Abstract
Trifluoromethyl sulfoxides are a new class of trifluoromethylthiolating reagent. The sulfoxides engage in metal‐free C−H trifluoromethylthiolation with a range of (hetero)arenes. The method is also applicable to the functionalization of important compound classes, such as ligand derivatives and polyaromatics, and in the late‐stage trifluoromethylthiolation of medicines and agrochemicals. The isolation and characterization of a sulfonium salt intermediate supports an interrupted Pummerer reaction mechanism.
Keywords: arenes, Pummerer reaction, reaction mechanisms, sulfoxides, trifluoromethylthiolation
May I interrupt? Taking advantage of the interrupted Pummerer reaction, trifluoromethyl sulfoxides engage in the metal‐free C−H trifluoromethylthiolation of (hetero)arenes, including drug molecules and natural products. This new class of trifluoromethylthiolating reagent exploits a new strategy for trifluoromethylthiolation in which sulfonium salts are assembled and selectively deconstructed.

Incorporating fluorine into organic compounds is a useful tool in drug design and development. The fluoro group is well known to improve the pharmacokinetic properties of a molecule and fluorine‐18 is an important radioisotope in molecular imaging.1, 2 Trifluoromethylthio (SCF3) groups are commonly found in drug molecules and veterinary medicines.3, 4 By combining a fluorinated moiety with a heteroatom, many have turned to the SCF3 group to impart useful properties, such as high lipophilicity, to a compound of interest.5
An attractive route for incorporating SCF3 groups into organic molecules is through the direct, metal‐free functionalization of C−H bonds.6 Early methods using trifluoromethylsulfenyl chloride have fallen from favor because of concerns over handling and toxicity of the reagent.7 This triggered a push to develop shelf‐stable, easy‐to‐handle trifluoromethylthiolating agents (Scheme 1 A).8 Despite the advantages of these reagents, they are generally limited to the C−H trifluoromethylthiolation of highly electron‐rich (hetero)arenes, such as indoles and phenols, whereas reactions involving less nucleophilic arenes, such as anisole and toluene, are scarce.8a, 8b, 8d, 8o, 8p Furthermore, few reports describe the use of these reagents for the late‐stage trifluoromethylthiolation of complex molecules.8a, 8b, 8j, 8m, 8n
Scheme 1.
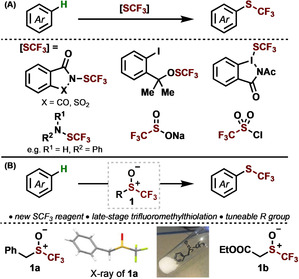
A) Current methods for transition metal‐free C−H trifluoromethylthiolation. B) This work: C−H trifluoromethylthiolation by an interrupted Pummerer reaction.
In recent years, our group9 and others10 have explored the so‐called interrupted Pummerer reaction of sulfoxides and its use for the functionalization of C−H bonds.11 For example, we have described the thioarylation of simple arenes using sulfoxides as sulfide precursors.9c Key to these reactions is the in situ formation of a highly electrophilic sulfonium salt, by activation of the sulfoxide with an acid anhydride, which is susceptible to reaction with a range of nucleophiles.
We were keen to assess whether underutilized trifluoromethyl sulfoxides would engage in C−H trifluoromethylthiolation. We reasoned that trifluoromethylsulfonium salts, generated from trifluoromethyl sulfoxides by an interrupted Pummerer reaction, would prove versatile intermediates en route to the incorporation of SCF3 into nucleophilic arenes. Herein, we present trifluoromethyl sulfoxides as novel, tuneable trifluoromethylthiolating agents (Scheme 1 B). The easy to prepare, bench‐stable and novel trifluoromethyl sulfoxides12 allow SCF3 incorporation into a variety of heteroarenes and arenes, including drug molecules, at the expense of C−H bonds. In contrast to current methods for trifluoromethylthiolation, which involve direct attack of an arene on an electrophilic SCF3 reagent, our unique strategy builds the desired connectivity to give sulfonium salts that are selectively deconstructed in situ to deliver trifluoromethylthiolated products.
Our first aim was to design and synthesize a sulfoxide suitable for general and selective trifluoromethylthiolation.13 Key to our mechanistic hypothesis for trifluoromethylthiolation is the selective loss of the R group, rather than the CF3 group, from the sulfoxide 1 (Scheme 1 B). As this step likely occurs by nucleophilic substitution in a sulfonium salt intermediate (see below), we identified the benzyl‐substituted trifluoromethyl sulfoxide 1 a as a candidate for enabling trifluoromethylthiolation: the activating effect of the adjacent π‐system, combined with the inhibitory effect of fluoro groups towards incoming nucleophiles,14 would make the benzyl group more susceptible to removal. We developed a new route for the synthesis of 1 a, which was obtained as a free‐flowing, bench‐stable, crystalline solid and has been characterized by X‐ray crystallographic analysis (Scheme 1 B).15
With a novel sulfoxide in hand, we attempted the trifluoromethylthiolation of indole (2 a; Scheme 2). The sulfoxide was activated using triflic anhydride9 to give the desired trifluoromethylthiolated indole 3 a in 70 % yield. The reaction tolerated substitution at all positions around the indole motif [C4 (3 b, 3 f, 3 h), C5 (3 d, 3 e, 3 i), C6 (3 c, 3 g), C7 (3 j) and C3 (3 k)], including various electron‐withdrawing (3 b–g) and electron‐donating (3 i–k) groups. We were pleased to find that functional groups that can undergo subsequent transformations, such as halides (3 b, 3 c), nitriles (3 d), esters (3 e–3 g), and boronate esters (3 h), were well tolerated. N‐methyl indoles also worked well in the procedure (3 l–n). A range of other heteroaromatic compounds also underwent efficient C−H trifluoromethylthiolation, such as benzothiophene (3 o), thiophenes (3 p, 3 q), benzofuran (3 r) and pyrroles (3 s, 3 t). The reaction was also executed on a gram scale without severe detriment to the yield (3 a).
Scheme 2.

Scope[a] of the metal‐free C−H trifluoromethylthiolation of heteroarenes. [a] Procedure A, conditions: i) 2 (0.3 mmol, 1.5 equiv), 1 a (0.2 mmol, 1.0 equiv), Tf2O (0.24 mmol, 1.2 equiv), MeCN (1.0 mL, 0.2 m) at 0 °C for 1 h. ii) Et2NH (0.5 mmol, 2.5 equiv). [b] Reaction run on a gram scale. [c] Numbers within parenthesis indicate ratio of C2 versus C3 trifluoromethylthiolation.
In comparison to heteroarenes, the trifluoromethylthiolation of arenes has received less attention.8a, 8b, 8d, 8o, 8p Initial results using 1 a gave poor yields of the desired trifluoromethylthiolated arenes, however, a novel ester‐derived trifluoromethyl sulfoxide, 1 b, showed good reactivity (Scheme 3). This outcome suggests that the structure of the sulfoxide can be tuned for optimization with a specific class of substrate.16 With 1 b, anisole, phenol and other alkylated arenes were responsive to trifluoromethylthiolation (5 a–e).17 Unfortunately, free amines were not tolerated in this reaction (5 f).18 A range of 1,2‐ (5 g–j) 1,3‐ (5 k) and 1,4‐disubstituted (5 l, 5 m) arenes, bearing various functionalities, such as halogens and esters, also performed well under our reaction conditions. The reaction was also applicable to trisubstituted arenes (5 n, 5 o) and naphthalenes (5 p, 5 q). Finally, we showcased our method using substrates relevant in catalysis, materials, medicine, and agriculture. We were able to trifluoromethylthiolate a BINOL derivative (5 r), pyrene (5 s), drugs (5 t), pesticides (5 u), and a natural product derivative (5 v).
Scheme 3.

Scope[a] of the metal‐free C−H trifluoromethylthiolation of arenes. [a] Procedure B, conditions: i) 4 (0.2 mmol, 1.0 equiv), 1 b (0.24 mmol, 1.2 equiv), Tf2O (0.3 mmol, 1.5 equiv), MeNO2 (1.0 mL, 0.2 m) at −25 °C for 10 min, then at RT for 3 h. ii) Et2NH (0.7 mmol, 3.5 equiv) at RT for 15 h. [b] Numbers within parenthesis indicate ratio of C4 versus C2 trifluoromethylthiolation. The major regioisomer is shown. [c] Procedure A (see Scheme 2). [d] See the Supporting Information for modified reaction stoichiometry. [e] Numbers within parenthesis indicate ratio of C4 versus C2 trifluoromethylthiolation. The major regioisomer is shown. [f] Numbers within parenthesis indicate ratio of C1 versus C2 trifluoromethylthiolation. The major regioisomer is shown.
A mechanistic proposal for the trifluoromethylthiolation is summarized in Scheme 4. The trifluoromethyl sulfoxides 1 are initially activated through reaction with Tf2O to produce the electrophilic intermediates 6. The intermediates 6 then undergo the so‐called interrupted Pummerer reaction with a (hetero)arene (e.g. 4) to give the sulfonium salts 7. Selective removal of the R group by Et2NH reveals the trifluoromethylthiolated products (e.g. 5). Experimental and computational studies provided support for our proposed mechanism. Firstly, the sulfonium salt 7 m was isolated from the reaction between p‐xylene (4 m) and 1 b.15, 19 We then modelled the dealkylation step using DFT calculations. These results showed that the transition state for attack of the amine (Et2NH) at the ‐CH2CO2Et group lies 40.8 kJ mol−1 lower in energy than the transition state for attack at the ‐CF3 group. In addition, the expected side‐product, Et2NCH2CO2Et (8), was detected by GCMS. It is likely that attack at the ‐CF3 group is disfavored because of unfavorable electrostatic interactions,14 though further studies are required to fully delineate the intricacies of this mechanism. These studies highlight our unique strategy for trifluoromethylthiolation; whereas current methods proceed through direct attack of an arene on an electrophilic SCF3 reagent,8 we have introduced alternative reactivity in which the desired connectivity is built, to give 7, before inducing controlled deconstruction and release of the desired trifluoromethylthiolated products.
Scheme 4.

A) Proposed mechanism for the trifluoromethylthiolation of (hetero)arenes using sulfoxides. B) Computational investigation of the chemoselective dealkylation. [a] The process was modelled using the cation of 7 m. See the Supporting Information for further details.
In summary, we have developed a new strategy for the metal‐free C−H trifluoromethylthiolation of (hetero)arenes. In this process, we utilize the interrupted Pummerer reaction to establish trifluoromethyl sulfoxides as novel trifluoromethylthiolating agents. Our method for incorporating SCF3 components exploits a build‐up/deconstruct strategy and is mechanistically distinct from current processes. A variety of (hetero)aromatic compounds underwent efficient trifluoromethylthiolation, including drug molecules and natural products. We expect trifluoromethyl sulfoxides to find application in other trifluoromethylthiolation reactions in the future.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank EPSRC (Postdoctoral Fellowship to D. W.—EP/S014128/1; Established Career Fellowship to D.J.P.—EP/M005062/1) and the University of Manchester (Lectureship to G.J.P.P.) for their generous support.
D. Wang, C. G. Carlton, M. Tayu, J. J. W. McDouall, G. J. P. Perry, D. J. Procter, Angew. Chem. Int. Ed. 2020, 59, 15918.
References
- 1. Purser S., Moore P. R., Swallow S., Gouverneur V., Chem. Soc. Rev. 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]
- 2. Preshlock S., Tredwell M., Gouverneur V., Chem. Rev. 2016, 116, 719–766. [DOI] [PubMed] [Google Scholar]
- 3. Pertusati F., Serpi M., Pileggi E., Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals, Elsevier, Amsterdam, 2019, pp. 141–180. [Google Scholar]
- 4. Sykes J. E., Papich M. G., Canine and Feline Infectious Diseases, Elsevier, Amsterdam, 2014, pp. 97–104. [Google Scholar]
- 5. Landelle G., Panossian A., Leroux F., Curr. Top. Med. Chem. 2014, 14, 941–951. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Shao X., Xu C., Lu L., Shen Q., Acc. Chem. Res. 2015, 48, 1227–1236; [DOI] [PubMed] [Google Scholar]
- 6b. Barata-Vallejo S., Bonesi S., Postigo A., Org. Biomol. Chem. 2016, 14, 7150–7182; [DOI] [PubMed] [Google Scholar]
- 6c. Chachignon H., Cahard D., Chin. J. Chem. 2016, 34, 445–454; [Google Scholar]
- 6d. Toulgoat F., Billard T., Modern Synthesis Processes and Reactivity of Fluorinated Compounds, Elsevier, Amsterdam, 2017, pp. 141–179. [Google Scholar]
- 7.See ref. [5] and
- 7a. Scribner R. M., J. Org. Chem. 1966, 31, 3671–3682; [Google Scholar]
- 7b. Haas A., Hellwig V., J. Fluorine Chem. 1975, 6, 521–532; [Google Scholar]
- 7c. Croft T. S., McBrady J. J., J. Heterocycl. Chem. 1975, 12, 845–849; [Google Scholar]
- 7d. Mirek J., Haas A., J. Fluorine Chem. 1981, 19, 67–70; [Google Scholar]
- 7e. Gerstenberger M. R. C., Haas A., Liebig F., J. Fluorine Chem. 1982, 19, 461–474; [Google Scholar]
- 7f. Gerstenberger M. R. C., Haas A., J. Fluorine Chem. 1983, 23, 525–540. [Google Scholar]
- 8.Recent selected reports:
- 8a. Ferry A., Billard T., Bacqué E., Langlois B. R., J. Fluorine Chem. 2012, 134, 160–163; [Google Scholar]
- 8b. Yang Y., Jiang X., Qing F.-L., J. Org. Chem. 2012, 77, 7538–7547; [DOI] [PubMed] [Google Scholar]
- 8c. Xu C., Ma B., Shen Q., Angew. Chem. Int. Ed. 2014, 53, 9316–9320; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 9470–9474; [Google Scholar]
- 8d. Alazet S., Billard T., Synlett 2015, 26, 76–78; [Google Scholar]
- 8e. Alazet S., Zimmer L., Billard T., J. Fluorine Chem. 2015, 171, 78–81; [Google Scholar]
- 8f. Glenadel Q., Alazet S., Billard T., J. Fluorine Chem. 2015, 179, 89–95; [Google Scholar]
- 8g. Honeker R., Ernst J. B., Glorius F., Chem. Eur. J. 2015, 21, 8047–8051; [DOI] [PubMed] [Google Scholar]
- 8h. Jereb M., Gosak K., Org. Biomol. Chem. 2015, 13, 3103–3115; [DOI] [PubMed] [Google Scholar]
- 8i. Wang Q., Qi Z., Xie F., Li X., Adv. Synth. Catal. 2015, 357, 355–360; [Google Scholar]
- 8j. Zhang P., Li M., Xue X.-S., Xu C., Zhao Q., Liu Y., Wang H., Guo Y., Lu L., Shen Q., J. Org. Chem. 2016, 81, 7486–7509; [DOI] [PubMed] [Google Scholar]
- 8k. Ernst J. B., Rakers L., Glorius F., Synthesis 2017, 49, 260–268; [Google Scholar]
- 8l. Kovács S., Bayarmagnai B., Goossen L. J., Adv. Synth. Catal. 2017, 359, 250–254; [Google Scholar]
- 8m. Nalbandian C. J., Miller E. M., Toenjes S. T., Gustafson J. L., Chem. Commun. 2017, 53, 1494–1497; [DOI] [PubMed] [Google Scholar]
- 8n. Bonazaba Milandou L. J. C., Carreyre H., Alazet S., Greco G., Martin-Mingot A., Nkounkou Loumpangou C., Ouamba J.-M., Bouazza F., Billard T., Thibaudeau S., Angew. Chem. Int. Ed. 2017, 56, 169–172; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 175–178; [Google Scholar]
- 8o. Horvat M., Jereb M., Iskra J., Eur. J. Org. Chem. 2018, 3837–3843; [Google Scholar]
- 8p. Nalbandian C. J., Brown Z. E., Alvarez E., Gustafson J. L., Org. Lett. 2018, 20, 3211–3214; [DOI] [PubMed] [Google Scholar]
- 8q. Lu S., Chen W., Shen Q., Chin. Chem. Lett. 2019, 30, 2279–2281; [Google Scholar]
- 8r. Liu S., Zeng X., Xu B., Asian J. Org. Chem. 2019, 8, 1372–1375; [Google Scholar]
- 8s. Shao X., Wang X., Yang T., Lu L., Shen Q., Angew. Chem. Int. Ed. 2013, 52, 3457–3460; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 3541–3544; [Google Scholar]
- 8t. Shao X., Xu C., Lu L., Shen Q., J. Org. Chem. 2015, 80, 3012–3021; [DOI] [PubMed] [Google Scholar]
- 8u. Ma B., Shao X., Shen Q., J. Fluorine Chem. 2015, 171, 73–77; [Google Scholar]
- 8v. Yang X.-G., Zheng K., Zhang C., Org. Lett. 2020, 22, 2026–2031; [DOI] [PubMed] [Google Scholar]
- 8w. Chachignon H., Maeno M., Kondo H., Shibata N., Cahard D., Org. Lett. 2016, 18, 2467–2470; [DOI] [PubMed] [Google Scholar]
- 8x. Yan Q., Jiang L., Yi W., Liu Q., Zhang W., Adv. Synth. Catal. 2017, 359, 2471–2480; [Google Scholar]
- 8y. Bu M.-J., Lu G.-P., Cai C., Org. Chem. Front. 2017, 4, 266–270; [Google Scholar]
- 8z. Zhao X., Zheng X., Tian M., Sheng J., Tong Y., Lu K., Tetrahedron 2017, 73, 7233–7238; [Google Scholar]
- 8aa. Sun D.-W., Jiang X., Jiang M., Lin Y., Liu J.-T., Eur. J. Org. Chem. 2017, 3505–3511; [Google Scholar]
- 8ab. Liu J., Zhao X., Jiang L., Yi W., Adv. Synth. Catal. 2018, 360, 4012–4016. [Google Scholar]
- 9.Interrupted Pummerer reactions with carbon nucleophiles:
- 9a. Eberhart A. J., Imbriglio J. E., Procter D. J., Org. Lett. 2011, 13, 5882–5885; [DOI] [PubMed] [Google Scholar]
- 9b. Eberhart A. J., Cicoira C., Procter D. J., Org. Lett. 2013, 15, 3994–3997; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Fernández-Salas J. A., Pulis A. P., Procter D. J., Chem. Commun. 2016, 52, 12364–12367; [DOI] [PubMed] [Google Scholar]
- 9d. Shrives H. J., Fernández-Salas J. A., Hedtke C., Pulis A. P., Procter D. J., Nat. Commun. 2017, 8, 14801; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9e. Šiaučiulis M., Sapmaz S., Pulis A. P., Procter D. J., Chem. Sci. 2018, 9, 754–759; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9f. He Z., Shrives H. J., Fernández-Salas J. A., Abengózar A., Neufeld J., Yang K., Pulis A. P., Procter D. J., Angew. Chem. Int. Ed. 2018, 57, 5759–5764; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 5861–5866; [Google Scholar]
- 9g. Šiaučiulis M., Ahlsten N., Pulis A. P., Procter D. J., Angew. Chem. Int. Ed. 2019, 58, 8779–8783; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 8871–8875; [Google Scholar]
- 9h. Yan J., Pulis A. P., Perry G. J. P., Procter D. J., Angew. Chem. Int. Ed. 2019, 58, 15675–15679; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 15822–15826; [Google Scholar]
- 9i. Aukland M. H., Šiaučiulis M., West A., Perry G. J. P., Procter D. J., Nat. Catal. 2020, 3, 163–169. [Google Scholar]
- 10.Selected intermolecular interrupted Pummerer chemistry with carbon nucleophiles:
- 10a. Nenajdenko V. G., Vertelezkij P. V., Balenkova E. S., Sulfur Lett. 1996, 20, 75–84; [Google Scholar]
- 10b. Nenajdenko V. G., Vertelezkij P. V., Gridnev I. D., Shevchenko N. E., Balenkova E. S., Tetrahedron 1997, 53, 8173–8180; [Google Scholar]
- 10c. Nenajdenko V. G., Vertelezkij P. V., Balenkova E. S., Synthesis 1997, 351–355; [Google Scholar]
- 10d. Shevchenko N. E., Karpov A. S., Zakurdaev E. P., Nenajdenko V. G., Balenkova E. S., Chem. Heterocycl. Compd. 2000, 36, 137–143; [Google Scholar]
- 10e. Shevchenko N. E., Nenajdenko V. G., Balenkova E. S., Synthesis 2003, 1191–1200; [Google Scholar]
- 10f. Matsuo J-I., Yamanaka H., Kawana A., Mukaiyama T., Chem. Lett. 2003, 32, 392–393; [Google Scholar]
- 10g. Shoji T., Higashi J., Ito S., Toyota K., Asao T., Yasunami M., Fujimori K., Morita N., Eur. J. Org. Chem. 2008, 1242–1252; [Google Scholar]
- 10h. Yoshida S., Yorimitsu H., Oshima K., Org. Lett. 2009, 11, 2185–2188; [DOI] [PubMed] [Google Scholar]
- 10i. Higuchi K., Tayu M., Kawasaki T., Chem. Commun. 2011, 47, 6728–6730; [DOI] [PubMed] [Google Scholar]
- 10j. Huang X., Patil M., Farès C., Thiel W., Maulide N., J. Am. Chem. Soc. 2013, 135, 7312–7323; [DOI] [PubMed] [Google Scholar]
- 10k. Tayu M., Higuchi K., Inaba M., Kawasaki T., Org. Biomol. Chem. 2013, 11, 496–502; [DOI] [PubMed] [Google Scholar]
- 10l. Tayu M., Higuchi K., Ishizaki T., Kawasaki T., Org. Lett. 2014, 16, 3613–3615; [DOI] [PubMed] [Google Scholar]
- 10m. Tayu M., Ishizaki T., Higuchi K., Kawasaki T., Org. Biomol. Chem. 2015, 13, 3863–3865; [DOI] [PubMed] [Google Scholar]
- 10n. Cowper P., Jin Y., Turton M. D., Kociok-Köhn G., Lewis S. E., Angew. Chem. Int. Ed. 2016, 55, 2564–2568; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 2610–2614; [Google Scholar]
- 10o. Tayu M., Suzuki Y., Higuchi K., Kawasaki T., Synlett 2016, 27, 941–945; [Google Scholar]
- 10p. Klose I., Misale A., Maulide N., J. Org. Chem. 2016, 81, 7201–7210; [DOI] [PubMed] [Google Scholar]
- 10q. Tayu M., Nomura K., Kawachi K., Higuchi K., Saito N., Kawasaki T., Chem. Eur. J. 2017, 23, 10925–10930; [DOI] [PubMed] [Google Scholar]
- 10r. Kawashima H., Yanagi T., Wu C.-C., Nogi K., Yorimitsu H., Org. Lett. 2017, 19, 4552–4555; [DOI] [PubMed] [Google Scholar]
- 10s. Hu G., Xu J., Li P., Org. Chem. Front. 2018, 5, 2167–2170; [Google Scholar]
- 10t. Higuchi K., Tago T., Kokubo Y., Ito M., Tayu M., Sugiyama S., Kawasaki T., Org. Chem. Front. 2018, 5, 3219–3225; [Google Scholar]
- 10u. Waldecker B., Kraft F., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2018, 57, 12538–12542; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 12718–12722; [Google Scholar]
- 10v. Zhang Z., He P., Du H., Xu J., Li P., J. Org. Chem. 2019, 84, 4517–4524; [DOI] [PubMed] [Google Scholar]
- 10w. Zhang Z., Luo Y., Du H., Xu J., Li P., Chem. Sci. 2019, 10, 5156–5161; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10x. Li X., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2019, 58, 9496–9500; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 9596–9600; [Google Scholar]
- 10y. Berger F., Plutschack M. B., Riegger J., Yu W., Speicher S., Ho M., Frank N., Ritter T., Nature 2019, 567, 223–228; [DOI] [PubMed] [Google Scholar]
- 10z. Kafuta K., Korzun A., Böhm M., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2020, 59, 1950–1955; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1966–1971. [Google Scholar]
- 11.
- 11a. Pulis A. P., Procter D. J., Angew. Chem. Int. Ed. 2016, 55, 9842–9860; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 9996–10014; [Google Scholar]
- 11b. Yanagi T., Nogi K., Yorimitsu H., Tetrahedron Lett. 2018, 59, 2951–2959; [Google Scholar]
- 11c. Kaiser D., Klose I., Oost R., Neuhaus J., Maulide N., Chem. Rev. 2019, 119, 8701–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulfoxide 1 b has not previously been prepared;
- 12a. DeMarinis R. M., Hoover J. R. E., Dunn G. L., Actor P., Uri J. V., Weisbach J. A., J. Antibiot. 1975, 28, 463–470; [DOI] [PubMed] [Google Scholar]
- 12b. Sokolenko L. V., Yagupolskii Y. L., Kumanetska L. S., Marrot J., Magnier E., Lipetskij V. O., Kalinin I. V., Tetrahedron Lett. 2017, 58, 1308–1311; [Google Scholar]
- 12c. Sokolenko L. V., Orlova R. K., Filatov A. A., Yagupolskii Y. L., Magnier E., Pégot B., Diter P., Molecules 2019, 24, 1249 For procedures related to the synthesis of 1 a see ref. [12c]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For the use of trifluoromethyl sulfoxides in trifluoromethylation, see:
- 13a. Prakash G. K. S., Hu J., Olah G. A., Org. Lett. 2003, 5, 3253–3256; [DOI] [PubMed] [Google Scholar]
- 13b. Li X., Zhao J., Zhang L., Hu M., Wang L., Hu J., Org. Lett. 2015, 17, 298–301. [DOI] [PubMed] [Google Scholar]
- 14. Martinez H., Rebeyrol A., Nelms T. B., Dolbier W. R., J. Fluorine Chem. 2012, 135, 167–175. [Google Scholar]
- 15. Deposition Numbers 1993042 (for 1a), and 1993043 (for 7m) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 16.See the Supporting Information for details of the optimization. When reacting the indole 2 l with 1 b, product 3 l was formed in 17 % yield. When reacting the arene 4 m with 1 a, product 5 m was formed in 7 % yield. This suggests that less nucleophilic arenes (4) require the presumably more electrophilic sulfoxide 1 b to react. However, further studies are required to fully understand the differing reactivity.
- 17.The reaction with benzene gave only a trace amount of product.
- 18.Preliminary results suggest that some amines, for example, NPh3, are tolerated. See the Supporting Information.
- 19.For a review on trifluoromethylsulfonium salts see: Shibata N., Matsnev A., Cahard D., Beilstein J. Org. Chem. 2010, 6, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


