is ∼70% lower than the ointment dose for an 18‐year‐old patient (48 900 mg). 21 Pediatric (aged ≥2 years) clearance can be calculated using the following allometric power model:
where CLpediatric is pediatric clearance and CLadult is adult clearance.
Using the above relationship, the ratio of clearance for a 2‐year‐old patient to an 18‐year‐old patient amounts to 0.29, corresponding to ∼71% lower clearance and approximating the reduction in ointment dose. Hence, for a given treated %BSA, the lower clearance of drug in a pediatric (aged ≥2 years) patient is offset by a similar‐in‐magnitude reduction in ointment dose. Theoretically, from PK‐first principles, systemic exposure is expected to be similar between adults and children as young as 2 years of age for topically applied agents at a given treated %BSA, assuming bioavailability does not change with age. Hence, the data for crisaborole provides evidence that topical bioavailability does not change across age for patients aged ≥2 years with AD.
This nonlinear regression model is limited by being based only on patients who had measurable PK parameters. Furthermore, it does not describe the entire PK profile and can only provide a mean expectation for a given set of conditions. A population PK analysis would be required to describe the relationship between dose and concentration over time, which would require more PK sampling than was available from clinical studies of crisaborole and a more complex absorption model that may include factors for time and/or skin barrier function improvement with treatment. Potential changes in skin barrier function and level of skin inflammation were not accounted for in the model given that the analysis is based on single assessments of PK parameters at steady state, and a sizable portion of the data were from healthy participants with assumed intact epidermal barrier function. Systemic exposure to topical calcineurin inhibitors decreases over time as skin inflammation resolves and barrier function improves. 22 A similar change in systemic exposure would be expected with crisaborole treatment, as it also reduces skin inflammation and improves skin barrier function as measured by transepidermal water loss. 11 However, studies with longer‐term treatment and multiple PK sample collections over time with crisaborole are not available to evaluate this potential effect.
Conclusions
The relationship between crisaborole ointment dose and systemic exposure parameters (AUCss and Cmax,ss) was adequately described using a nonlinear regression model that included body weight as an allometric function. Systemic exposure was higher in patients with AD or psoriasis compared with healthy participants and is predicted to be similar across age groups ≥2 years when treating the same %BSA. Crisaborole systemic exposures in children (aged ≥2 years) at maximum possible dose are unlikely to exceed the systemic exposures at the maximum possible dose in adults.
Conflicts of Interest
V.P., S.R., and H.T. are employees and shareholders of Pfizer Inc. W.P. was an employee of Pfizer Inc at the time of this analysis and is currently a shareholder of Pfizer Inc.
Data‐Sharing Statement
On request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
Supporting Information
Acknowledgments
Medical writing and editorial assistance under the guidance of the authors was provided by Julia Burke, PhD, Juan Sanchez‐Cortes, PhD, and Jennifer C. Jaworski, MS, at ApotheCom, San Francisco, California, and was funded by Pfizer Inc, New York, New York, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461‐464).
This analysis was funded by Pfizer Inc.
References
- 1. Li H, Zuo J, Tang W. Phosphodiesterase‐4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28(7):753‐763. [DOI] [PubMed] [Google Scholar]
- 3. Butler JM, Chan SC, Stevens S, Hanifin JM. Increased leukocyte histamine release with elevated cyclic AMP‐phosphodiesterase activity in atopic dermatitis. J Allergy Clin Immunol. 1983;71(5):490‐497. [DOI] [PubMed] [Google Scholar]
- 4. Grewe SR, Chan SC, Hanifin JM. Elevated leukocyte cyclic AMP‐phosphodiesterase in atopic disease: a possible mechanism for cyclic AMP‐agonist hyporesponsiveness. J Allergy Clin Immunol. 1982;70(6):452‐457. [DOI] [PubMed] [Google Scholar]
- 5. Otezla [package insert]. Summit, NJ: Celgene Corporation; 2019. [Google Scholar]
- 6. Wittmann M, Helliwell PS. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatol Ther (Heidelb). 2013;3(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eucrisa [package insert]. New York, NY: Pfizer Labs; 2018. [Google Scholar]
- 8. Zane LT, Chanda S, Jarnagin K, Nelson DB, Spelman L, Gold LS. Crisaborole and its potential role in treating atopic dermatitis: overview of early clinical studies. Immunotherapy. 2016;8(8):853‐866. [DOI] [PubMed] [Google Scholar]
- 9. Guttman‐Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 10. Freund YR, Akama T, Alley MR, et al. Boron‐based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012;586(19):3410‐3414. [DOI] [PubMed] [Google Scholar]
- 11. Bissonnette R, Pavel AB, Diaz A, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. J Allergy Clin Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 12. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494‐503.e496. [DOI] [PubMed] [Google Scholar]
- 13. Tom WL, Van Syoc M, Chanda S, Zane LT. Pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2% in adolescents with atopic dermatitis: an open‐label phase 2a study. Pediatr Dermatol. 2016;33(2):150‐159. [DOI] [PubMed] [Google Scholar]
- 14. Zane LT, Kircik L, Call R, et al. Crisaborole topical ointment, 2% in patients 2 to 17 years of age with atopic dermatitis: a phase 1b, open‐label, maximal‐use systemic exposure (MUSE) study. Pediatr Dermatol. 2016;33(4):380‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson BJ, Holford NH. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303‐332. [DOI] [PubMed] [Google Scholar]
- 16. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156(2):203‐221. [DOI] [PubMed] [Google Scholar]
- 17. Kobielak A, Boddupally K. Junctions and inflammation in the skin. Cell Commun Adhes. 2014;21(3):141‐147. [DOI] [PubMed] [Google Scholar]
- 18. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78(2):89‐94. [DOI] [PubMed] [Google Scholar]
- 19. Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups‐variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27(4):340‐357. [DOI] [PubMed] [Google Scholar]
- 20. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention, National Center for Health Statistics. Growth charts. September 9, 2010. https://www.cdc.gov/growthcharts/. Accessed July 31, 2018.
- 22. Meingassner JG, Aschauer H, Stuetz A, Billich A. Pimecrolimus permeates less than tacrolimus through normal, inflamed, or corticosteroid‐pretreated skin. Exp Dermatol. 2005;14(10):752‐757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


