Abstract
Background
Bleeding on brushing (BoB) is an important sign of gingival inflammation. Recently, the use of intelligent toothbrushes and oral health Apps has shown potential to improve oral and periodontal health. In the context of the introduction of an Internet of things network of intelligent power‐driven toothbrushes in a supportive periodontal care programme, the aim of this study was (a) to audit the adoption and retention of this new technology; and (b) to preliminarily assess the feasibility to gather data on BoB and associate them with clinical periodontal parameters.
Methods
100 subjects with different periodontal case diagnoses participating in supportive periodontal care (SPC) were provided with and instructed on the use of an intelligent power‐driven toothbrush connected with an App (I‐Brush). Brushing sessions and occurrence of BoB were recorded through the App and stored in a data protection compliant backend. Subject retention was audited over time. BoB recorded in the two weeks before the SPC appointment was associated with clinical parameters observed by the clinician blind to App data.
Results
75% of subjects provided data from using the power toothbrush and the App for a minimum of 10 brushing sessions over an average period of 362 days. Compared with baseline, subjects’ oral hygiene, bleeding on probing and prevalence of residual pockets improved gradually while using the I‐Brush. The number of BoB episodes in the two weeks leading to the SPC appointment and the number of residual pockets predicted BOP (p < .001) detected during the examination. App use in the previous two weeks was associated with lower plaque scores at SPC.
Conclusions
These preliminary observations indicate good adoption and retention of a mobile health system built around an intelligent power toothbrush in a SPC population. The App was able to gather clinically relevant information predicting the observed degree of gingival inflammation. Deployment of mHealth systems seems feasible in dental practice and may bring significant oral health benefits. More investigations are needed in this area.
Keywords: adherence, bleeding on brushing, bleeding on probing, gingival inflammation, mHealth, periodontitis, supportive periodontal care
Clinical Relevance.
Scientific rationale for the study: mHealth solutions have the potential to disrupt the current models of care and open new opportunities to improve adherence with self‐performed oral hygiene and preventive care.
Principal findings: Adult subjects in a periodontal supportive care programme showed good adoption and retention with a system built around an intelligent power toothbrush. The system was able to monitor patient adherence and gather clinically relevant information predicting gingival inflammation and level of plaque control.
Practical implications: Future studies assessing the cost benefits of mHealth interventions to improve adherence and effectiveness of secondary preventive programmes are needed.
1. INTRODUCTION
Support of subjects’ motivation and self‐performed oral hygiene is a priority and remains a critical challenge in dental and periodontal practice (Carra et al., 2020; Tonetti et al., 2015). Incomplete adherence with behavioural changes and interventions is a major issue in the management of chronic diseases. Recently, the potential effectiveness of applications running on personal digital platforms such as cell phones and tablets as a support to improve adherence and compliance has received a lot of attention and has shown significant advantages compared with the reliance of self‐management strategies alone (Lunde, Nilsson, Bergland, Kvaerner, & Bye, 2018; Peng et al., 2020; Perez‐Jover, Sala‐Gonzalez, Guilabert, & Mira, 2019).
Initial attempts in dentistry to improve adherence with oral hygiene instructions have used both short message services (SMS) text messaging and applications (App). Interventions have focused on adolescents (mostly orthodontic patients) and, in a systematic review, have resulted in significant improvements in oral hygiene parameters compared with the controls (Toniazzo, Nodari, Muniz, & Weidlich, 2019). The potential of mobile health applications (mHealth) in periodontal therapy and supportive periodontal care needs to be explored: for example, they may provide the capability of tracking and capturing key details of self‐performed oral hygiene routines in home setting. mHealth Apps may also provide unique opportunities in self‐monitoring and early detection of sentinel signs of periodontal diseases such as “gum bleeding” or bleeding on brushing (BoB). The association between BoB and periodontal health has been the subject of significant efforts since the 1990s (Kallio, 1996; Kallio, Nordblad, Croucher, & Ainamo, 1994; Wang et al., 2007; Weintraub, Finlayson, Gansky, Santo, & Ramos‐Gomez, 2013). Studies, however, have so far reported disappointing diagnostic accuracy of self‐reported “gum bleeding” or BoB. An observation that can perhaps be explained, at least in part, by its perception of normalcy, tendency of self‐neglect and recall bias of self‐reporting, variability in toothbrushing behaviours, perhaps due to lack of proper professional training, toothbrushing trauma or avoidance of specific areas, may lead to data distortion as well.
In principle, mHealth Apps may link healthcare professionals and patients through a two‐way communication allowing data gathering and delivery of simple interventions.
The aims of this clinical audit were i) to assess the feasibility of deployment of an Internet of things (IoT) network of intelligent oral hygiene instruments (I‐Brush) in a supportive care population in a periodontal specialty practice; and ii) to preliminarily assess the feasibility to gather data on BoB and associate them with clinical periodontal parameters.
2. MATERIALS AND METHODS
2.1. Study design
This clinical audit reports on the deployment, adoption and retention of use of an IoT network of intelligent power toothbrushes connected by an App in a consecutive sample of 100 subjects receiving periodontal supportive care (SPC) in a specialist periodontal practice in Genova, Italy (www.tonettidental.it). All subjects were required to have a compatible Android or iOS smartphone and to be willing to have the App installed on it. In the context of the clinical audit, the adoption and retention of use of the App were assessed as a basis for future deployment and study on the benefits of mHealth. Furthermore, the association between the occurrence of self‐reported bleeding on brushing (BoB) in the weeks leading to the SPC appointment, periodontal health and clinical inflammatory parameters was assessed as an example that such systems may assist in better monitoring of subjects in between SPC appointments. Subjects’ anonymity was ensured giving each power toothbrush serial number a consecutive code that could be matched to the individual subjects only by the attending hygienists or dentists. All subjects gave informed consent to participating in the pilot introduction of this technology and to the use of the data for research and audit purposes. The audit was open‐ended but aimed at obtaining a 12‐month follow‐up data for the majority of actual users. Data collection was between May 2017 and March 2019.
2.2. Experimental devices
Intelligent, power‐driven toothbrushes (Genius Power Toothbrush, D701, Oral‐B, Germany), connected via Bluetooth to an purpose‐designed App (Oral‐B 4.1.1‐clinical_study‐rc7 for Android or Oral‐B 4.1.2‐clinical‐study2‐rc3 for iOS) residing on the subject's personal smartphone and with a sensitive disposable head (EB60, Oral‐B, Germany), were provided to all participants (I‐Brush). The App was designed to record the following parameters: (a) date and time of each brushing session; (b) brushing duration in seconds; (c) the occurrence (number of events) and duration in seconds of overpressure (defined as a brushing pressure of >3 N); (d) user input of the occurrence of any noticeable bleeding on brushing after a prompt by the App at the completion of the brushing session; and v) user input of the performance of inter‐dental cleaning (brushing or flossing). The App was installed during a clinic visit and set‐up by a research team member who also individually instructed the patient on its use. Subjects were also instructed on the use of the I‐Brush by a dental hygienist in the context of the oral hygiene instructions and motivation of the regular SPC appointment and were invited to continue their use.
2.3. Internet of things network structure
Each I‐Brush was connected to the Internet through the smartphone of the subject and was programmed to save data on a customized backend running on Amazon (Frankfurt Region, Germany) and physically located within the European Union. The database was compliant with the relevant European data protection regulations (GDPR). The individual user was able to disconnect the data sharing option, and all subjects were instructed on how to do it. The attending dentist/hygienist was able to access the brushing data in the form of a consolidated report providing data on the brushing habits of the individual subject. In order to maintain blinding at the time of examination, access was allowed only after completion of the SPC appointment. No personal information was stored in the cloud database. The customized Amazon front end allowed the clinical team to see aggregated data of brushing events and BoB in an anonymous PDF report that could be matched to the individual subject ID.
2.4. Bleeding on brushing
Subjects were instructed to look for bleeding signs in the toothbrushing slurry during and at the end of brushing sessions. Upon completion of each brushing session, the App prompted the patient to self‐record the presence or absence of bleeding on brushing (BoB).
2.5. Periodontal assessment
In the context of SPC, each subject's periodontal health was monitored at each visit by two attending hygienists. Monitoring included number of teeth present, plaque control recorded after disclosing (O'Leary, 1972), bleeding on probing (BOP) recorded at 4 sites per tooth, presence and location of probing pocket depths 4 mm or deeper, and presence of furcation involvement. Dental implants were monitored with the same protocol. All sites were monitored with a UNC‐15 periodontal probe at a force of approximately 0.25N. Full‐mouth plaque and bleeding scores (FMPS, FMBS) were calculated as the percentage of sites with detectable plaque or BOP in the dentition.
2.6. Periodontal case diagnosis
Periodontal case diagnosis was determined integrating the clinical, radiographic and anamnestic data by a single clinician (MST) using the current international classification scheme of periodontal diseases (Caton et al., 2018; Papapanou et al., 2018; Tonetti et al., 2018; Tonetti and Sanz 2019).
2.7. Supportive periodontal care
All appointments had a 60‐min duration and followed the suggested flow that included review of medical history, periodontal assessment, oral hygiene instruction and motivation, supra‐ and subgingival instrumentation, application of local fluoride, desensitizing agents or chlorhexidine varnishes as indicated. The individualized suggested interval between SPC appointments ranged between 3 and 6 months.
2.8. Data analysis
Anonymized data comprising the periodontal assessments were entered in a personal computer and proofed for entry errors. They were subsequently merged with the brushing data collected by the App using the subject identifier number linking each subject to the serial number of the I‐Brush. For each participant, brushing data were aligned chronologically with the SPC appointments. In order to do so, brushing sessions occurring before 9:00 a.m. on the day of an appointment were assumed to have happened before the SPC visit, while those that happened later in the day afterwards. All analyses were performed using R 3.5.1 for windows and JMP pro 15.0. Unless specified differently, data are reported as mean ± standard deviation. The number of subjects continuing to use the App was determined and visualized for different points in time. In order to avoid patients with high numbers of brushing sessions having a higher impact on the outcome compared to patients with fewer brushing sessions, mean values (for continuous variables) and percentages (for categorical variables) were determined for the key endpoints for each patient individually and then averaged to give a global result. This ensures each patient has equal weight in the analysis, irrespective of the number of brushing sessions they contributed.
Respective variables were modelled using generalized linear mixed effect models, with subject as random effect. The differences between levels of the breakout variables were statistically tested by linear hypotheses within those models. FMPS and FMBS were separately modelled in a multiple linear mixed regression model against various parameters, and results from the final model are reported. As before, User ID was used as a random effect in those models.
3. RESULTS
3.1. Subject retention and bleeding on brushing
100 subjects 48.7 ± 12.8 (range: 18–71) years of age, 36% males, with an average of 27 ± 4 teeth (26 subjects had additionally 1 or more dental implant), were included. 17 of the 100 subjects who received the I‐Brush and the App never produced any data. The periodontal classification of the 83 subjects who kept the App transmission function activated included 15 non‐periodontitis subjects (healthy or localized gingivitis), 9 stage I periodontitis, 15 stage II, 39 stage III and 5 stage IV. Figure 1 shows the retention in I‐Brush and App use. 8 of the 83 subjects had a total of less than 10 App sessions and were excluded from further analyses. 85% of the 75 subjects considered further used the App for 100 days or more, and the average retention was 362 ± 204 days. Reasons for lack of adoption or retention are unknown.
FIGURE 1.
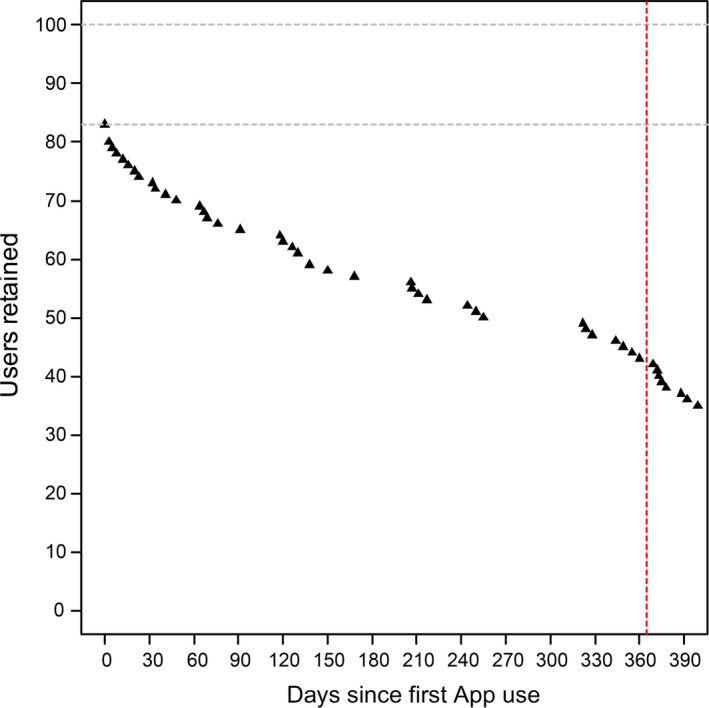
User retention. The figure illustrates subject retention up to 400 days since the time the I‐Brush and App were configured on their smartphones. Data collection was stopped in March 2019 while many users were still actively using the system. 14 subjects showed a retention of more than 600 days until that date [Colour figure can be viewed at wileyonlinelibrary.com]
The average across subjects of the individual average duration of a brushing session was 2 min and 58 s ± 39 s. On average across subjects, the average number of overpressure events in individual brushing session was 1.9 ± 6.5, with a duration of 1 ± 5 s. On average, subjects recorded the performance of inter‐dental cleaning in 60% of their respective sessions.
The periodontal conditions recorded during the SPC appointments at baseline and the 3 subsequent visits are illustrated in Table 1. The population showed good oral hygiene, low levels of FMBS and the presence of few residual periodontal pockets (4 mm or deeper) consistent with the advanced level of care that they had been receiving. A trend over time towards a decrease in periodontal inflammation was observed (p < .001).
TABLE 1.
Clinical presentation at the different SPC appointments (mean ± standard deviation)
| Outcomes | Baseline | SPC #1 | SPC #2 | SPC #3 |
|---|---|---|---|---|
| FMPS (%) | 19.7 ± 10 | 15.7 ± 8.9 | 17.9 ± 14.1 | 17.1 ± 15.4 |
| FMBS (%) | 5.9 ± 6.1 | 3.4 ± 3.5 | 2.9 ± 2.9 | 2.9 ± 3.2 |
| Number of pockets | 3.3 ± 5.3 | 3.2 ± 4.7 | 3.3 ± 4.7 | 4.1 ± 5.9 |
BoB was noted in 2% of the 26,755 brushing sessions recorded by the App. 61 of the 75 subjects reported BoB at least once during the study period. BoB was significantly more frequent for brushing sessions of longer duration, and the size of the effect, however, was small (data not shown).
A graphic representation of the brushing sessions of a representative subject based on the date and the time of the day is given in Figure 2. Each dot represents a brushing session. The subject SPC appointments are illustrated by the open triangles. Green dots display brushing sessions without detection of BoB, while red dots illustrate session in which BoB was recorded.
FIGURE 2.
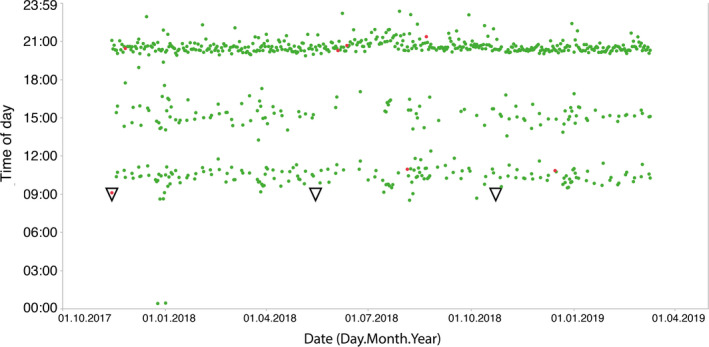
Recorded brushing sessions for a representative subject. Each dot in the time of the day versus date diagram represents a single recorded brushing session. The subject has been active for more than a year. Green dots represent brushing sessions without self‐reporting bleeding on brushing, while the red dots illustrate those with bleeding on brushing. The open triangles show the dates of the SPC appointments in the clinic [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Bleeding on brushing as a predictor of bleeding on probing at clinical presentation
A multiple linear mixed model was constructed considering two weeks of App data available prior to a SPC appointment to predict FMBS detected at the appointment (the subject base includes only those who had used the App in the two weeks before a SPC appointment). The model assessed the number of pockets, the counts of recorded BoB episodes in the two weeks prior to the appointment, the number of teeth and implants, the periodontitis case definition and stage, the age of the subjects, gender, smoking status, count of App sessions in previous two weeks, total App sessions by the user, overpressure events and length of recorded brushing duration, and it corrected for oral hygiene level (FMPS). Significant factors were selected by stepwise backwards elimination with SLS = 0.15. The adjusted R‐square of the final model was 0.636, and it was highly significant. Table 2 shows the significant factors in the model. Figure 3 illustrates the prediction plots of the level of FMBS based on the significant factors in the model. Predictions are based on the average of each parameter as observed in this population. Appendix 1 provides an interactive prediction profiler to explore the data trends.
TABLE 2.
Multiple linear mixed model predicting the level of full‐mouth BOP at a SPC appointment
| Parameters | Estimate ± SE | F Ratio | p value |
|---|---|---|---|
| Age (years) | 0.001 ± 0.001 | 4.334 | .0421 |
| Perio case diagnosis | * | 2.68 | .0420 |
| Number of pockets | 0.002 ± 0.001 | 11.467 | <.0001 |
| Count of BoB previous 2 weeks | 0.006 ± 0.002 | 6.271 | .0135 |
| Count of App sessions previous 2 weeks | −0.001 ± 0.001 | 2.727 | .1012 |
| Brushing duration | −0.0001 ± 7.3E−5 | 5.395 | .0234 |
No estimate is provided for the whole Perio case diagnosis.
FIGURE 3.
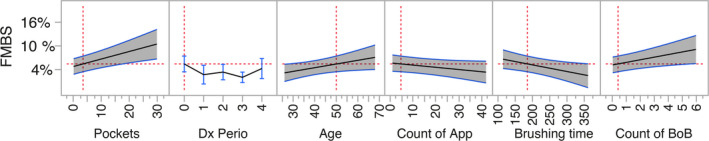
Prediction profiler based on multiple linear mixed model analysis with bleeding on probing at a SPC session as dependent variable. Prediction plots of the significant factors in the model after stepwise backwards elimination of non‐significant factors (p > .15). In each plot, the grey‐shaded area represents the 95% confidence interval of the prediction. The red dotted lines represent the reference point for the prediction and the confidence intervals; they are set at the population average for these plots (an interactive prediction profiler is available in Appendix 1). FMBS is the percentage of sites with bleeding on probing at the SPC appointment, Pockets is the number of sites with probing depths 4 mm or deeper, Dx Perio is the periodontal case definition (health/gingivitis = 0, stage I to stage IV periodontitis = 1–4), Age is in years, count of App sessions in the 2 weeks before the appointment, brushing time is in seconds as recorded by the App and count of BoB episodes recorded by the App in the 2 weeks before the SPC appointment [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Use of the app as a predictor of oral hygiene
Similarly, a multiple linear mixed model was constructed to test the effect of the factors included in the previous model on the level of oral hygiene observed at the SPC appointment. The final model had an adjusted R‐square of 0.444 and was highly significant (p = .0002). Significant factors were selected by backwards elimination with SLS = 0.15. The number of App use in the two weeks prior to the SPC appointment was associated with lower FMPS (p = .0007, estimate −0.002 ± 0.001). The periodontal case diagnosis and the number of teeth were also retained in the model (p = .103 and p = .134, respectively). A prediction plot for the model is displayed in Figure 4, and an interactive prediction profiler is available in Appendix 1.
FIGURE 4.
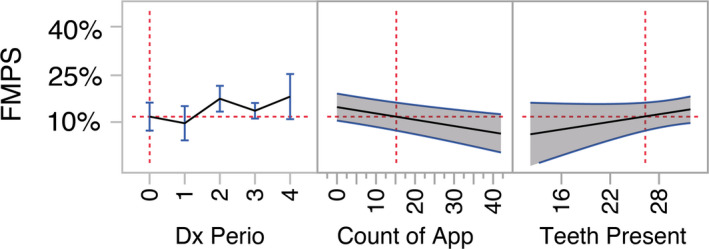
Prediction profiler based on multiple linear mixed model analysis with oral hygiene level (FMPS) at a SPC session as dependent variable. Prediction plot of the significant factor in the model after stepwise backwards elimination of non‐significant factors (p > .15). In the plot, the grey‐shaded area represents the 95% confidence interval of the prediction. The red dotted lines represent the reference point for the prediction and are set at the population average for count of app sessions in the past 2 weeks (P2W) (an interactive prediction profiler is available in Appendix 1). FMPS decreased significantly with greater number of use of the App in the previous two weeks leading to the SPC appointment. This analysis includes subjects without the use of the App (count of 0) in the two weeks before the SPC appointment [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This manuscript reports the feasibility to deploy an IoT network of connected intelligent toothbrushes and the possibility to gather clinically relevant information to inform better oral health management in an adult and older adult population. It extends previous evidence that mHealth‐based interventions in dentistry can be used to effectively communicate with subjects (Toniazzo et al., 2019) and supports the concept that two‐way communication systems can be engineered to bring oral health monitoring and simple interventions outside the dental practice and follow the patient home. In the architecture of such dental practice‐based systems, an intelligent power toothbrush may play a central role.
This audit reveals important issues related to adoption and retention. A critical one is the willingness of a subject to share brushing data with their carers, effectively allowing greater insight into their oral hygiene habits. 17% of subjects who received the I‐Brush never shared any data, and another 8% shared less than 10 brushing sessions. In this report, it is unclear what their motives were, and future studies should focus on the barriers observed in one out of 4 subjects. 75% of subjects used the App for a prolonged period of time in a pilot deployment that did not include professional feedback or communications from the carers, without a feedback mechanism that could provide increased perception of value. Future assessments of a full architecture (data collection and simple intervention) are needed.
The observed association of self‐reported BoB in the two weeks before a SPC appointment with the clinically assessed BOP during the appointment has potential importance. Firstly, it is a noteworthy example of the potential of mHealth architectures to gather clinically relevant data with the potential to improve oral health. Self‐reported BoB has shown promising diagnostic accuracy for screening of subjects with periodontal disease, but its retrospective assessment suffers from recall bias. The use of the App immediately after the brushing session may improve accuracy. The external applicability of these initial observations beyond a SPC population needs to be assessed using systems with full capability to both gather data and provide brief interventions to the individual subject. The potential to monitor gingival bleeding in the population by more objective means may prove an important tool for self‐detection and early intervention for gingival inflammation with important public health impact on the onset and progression of periodontitis (Chapple et al., 2015; Tonetti, Jepsen, Jin, & Otomo‐Corgel, 2017). Such efforts may be particularly important in the context of the recently published proceeds of the World Workshop on classification that have clearly defined periodontal health (Lang & Bartold, 2018) and the critical importance of gingival inflammation in the diagnosis of gingivitis and the monitoring of treatment outcomes for both gingivitis and periodontitis (Caton et al., 2018; Chapple et al., 2018; Papapanou et al., 2018).
Clinically, periodontal inflammation measured as BOP has long been suggested to be the critical parameter for monitoring of periodontal health after treatment (Lang, Joss, & Tonetti, 1996) and has been incorporated in risk assessment algorithms (Lang, Suvan, & Tonetti, 2015; Lang & Tonetti, 2003; Tonetti, 1998). If confirmed, the preliminary finding that BOP can be predicted with a mHealth approach opens new avenues in the design and optimization of secondary prevention programmes, and SPC in particular.
While the study reports improvements in some periodontal parameters and associates them with the use of the App, these should be interpreted with caution due to the clinical audit nature of this report. Further interventional studies are required to elucidate validity and effectiveness.
mHealth, IoT networks and artificial intelligence are changing health care. The recent WHO director general report to the 71st World Health Assembly has provided the social and technical framework for the use of appropriate digital technologies for public health and the overall priority areas from the perspective of strengthening the health systems and moving towards universal health coverage (WHO‐Director‐General, 2018). Several healthcare fields have seen tremendous progress and efforts, and this has led to the accumulation of significant scientific evidence on the impact of mHealth to radically change systems. These have been recently summarized through a formal evidence‐based guideline by the WHO (Who, 2019). In the recommendations, which embrace implementation in a series of priority areas of application such as maternal, reproductive and child health, few aspects seem to be particularly relevant for developments in oral health care: (a) improving continuity of care; (b) providing tailored support, guidance and information and giving a sense of direction, reassurance and motivation; and (c) increasing participation, independence and self‐care. One of the key issues is in the field of preventive services: both self‐performed and professional. The recent S3 evidence‐based EFP guidelines on the treatment of periodontitis recommend emphasizing behavioural change for self‐performed oral hygiene, risk factor control and adherence to a professional SPC for long‐term periodontal health (Sanz et al., 2020). Adherence with SPC appointments, as an example, has been limited even among periodontal patients (Echeverria, Echeverria, & Caffesse, 2019). mHealth interventions and digital architectures could prove decisive to address these barriers.
CONFLICT OF INTEREST
MST is a member of the European Advisory Board of the Procter & Gamble Company, the manufacturer of the intelligent power toothbrush and App that has been used in this study and has received personal fees not related to this work. Susanne Thurnay is an employee of the Procter & Gamble Company. All other authors report no conflict of interest with this study.
Appendix 1.
An interactive prediction profiler for FMBS or FMPS at the SPC appointment as a function of the significant variables in the multiple linear mixed model analysis is available to further explore the data presented in Figures 3 and 4. Click on the icon to launch an html interactive tool.
Tonetti MS, Deng K, Christiansen A, et al. Self‐reported bleeding on brushing as a predictor of bleeding on probing: Early observations from the deployment of an internet of things network of intelligent power‐driven toothbrushes in a supportive periodontal care population. J Clin Periodontol. 2020;47:1219–1226. 10.1111/jcpe.13351
Funding information
This was an investigator‐initiated study that was funded in part by the European Research Group on Periodontology, a Swiss public good foundation, and grant # 07182796 from the Human Medical Research Fund of Hong Kong. The intelligent toothbrushes and the App used in this study were provided by the Procter & Gamble Company.
REFERENCES
- Carra, M. C. , Detzen, L. , Kitzmann, J. , Woelber, J. P. , Ramseier, C. A. , & Bouchard, P. (2020). Promoting behavioural changes to improve oral hygiene in patients with periodontal diseases: A systematic review. Journal of Clinical Periodontology, 47(S22), 72–89. 10.1111/jcpe.13234 [DOI] [PubMed] [Google Scholar]
- Caton, J. , Armitage, G. , Berglundh, T. , Chapple, I. L. C. , Jepsen, S. K. S. B. L. M. , Papapanou, P. N. , & Sanz, M. M. (2018). A new classification scheme for periodontal and peri‐implant diseases and conditions ‐ introduction and key changes from the 1999 classification. Journal of Clinical Periodontology, 45(Suppl 20), S1–S8. [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Mealey, B. L. , Van Dyke, T. E. , Bartold, P. M. , Dommisch, H. , Eickholz, P. , … Yoshie, H. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S68–S77. [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. , Van Der Weijden, F. , Doerfer, C. , Herrera, D. , Shapira, L. , Polak, D. , … Graziani, F. (2015). Primary prevention of periodontitis: Managing gingivitis. Journal of Clinical Periodontology, 42(Suppl 16), S71–S76. [DOI] [PubMed] [Google Scholar]
- Echeverria, J. J. , Echeverria, A. , & Caffesse, R. G. (2019). Adherence to Supportive Periodontal Treatment. Periodontology, 2000(79), 200–209. [DOI] [PubMed] [Google Scholar]
- Kallio, P. (1996). Self‐assessed bleeding in monitoring gingival health among adolescents. Community Dentistry and Oral Epidemiology, 24, 128–132. [DOI] [PubMed] [Google Scholar]
- Kallio, P. , Nordblad, A. , Croucher, R. , & Ainamo, J. (1994). Self‐reported gingivitis and bleeding gums among adolescents in Helsinki. Community Dentistry and Oral Epidemiology, 22, 277–282. [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , & Bartold, P. M. (2018). Periodontal health. Journal of Clinical Periodontology, 45(Suppl 20), S9–S16. [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Joss, A. , & Tonetti, M. S. (1996). Monitoring disease during supportive periodontal treatment by bleeding on probing. Periodontology, 2000(12), 44–48. [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Suvan, J. E. , & Tonetti, M. S. (2015). Risk factor assessment tools for the prevention of periodontitis progression a systematic review. Journal of Clinical Periodontology, 42(Suppl 16), S59–S70. [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , & Tonetti, M. S. (2003). Periodontal risk assessment (Pra) for patients in supportive periodontal therapy (Spt). Oral Health & Preventive Dentistry, 1, 7–16. [PubMed] [Google Scholar]
- Lunde, P. , Nilsson, B. B. , Bergland, A. , Kvaerner, K. J. , & Bye, A. (2018). The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: Systematic review and meta‐analyses. Journal of Medical Internet Research, 20, E162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleary, T. J. , Drake, R. B. , & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43, 38. [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , Sanz, M. , Buduneli, N. , Dietrich, T. , Feres, M. , Fine, D. H. , … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S162–S170. [DOI] [PubMed] [Google Scholar]
- Peng, Y. , Wang, H. , Fang, Q. , Xie, L. , Shu, L. , Sun, W. , & Liu, Q. (2020). Effectiveness of mobile applications on medication adherence in adults with chronic diseases: A systematic review and meta‐analysis. Journal of Managed Care & Specialty Pharmacy, 26, 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Jover, V. , Sala‐Gonzalez, M. , Guilabert, M. , & Mira, J. J. (2019). Mobile apps for increasing treatment adherence: Systematic review. Journal of Medical Internet Research, 21, E12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, M. , Herrera, D. , Kebschull, M. , Chapple, I. L. C. , Jepsen, S. , Berglundh, T. , … Tonetti, M. S. (2020). Treatment of stage I‐Iii periodontitis –the Efp S3 level clinical practice guideline. Journal of Clinical Periodontology, 47(Suppl 22), 4–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti, M. S. (1998). Cigarette smoking and periodontal diseases: Etiology and management of disease. Annals of Periodontology, 3, 88–101. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Eickholz, P. , Loos, B. G. , Papapanou, P. , Van Der Velden, U. , Armitage, G. , … Suvan, J. E. (2015). Principles In prevention of periodontal diseases: consensus report of group 1 of the 11th european workshop on periodontology on effective prevention of periodontal and peri‐implant diseases. Journal of Clinical Periodontology, 42(Suppl 16), S5–S11. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Jepsen, S. , Jin, L. , & Otomo‐Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. [DOI] [PubMed] [Google Scholar]
- Tonetti, Maurizio S. , Henry, Greenwell , & Kornman, Kenneth S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45, S149–S161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Tonetti, Maurizio S. , & Mariano, Sanz (2019). Implementation of the new classification of periodontal diseases: Decision‐making algorithms for clinical practice and education. Journal of Clinical Periodontology, 46(4), 398–405. 10.1111/jcpe.13104 [DOI] [PubMed] [Google Scholar]
- Toniazzo, M. P. , Nodari, D. , Muniz, F. , & Weidlich, P. (2019). Effect of mhealth in improving oral hygiene: A systematic review with meta‐analysis. Journal of Clinical Periodontology, 46, 297–309. [DOI] [PubMed] [Google Scholar]
- Wang, Q. T. , Wu, Z. F. , Wu, Y. F. , Shu, R. , Pan, Y. P. , & Xia, J. L. (2007). Epidemiology and preventive direction of periodontology in China. Journal of Clinical Periodontology, 34, 946–951. [DOI] [PubMed] [Google Scholar]
- Weintraub, J. A. , Finlayson, T. L. , Gansky, S. A. , Santo, W. , & Ramos‐Gomez, F. (2013). Clinically determined and self‐reported dental status during and after pregnancy among low‐income hispanic women. Journal of Public Health Dentistry, 73, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2019). Who Guideline: Recommendations On Digital Interventions For Health System Strengthening, Geneva: https://www.who.int/reproductivehealth/publications/digital‐interventions‐health‐system‐strengthening/en/ [PubMed] [Google Scholar]
- WHO‐Director‐General (2018). Mhealth. Use Of Appropriate Digital Technologies For Public Health. Report To The 71st World Health Assembly. World Health Organization.https://apps.who.int/iris/handle/10665/274134 [Google Scholar]


