Abstract
Aim
To determine the glucose‐independent effect of the dipeptidyl peptidase‐4 (DPP‐4) inhibitor linagliptin versus the sulphonylurea glimepiride on systemic haemodynamics in the fasting and postprandial state in patients with type 2 diabetes (T2D).
Materials and Methods
In this prespecified secondary analysis of a phase IV, double‐blind trial, 46 metformin‐treated, overweight patients with T2D were included and randomly assigned (1:1) to once‐daily linagliptin (5 mg) or glimepiride (1 mg) for 8 weeks. In a sub‐study involving 26 patients, systemic haemodynamics were also assessed following a standardized liquid meal (Nutridrink Yoghurt style). Systemic haemodynamics (oscillometric device and finger photoplethysmography), arterial stiffness (applanation tonometry) and cardiac sympathovagal balance (heart rate variability [HRV]) were measured in the fasting state and repetitively following the meal. Ewing tests were performed in the fasting state.
Results
From baseline to week 8, linagliptin compared with glimepiride did not affect systemic haemodynamics, arterial stiffness or HRV in the fasting state. Linagliptin increased parasympathetic nervous activity, as measured by the Valsalva manoeuvre (P = .021) and deep breathing test (P = .027) compared with glimepiride. Postprandially, systolic blood pressure (SBP) dropped an average of 7.6 ± 1.6 mmHg. Linagliptin reduced this decrease to 0.7 ± 2.3 mmHg, which was significant to glimepiride (P = .010).
Conclusions
When compared with glimepiride, linagliptin does not affect fasting blood pressure. However, linagliptin blunted the postprandial drop in SBP, which could benefit patients with postprandial hypotension.
Keywords: DPP‐4 inhibitor, glimepiride, haemodynamics, heart rate, linagliptin, sulphonylurea, sympathetic nervous system, type 2 diabetes
1. INTRODUCTION
Inhibitors of the enzyme dipeptidyl peptidase‐4 (DPP‐4) are widely used for the treatment of hyperglycaemia in type 2 diabetes (T2D). These agents reduce the degradation of, among others, glucagon‐like peptide‐1 (GLP‐1), thereby increasing insulin and reducing glucagon secretion. Although developed for glucose lowering, DPP‐4 inhibitors have been shown to exert several pleiotropic effects, which include modest reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of ~3 and 1.5 mmHg, respectively. 1
Whether DPP‐4 inhibitors decrease postprandial blood pressure (BP) (and potentially augment postprandial hypotension [PPH], as we previously showed with sitagliptin 2 ), or improve the postprandial BP drop (as described in two case reports in older patients and patients with dementia 3 , 4 ) is unknown. PPH is defined as a decrease in SBP of ≥20 mmHg or a decrease below 90 mmHg from ≥100 mmHg within 2 hours after a meal, is highly prevalent in patients with T2D, 5 and is associated with postprandial dizziness and collapse. 5 Moreover, PPH is a risk factor for arteriosclerosis, and seems to increase the cardiovascular and all‐cause mortality risk. 5 Although the pathophysiology has yet to be fully delineated, PPH is believed to result from inadequate cardiovascular compensation of the normal postmeal decrease in vascular resistance. 3 , 6 In diabetes, a lack of sympathetic activation may drive this inadequate response. 6
Mechanisms that underlie a potential effect of DPP‐4 inhibitors on BP or PPH in patients with T2D have not been studied systematically, but may include effects that affect arterial stiffness, urinary sodium excretion, or the cardiovascular autonomic nervous system (ANS).
While GLP‐1 receptor‐mediated effects may underlie the actions of this drug class, vasopressor actions may also be effectuated by preservation of active forms of the numerous other substrates that are degraded by DPP‐4 (such as the vasoactive stromal cell‐derived factor [SDF]‐1α, neuropeptide Y [NPY] and substance‐P). 7 , 8 , 9
In previous trials we assessed the effect of the DPP‐4 inhibitor sitagliptin on systemic haemodynamics in patients with T2D compared with placebo. However, because glucose lowering per se could influence systemic haemodynamics, given that glucose appears to be a vasodilator, 10 the former design does not allow sufficient interpretation of drug‐specific effects. As such, to attain glycaemic equipoise, and to allow for clinically relevant comparisons, the sulfonylurea (SU) glimepiride was selected as an active comparator in the current trial. Therefore, we assessed the effects of 8 weeks of treatment with linagliptin compared with glimepiride on fasting and postprandial haemodynamics in overweight patients with T2D.
2. MATERIALS AND METHODS
2.1. Trial design
This is a secondary analysis of the RENALIS (RENoprotection in diAbetes by LInagliptin versus Sulfonylurea) trial. RENALIS was a phase IV, randomized, double‐blind, comparator‐controlled, parallel‐group, mechanistic intervention study conducted at the Amsterdam University Medical Center (location VUmc) in Amsterdam, the Netherlands, between May 2014 and April 2016. The co‐primary endpoint was change in glomerular filtration rate and effective renal plasma flow; the systemic haemodynamic endpoints were assessed as secondary. The study was registered at ClinicalTrials.gov (NCT02106104), where all endpoints are described. After screening, inclusion, and a 6‐week run‐in period, baseline testing was performed as described below. Then 8 weeks of treatment commenced, followed by endpoint measurements. All participants underwent fasting measurements; following a study amendment, postprandial assessments were added for the remaining enrolled patients (N = 26). The trial protocol and its amendments were approved by the local institutional review and ethics committee, competent local authorities, and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice. All participants provided written informed consent before participation.
2.2. Study population
Forty‐eight Caucasian males and postmenopausal females with T2D were recruited using advertisements. Relevant inclusion criteria were age 35 to 75 years, an HbA1c of 6.5%‐9.0% (48‐75 mmol/mol) and body mass index (BMI) ≥25 kg/m2. Patients were treated with metformin alone (or low‐dose SU derivative that could be safely washed out at the investigators discretion). In case of hypertension (defined as >140/90 mmHg) and/or albuminuria, treatment included a renin‐angiotensin‐aldosterone‐blocker (stable dose) for ≥3 months. Exclusion criteria included a history of pancreatic, active liver or malignant disease, diagnosis of a major cardiovascular event in the 6 months before screening, estimated GFR (eGFR) <60 mL/minute/1.73m2, or use of diuretics that could not be stopped 3 months prior to or during the intervention period.
2.3. Intervention and randomization
After baseline measurements, participants were randomly assigned in a 1:1 ratio (with a block size of four; performed by an independent trial pharmacist using computer‐generated numbers) to linagliptin 5 mg QD (Trajenta, Boehringer Ingelheim Pharma GmbH & Co., Ingelheim am Rhein, Germany) or glimepiride 1 mg QD added to ongoing metformin (dose unchanged throughout the study). Patients were instructed to take their study drug daily at the same time in the evening with water. The investigational medicinal products were over‐encapsulated, producing visually identical oral capsules (ACE Pharmaceuticals, Zeewolde, the Netherlands); patients and investigators remained blinded to treatment status until database lock.
2.4. Study protocol and endpoint measurements
The study protocol and endpoint measurements are detailed in Appendix S1 (see the supporting information). In brief, after an overnight fast, endpoint measurements were performed. In 26 patients, these measurements were repeated after intake of a standardized liquid meal (Nutridrink Yoghurt style, Nutricia; energy 150 kcal, 5.8 g fat, 18.7 g carbohydrates and 5.9 g protein).
2.4.1. BP and heart rate
SBP and DBP, mean arterial pressure and heart rate (HR) were measured by a trained observer using an automatic oscillometric device (Dinamap, GE Healthcare, Little Chalfont, UK).
2.4.2. Systemic haemodynamic functions
Stroke volume (SV), cardiac output (CO) and systemic vascular resistance (SVR) were calculated non‐invasively by a beat‐to‐beat finger arterial photoplethysmography BP‐monitoring device (Nexfin, Amsterdam, The Netherlands). These variables were normalized to body surface area, to acquire SV index (SVI), cardiac index (CI) and SVR index (SVRI), respectively.
2.4.3. Pulse wave analysis
Pulse wave analysis (PWA) was performed at the level of the radial artery using applanation tonometry with a high‐fidelity micromanometer (SPT‐301, Millar Instruments, Houston, TX, USA) coupled to a SphygmoCor apparatus and version 6.31 software (Atcor Medical Pty Ltd, West Ryde, Australia).
2.4.4. Heart rate variability assessments
Using an electrocardiogram (ECG)‐equipped Nexfin device, 5‐minute recordings were obtained in the resting state. These ECG strips were entered into Kubios HRV Analysis Software 2.1 (University of Eastern Finland, Biosignal Analysis and Medical Imaging Group, Kuopio, Finland), where fast Fourier spectral analyses were performed on intervals between R‐peaks to obtain cardiac ANS balance.
2.4.5. Cardiovascular reflex tests
In the fasting state, cardiovascular reflex tests (CARTs) were performed after the heart rate variability (HRV) assessment. The following CARTs were employed in this study: cyclic deep breathing, Valsalva manoeuvre and the orthostatic test. HR and BP were recorded using the Nexfin device.
2.4.6. Renal sodium excretion
Inulin‐based fractional sodium excretion (FENA) was measured, as described previously. 11
2.4.7. Laboratory measurements
Venous plasma glucose, sodium, inulin, insulin, glucagon, SDF‐1α, NPY (pro and active) and substance‐P were measured as described in Appendix S1.
2.5. Sample size calculation, data management and statistics
As current data are secondary and exploratory endpoints, no formal a priori power calculation was performed for these objectives. However, for the primary endpoints, it was calculated that 21 subjects per treatment arm would be needed (to detect a change in GFR of 15%, with an assumed standard deviation of 10 mL/minute, α = 0.05 and power [1‐β] of 80%). We increased this to 24 to allow for potential drop‐outs. All data were double‐entered into an electronic data management system (OpenClinica LLC, version 3.6, Waltham, MA, USA) and transferred to the final study database.
Paired t‐tests (Gaussian distributed data) or Wilcoxon signed rank tests (non‐Gaussian distributed data) were carried out for within‐group comparisons. Multivariable linear regression models were used to examine the effects of linagliptin versus glimepiride. Corresponding baseline values were added as independent variables, to correct for potential between‐group baseline differences. For the postprandial data, linear mixed models were used. Intervention and time were added as fixed factors, and the intervention‐by‐time interaction was the variable of interest. With these analyses, any difference in baseline values is taken into account and, moreover, it corrects for repeated measurements. In addition, the interaction with angiotensin‐converting enzyme (ACE) inhibitors was explored by adding ACE inhibitor use and the interaction between treatment allocation and ACE inhibitor use to the model. Spearman’s signed‐rank test was used to analyse associations between changes in variables. All analyses were performed using SPSS 22.0 (IBM SPSS, Chicago, IL, USA), and a two‐sided P‐value of <.05 was considered statistically significant. Data are presented as means ± SEM, median (IQR) or mean difference with a two‐sided 95% confidence interval, unless stated otherwise.
3. RESULTS
Demographic and clinical characteristics were well balanced between treatment groups (Table 1). Most patients received other treatments in addition to metformin, most relevantly antihypertensive agents. For the fasting study, the prescription pattern was similar in both treatment groups. For the postprandial study, the linagliptin‐assigned patients used more ACE inhibitors, beta‐blockers and calcium‐blockers.
TABLE 1.
Demographic and baseline clinical characteristics in the main study, and postprandial sub‐study
| Variable | Fasting (main study) | Postprandial (sub‐study) | ||
|---|---|---|---|---|
| Linagliptin 5 mg (N = 23) | Glimepiride 1 mg (N = 23) | Linagliptin 5 mg (N = 13) | Glimepiride 1 mg (N = 13) | |
| Age, y | 62.4 ± 9.2 | 63.5 ± 7.9 | 62.8 ± 7.5 | 66.4 ± 6.4 |
| Male, n (%) | 20 (87.0) | 18 (78.3) | 13 (100) | 9 (69) |
| Current smoker, n (%) | 5 (21.7) | 5 (21.7) | 3 (23.1) | 2 (15.4) |
| Diabetes duration, y | 7.6 ± 4.1 | 6.4 ± 5.3 | 7.7 ± 4.7 | 7.5 ± 5.5 |
| Body weight, kg | 101.5 ± 16.1 | 95.0 ± 14.5 | 104.9 ± 15.1 | 92.8 ± 15.0 |
| BMI, kg/m2 | 31.3 ± 4.2 | 30.1 ± 3.5 | 31.6 ± 4.9 | 30.4 ± 3.9 |
| HbA1c, % | 7.0 (6.6‐7.6) | 7.0 (6.7‐7.7) | 7.3 ± 0.9 | 7.4 ± 1.2 |
| HbA1c, mmol/mol | 53 (49‐60) | 53 (50‐61) | 56.5 ± 10.4 | 56.8 ± 12.6 |
| Fasting plasma glucose, mmol/L | 7.90 (7.30‐9.20) | 8.50 (7.00‐9.80) | 7.23 (6.76‐8.86) | 8.39 (6.84‐9.55) |
| eGFR‐MDRD, mL/minute/1.73m2 | 95.5 ± 17.2 | 91.3 ± 13.3 | 97.7 ± 16.1 | 90.5 ± 11.9 |
| Albumin‐creatinine ratio, mg/mmol | 0.80 (0.49‐3.60) | 1.11 (0.47‐3.71) | 0.63 (0.45‐5.25) | 0.89 (0.45‐2.53) |
| Metformin dose, mg | 1748 ± 764 | 1696 ± 726 | 1603 ± 694 | 1576 ± 772 |
| Use of | ||||
| ACE inhibitor (n [%]) | 8 (34.8) | 6 (26.1) | 6 (42.6) | 3 (23.1) |
| ARB (n [%]) | 8 (34.8) | 5 (21.7) | 4 (30.8) | 4 (30.8) |
| Beta‐blockers (n [%]) | 6 (26.1) | 5 (21.7) | 3 (23.1) | 1 (7.7) |
| Calcium‐blockers (n [%]) | 4 (17.4) | 4 (17.4) | 3 (23.1) | 2 (15.4) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐II receptor blocker; BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; HR, heart rate; SBP, systolic blood pressure; T2D, type 2 diabetes.
Data are mean ± SD or median (IQR), unless stated otherwise.
Of 70 patients screened, 48 were included and randomized (Appendix S2). In the linagliptin arm, one patient was excluded because of initiation of a disallowed co‐medication (oral corticosteroids) to manage an adverse effect (generalized pruritus) during the intervention period. In the glimepiride group, one participant was excluded because of technical issues during the testing days (Appendix S2). In a subset of 26 patients (N = 13 per treatment group), postprandial systemic haemodynamic data were available.
3.1. Fasting state (main study)
Both linagliptin and glimepiride tended to numerically reduce SBP and DBP from baseline to week 8, although this did not reach statistical significance (between‐group mean difference SBP 1.5 ± 2.7 mmHg, P = .583; and DBP 0.8 ± 1.6 mmHg, P = .514) (Figure 1 and Appendix S3). Similarly, no difference between linagliptin and glimepiride was seen at the safety visit after 4 weeks of treatment (Figure 1). There were no within‐group or between‐group differences in HR, SVI, CI, SVRI and augmentation index normalized to an HR of 75 beats/minute (AIX@HR75) from baseline to week 8.
FIGURE 1.
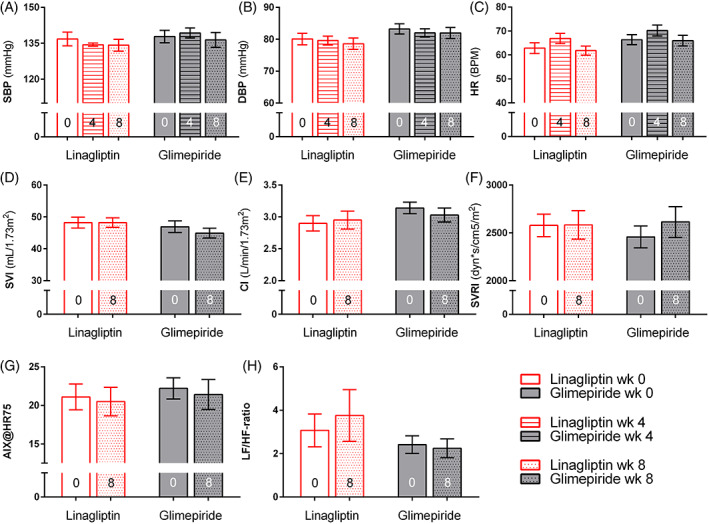
Effects in fasting study. Effects of linagliptin (red bars) and glimepiride (grey bars) on fasting haemodynamics: (A) systolic blood pressure; (B) diastolic blood pressure; (C) heart rate (HR); (D) stroke volume index (SVI); (E) cardiac index (CI); (F) systemic vascular resistance index (SVRI); (G) augmentation index normalized to an HR of 75 beats/minute (AIX@HR75); (H) ratio of low frequency to high frequency (LF/HF‐ratio); all values are expressed as mean ± SEM
Neither linagliptin (0.5 ± 1.5, P = .759) nor glimepiride (0.1 ± 0.4, P = .693) affected the LF/HF‐ratio (Figure 1 and Appendix S3). With linagliptin, the Valsalva ratio (median effect linagliptin −0.04 [IQR −0.22 to 0.08]; glimepiride −0.07 [−0.14 to 0.07]; P = .021) and E/I ratio (median effect linagliptin −0.01 [IQR −0.05 to 0.02]; glimepiride −0.02 [−0.24 to 0.01]; P = .027) decreased less compared with glimepiride. The other CARTs were not altered by either treatment.
Reductions in HbA1c were similar in the linagliptin (−0.45% ± 0.09%) and glimepiride (−0.65% ± 0.10%) groups after 8 weeks of administration (between‐group mean difference 0.17 ± 0.40; P = .101). At week 8, mean decreases in fasting plasma glucose were −1.17 ± 0.34 mmol/L with linagliptin and −1.54 ± 0.40 mmol/L with glimepiride (between‐group P = .817). Body weight slightly but non‐significantly increased with linagliptin (P = .059), although this was statistically less compared with glimepiride relative to baseline (between‐group mean difference − 0.8 kg; −1.5 to −0.1 kg; P = .022).
Only linagliptin increased FENA (+17 ± 7%; P = .050) after 8 weeks of administration, although this did not reach statistical significance compared with glimepiride relative to baseline. SDF‐1α was significantly reduced with linagliptin compared with glimepiride (−838 ± 65, P < .001) (Figure 2). Insulin, glucagon, NPY and substance‐P were not affected by either agent.
FIGURE 2.
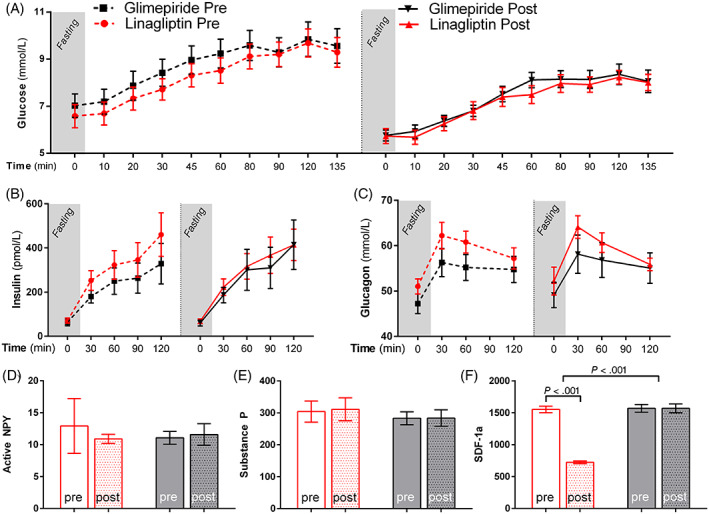
Effects on metabolic variables. Effects of linagliptin (red line, red bars) and glimepiride (black line, black bars) on glucose, hormones and peptides; the dashed lines indicate pretreatment values: (A) glucose, (B) insulin, (C) glucagon, (D) active NPY, (E) substance‐P and (F) SDF‐1α
Correction for the use of ACE inhibitors did not yield different results. Because no significant changes in BP were observed, no correlation analyses were performed.
3.2. Postprandial state (sub‐study)
Before randomization, in all patients meal ingestion induced a decrease in SBP and DBP of −7.6 ± 1.6 and −13.7 ± 2.7 mmHg, respectively. At baseline, only one patient (randomized to linagliptin) showed a drop in SBP of ≥20 mmHg, and as such could be classified as having PPH. The postprandial drop in BP at week 0 was accompanied by a decrease in SVRI (maximum decrease −572.3 ± 122.3 dyn*s/cm5/m2), while HR and SVI increased in all patients (maximum increase +11.6 ± 0.8 beats per minute [BPM], and 2.9 ± 0.7 mL/1.73m2, respectively; Appendix S4).
Linagliptin treatment for 8 weeks significantly blunted the maximum postprandial decrease in SBP (from −7.8 ± 2.3 to −0.7 ± 2.3 mmHg, P = .009; Figure 3 and Appendix S5); this was significant compared with glimepiride (between‐group difference: 8.4 ± 3.2 mmHg, P = .010). The patient with PPH did not meet the definition after 8 weeks of treatment with linagliptin. DBP was not affected by either linagliptin or glimepiride. Linagliptin increased HR compared with glimepiride in the postprandial state (maximum meal‐induced difference between linagliptin and glimepiride: 9.2 ± 2.5 BPM, P < .001), without affecting SVI or CI. SVR was not affected by either treatment. Although the mean AIX@HR75 was significantly higher in the glimepiride group at the start of each testing day, the meal‐induced changes from baseline were not significantly different between groups. Furthermore, no differences were observed in the LF/HF ratio.
FIGURE 3.
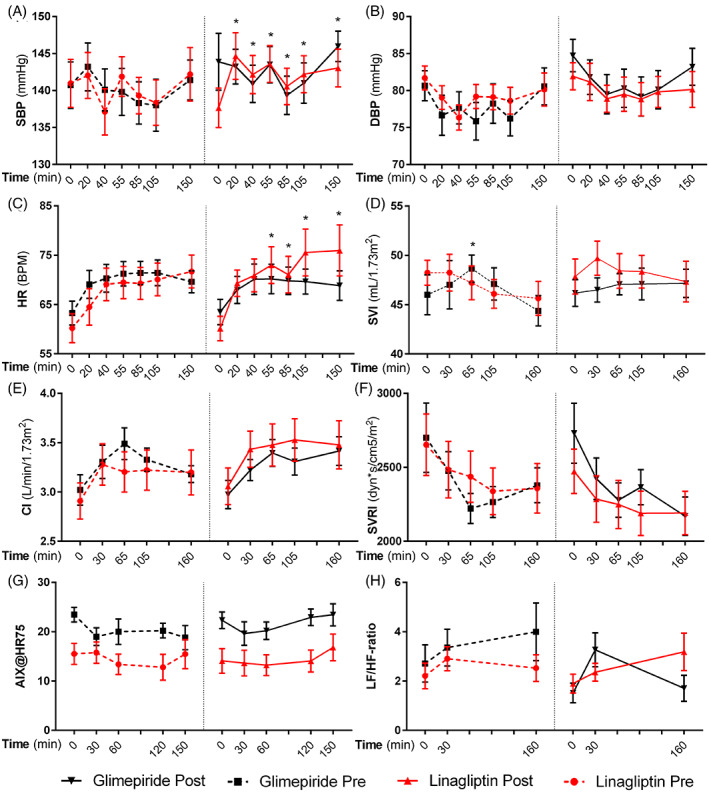
Effects in postprandial study. Effects of linagliptin (red line) and glimepiride (black line) on postprandial haemodynamics; the dashed lines indicate pretreatment values. The liquid meal was administered immediately after time point zero: (A) systolic blood pressure; (B) diastolic blood pressure; (C) heart rate (HR); (D) stroke volume index (SVI); (E) cardiac index (CI); (F) systemic vascular resistance index (SVRI); (G) augmentation index normalized to an HR of 75 beats/minute (AIX@HR75); (H) ratio of low frequency to high frequency (LF/HF ratio); all values are expressed as mean ± SEM. Significant differences between linagliptin and glimepiride are denoted as * (P < .05), within‐group differences (before and after treatment) as # (P < .05)
Although in this subgroup analysis both linagliptin and glimepiride improved HbA1c, this effect was more pronounced in the glimepiride‐treated patients (between‐group difference −0.3% ± 0.1%; P = .012). Correction for the use of ACE inhibitors was not applied because of low numbers of patients for this analysis. However, visually, the use of ACE inhibitors was not associated with different results. In order to try to understand why a decrease in SBP drop was seen with linagliptin, correlation analyses were performed (see Appendix S6). Apart from an inverse relation between SVI and DBP (R −0.727, P = .011) in the linagliptin‐treated group, these were not significant.
4. DISCUSSION
The current secondary analysis of a head‐to‐head mechanistic intervention study is the first to assess glucose‐independent effects of a DPP‐4 inhibitor versus an SU in patients with T2D without chronic kidney disease. We did not observe a significant difference in BP or HR in the fasting or postprandial state between linagliptin and glimepiride after 8 weeks of treatment; only modest numerical reductions in fasting BP were observed with both treatments. Interestingly, linagliptin reduced a postprandial decrease in SBP, which could make it a potentially interesting agent for postprandial hypotension to prevent symptoms and morbidity.
The current study was designed to not only assess effects on BP per se, but to focus on potential underlying mechanisms. Physiologically, BP is determined by the product of CO and SVR. Using non‐invasive techniques, we found no effect of prolonged linagliptin or glimepiride treatment on any of these variables in patients with T2D with well‐controlled baseline BP. A possible explanation could be that the observed effect size on BP in our study is too small to dissect changes in underlying mechanisms. Nevertheless, as per our design, we additionally assessed several variables which could change CO or SVR. First, linagliptin modestly increased renal sodium excretion, although this change was not significant compared with glimepiride. An effect of DPP‐4 inhibitors on FENA remains under debate as studies have provided conflicting results. 11 , 12 Interestingly, we previously observed that sitagliptin increased FENA after 2 weeks, yet this effect seemed to disappear after 12 weeks. 11 Because no effect of linagliptin on BP was seen in the within‐group analysis, and change from baseline in FENA was not correlated with change in BP (data not shown), we believe that the modest effect on FENA is probably not responsible for the reduction in BP as seen in meta‐analyses. 1 Second, we observed changes in the ANS. Although the LF/HF ratio was not affected, linagliptin increased the E/I ratio and the Valsalva ratio. Both reflect increased vagal activity during the CARTs. An increase in vagal tone could explain a reduction in BP, yet one would either expect a reduction in vascular tone or cardiac output, neither of which was observed here. Finally, the observed reduction in total SDF‐1α with linagliptin could explain a reduction in BP, as this peptide potentiates sympathetic activity. 8 However, DPP‐4 inhibition may be expected to increase, rather than decrease, active SDF‐1α, as previously shown. 12 , 13 Potentially, the currently observed decrease in SDF‐1α is an assay‐specific effect, because several other trials (vildagliptin, 14 , 15 sitagliptin 16 , 17 ) observed a similar decrease using the same ELISA as used here. Further studies on the effect of DPP‐4 inhibition on SDF‐1α and the relation with BP are warranted.
In addition to systemic haemodynamics, we measured variables of arterial stiffness by PWA. After 8 weeks, we observed no effects of linagliptin on AIX@HR75, a widely used standardized marker of arterial stiffness, which contrasts with the results of previous studies. For example, linagliptin and alogliptin reduced aortic pulse wave velocity, indicating reduced vascular stiffness, 18 , 19 while vildagliptin and sitagliptin improved AIX@HR75 in patients with T2D compared with baseline. 20 Potential explanations for the different results are treatment duration (only 8 weeks in our study, compared with 3 months or more in previous trials), and the use of an active comparator in the current trial.
Based on previous and current data, DPP‐4 inhibitors have at best only limited systemic haemodynamic effects in patients with T2D that do not differ from traditional glucose‐lowering drugs such as SUs. It is important to note that the small effect sizes observed in our trial are in the same range as in a large meta‐analysis 1 and cardiovascular outcome trials (CVOTs). It is probable that the minor changes do not have clinical consequences for patients at high cardio‐renal risk. Although primarily designed as safety studies, the landmark CVOTs involving DPP‐4 inhibitors versus placebo (CARMELINA for linagliptin, EXAMINE for alogliptin, SAVOR‐TIMI 53 for saxagliptin and TECOS for sitagliptin) did not find a beneficial effect on cardiovascular events or hard renal endpoints. 21 , 22 , 23 , 24 Moreover, in the recent landmark CAROLINA trial involving 6033 high‐risk patients with T2D, linagliptin was non‐inferior to glimepiride when added to usual care after a median follow‐up of 6.3 years. 25 The current study is in line with these large trials, as we found no mechanistic signs that linagliptin would be beneficial for or detrimental to the cardiovascular system.
Significant postprandial reductions in BP frequently occur in patients with T2D, and PPH may occur in up to 70% of patients. 26 PPH is a condition with symptoms ranging from postprandial dizziness to collapse and cardiovascular death. 5 A recent study found PPH to be associated with a hazard ratio of 11.2 to develop cardiovascular disease. 27 Despite its prevalence and consequences, no therapeutic strategy is available. Because two case reports have suggested the efficacy of vildagliptin and sitagliptin for improving PPH symptoms, 3 , 4 we performed a subgroup analysis to assess the effects of linagliptin on postprandial BP. Here, the decrease in SBP following the standardized liquid meal was blunted. Moreover, this effect was statistically significant compared with glimepiride. Theoretically, a decreased drop in SBP might be explained by an increase in CI and/or SVRI. However, in this study, CI and SVRI were not significantly affected by the study treatment (nor was a trend visible), and moreover, changes in these variables were not correlated with changes in BP. Changes in measured hormones were also not correlated with BP, with the caveat that we did not assess these hormones in the postprandial setting, nor did we assess concentrations in the portal vein (where they may directly affect neuronal pathways involved in systemic haemodynamic regulation). 28 Whether GLP‐1, the most studied hormone when regarding DPP‐4 inhibition, might be involved is unclear, as data with endogenous GLP‐1 or the GLP‐1 receptor antagonist exendin (9‐39) are lacking. Interestingly, infusion of GLP‐1 peptide to reach postprandial levels (~30 pmol/L), 29 as well as treatment with the short‐acting GLP‐1 receptor agonist liraglutide, 30 decreases the postprandial drop in BP. However, this is presumably caused by reducing gastric emptying speed. It is important to note that gastric emptying has been identified as a major target for PPH, as a reduced glucose delivery to the duodenum reduces PPH symptoms. 31 However, all current data argue against an inhibiting effect of DPP‐4 inhibitors on gastric emptying rate. 32 To understand which hormone might be involved in preventing PPH, we need studies with receptor antagonists after meal ingestion during DPP‐4 inhibition. Finally, it is important to observe that the glimepiride‐treated patients used fewer antihypertensive agents, and had better glycaemic control after 8 weeks. Both variables are associated with less PPH, indicating that the difference between linagliptin and glimepiride might even be bigger.
Fascinatingly, although case reports suggest beneficial effects of DPP‐4 inhibitors on PPH, 3 , 4 the available trials are conflicting. Stevens et al observed no effect on postprandial BP after two doses of sitagliptin. 33 Our own group previously showed that 12 weeks of sitagliptin versus placebo augments the postprandial drop in DBP. 2 Moreover, Wilson et al recently reported that 1 week of sitagliptin augments the drop in mean arterial pressure compared with placebo, but only when combined with an ACE inhibitor, and not during angiotensin II or calcium channel blockage. 34 This interaction probably depends on hormones/peptides degraded by both DPP‐4 and ACE, such as substance‐P and NPY. Finally, in the current trial, linagliptin diminishes the meal‐induced drop in DBP. The reason behind these very different trial results remains unclear. One explanation could be the use of co‐medication. ACE inhibitors are an obvious option 34 ; however, arguing against this, we found that sitagliptin augments while linagliptin diminishes the postprandial BP drop, while the sitagliptin group used fewer ACE inhibitors (25% vs. 42%, respectively). 2 Other co‐medication, such as beta‐blockers, might still be involved. Another explanation could lie in differences in the meal used in the study (e.g. meal size, 35 differences in macronutrients, 36 and possibly meal consistency 37 ), which is known to affect BP response. 5 Also, compound‐specific effects might be involved, i.e. effects not generalizable to all DPP‐4 inhibitors, and comprise (for example) structural differences and different selectivity to DPP‐4. 38 However, because this is the first study with linagliptin, and trials with many other DPP‐4 inhibitors are lacking, conclusions cannot be drawn yet. As the burden of PPH in patients with T2D is high, and no therapy for this condition is currently at hand, further studies that assess the effects of DPP‐4 inhibitors on postprandial systemic haemodynamics in different populations are warranted.
Our study has several limitations that merit consideration. First, all employed vascular measurement techniques are non‐invasive. Importantly, for determination of CI, the non‐invasive arterial BP monitoring device has been well validated against intra‐arterial measurements. 39 Second, we limited the measurements of ANS activity to Ewing tests and HRV. Other measures of sympathetic activity (e.g. muscle sympathetic nervous activity or plasma norepinephrine) are possible. Finally, the sample size was comparatively small, especially for the postprandial sub‐study. This could have caused type II statistical errors. To reduce any further risk of false‐negative findings, we decided not to correct for multiple testing, allowing the possibility that the observed changes are the result of a type I error.
We conclude that 8 weeks of treatment with the DPP‐4 inhibitor linagliptin or SU glimepiride in overweight patients with T2D has no effect upon fasting BP. Interestingly, in the postprandial state, linagliptin blunted the drop in SBP, which could decrease postprandial dizziness, collapse and cardiovascular mortality in patients with PPH, and thus requires further study.
CONFLICT OF INTEREST
M.H.A.M. is a speaker/consultant for AstraZeneca, Eli Lilly & Co., Novo Nordisk and Sanofi. Through M.H.H.K., the Amsterdam University Medical Centers, location VUmc, received research grants from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi‐Aventis. D.H.v.R. serves on advisory boards of Sanofi‐Aventis and Merck Sharp & Dohme. All the authors declare that they did not receive personal fees in connection with the roles described above; all honoraria were paid to their employer (the Amsterdam University Medical Centers, location VUmc). No other potential conflicts of interest relevant to this article are reported.
AUTHOR CONTRIBUTIONS
M.H.A.M. participated in the design and planning of the study, coordinated the test visits and performed the measurements, contributed to the discussion and manuscript writing. J.K. analysed the data and drafted the manuscript. M.M.S. helped with data collection, analysed the data and drafted the manuscript. L.T. and D.M.O. helped with data collection, and contributed to the discussion and manuscript writing. M.H.H.K. and D.H.v.R. contributed to the discussion and edited the manuscript. All authors have approved the final version of this manuscript. All authors had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors extend their gratitude to all study participants who took part in this study for their time and commitment to the demanding protocol. We thank Prof. Michaela Diamant for her commitment to this study up to her passing in 2014. Furthermore, we thank the study nurses for their excellent practical support during the conduct of this study, with special thanks to Sandra Gassman and Jeannette Boerop (Diabetes Center, Department of Internal Medicine, Amsterdam University Medical Centers, location VUmc, Amsterdam, The Netherlands). Funding for this investigator‐initiated study was provided by Boehringer Ingelheim. The funder had no role in the study design, the analyses or interpretation of the data, or drafting the manuscript. The funder had no role in the decision to submit this manuscript for publication.
Kraaijenhof J, Muskiet MHA, Tonneijck L, et al. Effects of dipeptidyl peptidase‐4 inhibitor linagliptin versus sulphonylurea glimepiride on systemic haemodynamics in overweight patients with type 2 diabetes: A secondary analysis of an 8‐week, randomized, controlled, double‐blind trial. Diabetes Obes Metab. 2020;22:1847–1856. 10.1111/dom.14107
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14107.
Funding information Funding for this investigator‐initiated study was provided by Boehringer Ingelheim.
REFERENCES
- 1. Zhang X, Zhao Q. Effects of dipeptidyl peptidase‐4 inhibitors on blood pressure in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hypertens. 2016;34(2):167‐175. [DOI] [PubMed] [Google Scholar]
- 2. Smits MM, Tonneijck L, Muskiet MHA, et al. The effects of GLP‐1 based therapies on postprandial haemodynamics: two randomised, placebo‐controlled trials in overweight type 2 diabetes patients. Diabetes Res Clin Pract. 2017;124:1‐10. [DOI] [PubMed] [Google Scholar]
- 3. Yonenaga A, Ota H, Honda M, et al. Marked improvement of elderly postprandial hypotension by dipeptidyl peptidase IV inhibitor. Geriatr Gerontol Int. 2013;13(1):227‐229. [DOI] [PubMed] [Google Scholar]
- 4. Saito Y, Ishikawa J, Harada K. Postprandial and orthostatic hypotension treated by Sitagliptin in a patient with dementia with Lewy bodies. Am J Case Rep. 2016;17:887‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trahair LG, Horowitz M, Jones KL. Postprandial hypotension: a systematic review. J Am Med Dir Assoc. 2014;15(6):394‐409. [DOI] [PubMed] [Google Scholar]
- 6. Smits MM, Muskiet MHA, Tushuizen ME, et al. Uncomplicated human type 2 diabetes is associated with meal‐induced blood pressure lowering and cardiac output increase. Diabetes Res Clin Pract. 2014;106(3):617‐626. [DOI] [PubMed] [Google Scholar]
- 7. Unger T, Rascher W, Schuster C, et al. Central blood pressure effects of substance P and angiotensin II: role of the sympathetic nervous system and vasopressin. Eur J Pharmacol. 1981;71(1):33‐42. [DOI] [PubMed] [Google Scholar]
- 8. Wei S‐G, Zhang Z‐H, Yu Y, Weiss RM, Felder RB. Central actions of the chemokine stromal cell‐derived factor 1 contribute to Neurohumoral excitation in heart failure rats. Hypertension. 2012;59(5):991‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westfall TC, Martin J, Chen X, et al. Cardiovascular effects and modulation of noradrenergic neurotransmission following central and peripheral administration of neuropeptide Y. Synapse. 1988;2(3):299‐307. [DOI] [PubMed] [Google Scholar]
- 10. van Veen S, Frölich M, Chang PC. Acute hyperglycaemia in the forearm induces vasodilation that is not modified by hyperinsulinaemia. J Hum Hypertens. 1999;13(4):263‐268. [DOI] [PubMed] [Google Scholar]
- 11. Tonneijck L, Smits MM, Muskiet MHA, et al. Renal effects of DPP‐4 inhibitor sitagliptin or GLP‐1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2016;39(11):2042‐2050. [DOI] [PubMed] [Google Scholar]
- 12. Lovshin JA, Rajasekeran H, Lytvyn Y, et al. Dipeptidyl peptidase 4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF‐1α 1‐67 in patients with type 2 diabetes. Diabetes Care. 2017;40(8):1073‐1081. [DOI] [PubMed] [Google Scholar]
- 13. Fadini GP, Bonora BM, Cappellari R, et al. Acute effects of Linagliptin on progenitor cells, monocyte phenotypes, and soluble mediators in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):748‐756. [DOI] [PubMed] [Google Scholar]
- 14. Dei Cas A, Spigoni V, Cito M, et al. Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: a 12‐month randomized controlled trial in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park KS, Kwak S, Cho YM, et al. Vildagliptin reduces plasma stromal cell‐derived factor‐1α in patients with type 2 diabetes compared with glimepiride. J Diabetes Investig. 2017;8(2):218‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aso Y, Jojima T, Iijima T, et al. Sitagliptin, a dipeptidyl peptidase‐4 inhibitor, increases the number of circulating CD34+CXCR4+ cells in patients with type 2 diabetes. Endocrine. 2015;50(3):659‐664. [DOI] [PubMed] [Google Scholar]
- 17. Goodwin SR, Reeds DN, Royal M, Struthers H, Laciny E, Yarasheski KE. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J Clin Endocrinol Metab. 2013;98(2):743‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Boer SA, Heerspink HJL, Juárez Orozco LE, et al. Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double‐blind, controlled 26‐week trial (RELEASE). Diabetes Obes Metab. 2017;19(8):1147‐1154. [DOI] [PubMed] [Google Scholar]
- 19. Kishimoto S, Kinoshita Y, Matsumoto T, et al. Effects of the dipeptidyl peptidase 4 inhibitor Alogliptin on blood pressure in hypertensive patients with type 2 diabetes mellitus. Am J Hypertens. 2019;32(7):695‐702. [DOI] [PubMed] [Google Scholar]
- 20. Duvnjak L, Blaslov K. Dipeptidyl peptidase‐4 inhibitors improve arterial stiffness, blood pressure, lipid profile and inflammation parameters in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2016;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of Linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk. JAMA. 2019;321(1):69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White W, Cannon C. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 23. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 24. Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232‐242. [DOI] [PubMed] [Google Scholar]
- 25. Rosenstock J, Kahn SE, Johansen OE, et al. Effect of Linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes. JAMA. 2019;322(12):1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanakaya M, Takahashi N, Takeuchi K, et al. Postprandial hypotension due to a lack of sympathetic compensation in patients with diabetes mellitus. Acta Med Okayama. 2007;61(4):191‐197. [DOI] [PubMed] [Google Scholar]
- 27. Jang A. Postprandial hypotension as a risk factor for the development of new cardiovascular disease: a prospective cohort study with 36 month follow‐up in community‐dwelling elderly people. J Clin Med. 2020;9(2):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh A. Dipeptidyl peptidase‐4 inhibitors: novel mechanism of actions. Indian J Endocrinol Metab. 2014;18(6):753‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trahair LG, Horowitz M, Stevens JE, et al. Effects of exogenous glucagon‐like peptide‐1 on blood pressure, heart rate, gastric emptying, mesenteric blood flow and glycaemic responses to oral glucose in older individuals with normal glucose tolerance or type 2 diabetes. Diabetologia. 2015;58(8):1769‐1778. [DOI] [PubMed] [Google Scholar]
- 30. Tonneijck L, Muskiet MHA, Twisk JW, et al. Lixisenatide versus insulin Glulisine on fasting and postbreakfast systemic hemodynamics in type 2 diabetes mellitus patients. Hypertension. 2018;72(2):314‐322. [DOI] [PubMed] [Google Scholar]
- 31. Jones KL, Tonkin A, Horowitz M, et al. Rate of gastric emptying is a determinant of postprandial hypotension in non‐insulin‐dependent diabetes mellitus. Clin Sci. 1998;94(1):65‐70. [DOI] [PubMed] [Google Scholar]
- 32. Smits MM, van Raalte DH, Tonneijck L, Muskiet MHA, Kramer MHH, Cahen DL. GLP‐1 based therapies: clinical implications for gastroenterologists. Gut. 2016;65(4):702‐711. [DOI] [PubMed] [Google Scholar]
- 33. Stevens JE, Buttfield M, Wu T, et al. Effects of sitagliptin on gastric emptying of, and the glycaemic and blood pressure responses to, a carbohydrate meal in type 2 diabetes. Diabetes Obes Metab. 2020;22(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 34. Wilson JR, Kerman SJ, Hubers SA, et al. Dipeptidyl peptidase 4 inhibition increases postprandial norepinephrine via substance P (NK1 receptor) during RAAS inhibition. J Endocr Soc. 2019;3(10):1784‐1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puvi‐Rajasingham S, Mathias CJ. Effect of meal size on post‐prandial blood pressure and on postural hypotension in primary autonomic failure. Clin Auton Res. 1996;6(2):111‐114. [DOI] [PubMed] [Google Scholar]
- 36. Vloet LC, Mehagnoul‐Schipper DJ, Hoefnagels WH, Jansen RW. The influence of low‐, normal‐, and high‐carbohydrate meals on blood pressure in elderly patients with postprandial hypotension. J Gerontol A Biol Sci Med Sci. 2001;56(12):M744‐M748. [DOI] [PubMed] [Google Scholar]
- 37. Berry MK, Russo A, Wishart JM, Tonkin A, Horowitz M, Jones KL. Effect of solid meal on gastric emptying of, and glycemic and cardiovascular responses to, liquid glucose in older subjects. Am J Physiol Liver Physiol. 2003;284(4):G655‐G662. [DOI] [PubMed] [Google Scholar]
- 38. Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase‐4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18(4):333‐347. [DOI] [PubMed] [Google Scholar]
- 39. Bogert LWJ, Wesseling KH, Schraa O, et al. Pulse contour cardiac output derived from non‐invasive arterial pressure in cardiovascular disease. Anaesthesia. 2010;65(11):1119‐1125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


