Abstract
The aim was to evaluate the association of molecular‐level human leukocyte antigen (HLA) mismatching with post‐transplant graft survival, rejection, and cardiac allograft vasculopathy (CAV). We retrospectively analyzed all primary cardiac transplant recipients between 01/1984‐06/2016. 1167 patients fulfilled inclusion criteria and had HLA typing information available. In 312 donor‐recipient pairs, typing at serological split antigen level was available. We used the Epitope MisMatch Algorithm to calculate the number of amino acid differences in antibody‐verified HLA eplets (amino acid mismatch load (AAMM)) between donor and recipient. Patients with a higher HLA‐DR AAMM load had inferior 1‐year graft survival (hazard ratio [HR], 1.14; 95% confidence interval [CI], 1.01–1.28). The HLA‐AB AAMM load showed no impact on graft survival. In the subgroup with available split‐level information, we observed an inferior graft survival for a higher HLA‐DR AAMM load 3 months after transplantation (HR, 1.22; 95% CI, 1.04–1.44) and a higher risk for rejection for an increasing HLA‐AB (HR, 1.70; 95% CI, 1.29–2.24) and HLA‐DR (HR, 1.32; 95% CI, 1.09–1.61) AAMM load. No impact on the development of CAV was found. Molecular‐level HLA mismatch analysis could serve as a tool for risk stratification after heart transplantation and might take us one step further into precision medicine.
Keywords: allograft rejection, antibody‐verified HLA eplets, cardiac allograft vasculopathy, graft survival, heart transplantation, precision medicine
Introduction
In everyday clinical practice, transplant physicians have to make difficult decisions, especially if complications force them to lower immunosuppression. Despite great improvements in post‐transplant surveillance, it is still not clear in which recipients an immunosuppression minimization can be safely achieved. The fundamental problem is that one cannot predict the risk that a heart transplant (HTx) recipient elicits a detrimental alloimmune response. One potential approach to tackle this problem is to consider the degree of HLA matching.
At population level, it has been shown that a greater number of HLA allele mismatches between donor and recipient is associated with an inferior outcome [1, 2, 3]. However, a disadvantage of counting the conventional HLA allele mismatches is that the immunogenicity of individual HLA mismatches differ [4, 5]. Due to its lack of precision, clinical decision‐making based on the degree of HLA allele or antigen matching is therefore practically not feasible. A novel and more accurate approach are to analyze the degree of HLA matching at the molecular‐level. The alloimmune response is directed against specific epitopes on the surface of the HLA molecules and especially epitopes that are potential antibody recognition sites seem to be crucial. Key elements of these epitopes are polymorphic amino acid residues [6]. Each structural epitope features a functional epitope, termed “eplet,” which is composed of a few amino acids which can be recognized by an antibody [7]. It is noteworthy that only a proportion of the eplets have been verified to cause antibody reactivity [8]. With the help of the Epitope MisMatch Algorithm [9, 10], it has now become possible to calculate the number of potentially mismatched amino acids in previously antibody‐verified eplets [8] between donor and recipient, thus allowing for a more precise assessment of histocompatibility.
The aim of this exploratory study is to describe associations of molecular‐level HLA mismatching with graft survival, rejection, and cardiac allograft vasculopathy (CAV) in order to answer the question whether quantifying the molecular‐level HLA mismatch can serve as a tool for post‐transplant risk stratification.
Materials and methods
Study population
Approval for this study was obtained from the Ethics Review Board of the Medical University of Vienna (EK no. 1130/2018). We retrospectively analyzed all primary cardiac transplant recipients transplanted between January 1984 and June 2016 within the Heart Transplant Program Vienna. Patients with combined heart and lung transplantation, retransplantation, or with missing HLA typing information were excluded from the analyses (Figure S1). All patients were followed up from their transplantation date until the 1st of May 2018. Patients still alive at this date were considered censored observations.
Endpoints
The primary endpoint was graft loss either due to all‐cause mortality or due to retransplantation. We analyzed the collective’s long‐term and 1‐year graft survival. The secondary clinical endpoints were the development of allograft rejection and cardiac allograft vasculopathy.
Rejection was diagnosed during follow‐up by histopathological examination of either endomyocardial biopsy samples or samples taken in autopsy or retransplantation. In the first year following heart transplantation, patients underwent a minimum of seven endomyocardial routine biopsies and further endomyocardial biopsies whenever clinically indicated. In some rare cases, treatment for rejection was initiated even though histopathological information was not available, but symptoms indicative of an acute rejection were present. Rejection grading was done at our core pathology unit according to the International Society for Heart and Lung Transplantation (ISHLT) 2004 and 2013 nomenclature [11, 12].
Cardiac allograft vasculopathy was diagnosed through coronary angiography performed routinely at 1, 3, 5, 7, and 10 years after HTx or whenever clinically indicated. Grading of CAV was according to the ISHLT 2010 nomenclature [13].
Molecular‐level HLA matching
Following René Duquesnoy’s eplet version of the HLAMatchmaker program (http://www.epitopes.net) [6, 7] a new algorithm referred to as EMMA (Epitope MisMatch Algorithm), was developed at Leiden University Medical Center, the Netherlands [9, 10].
We used the HLA EMMA version 2.01 for calculating the number of amino acid differences in antibody‐verified HLA eplets between donor and recipient. First, the available HLA typing information was entered in the program and all available alleles (including broad and split‐level typed alleles) were translated into high‐resolution alleles, based on the most common alleles in the Eurotransplant reference panel (http://www.allelefrequencies.net) [14]. Then, after assigning the respective HLA epitopes, only previously antibody‐verified HLA eplets included in the HLA Epitope Registry (http://www.epregistry.com.br) [8] were selected for further analysis. The final matching process concerned only the mismatched amino acids that are part of an antibody‐verified HLA eplet. The mismatches between donor and recipient are reflected in the number of mismatched amino acids in antibody‐verified HLA eplets (abbreviated “AAMM” in the following). Comparisons for HLA class I alleles (HLA‐A and HLA‐B) were performed by interlocus subtraction [15], because of shared eplets between both loci. For this reason the number of HLA‐A and HLA‐B AAMM are analyzed together.
Statistical analyses
Patient characteristics are given in absolute numbers (percentages) for categorical variables and in medians (quartiles) in case of continuous variables. The Kaplan–Meier method was used to estimate 1‐year and long‐term graft survival probabilities, and the log‐rank test was calculated for group comparisons. Competing risk analyses were performed to estimate the cumulative incidence of allograft rejection and cardiac allograft vasculopathy, respectively, accounting for death as a competing event. The Gray test was then applied for group comparisons. To evaluate the potential influence of the number of AAMM on primary and secondary endpoints, univariate, and multivariable Cox proportional hazards regression models were applied. Covariates that have been shown in the ISHLT registry [16] to be of relevance for the respective endpoints were included in the multivariable models and are listed below each table. 1‐year graft survival was adjusted for transplant era (1984–1999 vs 2000–2008, 1984–1999 vs 2009–2012, 1984–1999 vs 2013–2016), primary graft dysfunction, IMPACT score [17], donor age, preoperative admission to intensive care unit versus non‐ICU, previous cardiac surgery. The IMPACT score includes the following preoperative recipient variables: age, bilirubin, creatinine clearance, sex, heart failure etiology, infection, intra‐aortic balloon pump, mechanical ventilation, ethnicity, temporary circulatory support and ventricular assist device. Long‐term graft survival was adjusted for transplant era, recipient age, diabetes mellitus, serum creatinine, preoperative admission to intensive care unit, heart failure etiology, and donor‐to‐recipient sex mismatch. Rejection was adjusted for transplant era, recipient sex, recipient age, and immunosuppression regimen (Cyclosporin A vs Tacrolimus). CAV was adjusted for transplant era, donor age, recipient sex, previous cardiac surgery, and recipient age. All models were calculated separately for the combined loci of HLA‐AB and of HLA‐DR. The number of AAMM was considered both as a continuous factor (log2‐transformed), and as a binary factor, dichotomized according to the median number in the total study population (“All patients”; median HLA‐AB AAMM number = 8; median HLA‐DR AAMM number = 5). The strength of the effects on the outcomes is described by hazard ratios (HR) with 95% confidence intervals (CI). In case of the continuous log2‐transformed factors, the hazard ratios refer to a doubling of the HLA AAMM. With respect to the primary outcome,that is, long‐term graft survival, potential time‐varying effects were tested within the Cox regression models. In case of statistical significance, the time‐dependent effect is presented by estimated hazard ratios at selected time points. Two‐sided P‐values < 0.05 were defined as statistically significant. The software SAS (SAS Institute Inc. (2012), Cary, NC, USA) was used for all statistical analyses.
Results
Study population
The study population included 1167 patients with HLA typing information for donors and recipients (denoted as All Patients). Of those patients, a subgroup of 312 donor‐recipient pairs had HLA typing information available at serological split antigen level for HLA‐A, ‐B, and the ‐DR locus (denoted as Split‐level Typed Patients). This subgroup was also analyzed separately. The main recipient and donor characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Variables | All patients (n = 1167) | Split–level typed patients (n = 312) |
|---|---|---|
| Recipient characteristics | ||
| Age at HTx, years, median (IQR) | 53.8 (45.7–60.2) | 56 (45.2–62.3) |
| Male sex | 949 (81.3) | 249 (79.8) |
| Weight, kg, median (IQR) | 75 (66–83) | 77 (67–85) |
| Height, cm, median (IQR) | 174 (168–179) | 174 (168–180) |
| Heart failure etiology | ||
| Dilative cardiomyopathy | 712 (61) | 188 (60.3) |
| Ischemic cardiomyopathy | 373 (32) | 90 (28.8) |
| Congenital heart disease | 25 (2.1) | 15 (4.8) |
| Other | 57 (4.9) | 19 (6.1) |
| Previous cardiac surgery | 447 (38.4) | 130 (41.9) |
| Diabetes mellitus | 231 (19.9) | 58 (18.7) |
| Serum bilirubin, mg/dl, median (IQR) | 0.9 (0.6–1.6) | 0.9 (0.6–1.4) |
| Serum creatinine, mg/dl, median (IQR) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) |
| Renal replacement therapy | 37 (3.2) | 12 (3.9) |
| Pre–transplant admission status | ||
| At home | 698 (60) | 194 (62.2) |
| Hospitalized | 295 (25.4) | 72 (23.1) |
| ICU | 170 (14.6) | 46 (14.7) |
| VAD support | 150 (12.9) | 62 (19.9) |
| ECMO support | 20 (1.7) | 8 (2.6) |
| IABP support | 2 (0.2) | 0 (0) |
| Mechanical ventilation | 26 (2.2) | 7 (2.2) |
| Infection | 32 (2.8) | 20 (3.2) |
| IMPACT–Score26, median (IQR) | 4 (2–6) | 4.5 (2–7) |
| Donor characteristics | ||
| Age, years, median (IQR) | 35 (24–45) | 38.5 (27–48) |
| Weight, kg, median (IQR) | 75 (67–85) | 77 (70–85) |
| Height, cm, median (IQR) | 175 (170–180) | 176 (170–182) |
| Male sex | 819 (70.2) | 215 (68.9) |
| Cause of death: Trauma | 578 (50) | 139 (45) |
| HTx era | ||
| 1984–1999 | 623 (53.4) | 80 (25.6) |
| 2000–2008 | 265 (22.7) | 81 (26) |
| 2009–2012 | 126 (10.8) | 63 (20.2) |
| 2013–2016 | 153 (13.1) | 88 (28.2) |
| Combined heart‐kidney‐Tx | 16 (1.4) | 6 (1.9) |
| Primary graft dysfunction | 157 (13.5) | 51 (16.4) |
| Immunosuppressive regimen | ||
| Cyclosporin A | 933 (79.9) | 198 (64.3) |
| Tacrolimus | 226 (19.4) | 110 (35.7) |
| Number of amino acid mismatches for HLA‐AB, median (IQR) | 8 (4–12) | 8 (5–13) |
| Number of amino acid mismatches for HLA‐DR, median (IQR) | 5 (2.25–7) | 4 (2–7) |
| Follow–up, years, median (IQR) | 8 (3–15) | 5.8 (2.8–11.7) |
Values are n (%) unless indicated otherwise. ECMO, extracorporeal membrane oxygenation; HLA, human leukocyte antigen; HTx, heart transplantation; IABP, intra‐aortic balloon pump; ICU, intensive care unit; IQR, inter‐quartile range; Tx, transplantation; VAD, ventricular assist device.
Results of the binary analyses are provided in Tables S2‐S5.
Graft survival
The endpoint graft loss was reached by a total of 697 patients (59.7% of All patients) in the long‐term graft survival analysis. In the first year after transplantation, 188 (16.1%) events occurred. The endpoint graft loss was reached by 128 Split‐level Typed Patients (41.0%) during long‐term follow‐up and by 49 (15.7%) in the first year after transplantation.
A higher HLA‐AB AAMM load showed no impact on 1‐year and long‐term graft survival, neither in the Kaplan–Meier estimates (Fig. 1a/c and Fig. 2a/c) nor in the univariate or multivariable Cox regression analyses (Tables 2 and 3).
Figure 1.
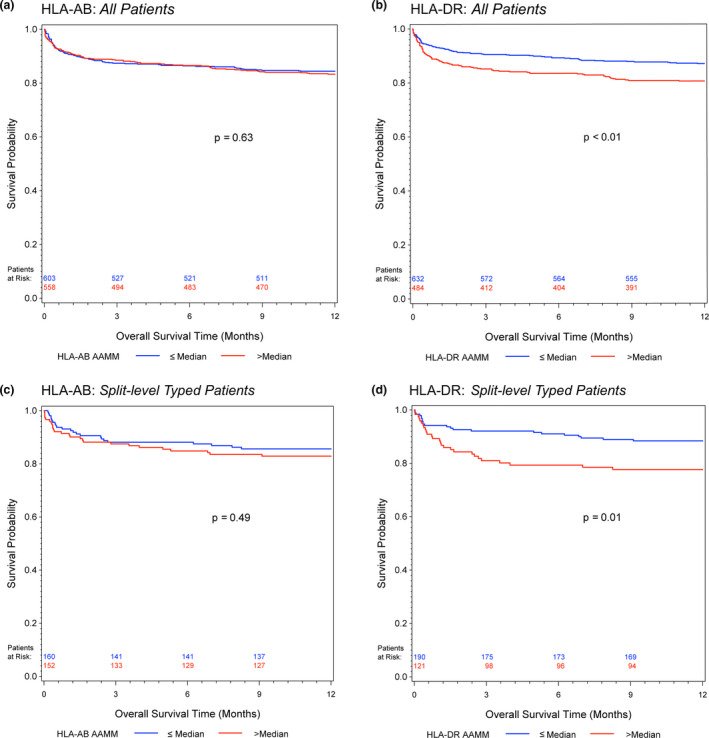
Kaplan–Meier estimates of 1‐year graft survival for (a) HLA‐AB and (b) HLA‐DR AAMM in all patients; (c) HLA‐AB and (d) HLA‐DR AAMM in split‐level typed patients. Log‐rank test was calculated for group comparisons
Figure 2.
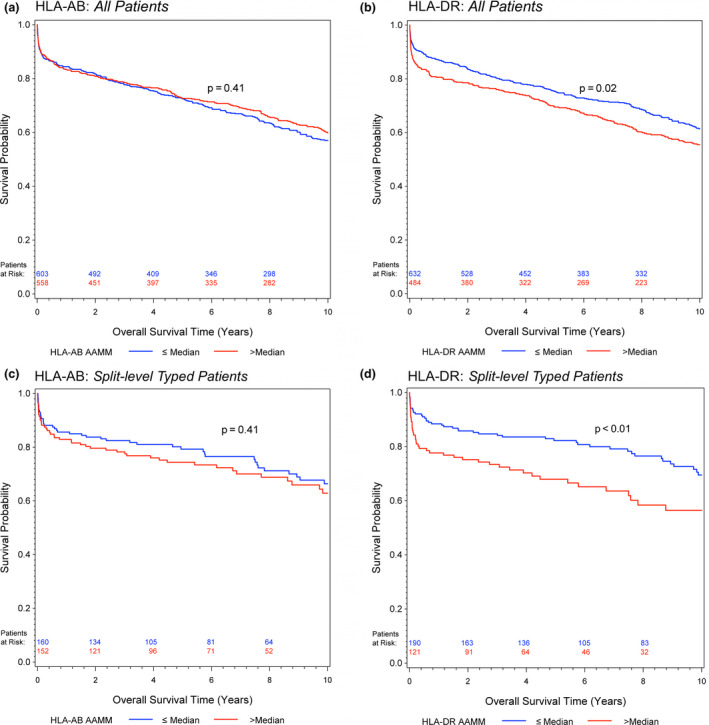
Kaplan–Meier estimates of long‐term (up to 10 years) graft survival for (a) HLA‐AB and (b) HLA‐DR AAMM in all patients; (c) HLA‐AB and (d) HLA‐DR AAMM in split‐level typed patients. Log‐rank test was calculated for group comparisons
Table 2.
One‐year graft survival. Univariate and multivariable Cox proportional hazards regression models
| AAMM | Univariate | Multivariable†, ‡ | ||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | P‐value | HR | CI (95%) | P‐value | |
| HLA–AB – all patients | ||||||
| Continuous* | 1.03 | 0.91–1.17 | 0.59 | 1.04 | 0.92–1.18 | 0.55 |
| HLA–AB – split–level typed patients | ||||||
| Continuous* | 1.07 | 0.84–1.36 | 0.57 | 1.07 | 0.86–1.34 | 0.53 |
| HLA–DR – all patients | ||||||
| Continuous* | 1.14 | 1.01–1.28 | 0.03 | 1.11 | 0.99–1.26 | 0.08 |
| HLA–DR – split–level typed patients | ||||||
| Continuous* | 1.22 | 0.99–1.51 | 0.06 | 1.19 | 0.97–1.47 | 0.10 |
Bold values are P‐values considered statistically significant, with a value P < 0.05.
log2‐transformed; HRs refer to a doubling of the HLA AAMM number.
All Patients adjusted for: transplant era (1984–1999 vs 2000–2008, 1984–1999 vs 2009–2012, 1984–1999 vs 2013–2016), primary graft dysfunction, IMPACT score26, donor age, preoperative admission to intensive care unit vs non‐ICU, previous cardiac surgery.
Split‐level Typed Patients adjusted for: transplant era, primary graft dysfunction.
Table 3.
Long‐term graft survival. Univariate and multivariable Cox proportional hazards regression models
| AAMM | Univariate | Multivariable† | ||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | P‐value | HR | CI (95%) | P‐value | |
| HLA–AB – all patients | ||||||
| Continuous* | 1.00 | 0.94–1.07 | 0.93 | 1.00 | 0.94–1.07 | 0.93 |
| HLA–AB – split–level typed patients | ||||||
| Continuous* | 1.13 | 0.97–1.32 | 0.11 | 1.11 | 0.95–1.29 | 0.18 |
| HLA–DR – all patients | ||||||
| Continuous* | ||||||
| 3 months | 1.08 | 1.00–1.17 | 0.07 | 1.10 | 1.01–1.20 | 0.047 |
| 6 months | 1.06 | 0.99–1.14 | 1.08 | 1.00–1.16 | ||
| 12 months | 1.04 | 0.98–1.11 | 1.05 | 0.99–1.13 | ||
| 120 months | 0.97 | 0.91–1.04 | 0.98 | 0.91–1.05 | ||
| HLA–DR – split–level typed patients | ||||||
| Continuous* | ||||||
| 3 months | 1.22 | 1.04–1.44 | 0.04 | 1.26 | 1.05–1.50 | 0.03 |
| 6 months | 1.18 | 1.02–1.36 | 1.20 | 1.03–1.40 | ||
| 12 months | 1.13 | 0.99–1.29 | 1.15 | 0.99–1.32 | ||
| 120 months | 1.00 | 0.85–1.17 | 0.98 | 0.82–1.17 | ||
Bold values are P‐values considered statistically significant, with a value P < 0.05.
log2‐transformed; HRs refer to a doubling of the HLA AAMM number.
Adjusted for: transplant era (1984–1999 vs 2000–2008, 1984–1999 vs 2009–2012, 1984–1999 vs 2013–2016), recipient age, diabetes mellitus, serum creatinine, preoperative admission to intensive care unit, heart failure etiology, donor‐to‐recipient sex mismatch.
On the other hand, patients with a higher HLA‐DR AAMM load had worsened graft survival (Fig. 1b/d and Fig. 2b/d). The long‐term graft survival analyses showed a time‐dependent effect of the HLA‐DR AAMM load, with the biggest impact in the early period (All Patients, 3 months after transplantation, univariate analysis: HR, 1.08; 95% CI, 1.00–1.17) and most evidently in Split‐level Typed Patients (3 months, univariate HR, 1.22; 95% CI, 1.04–1.44) (Table 3).
Rejection
In 269 (23.1%) of All Patients, a rejection episode was observed. Of all Split‐level Typed Patients 61 (19.6%) had a rejection episode.
Competing risk analyses of All Patients showed a slightly higher cumulative incidence of rejection in transplants with an HLA‐AB and HLA‐DR AAMM load above the respective medians (Fig. 3a/b). In Split‐level Typed Patients, this difference was more pronounced (Fig. 3c/d).
Figure 3.
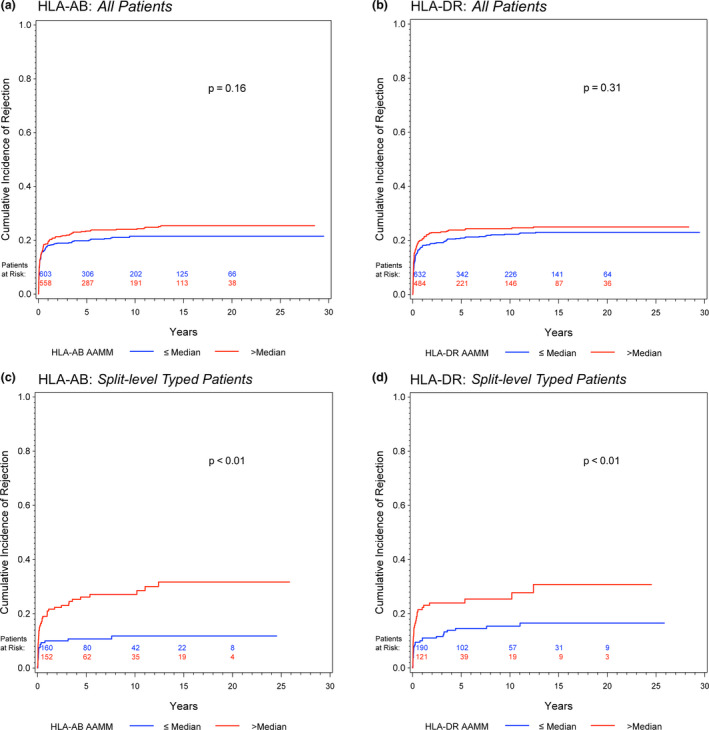
Competing risk analyses to estimate the cumulative incidence of allograft rejection accounting for death as a competing event for (a) HLA‐AB and (b) HLA‐DR AAMM in all patients; (c) HLA‐AB and (d) HLA‐DR AAMM in split‐level typed patients. Gray test was calculated for group comparisons
Evaluating the HLA AAMM number as a continuous risk factor, univariate, and multivariable Cox regression analyses showed a higher risk of rejection in All Patients with a higher HLA‐DR AAMM load (univariate analysis: HR, 1.10; 95% CI, 1.00–1.21). Once more, the adverse impact of a higher HLA‐AB and HLA‐DR AAMM load was most evident in Split‐level Typed Patients, shown in univariate (HR, 1.70; 95% CI, 1.29–2.24; HR, 1.32, 95% CI, 1.09–1.61; respectively) as well as in multivariable regression analyses (Table 4).
Table 4.
Rejection. Univariate and multivariable Cox proportional hazards regression models
| AAMM | Univariate | Multivariable†, ‡ | ||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | P‐value | HR | CI (95%) | P‐value | |
| HLA–AB – all patients | ||||||
| Continuous* | 1.08 | 0.97–1.20 | 0.14 | 1.08 | 0.97–1.20 | 0.15 |
| HLA–AB – split–level typed patients | ||||||
| Continuous* | 1.70 | 1.29–2.24 | <0.01 | 1.63 | 1.23–2.14 | <0.01 |
| HLA–DR – all patients | ||||||
| Continuous* | 1.10 | 1.00–1.21 | 0.045 | 1.10 | 1.00–1.21 | 0.045 |
| HLA–DR – split–level typed patients | ||||||
| Continuous* | 1.32 | 1.09–1.61 | 0.01 | 1.37 | 1.12–1.67 | <0.01 |
Bold values are P‐values considered statistically significant, with a value P < 0.05.
log2‐transformed; HRs refer to a doubling of the HLA AAMM number.
All Patients adjusted for: transplant era (1984–1999 vs 2000–2008, 1984–1999 vs 2009–2012, 1984–1999 vs 2013–2016), recipient sex, recipient age at transplant, immunosuppression regimen (Cyclosporin A vs Tacrolimus).
Split‐level Typed Patients adjusted for: transplant era, immunosuppression regimen (Cyclosporin A vs Tacrolimus).
Cardiac allograft vasculopathy
283 (24.3%) of All Patients developed cardiac allograft vasculopathy, and 82 (26.3%) of the Split‐level Typed Patients.
Neither in the cumulative incidence functions (Figure S2) nor in univariate analysis or after multivariable adjustment were we able to see an impact of the HLA‐AB or HLA‐DR AAMM load on the development of CAV (Table S1).
Discussion
The results of our exploratory study provide evidence for the usefulness of quantifying the degree of the molecular‐level HLA mismatch as a tool for post‐transplant risk stratification. We demonstrate that in heart transplant recipients a higher level of mismatched amino acids in antibody‐verified HLA eplets of the donor is independently associated with rejection (at HLA‐AB and HLA‐DR) and with worsened graft survival (at HLA‐DR).
It has previously been shown that a higher HLA allele mismatch adversely affects survival after heart transplantation [1, 3, 18]. Since then, HLA matching has evolved, due to new technologies and better understanding. More recently, the concept of HLA epitope matching has emerged, initially introduced to search for compatible donors for highly sensitized kidney recipients [6]. Instead of looking at the HLA allele as a whole, the principle behind epitope matching is that potential targets of antibody reactivity on the antigen’s surface are matched. This is a more precise way of matching, enabling discrimination of very immunogenic and less immunogenic HLA allele mismatches on the basis of the number of epitopes present on the donor HLA, which are not shared by the HLA antigens of the recipient. Each epitope features a centrally located area, the functional epitope, also termed “eplet,” composed by a few amino acids that can be potentially recognized by an antibody [7].
A number of studies have examined the clinical impact of HLA eplet mismatching. The majority has been performed in the field of renal transplantation. Sapir‐Pichhadze et al. [19] identified an increased class II eplet mismatch load as an independent risk factor for transplant glomerulopathy. Wiebe and colleagues have shown that an increased class II (separately for HLA‐DR and HLA‐DQ) eplet mismatch is an independent risk factor for class II de novo Donor Specific Antibody (DSA) formation [20]. The group proceeded with a prospective trial where they showed that poor medication adherence acted synergistically with a higher eplet mismatch load (of HLA‐DR and HLA‐DQ) on the development of rejection and incidence of graft loss [21]. After taking a closer look at their recipients’ tacrolimus trough levels, they concluded that recipients with a higher HLA eplet mismatch score were less likely to tolerate low tacrolimus trough levels without developing de novo DSAs [22]. In lung transplant recipients, Walton et al. [23] have found that class II eplet mismatching (in contrast to class I) is predictive for the risk of chronic lung allograft dysfunction.
In cardiac transplantation these types of analyses have rarely been done. There has been only one study in heart transplantation, specifically pediatric heart transplantation, by Sullivan and colleagues [24]. They investigated a large cohort in the Scientific Registry of Transplant Recipients database and showed an increase in long‐term graft loss for recipients with a higher number of HLA class I eplet mismatches (HLA‐AB). In this study, an impact of class II mismatches (HLA‐DR) was not observed. Rejection events and incidence of CAV were not documented in the database. Interestingly, in our cohort, the class I effect that we were able to see was limited to rejection events, but we were not able to see an effect on graft survival. Instead, we saw an effect of HLA‐DR on rejection and on graft survival. Based on their results, Sullivan et al. hypothesize that class I might play a more relevant role in pediatric heart transplantation than class II. The lack of an observed class II effect could also have been due to the fact that typing information was mostly available at serological level.
The obtained results of the above studies strongly underscore the clinical association of HLA eplet mismatching with post‐transplant alloimmunity. However, it has been pointed out as a major limitation in these studies that the approach of matching all known HLA eplets considers not only antibody‐verified eplets but also eplets that are only considered theoretical eplets [25]. We tried to overcome this possible distortion by focusing only on eplets that have been previously described as antibody‐verified [8].
Our study is the first to investigate the association of molecular‐level HLA mismatching with the outcomes of adult heart transplant recipients and the first in cardiac transplantation to investigate amino acid mismatches. Our results show that an increased HLA‐DR AAMM load is an independent risk factor for graft loss. This risk is highest in the first 6 months after transplantation, which is reflected in time‐dependent and increased hazard ratios in adjusted and unadjusted Cox proportional hazards regression models and depicted in the Kaplan–Meier estimates. Concerning risk assessment for rejection, HLA‐AB and HLA‐DR AAMM loads are independent risk factors if increased. The biggest effect was observed in the first year after transplantation.
Instead of trying to search for a cutoff value, we chose to divide our patient cohort at the respective AAMM medians. One must bear in mind when using mismatch loads that it is not a simple “numbers game”, because immune responses can be very different for the same quantity of mismatches [26]. Some polymorphic sites have the potential to cause severe reactions and others not. To address this key issue, a group effort is currently underway in order to grade the varying immunogenicity of individual epitopes [9].
Prospective HLA matching in heart transplantation, unlike in kidney transplantation, has not been feasible so far due to the scarcity of donor organs, shorter tolerable ischemic times and clinical needs of end‐stage heart failure patients. As long as these factors remain as they are, we do not believe that there will be much potential for prospective molecular‐level HLA matching in cardiac transplantation. At present, the main area of potential application is post‐transplant risk stratification. A patient after heart transplantation could be retrospectively matched with the donor on a molecular‐level in order to help stratify the patient’s personal risk of suffering an alloimmune response. The obtained mismatch result could inform the clinician about a potentially increased risk of graft loss and occurrence of any rejection episode, which could be counteracted through increased patient surveillance and tailored immunosuppressive therapy. We could imagine that immunosuppression tailoring could be performed after creating a certain “immunological risk profile” by adding this result to other already established parameters, so that clinicians would gain a more comprehensive view of the patient’s alloimmune risk. Future prospective clinical trials in heart transplantation should implement molecular‐level matching information in order to confirm its clinical usefulness.
Limitations
All reported patients were transplanted at our institution in central Europe, which is part of Eurotransplant. There is very little ethnic diversity and a predominantly Caucasian population. Our results may therefore not be directly applicable to ethnically different or more diverse patient populations. We did not observe an impact of a higher HLA‐DR AAMM load on the development of CAV, even though it has been reported that a higher number of HLA‐DR allele mismatches increased the risk of developing CAV after 8 years [27]. It has to be taken into account that CAV is a multifactorial disease and its development is also driven by several factors, such as age, sex, recipient BMI, ischemic cardiomyopathy prior to transplant, etc [28]. Our study included patients transplanted before HLA typing through DNA techniques was readily available, and additionally, there were some discrepancies in typing quality especially between different donor‐tissue‐typing laboratories. Therefore, we decided to focus in our results on the loci with higher typing quality, namely HLA‐A, HLA‐B, and HLA‐DR. The adverse impact of a higher mismatch load was most evident in donor‐recipient pairs in which HLA typing had been performed at split antigen level. A major limitation of our study was the fact that multivariable analysis of 1‐year graft survival of split‐level typed patients could only be corrected for transplant era and primary graft dysfunction. The model could not be corrected for all covariates, due to the low number of events in the first year after transplantation, which precludes a full bias correction. The higher the resolution of the obtained typing information, the more accurate is the assignment of the respective amino acid sequences. We, therefore, recommend performing high‐resolution HLA typing through molecular methods if further epitope matching is to be carried out.
Conclusions
A higher level of mismatched amino acids between antibody‐verified HLA eplets of donor and recipient is independently associated with rejection (at combined loci HLA‐AB and at locus HLA‐DR) and with worsened graft survival (at locus HLA‐DR). Molecular‐level HLA mismatch analysis could therefore serve as a tool for risk stratification after heart transplantation and might take us one step further into precision medicine.
Authorship
EO‐J, AZ, and AZA‐Z: participated in research design, writing of the paper, performance of the research, and data analysis. GWH and FHJC: participated in research design, writing of the paper, contributed analytic tools, and participated in performance of the research and data analysis. AK: participated in research design, writing of the paper and data analysis. A‐KS, TH, JG, PA, and RM: participated in performance of the research and data analysis. GFF: contributed analytic tools and participated in performance of the research. GL: participated in research design and data analysis.
Funding
There was no funding for this work.
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Figure S1 Patient collective.
Figure S2 Cumulative incidence of cardiac allograft vasculopathy.
Table S1 Cardiac allograft vasculopathy.
Table S2 Cardiac allograft vasculopathy – Binary analyses.
Table S3 1‐year graft survival – Binary analyses.
Table S4 Long‐term graft survival – Binary analyses.
Table S5 Rejection – Binary analyses.
Acknowledgements
None.
References
- 1. Hosenpud JD, Edwards EB, Lin H‐M, Daily OP. Influence of HLA matching on thoracic transplant outcomes. Circulation 1996; 94: 170. [DOI] [PubMed] [Google Scholar]
- 2. Lund LH, Khush KK, Cherikh WS, et al The registry of the international society for heart and lung transplantation: thirty‐fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017; 36: 1037. [DOI] [PubMed] [Google Scholar]
- 3. Opelz G, Wujciak T. The influence of HLA compatibility on graft survival after heart transplantation. The Collaborative Transplant Study. N Engl J Med 1994; 330: 816. [DOI] [PubMed] [Google Scholar]
- 4. Claas FHJ, Dankers MK, Oudshoorn M, et al Differential immunogenicity of HLA mismatches in clinical transplantation. Transpl Immunol 2005; 14: 187. [DOI] [PubMed] [Google Scholar]
- 5. Lucas DP, Leffell MS, Zachary AA. Differences in immunogenicity of HLA antigens and the impact of cross‐reactivity on the humoral response. Transplantation 2015; 99: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duquesnoy RJ. HLAMMATCHMAKER: a molecularly based donor selection algorithm for highly alloimmunized patients. Transplant Proc 2001; 33: 493. [DOI] [PubMed] [Google Scholar]
- 7. Duquesnoy RJ. A structurally based approach to determine HLA compatibility at the humoral immune level. Hum Immunol 2006; 67: 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duquesnoy RJ, Marrari M, Sousa LCD, et al 16th IHIW: a website for antibody‐defined HLA epitope Registry. Int J Immunogenet 2013; 40: 54. [DOI] [PubMed] [Google Scholar]
- 9. Kramer C, Heidt S, Claas FHJ. Towards the identification of the relative immunogenicity of individual HLA antibody epitopes. Hum Immunol 2019; 80: 218. [DOI] [PubMed] [Google Scholar]
- 10. Kramer CS, Koster J, Haasnoot GW, Roelen DL, Claas FH, Heidt S. A novel tool to define the immunogenicity of HLA mismatches. Transplantation 2018; 102: S157. [Google Scholar]
- 11. Berry GJ, Burke MM, Andersen C, et al The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody‐mediated rejection in heart transplantation. J Heart Lung Transplant 2013; 32: 1147. [DOI] [PubMed] [Google Scholar]
- 12. Stewart S, Winters GL, Fishbein MC, et al Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24: 1710. [DOI] [PubMed] [Google Scholar]
- 13. Mehra MR, Crespo‐Leiro MG, Dipchand A, et al International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy‐2010. J Heart Lung Transplant 2010; 29: 717. [DOI] [PubMed] [Google Scholar]
- 14. González‐Galarza Faviel F, Takeshita Louise YC, Santos Eduardo JM, et al Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res 2015; 43: D784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duquesnoy RJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. I. Description of the algorithm. Hum Immunol. 2002; 63: 339. [DOI] [PubMed] [Google Scholar]
- 16. Khush KK, Cherikh WS, Chambers DC, et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 2019; 38: 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss ES, Allen JG, Arnaoutakis GJ, et al Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011; 92: 914. [DOI] [PubMed] [Google Scholar]
- 18. Frist WH, Oyer PE, Baldwin JC, Stinson EB, Shumway NE. HLA Compatibility and Cardiac Transplant Recipient Survival. Ann Thorac Surg 1987; 44: 242. [DOI] [PubMed] [Google Scholar]
- 19. Sapir‐Pichhadze R, Tinckam K, Quach K, et al HLA‐DR and ‐DQ eplet mismatches and transplant glomerulopathy: a nested case‐control study. Am J Transplant 2015; 15: 137. [DOI] [PubMed] [Google Scholar]
- 20. Wiebe C, Pochinco D, Blydt‐Hansen TD, et al Class II HLA epitope matching‐A strategy to minimize de novo donor‐specific antibody development and improve outcomes. Am J Transplant 2013; 13: 3114. [DOI] [PubMed] [Google Scholar]
- 21. Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The synergistic effect of class II HLA epitope‐mismatch and nonadherence on acute rejection and graft survival. Am J Transplant 2015; 15: 2197. [DOI] [PubMed] [Google Scholar]
- 22. Wiebe C, Rush DN, Nevins TE, et al Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor‐specific antibody development. J Am Soc Nephrol 2017; 28: 3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walton DC, Hiho SJ, Cantwell LS, et al HLA matching at the eplet level protects against chronic lung allograft dysfunction. Am J Transplant 2016; 16: 2695. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan PM, Warner P, Kemna MS, et al HLA molecular epitope mismatching and long‐term graft loss in pediatric heart transplant recipients. J Heart Lung Transplant 2015; 34: 950. [DOI] [PubMed] [Google Scholar]
- 25. Sypek M, Kausman J, Holt S, Hughes P. HLA epitope matching in kidney transplantation: an overview for the general nephrologist. Am J Kidney Dis 2018; 71: 720. [DOI] [PubMed] [Google Scholar]
- 26. Claas FHJ, Heidt S. Epitope‐based HLA matching: a useful strategy with many shortcomings to overcome. Transplantation 2017; 101: 1744. [DOI] [PubMed] [Google Scholar]
- 27. Taylor DO, Stehlik J, Edwards LB, et al Registry of the international society for heart and lung transplantation: twenty‐sixth official adult heart transplant report—2009. J Heart Lung Transplant 2009; 28: 1007. [DOI] [PubMed] [Google Scholar]
- 28. Khush KK, Cherikh WS, Chambers DC, et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐fifth Adult Heart Transplantation Report‐2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant 2018; 37: 1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Patient collective.
Figure S2 Cumulative incidence of cardiac allograft vasculopathy.
Table S1 Cardiac allograft vasculopathy.
Table S2 Cardiac allograft vasculopathy – Binary analyses.
Table S3 1‐year graft survival – Binary analyses.
Table S4 Long‐term graft survival – Binary analyses.
Table S5 Rejection – Binary analyses.


