Abstract
Hospital-acquired pneumonia (HAP) is a significant nosocomial infection; data on the distribution and antimicrobial resistance profiles of HAP in China are limited. We included 2827 adult patients with HAP from the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections network admitted in 15 Chinese teaching hospitals between 2007 and 2016. Clinical data and antimicrobial susceptibility of isolated pathogens were obtained from the medical records and central laboratory, respectively. Multivariable logistic regression was performed to determine the risk factors for mortality and multidrug resistance (MDR). A total of 386 (13.7%) patients died in the hospital, while 1181 (41.8%) developed ventilator-associated pneumonia (VAP). Active immunosuppressant therapy (OR 1.915 (95% CI 1.475–2.487)), solid tumor (OR 1.860 (95% CI 1.410–2.452)), coma (OR 1.783 (95% CI 1.364–2.333)), clinical pulmonary infection score ≥7 (OR 1.743 (95% CI 1.373–2.212)), intensive care unit stay (OR 1.652 (95% CI 1.292–2.111)), age ≥65 years (OR 1.621 (95% CI 1.282–2.049)), and tracheal cannula insertion (OR 1.613 (95% CI 1.169–2.224)) were independent risk factors for in-hospital mortality. Liver cirrhosis (OR 3.120 (95% CI 1.436–6.780)) and six other variables were independent predictors of MDR. Acinetobacter baumannii (25.6%), Pseudomonas aeruginosa (20.1%), Klebsiella pneumoniae (15.4%), and Staphylococcus aureus (12.6%) were the most common pathogens (MDR prevalence 64.9%). Isolates from VAP patients showed more A. baumannii and less K. pneumoniae and E. coli strains (p < 0.001, respectively) than those from patients without VAP. The proportion of methicillin-resistant S. aureus strains decreased; that of carbapenem-resistant A. baumannii and Enterobacterales strains increased. There had been changes in the antibiotic resistance profiles of HAP pathogens in China. Risk factors for mortality and MDR are important for the selection of antimicrobials for HAP in China.
Electronic supplementary material
The online version of this article (10.1007/s10096-020-04046-9) contains supplementary material, which is available to authorized users.
Keywords: Hospital-acquired pneumonia, Antimicrobial resistance, Multidrug resistance, Risk factor, Mortality
Introduction
Hospital-acquired pneumonia (HAP) is the second most frequent hospital-acquired infection and the main cause of mortality from nosocomial infections [1]. HAP and ventilator-associated pneumonia (VAP) are significantly related to prolonged hospital stay and increased healthcare costs [2, 3]. The distribution and antimicrobial susceptibilities of causative pathogens isolated from patients with HAP differ in each region and individual situation [4–6]. Empiric antibiotic treatments should be selected based on the local data as recommended in the latest American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines published in 2016 [5]. However, there is limited literature on the distribution and antimicrobial resistance profiles of HAP in China.
The Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) is a nationwide surveillance program established in 2007 aimed at investigating the antibiotic resistance profiles of pathogens causing hospital-acquired infections in China. The results of this program will be used as a basis for developing and implementing Chinese guidelines. Here, we aimed to report the clinical and microbiological characteristics of adults with HAP from this 10-year prospective observational study in China, and provide more information on the risk factors for mortality and multidrug-resistant (MDR) infection.
Methods
Setting and participants
The present study included patients with HAP who were aged ≥18 years from the CARES network and were admitted in 15 teaching hospitals between January 2007 and December 2016. All patients had radiographically confirmed pneumonia and appropriate clinical findings. Patients with other kind of infiltrate were excluded. HAP that occurred 48 h or more after hospital admission and VAP that occurred >48 h after endotracheal intubation were defined according to the ATS/IDSA guidelines [5]. Data were collected from each hospital’s electronic health record system and included demographic characteristics, pre-existing medical conditions, clinical presentations, antimicrobial therapy administration, and outcomes. Duplicate cases or duplicate isolates from the same patient were excluded. The present study was approved by the Research Ethics Board at Peking University People’s Hospital (Beijing, China).
Microbiological methods
HAP pathogens were isolated and identified in each participating center following the standard operating procedures. Then, all isolates were processed and antibiotic susceptibility testing were performed at the Clinical Microbiology Laboratory of Peking University People’s Hospital. The agar dilution method and broth microdilution method (tigecycline and polymyxin B) were used to measure the susceptibilities of the bacterial strains, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines [7]. The results were interpreted based on the latest CLSI breakpoints [8]. The tigecycline test was performed in accordance with the Food and Drug Administration standards. The tested antimicrobial agents included amikacin, ceftazidime, ceftazidime/clavulanic acid, cefotaxime, cefotaxime/clavulanic acid, cefepime, cefoxitin, chloramphenicol, clindamycin, erythromycin, levofloxacin, minocycline, moxifloxacin, polymyxin B, rifampicin, teicoplanin, trimethoprim–sulfamethoxazole, vancomycin (National Institute for Food and Drug Control of China, China), cefoperazone/sulbactam, linezolid, piperacillin/tazobactam, tigecycline (Pfizer, Inc., USA), ceftriaxone (Hoffmann-La Roche Ltd., Switzerland), ciprofloxacin (Bayer AG, Germany), daptomycin (Cubist Pharmaceuticals, Inc., USA), imipenem (Merck & Co., Inc., USA), and meropenem (Sumitomo Dainippon Pharma, Japan). The reference isolates Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853 were used as quality control isolates. The isolates resistant to at least three different antimicrobial classes were considered as MDR and all methicillin-resistant S. aureus (MRSA) isolates are defined as MDR [9, 10]. A patient isolated with multiple organisms was considered to have an MDR infection when one of the isolates was MDR. Isolates resistant to imipenem or meropenem were classified as carbapenem resistant.
Statistical methods
Continuous variables were expressed as median (IQR) and compared using one-way ANOVA; categorical variables were expressed as frequency counts (%) and compared using the χ2 test or Fisher’s exact test. The threshold for statistical significance was p ≤0.05, and all tests were two tailed. A stepwise conditional logistic regression analysis was performed in survivor versus non-survivor and MDR versus non-MDR to determine the independent risk factors [11]. The variables about recent antibiotic exposure and bacteria species and resistance were excluded in the logistic regression analysis of survivor versus non-survivor and MDR versus non-MDR, respectively. A univariate logistic regression analysis was performed to estimate the ORs and corresponding 95% CIs. All variables with p values ≤0.05 were included in the multivariable model. A logistic regression was carried out using IBM SPSS Statistics version 20.0 (IBM, Armonk, NY).
Results
Demographic and clinical characteristics of patients with HAP
During the 10-year study period, 2827 cases fulfilled our inclusion criteria, and their demographic, clinical, and microbiological characteristics are shown in Table 1. More patients in general wards (62.2%) were diagnosed with HAP than in intensive care units (ICUs; 37.8%). VAP accounted for 40.7% (1152/2827) of patients with HAP. Antibiotics were used within the last 30 days in 60.7% (1717/2827) of patients. The all-cause mortality rate in hospitals was 13.7% (386/2827). Patients in the ICU (20.0%) had a higher in-hospital all-cause mortality rate than those in non-ICU settings (9.8%) (p < 0.001). The in-hospital all-cause mortality rate in patients with VAP (20.7%) was significantly higher than that in patients with non-VAP (8.8%) (p < 0.001).
Table 1.
Demographic, clinical, and microbiological characteristics of 2827 HAP patients
| Variable | Survivora n = 2441(%) |
Non-survivorb n = 386 (%) |
p value |
|---|---|---|---|
| Demographics | |||
| Age | 65.0 (52.0–76.0) | 70.0 (57.0–79.0) | <0.001* |
| Male sex | 1705 (69.8) | 272 (70.5) | 0.806 |
| Smoking habit (current or former) | 663 (27.2) | 117 (30.3) | 0.198 |
| Alcohol abuse (current or former) | 415 (17.0) | 77 (19.9) | 0.156 |
| Pre-existing medical conditions | |||
| Structural lung disease | 424 (17.4) | 79 (20.5) | 0.139 |
| Congestive heart failure | 78 (3.2) | 15 (3.9) | 0.480 |
| Renal disease requiring dialysis | 25 (1.0) | 7 (1.8) | 0.173 |
| Use of an active immunosuppressant agent | 410 (16.8) | 121 (31.3) | <0.001* |
| Diabetes | 392 (16.1) | 79 (20.5) | 0.031* |
| Autoimmune disease | 100 (4.1) | 24 (6.2) | 0.059 |
| Liver cirrhosis | 32 (1.3) | 6 (1.6) | 0.700 |
| Solid tumor | 390 (16.0) | 93 (24.1) | <0.001* |
| Hematopoietic tumor | 62 (2.5) | 11 (2.8) | 0.722 |
| Coma | 435 (17.8) | 137 (35.5) | <0.001* |
| Absolute neutrophil count <500 cells/μL | 54 (2.2) | 11 (2.8) | 0.438 |
| Splenectomy | 12 (0.5) | 1 (0.3) | 0.531 |
| CPIS score | 6.0 (5.0–8.0) | 7.0 (6.0–9.0) | <0.001* |
| Infection occurred within 72 h of admission | 476 (19.5) | 78 (20.2) | 0.745 |
| Invasive procedure | |||
| Tracheal cannula | 914 (37.4) | 238 (61.7) | <0.001* |
| Time of tracheal cannula ≥7 days | 634 (26.0) | 169 (43.8) | <0.001* |
| Hospitalizations within the last 90 days | 765 (31.3) | 151 (39.1) | 0.002* |
| Infection occurred in ICU | 854 (35.0) | 214 (55.4) | <0.001* |
| Surgery within the last 30 days | 609 (24.9) | 104 (26.9) | 0.402 |
| Transferred from other hospitals | 742 (30.4) | 153 (39.6) | <0.001* |
| Bacteria species and resistance | |||
| Acinetobacter baumannii | 645 (26.4) | 104 (26.9) | 0.830 |
| Pseudomonas aeruginosa | 518 (21.2) | 68 (17.6) | 0.105 |
| Enterobacterales | 826 (33.8) | 107 (27.7) | 0.018* |
| Staphylococcus aureus | 306 (12.5) | 62 (16.1) | 0.056 |
| MDR bacteria | 1172 (48.0) | 216 (56.0) | <0.001* |
| CRAB | 420 (17.2) | 75 (19.4) | 0.286 |
| CRPA | 212 (8.7) | 27 (7.0) | 0.268 |
| CRE | 31 (1.3) | 3 (0.8) | 0.409 |
| MRSA | 219 (9.0) | 51 (13.2) | 0.008* |
| Recent antibiotic exposure (<30 days) | |||
| First- or second-generation cephalosporins | 233 (9.5) | 53 (13.7) | 0.016* |
| Third- or fourth-generation cephalosporins | 634 (26.0) | 111 (28.8) | 0.306 |
| Penicillins | 221 (9.1) | 45 (11.7) | 0.103 |
| Aminoglycosides | 146 (6.0) | 14 (3.6) | 0.063 |
| Quinolones | 458 (18.8) | 94 (24.4) | 0.010* |
| Macrolides | 96 (3.9) | 19 (4.9) | 0.361 |
| Tetracyclines | 27 (1.1) | 4 (1.0) | 0.902 |
| Carbapenems | 440 (18.0) | 95 (24.6) | 0.002* |
| Glycopeptides | 246 (10.1) | 52 (13.5) | 0.044* |
| Antibiotic combination | 488 (20.0) | 95 (3.9) | 0.037* |
HAP hospital-acquired pneumonia, CPIS clinical pulmonary infection score, ICU intensive care unit, MDR multidrug-resistant, CRAB carbapenem-resistant Acinetobacter baumannii, CRPA carbapenem-resistant Pseudomonas aeruginosa, CRE carbapenem-resistant Enterobacterales, MRSA methicillin-resistant Staphylococcus aureus
*Statistically significant (p < 0.05)
a,bData are presented as number (%) or median (IQR)
Distribution and antimicrobial resistance of bacterial isolates from patients with HAP
From 2007 to 2016, 2930 isolates were isolated from 2827 patients with HAP. A total of 101 patients (3.6%) had multiple isolates (99 patients with 2 isolates and 2 patients with 3 isolates). The pathogens were isolated from sputum (62.9%), tracheal aspirates (31.7%), bronchoalveolar lavage (1.1%), protected brush catheter (0.5%), and other samples. Among the 2930 isolates, the proportion of gram-negative isolates (2480/2930 (84.6%)) were higher than that of gram-positive ones (450/2930 (15.4%)). Acinetobacter baumannii (25.6%) and P. aeruginosa (20.1%) were the most frequent pathogens followed by Klebsiella pneumoniae (15.4%), S. aureus (12.6%), and E. coli (7.5%) (Table 2).
Table 2.
Species distributiona of pathogens from VAP and non-VAP patients of the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections network, 2007–2016
| Speciesa | VAP n = 1181(%) |
Non-VAP n = 1749 (%) |
Total n = 2930 (%) |
p value |
|---|---|---|---|---|
| Acinetobacter baumannii | 374 (31.7) | 375 (21.4) | 749 (25.6) | <0.001* |
| Pseudomonas aeruginosa | 250 (21.2) | 338 (19.3) | 588 (20.1) | 0.240 |
| Klebsiella pneumoniae | 142 (12.0) | 310 (17.7) | 452 (15.4) | <0.001* |
| Staphylococcus aureus | 156 (13.2) | 212 (12.1) | 368 (12.6) | 0.415 |
| Escherichia coli | 60 (5.1) | 160 (9.1) | 220 (7.5) | <0.001* |
| Pseudomonas maltophilia | 57 (4.8) | 77 (4.4) | 134 (4.6) | 0.654 |
| Enterobacter cloaca | 38 (3.2) | 81 (4.6) | 119 (4.1) | 0.071 |
| Serratia marcescens | 11 (0.9) | 25 (1.4) | 36 (1.2) | 0.303 |
| Burkholderia cepacia | 25 (2.1) | 10 (0.6) | 35 (1.2) | <0.001* |
| Enterobacter aerogenes | 7 (0.6) | 22 (1.3) | 29 (1.0) | 0.112 |
| Streptococcus pneumoniae | 2 (0.2) | 22 (1.3) | 24 (0.8) | 0.003 |
| Citrobacter freundii | 8 (0.7) | 13 (0.7) | 21 (0.7) | 0.987 |
| Proteus mirabilis | 7 (0.6) | 14 (0.8) | 21 (0.7) | 0.667 |
| Klebsiella oxytoca | 7 (0.6) | 12 (0.7) | 19 (0.6) | 0.941 |
| Staphylococcus haemolyticus | 2 (0.2) | 9 (0.5) | 11 (0.4) | 0.234 |
| Staphylococcus epidermidis | 1 (0.1) | 9 (0.5) | 10 (0.3) | 0.102 |
VAP ventilator-associated pneumonia
*Statistically significant (p < 0.05)
aOnly species with ≥10 isolates are listed in the table
The antibiotic resistance profiles (susceptibility rates, MIC50, MIC90, and MIC ranges) of the major bacterial pathogens are shown in Tables A1–4. Among the 2930 isolates, 1417 MDR isolates were detected in all patients; MDR isolates (766/1181 (64.9%)) were more frequent in patients with VAP than in those without VAP. The MDR rates among A. baumannii and S. aureus isolates were 74.6% and 70.9%. The MDR rates among P. aeruginosa, K. pneumoniae, and E. coli were 27.9%, 29.6%, and 44.5%, respectively. The MDR profiles of A. baumannii, K. pneumoniae, and P. aeruginosa are shown in Table A5.
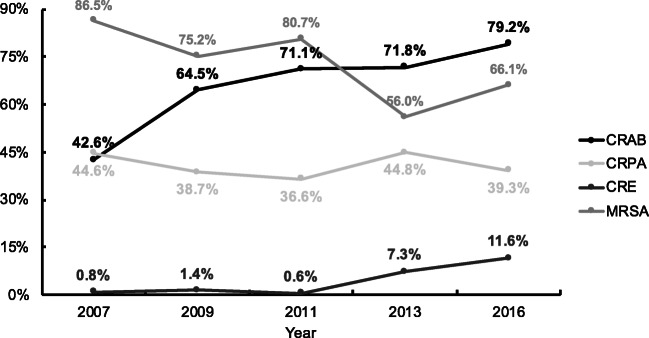
Among the isolates in patients with HAP, MRSA markedly decreased from 86.5% in 2007 to 66.1% in 2016 in our study (Fig. 1). Meanwhile, marked changes were shown: the proportions of carbapenem-resistant A. baumannii (CRAB) and carbapenem-resistant Enterobacterales (CRE) increased from 42.6% and 0.8% in 2007 to 79.2% and 11.6% in 2016, respectively. The carbapenem resistance rates in P. aeruginosa were relatively stable in the same period (36.6–44.8%). Meanwhile, significant differences were observed in the rates of MRSA, CRAB, CRE, carbapenem-resistant P. aeruginosa (CRPA), and MDR (p < 0.05, respectively).
Fig. 1.
Prevalence of CRAB, CRPA, CRE, and MRSA in HAP patients from 2007 to 2016. CRAB, carbapenem-resistant Acinetobacter baumannii; CRPA carbapenem-resistant Pseudomonas aeruginosa; CRE, carbapenem-resistant Enterobacterales; MRSA, methicillin-resistant Staphylococcus aureus
Independent risk factors associated with in-hospital mortality
Our univariate logistic regression analysis showed that 14 study variables were significantly related to in-hospital mortality (Table 3). The final multivariate logistic regression models identified seven independent risk factors associated with in-hospital mortality using stepwise variable selection (Table 3): active immunosuppressant therapy (OR 1.915 (95% CI 1.475–2.487)), solid tumor (OR 1.860 (95% CI 1.410–2.452)), coma (OR 1.783 (95% CI 1.364–2.333)), clinical pulmonary infection score (CPIS) ≥7 (OR 1.743 (95% CI 1.373–2.212)), infection occurred in ICU (OR 1.652 (95% CI 1.292–2.111)), age ≥65 years (OR 1.621 (95% CI 1.282–2.049)), and tracheal cannula insertion (OR 1.613 (95% CI 1.169–2.224)).
Table 3.
Univariate and multivariate regression analyses of the risk factors associated with in-hospital all-cause mortality for HAP patients
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age ≥65 years | 1.446 (1.162–1.800) | 0.001 | 1.621 (1.282–2.049) | <0.001 |
| Use of an active immunosuppressant agent | 2.262 (1.780–2.875) | <0.001 | 1.915 (1.475–2.487) | <0.001 |
| Diabetes | 1.345 (1.027–1.762) | 0.031 | ||
| Solid tumor | 1.669 (1.291–2.159) | <0.001 | 1.860 (1.410–2.452) | <0.001 |
| Coma | 2.537 (2.010–3.202) | <0.001 | 1.783 (1.364–2.333) | <0.001 |
| CPIS score ≥7 | 2.262 (1.807–2.832) | <0.001 | 1.743 (1.373–2.212) | <0.001 |
| Tracheal cannula | 2.687 (2.154–3.351) | <0.001 | 1.613 (1.169–2.224) | 0.004 |
| Time of tracheal cannula (≥7 days) | 2.220 (1.780–2.767) | <0.001 | ||
| Hospitalizations within the last 90 days | 1.408 (1.128–1.757) | 0.002 | ||
| Infection occurred in ICU | 2.312 (1.861–2.873) | <0.001 | 1.652 (1.292–2.111) | <0.001 |
| Transferred from other hospitals | 1.504 (1.205–1.876) | <0.001 | ||
| Enterobacterales infection | 0.075 (0.591–0.951) | 0.018 | ||
| MDR bacterium infection | 1.376 (1.108–1.708) | 0.004 | ||
| MRSA infection | 1.545 (1.115–2.139) | 0.009 | ||
HAP hospital-acquired pneumonia, CPIS clinical pulmonary infection score, ICU intensive care unit, MDR multidrug-resistant, CRAB carbapenem-resistant Acinetobacter baumannii, CRPA carbapenem-resistant Pseudomonas aeruginosa, CRE carbapenem-resistant Enterobacterales, MRSA methicillin-resistant Staphylococcus aureus
Independent risk factors associated with MDR infection
The multivariate logistic regression model analysis showed that liver cirrhosis (OR 3.120 (95% CI 1.436–6.780)), infection that occurred in the ICU (OR 1.555 (95% CI 1.304–1.854)), previous carbapenem use (OR 1.532 (95% CI 1.228–1.911)), previous glycopeptide use (OR 1.335 (95% CI 1.006–1.770)), transfer from other hospitals (OR 1.284 (95% CI 1.064–1.551)), previous treatment with third- or fourth-generation cephalosporins (OR 1.226 (95% CI 1.012–1.485)), and solid tumor (OR 0.760 (95% CI 0.614–0.941)) were significantly connected with MDR infection (Table 4).
Table 4.
Univariate and multivariable regression analysis of predictors of MDR infection among patients with HAP
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Liver cirrhosis | 2.575 (1.273–5.212) | 0.009 | 3.120 (1.436–6.780) | 0.004 |
| Solid tumor | 0.754 (0.619–0.918) | 0.005 | 0.760 (0.614–0.941) | 0.012 |
| CPIS score ≥7 | 1.195 (1.031–1.385) | 0.018 | ||
| Infection occurred within 72 h of admission | 0.784 (0.650–0.945) | 0.011 | ||
| Tracheal cannula | 1.577 (1.356–1.834) | <0.001 | ||
| Time of tracheal cannula (≥7 days) | 1.573 (1.334–1.855) | <0.001 | ||
| Hospitalizations within the last 90 days | 1.177 (1.006–1.378) | 0.042 | ||
| Infection occurred in ICU | 1.797 (1.541–2.096) | <0.001 | 1.555 (1.304–1.854) | <0.001 |
| Transferred from other hospitals | 1.313 (1.120–1.539) | 0.001 | 1.284 (1.064–1.551) | 0.009 |
| Previous treatment with third- or fourth-generation cephalosporins | 1.459 (1.230–1.731) | <0.001 | 1.226 (1.012–1.485) | 0.037 |
| Previous treatment with penicillin | 1.292 (1.003–1.665) | 0.048 | ||
| Previous treatment with aminoglycosides | 1.471 (1.065–2.032) | 0.019 | ||
| Previous treatment with quinolones | 1.435 (1.190–1.731) | <0.001 | ||
| Previous treatment with carbapenem | 1.952 (1.609–2.368) | <0.001 | 1.532 (1.228–1.911) | <0.001 |
| Previous treatment with glycopeptides | 1.833 (1.432–2.437) | <0.001 | 1.335 (1.006–1.770) | 0.045 |
| Previous treatment with a combination of antibiotics | 1.514 (1.260–1.820) | <0.001 | ||
HAP hospital-acquired pneumonia, CPIS clinical pulmonary infection score, ICU intensive care unit, MDR multidrug-resistant, CRAB carbapenem-resistant Acinetobacter baumannii, CRPA carbapenem-resistant Pseudomonas aeruginosa, CRE carbapenem-resistant Enterobacterales, MRSA methicillin-resistant Staphylococcus aureus
Discussion
This prospective observational multicenter study demonstrated clinical and microbiological characteristics of 2827 patients with HAP from 15 teaching hospitals during a 10-year period. In the present study, we demonstrated the updated distribution and antimicrobial susceptibility of the isolated pathogens and investigated the risk factors for the HAP-related mortality and harboring MDR pathogen.
The local distribution and antibiotic resistance profile of pathogens causing HAP are significant for the selection of empiric antimicrobial therapy [12]. Moreover, the resistance profiles differed among the institutions. Similar to previous studies [6, 13], gram-negative bacteria, especially non-fermentative bacteria, were the most frequent causative pathogens of HAP in the present study. A. baumannii was the most frequent pathogen and most of them is MDR. The distribution of pathogens causing HAP remained stable. Our results suggest that non-fermentative bacteria should be considered when selecting empiric antimicrobials in China. The trends of CRAB, CRPA, CRE, and MRSA from 2007 to 2016 reported in our study were consistent with the data from the China Antimicrobial Surveillance Network, which is a well-known Chinese national surveillance network for bacterial resistance [14].
The all-cause mortality rate associated with HAP was 13.7% in the present study, which is lower than that reported in previous studies conducted in China. The HAP clinical survey results of 13 large Chinese teaching hospitals showed that the average all-cause mortality rate of HAP was 22.3%, of which that of VAP was 34.5% [13]. The all-cause mortality rate of VAP in the present study was 20.7%, which was also lower than that reported in a recent study conducted in China (45%) [6]. The difference in the in-hospital mortality reported in our study and the 28-day mortality reported in other studies may explain the lower mortality rates. However, a recent 3-year prospective multicenter cohort study conducted in Japan and a recent retrospective study conducted in China showed HAP mortality rate (13.6% and 14.5%, respectively) similar with those reported in our study [15, 16].
Older age, ICU, and tracheal cannula were associated with higher mortality due to HAP, which is consistent with the finding of previous studies [15–17]. Although the CPIS has shown a low diagnostic performance in recent studies [18, 19], CPIS ≥7 was a risk factor for mortality due to HAP in our study. Patients receiving immunosuppressants, including the patients using active immunosuppressant and having solid tumors, had significantly higher mortality rate than those receiving non-immunosuppressant agents. These results are consistent with those of previous studies showing that HAP in transplant patients was common and was linked to increased mortality [20]. Unlike other previous studies, the mortality rates did not increase in patients with MDR pathogens [15, 16].
The risk factors for MDR pathogens should be evaluated in order to determine the appropriate clinical empiric therapy for suspected VAP and HAP in accordance with the 2016 ATS/IDSA guidelines [5]. The timely identification of MDR pathogens can improve the patient’s clinical outcomes and prevent the necessary administration of superfluous antimicrobial agents that may result in adverse drug effects, Clostridium difficile infections, antibiotic resistance, and extra economic costs. The risk factors for MDR pathogens detected in our study were similar to those observed in previous studies and partially overlap, including the duration of ICU stay, prior antimicrobial therapy [21], and chronic liver disease [15]. Previous studies also defined some important risk factors, including colonized with MDR [22], age [15], and chronic obstructive pulmonary disease [23]. Transferred from other hospitals was associated with MDR in our study. A possible explanation is that the patients transferred from other hospitals had a longer duration of hospitalization.
Our study has several limitations. First, our study was conducted in 15 tertiary care hospitals; hence, the results cannot be generalized to other smaller Chinese hospitals. In addition, the pathogens identified in the present study were obtained from sputa sample, accounting for 60%, which may not have been the cause of pneumonia. Third, the appropriateness of antimicrobial treatments was not evaluated in our study. Finally, as for other multicenter studies based mainly on electronic health records, some risk factors were not adequately reported as the reporting procedures were not fully assessed.
In conclusion, distribution of pathogens causing HAP in China remained stable, while their antibiotic resistance profiles gradually changed in the last decade, indicating that close monitoring of those pathogens is crucial for preventing further emergence of resistance, the development of treatment guidelines, and improving clinical therapy. The risk factors for HAP mortality and MDR pathogens will serve as a basis to appropriately manage high-risk populations in the future.
Electronic supplementary material
(DOCX 25 kb)
Acknowledgments
The authors would like to thank the participating investigators and institutions of the CARES network. The members from 16 hospitals (principal collaborators) of the CARES network are as follows: Chunxia Yang, Beijing Chao-Yang Hospital; Bin Cao, Yingmei Liu, China–Japan Friendship Hospital; Yanping Luo, Chinese PLA General Hospital; Hongli Sun, Peking Union Medical College Hospital; Hui Wang, Peking University People’s Hospital; Yongzhong Ning, Peking University Third Hospital; Wenen Liu, Xiangya Hospital of Central South University; Kang Liao, First Affiliated Hospital of Sun Yat-sen University; Chao Zhuo, Guangzhou Institute of Respiratory Disease; Rong Zhang, The Second Affiliated Hospital of Zhejiang University School of Medicine; Yan Jin, Shandong Provincial Hospital; Bijie Hu, Zhongshan Hospital of Fudan University; Yunzhuo Chu, The First Hospital of China Medical University; Zhidong Hu, Tianjin Medical University General Hospital; Ji Zeng, Affiliated Puai Hospital of Tongji Medical College, Huazhong University of Science and Technology; and Xiuli Xu, Xijing Hospital, The Fourth Military Medical University.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
H.W. conceived and designed the study. C.Z., H.L., L.J., Q.W., R.W., Y.Z., and J.Z. led the data collection and performed the susceptibility testing. Y.Y. analyzed the data and wrote the manuscript. H.W. critically reviewed and edited the manuscript. All coauthors have read, commented, and approved the final version of the article.
Funding
This study (part of the CARES program) was supported by a research grant from Pfizer Inc. The funders had no role in the study design, data collection and interpretation, or decision to submit this work for publication.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Research Ethic Board at Peking University People’s Hospital.
Consent to participate
Informed consent was not required because the medical records and patient information were anonymously reviewed and collected in this observational study. For the participating hospitals, administrative permissions to access the raw samples were granted by the Research Department of the hospital.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuyao Yin, Email: yuyaoy@bjmu.edu.cn.
Chunjiang Zhao, Email: chunkiang@163.com.
Henan Li, Email: lhnpku@163.com.
Longyang Jin, Email: jly@bjmu.edu.cn.
Qi Wang, Email: wangqi99887@sina.com.
Ruobing Wang, Email: ruobing_wang@bjmu.edu.cn.
Yawei Zhang, Email: z_yw1990@163.com.
Jiangang Zhang, Email: zjg@bjmu.edu.cn.
Hui Wang, Email: whuibj@163.com.
CARES network, Email: CARESnetwork@163.com.
CARES network:
Chunxia Yang, Bin Cao, Yingmei Liu, Yanping Luo, Hongli Sun, Hui Wang, Yongzhong Ning, Wenen Liu, Kang Liao, Chao Zhuo, Rong Zhang, Yan Jin, Bijie Hu, Yunzhuo Chu, Zhidong Hu, Ji Zeng, and Xiuli Xu
References
- 1.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 50(3). 10.1183/13993003.00582-2017 [DOI] [PubMed]
- 2.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(3):250–256. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 3.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 4.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang CI, Peck KR. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 5.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Yang Y, Huang Y, Kang Y, Xu Y, Ma X, Wang X, Liu J, Wu D, Tang Y, Qin B, Guan X, Li J, Yu K, Liu D, Yan J, Qiu H. The current epidemiological landscape of ventilator-associated pneumonia in the intensive care unit: a multicenter prospective observational study in China. Clin Infect Dis. 2018;67(suppl_2):S153–s161. doi: 10.1093/cid/ciy692. [DOI] [PubMed] [Google Scholar]
- 7.Clinical & Laboratory Standards Institute . M07 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11. Wayne: CLSI; 2018. [Google Scholar]
- 8.Clinical & Laboratory Standards Institute . M100 Performance standards for antimicrobial susceptibility testing. 27. Wayne: CLSI; 2017. [Google Scholar]
- 9.Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, Wilson APR. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y, Tu B, Xu Z, Zhang X, Bi J, Zhao M, Chen W, Shi L, Zhao P, Bao C, Qin E, Xu D. Bacterial distributions and prognosis of bloodstream infections in patients with liver cirrhosis. Sci Rep. 2017;7(1):11482. doi: 10.1038/s41598-017-11587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Loeches I, Rodriguez AH, Torres A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr Opin Crit Care. 2018;24(5):347–352. doi: 10.1097/mcc.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Chen H, Wang H, Liu W, Zhuo C, Chu Y, Zeng J, Jin Y, Hu Z, Zhang R, Cao B, Liao K, Hu B, Xu X, Luo Y, Zou M, Su D, Wang Y, Tian B, Zhou H, Liu Y, Guo P, Zhou C, Chen X, Wang Z, Zhang F. Analysis of pathogen spectrum and resistance of clinical common organisms causing bloodstream infections, hospital-acquired pneumonia and intra-abdominal infections from thirteen teaching hospitals in 2013. Zhonghua Yi Xue Za Zhi. 2015;95(22):1739–1746. [PubMed] [Google Scholar]
- 14.Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–s134. doi: 10.1093/cid/ciy657. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama T, Fujisawa T, Ishida T, Ito A, Oyamada Y, Fujimoto K, Yoshida M, Maeda H, Miyashita N, Nagai H, Imamura Y, Shime N, Suzuki S, Amishima M, Higa F, Kobayashi H, Suga S, Tsutsui K, Kohno S, Brito V, Niederman MS. A therapeutic strategy for all pneumonia patients: a 3-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2019;68(7):1080–1088. doi: 10.1093/cid/ciy631. [DOI] [PubMed] [Google Scholar]
- 16.Feng DY, Zhou YQ, Zou XL, Zhou M, Wu WB, Chen XX, Wang YH, Zhang TT. Factors influencing mortality in hospital-acquired pneumonia caused by Gram-negative bacteria in China. J Infect Public Health. 2019;12(5):630–633. doi: 10.1016/j.jiph.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Ahn JH, Lee KH, Chung JH, Shin KC, Lee CK, Kim HJ, Choi EY. Clinical characteristics and prognostic risk factors of healthcare-associated pneumonia in a Korean tertiary teaching hospital. Medicine. 2017;96(42):e8243. doi: 10.1097/md.0000000000008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51(Suppl 1):S131–S135. doi: 10.1086/653062. [DOI] [PubMed] [Google Scholar]
- 19.Zhou XY, Ben SQ, Chen HL, Ni SS. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis. 2015;30:144–147. doi: 10.1016/j.ijid.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Gudiol C, Sabé N, Carratalà J. Is hospital-acquired pneumonia different in transplant recipients? Clin Microbiol Infect. 2019;25(10):1186–1194. doi: 10.1016/j.cmi.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care. 2008;23(1):18–26. doi: 10.1016/j.jcrc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2018;7:79. doi: 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Barat L, Ferrer M, De Rosa F, Gabarrús A, Esperatti M, Terraneo S, Rinaudo M, Li Bassi G, Torres A. Intensive care unit-acquired pneumonia due to Pseudomonas aeruginosa with and without multidrug resistance. J Infect. 2017;74(2):142–152. doi: 10.1016/j.jinf.2016.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 25 kb)
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.