Abstract
Aims
Heart failure is traditionally classified by left ventricular ejection fraction (LVEF), rather than by left ventricular (LV) geometry, with guideline‐recommended therapies in heart failure with reduced ejection fraction (HFrEF) but not heart failure with preserved ejection fraction (HFpEF). Most patients with HFrEF have eccentric LV hypertrophy, but some have concentric LV hypertrophy. We aimed to compare clinical characteristics, biomarker patterns, and response to treatment of patients with HFrEF and eccentric vs. concentric LV hypertrophy.
Methods and results
We performed a retrospective post‐hoc analysis including 1015 patients with HFrEF (LVEF <40%) from the multinational observational BIOSTAT‐CHF study. LV geometry was classified using two‐dimensional echocardiography. Network analysis of 92 biomarkers was used to investigate pathophysiologic pathways. Concentric LV hypertrophy was present in 142 (14%) patients, who were on average older and more likely hypertensive compared to those with eccentric LV hypertrophy. Network analysis revealed that N‐terminal pro‐B‐type natriuretic peptide was an important hub in eccentric hypertrophy, whereas in concentric hypertrophy, tumour necrosis factor receptor 1, urokinase plasminogen activator surface receptor, paraoxonase and P‐selectin were central hubs. Up‐titration of beta‐blockers was associated with a mortality benefit in HFrEF with eccentric but not concentric LV hypertrophy (P‐value for interaction ≤0.001). For angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, the hazard ratio for mortality was higher in concentric hypertrophy, but the interaction was not significant.
Conclusion
Patients with HFrEF with concentric hypertrophy have a clinical and biomarker phenotype that is distinctly different from those with eccentric hypertrophy. Patients with concentric hypertrophy may not experience similar benefit from up.‐titration of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and beta‐blockers compared to patients with eccentric hypertrophy.
Keywords: Heart failure, Heart failure with reduced ejection fraction, Echocardiography, Left ventricular geometry, Concentric hypertrophy, Eccentric hypertrophy, Medical therapy, Biomarkers
Introduction
Remodelling of the left ventricle is one of the most important pathophysiological processes in heart failure. Four basic patterns of left ventricular (LV) remodelling have been recognized: normal geometry, concentric remodelling, concentric hypertrophy and eccentric hypertrophy. Current recommendations on LV chamber quantification advise to classify LV geometry by using echocardiographically determined LV mass index (LVMI) and relative wall thickness (RWT).1 Normal geometry is characterized by a normal LMVI and RWT. In concentric remodelling, only RWT is increased. In both concentric and eccentric hypertrophy, LVMI is increased, with a normal RWT in eccentric hypertrophy and an increased RWT in concentric hypertrophy. These four categories are illustrated in Figure 1. In this paper, we analysed differences between the two types of hypertrophy. Eccentric hypertrophy, associated with chronic volume overload, is characterized by increasing myocyte length, which ultimately results in a dilated left ventricle with thin walls.2 In contrast, in concentric hypertrophy, myocytes mainly increase in short‐axis diameter.3
Figure 1.
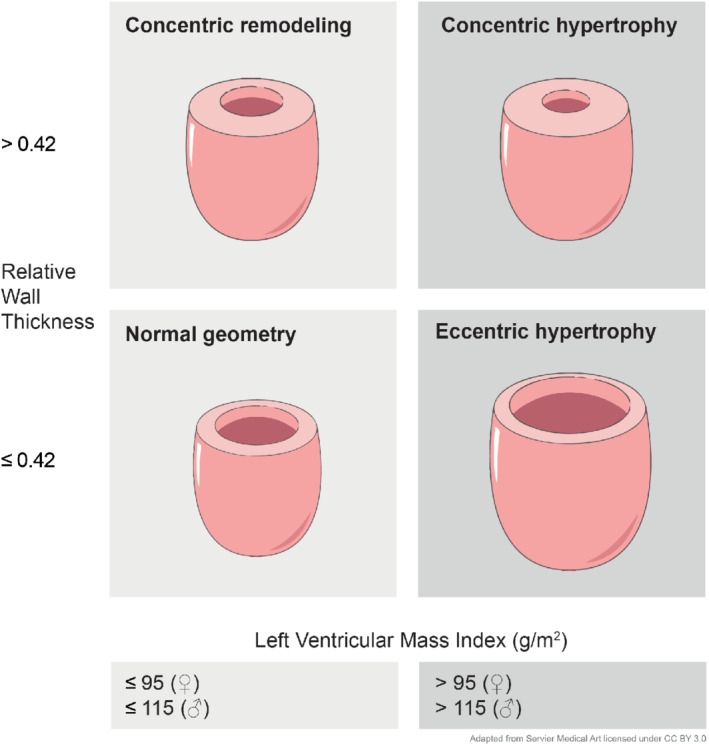
Basic patterns of left ventricular remodelling.
Despite recognition of the importance of LV remodelling, heart failure has traditionally been classified by LV ejection fraction (LVEF) rather than LV geometry. Recently it has been argued that more attention should be given to phenotyping heart failure beyond ejection fraction alone.4 The most common type of LV remodelling in heart failure with a reduced ejection fraction (HFrEF) is eccentric hypertrophy. However, a subset of HFrEF patients appear to have concentric hypertrophy.5 We aimed to investigate the clinical characteristics between these two patterns of LV hypertrophy in patients with HFrEF. In addition, we performed a network analysis using circulating biomarkers to gain insight into the underlying biological processes in the two LV geometry groups. Finally, we assessed whether the type of LV remodelling was associated with a differential response to guideline‐directed medical therapy in HFrEF.
Methods
Study population
We retrospectively analysed data from the BIOSTAT‐CHF (A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure) study. BIOSTAT‐CHF recruited patients with worsening heart failure from 11 European countries, between 2010 and 2015. Patients had to be anticipated to be up‐titrated with angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) and/or beta‐blockers to be eligible.6 The study complies with the Declaration of Helsinki and was approved by the medical ethics committees of all participating centres. All patients provided written informed consent. The index cohort consisted of a total of 2516 patients with heart failure, of which a subset of 1819 patients had an LVEF <40%. The echocardiographic variables that define LV geometry (LVMI and RWT) were present in 1304 of these patients (72%). Hypertrophy of either concentric or eccentric type was present in 1015 patients. Patients with normal geometry or concentric remodelling were not included in the present analysis since they are less common, and we were specifically interested in the pathophysiological differences between the two phenotypes of hypertrophy. Baseline characteristics of patients with normal geometry or concentric remodeling are provided in supplementary Table S3. A flowchart of patient selection is provided in the online supplementary Figure S6 . We validated the results of the network analysis in an independent validation cohort, consisting of 1738 patients from six centres in Scotland. The validation cohort included 730 patients with a LVEF <40%. Data on LV geometry were present in 328 of these patients (45%). Baseline characteristics of the validation cohort are provided in supplementary Table S1.
Echocardiography
Patients underwent two‐dimensional echocardiography using a commercially available echocardiograph with a 3.5 MHz probe. There was a median of two days between echocardiography and blood sampling. The examination was performed according to current guidelines and comprised: quantification of chamber and atrial dimensions and LV mass. Chamber function was calculated using LVEF according to the modified Simpson rule. Valvular function was evaluated using two‐dimensional and colour Doppler echocardiography. According to current guidelines, eccentric hypertrophy was defined as LVMI >95 g/m2 for women and >115 g/m2 for men with an RWT ≤0.42; concentric hypertrophy was defined as LVMI >95 g/m2 for women and >115 g/m2 for men with RWT >0.42.1
Laboratory analyses
A panel consisting of 92 cardiovascular disease‐related biomarkers (Olink CVD III panel) was measured in both the index and validation cohorts. Measurements were performed by Olink Bioscience (Uppsala, Sweden). The panel consists of known biomarkers of cardiovascular disease and inflammation, as well as several promising new biomarkers, selected to further elucidate cardiovascular pathophysiology. The panel uses a proximity extension assay, in which 92 oligonucleotide‐labelled antibodies can bind to their targets.7 Real‐time polymerase chain reaction was performed thereafter, resulting in semi‐quantitative normalized protein expression levels.
Statistical analyses
Network analysis
Network analysis of protein–protein correlations was performed using a method previously described.8 In brief, pairwise correlations between were calculated in the subsets with concentric and eccentric hypertrophy. P‐values were corrected for multiple testing using Benjamini–Hochberg procedure. Next, only significant correlations in both index and validation cohorts were retained. Because the concentric and eccentric subgroups had a large difference in numbers, an additional cut‐off based on correlation strength (R2 >0.4) was used. Network analysis with these lists of protein–protein interactions were performed using Cytoscape version 3.6.1 (Cytoscape Consortium, New York, NY, USA).9
Up‐titration
BIOSTAT included heart failure patients aged ≥18 years, who were using oral or intravenous diuretics at a dose of furosemide ≥40 mg or equivalent. At the time of inclusion, patients should not have been previously treated with evidence‐based therapies (ACEi/ARBs and beta‐blockers) or were receiving less than 50% of the target doses of these drugs. In addition, initiation or up‐titration of ACEi/ARBs or beta‐blockers needed to be anticipated by the treating physician. For this analysis, patients were considered to be successfully up‐titrated if they achieved at least 50% or more of the recommended treatment dose for ACEi/ARBs or beta‐blockers in the current European Society of Cardiology guidelines. To correct for treatment indication bias in all analyses estimating the effects of up‐titration of ACEi/ARBs and beta‐ blockers, we inversely weighted for the probability (IPW) of achieving the treatment.10 To determine IPW weights we first imputed missing values five times using R package mice. We then performed logistic regression on all five imputed datasets, using LASSO penalization to obtain parsimonious logistic models from an initial comprehensive list of 20 clinical and laboratory variables and the 92 Olink biomarkers. To find the optimal lambda in our LASSO regression, we performed 10 cross validations to increase robustness. The IPW weights were calculated by the average probability of achieving recommended treatment dose, determined by the LASSO regression models from all five imputed datasets for both ACEi/ARBs and beta‐blockers, separately. These IPW weights were then used in our final Cox proportional hazards models comparing survival in concentric vs. eccentric hypertrophy for both ACEi/ARBs and beta‐blockers.
Results
Baseline characteristics
Concentric hypertrophy was found in 142 of the 1015 patients (14%) (Figure 2). Baseline characteristics of patients with concentric vs. eccentric hypertrophy are shown in Table 1. Compared to eccentric hypertrophy, HFrEF patients with concentric hypertrophy were on average older (70 ± 11 vs. 67 ± 12 years, P = 0.005) and more likely to have a history of hypertension (77% vs. 58%, P < 0.001). Likewise, hypertension was also the common primary aetiology in concentric hypertrophy (20.4% vs. 8.3%, P < 0.001). In contrast, a primary aetiology of cardiomyopathy was more prevalent in eccentric hypertrophy (32.4% vs. 20.4%, P = 0.003). Primary aetiologies of ischaemic heart disease and valvular heart disease were not different between groups. Smoking history was comparable between concentric and eccentric hypertrophy as well. Renal disease [defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] was more prevalent (31% vs. 23%, P = 0.046) and mean eGFR was lower in patients with concentric hypertrophy (57.7 mL/min/1.73 m2 vs. 61.9 mL/min/1.73 m2, P = 0.037). The prevalence of atrial fibrillation was similar in concentric vs. eccentric hypertrophy (45.1% vs. 40.4%, P = 0.298). Mean ejection fraction was higher in concentric hypertrophy (30.2 ± 6.2 vs. 26.5 ± 7.0, P < 0.001) although all patients had LVEF <40% by definition. The prevalence of moderate–severe mitral valve regurgitation was lower in concentric hypertrophy (42% vs. 58% in eccentric hypertrophy, P < 0.001). Overal survival stratified by geometry group is given in supplementary Figure S1 (index cohort) and supplementary Figure S2 (validation cohort).
Figure 2.
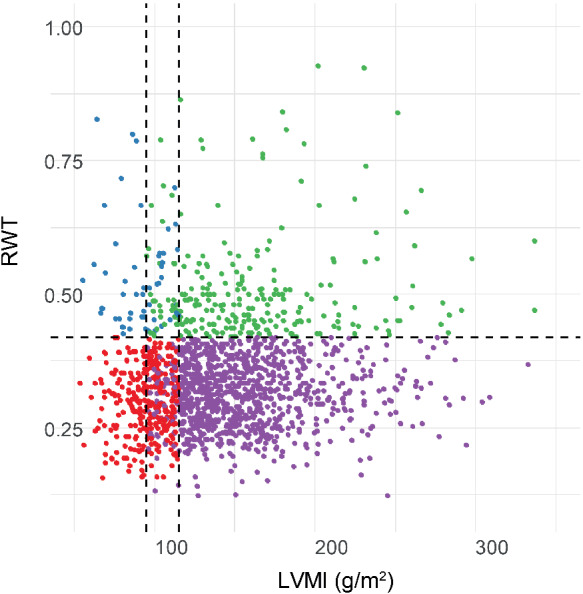
Scatterplot of 1015 patients with heart failure with reduced ejection fraction classified as normal (red), concentric remodelling (blue), concentric hypertrophy (green) and eccentric hypertrophy (purple). RWT, relative wall thickness; LVMI, left ventricular mass index.
Table 1.
Baseline characteristics
| Concentric hypertrohy (n = 142) | Eccentric hypertrophy (n = 873) | P‐value | |
|---|---|---|---|
| Age, years | 69.7 (11.4) | 66.5 (11.8) | 0.005 |
| Female sex | 43 (30.3%) | 220 (25.2%) | 0.200 |
| BMI, kg/m2 | 28.4 (5.3) | 27.5 (4.9) | 0.059 |
| SBP, mmHg | 129.4 (21.1) | 123.9 (21.3) | <0.001 |
| DBP, mmHg | 78.3 (12.9) | 75.7 (12.7) | 0.028 |
| NYHA class | 0.683 | ||
| I | 12 (8.5%) | 65 (7.4%) | |
| II | 62 (43.7%) | 419 (48.0%) | |
| III | 51 (35.9%) | 266 (30.5%) | |
| IV | 4 (2.8%) | 25 (2.9%) | |
| Not assessed | 13 (9.2%) | 98 (11.2%) | |
| Heart rate, bpm | 80.5 (18.0) | 80.1 (17.7) | 0.705 |
| Medical history | |||
| Myocardial infarction | 44 (31.0%) | 332 (38.0%) | 0.107 |
| PCI | 31 (21.8%) | 176 (20.2%) | 0.647 |
| CABG | 22 (15.5%) | 138 (15.8%) | 0.924 |
| Valvular surgery | 11 (7.7%) | 55 (6.3%) | 0.517 |
| Moderate–severe mitral regurgitation | 59 (41.5%) | 504 (57.9%) | <0.001 |
| Atrial fibrillation (history) | 64 (45.1%) | 353 (40.4%) | 0.298 |
| Atrial fibrillation (ECG) | 48 (33.8%) | 258 (29.7%) | 0.318 |
| Hypertension | 109 (76.8%) | 526 (60.3%) | <0.001 |
| Diabetes | 48 (33.8%) | 259 (29.7%) | 0.320 |
| COPD | 29 (20.4%) | 148 (17.0%) | 0.312 |
| Renal disease | 44 (31.0%) | 203 (23.3%) | 0.046 |
| Current malignancy | 5 (3.5%) | 16 (1.8%) | 0.190 |
| Device therapy | 0.025 | ||
| Pacemaker | 9 (6.3%) | 56 (6.4%) | |
| ICD | 4 (2.8%) | 90 (10.3%) | |
| CRT‐P | 0 (0%) | 17 (1.9%) | |
| CRT‐D | 7 (4.9%) | 47 (5.4%) | |
| Smoking history | 0.769 | ||
| None | 56 (39.4%) | 318 (36.5%) | |
| Past | 63 (44.4%) | 413 (47.4%) | |
| Current | 23 (16.2%) | 141 (16.2%) | |
| Primary aetiology | |||
| Ischaemic heart disease | 65 (45.7%) | 386 (44.2%) | 0.833 |
| Hypertension | 29 (20.4%) | 73 (8.3%) | <0.001 |
| Cardiomyopathy | 29 (20.4%) | 283 (32.4%) | 0.003 |
| Valvular heart disease | 7 (4.9%) | 50 (5.7%) | 0.676 |
| Other | 12 (8.4%) | 71 (8.1%) | 0.799 |
| Type of visit | 0.108 | ||
| Outpatient (scheduled) | 35 (24.6%) | 259 (29.7%) | |
| Outpatient (unscheduled) | 6 (4.2%) | 67 (7.7%) | |
| Inpatienthospitalization | 101 (71.1%) | 547 (62.7%) | |
| NT‐proBNP, ng/L | 3862 | 2493 | 0.006 |
| eGFR, mL/min/1.73 m2 | 57.7 | 61.9 | 0.037 |
| Urea, mmol/L | 12.4 | 11.6 | 0.140 |
| Haemoglobin, g/dL | 13.4 (2.0) | 13.4 (1.8) | 0.762 |
| ACEi/ARB use | 99 (69.7%) | 649 (74.3%) | 0.246 |
| Beta‐blocker use | 111 (78.2%) | 742 (85.0%) | 0.039 |
| MRA use | 71 (50.0%) | 524 (60.0%) | 0.025 |
| Diuretic use | 142 (100.0%) | 872 (99.9%) | 0.687 |
| LVEF (%) | 30.2 (6.2) | 26.5 (7.0) | <0.001 |
| LVEDD (mm) | 56.0 | 65.0 | <0.001 |
| LVMI (g/m2) | 157.1 | 147.8 | <0.001 |
| RWT | 0.47 | 0.31 | <0.001 |
| E/A ratio | 1.0 (0.7, 2.0) | 1.4 (0.7, 2.3) | 0.717 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacemaker; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RWT, relative wall thickness; SBP, systolic blood pressure.
Network analysis
Patients with concentric hypertrophy showed 37 significant correlations that could be successfully validated; of the total 37 correlations, 15 were unique to concentric hypertrophy. Patients with eccentric hypertrophy showed 28 significant correlations that could be successfully validated; of the total 37 correlations, 8 were unique to eccentric hypertrophy. Heatmaps of all protein–protein correlations are provided in the online supplementary Figures S4 and S5 . Protein–protein correlations that are unique to concentric and eccentric hypertrophy, respectively, are presented in Figure 3. For instance, the correlation between tumour necrosis factor receptor 1 (TNF‐R1) and interleukin‐2 receptor alpha was only present in the network analysis of concentric hypertrophy. The size of the 'hub' is related to the centrality of the hub in the network. In other words, proteins that are implicated in a high number of other protein–protein correlations are more important in the network, and plotted as larger hubs. The most important hubs in eccentric hypertrophy were N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and junctional adhesion molecule A (JAM‐A). In concentric hypertrophy, the main hubs were TNF‐R1, urokinase plasminogen activator surface receptor (U‐PAR), paraoxonase (PON3) and P‐selectin (SELP). The levels of the individual biomarkers used for the network analysis are presented in the online supplementary Table S2 .
Figure 3.

Results of network analyses depicting unique protein–protein correlations in heart failure with reduced ejection fraction HFrEF with concentric hypertrophy (A) and heart failure with reduced ejection fraction with eccentric hypertrophy (B). The size of the hub corresponds to the betweenness centrality, which signified the importance of the hub in the network. The larger the hub, the more important it is to the network. The edges (dotted lines) between the nodes represent the correlations with the corresponding coefficients. CASP‐3, caspase‐3; EPHB4, ephrin type B receptor 4 precursor; IGFBP, insulin‐like growth factor binding protein; ITGB2, integrin beta‐2 precursor; JAM‐A, junctional adhesion molecule A; LDL, low‐density lipoprotein; LTBR, lymphotoxin beta receptor; MMP2, matrix metalloproteinase 2; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI, plasminogen activator inhibitor; PDGF, platelet‐derived growth factor; PGLYRP‐1, peptidoglycan recognition protein‐1; PON3, paraoxonase 3; RETN, resistin; SELP, P‐selectin; TFF3, trefoil factor 3; TLT‐2, TREM‐like transcript 2; TNF‐R1, tumour necrosis factor receptor 1; TNF‐R2, tumour necrosis factor receptor 2; t‐pA, tissue‐type plasminogen activator; TR‐AP, tartrate‐resistant acid phosphatase; U‐PAR, urokinase plasminogen activator surface receptor; vWF, von Willebrand factor.
Effect of up‐titration
Figure 4 shows model estimated probability of survival, comparing successfully up‐titrated patients (having achieved ≥50% of guideline‐recommended dose) with patients who reached only <50% of guideline‐recommended dose for both beta‐blockers and ACEi. Unweighted Kaplan‐Meier curves are provided in supplementary Figure S3. Up‐titration of beta‐blockers was significantly associated with a survival benefit in patients with eccentric hypertrophy [hazard ratio 0.48, 95% confidence interval (CI) 0.40–0.58, P ≤ 0.001]. Such an effect could not be established in patients with concentric hypertrophy (hazard ratio 0.87, 95% CI 0.62–1.23, P = 0.41; P‐value for interaction <0.001). For ACEi/ARBs, successful up‐titration resulted in a hazard ratio of 0.38 (95% CI 0.31–0.45, P < 0.001) for all‐cause mortality in eccentric hypertrophy compared to 0.63 (95% CI 0.43–0.94, P = 0.02) for concentric hypertrophy. In contrast to beta‐blockers, there was no significant interaction between remodelling type and effects of up‐titration of ACEi/ARBs on outcome (P‐value for interaction = 0.68).
Figure 4.
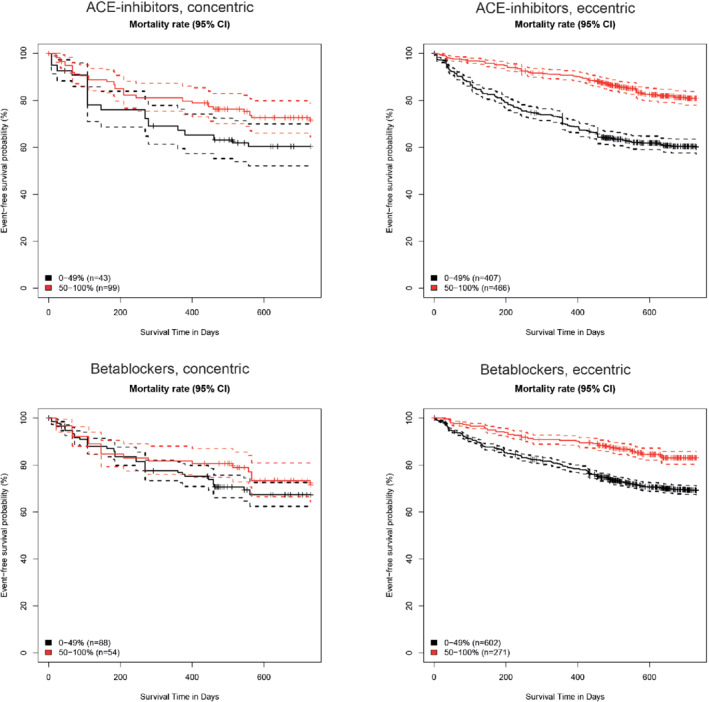
Adjusted mortality rate for patients receiving 0–49% or 50–100% of the recommended angiotensin‐coverting enzyme (ACE) inhibitor/angiotensin receptor blocker (top) or beta‐blocker dose (bottom). CI, confidence interval.
Discussion
From a large multinational longitudinal study we conclude that patients with HFrEF vary in clinical characteristics and pathophysiology, and might respond different to medical therapy according to LV geometry. The minority of patients with HFrEF had concentric LV hypertrophy. The classic depiction of the natural history of HFrEF begins with an initial index event that damages the myocardium, followed by activation of compensatory mechanisms that include LV hypertrophy, and attempts to stabilize contractility and cardiac output through neurohormonal activation, which lead to further LV systolic dysfunction. Progressive LV dilatation, wall thinning and eccentric remodelling, occurring along with a decline in LVEF, has been proposed as a therapeutic target in and of itself in the biomechanical model of heart failure.11 It is therefore surprising that a sizeable proportion of our patients with HFrEF showed a clear reduction in LVEF without eccentric remodelling, and had concentric remodelling instead. Patients with concentric hypertrophy had similarly advanced New York Heart Association functional class and even higher NT‐proBNP and worse renal function, compared to those with eccentric hypertrophy — thus arguing against concentric hypertrophy being an earlier or milder form of HFrEF. Instead, patients with HFrEF and concentric hypertrophy were more predominantly older hypertensive women — a profile usually indicative for HFpEF. Of interest, the prevalence of moderate or severe mitral regurgitation was higher in patients with eccentric hypertrophy, even though valvular heart disease as aetiology was not different between groups. These findings might be explained by secondary mitral annular dilatation, which is often seen in dilated, eccentric remodelled hearts leading, to malcoaptation of mitral leaflets.
Results of the network analyses of circulating biomarkers provided independent evidence of relevant differences between the two LV geometry groups of patients with HFrEF. In patients with eccentric hypertrophy, NT‐proBNP was the most important central hub. This is in line with our understanding that in eccentric hypertrophy increased wall stress is an important pathophysiological mechanism. In our subgroup of HFrEF patients with eccentric geometry, one of the matrix metalloproteinases (MMP2) was an important hub. This is not surprising as matrix metalloproteinases are key enzymes involved in post‐myocardial infarction remodelling of the left ventricle.12 Little is known about the role of JAM‐A in heart failure. Hyperreactivity of JAM‐A has been shown to accelerate atherosclerosis in mice.13 In contrast, in HFrEF patients with concentric hypertrophy, U‐PAR, PON3, SELP, TNF‐R1 and TNF‐R2 were important hubs. We will briefly discuss these proteins below. U‐PAR is a novel biomarker of chronic low‐grade inflammation. Elevated levels of U‐PAR are independently associated with the incidence of common co‐morbidities in heart failure, such as chronic kidney disease.14 Its soluble form has been associated with diastolic dysfunction in HFpEF patients.15 TNF‐R1 and TNF‐R2 are both cytokine receptors active in the TNF‐alpha axis. The effects of this pro‐inflammatory cytokine are complex and incompletely understood. In mice, overexpression of the transmembrane form of TNF leads to a phenotype of concentric hypertrophy.16 It is hypothesized that the ratio between TNF‐R1 and TNF‐R2 is responsible for the type of hypertrophy that develops. Interestingly, circulating levels of TNF‐R2 are known to be increased in heart failure with preserved ejection fraction (HFpEF) relative to HFrEF in humans.17 P‐selectin is a member of the selectins, a family of cell adhesion molecules that are involved leucocyte migration. P‐selectin is found in platelets and endothelial cells. PON3 is a member of the paraoxonase family, present in high‐density lipoproteins, mitochondria and the endoplasmic reticulum. Paraoxonases have a strong anti‐oxidative effect and are believed to slow the initiation and progression of atherosclerosis. At the time of writing there are little data on its role in heart failure.18 Overall, from our network analyses we conclude that the biomarker profile of HFrEF patients with concentric hypertrophy is characterized by markers of oxidative stress and inflammation.
In addition, we explored the differential association between LV geometry and effects of up‐titration of medical therapy. Our data suggest that for beta‐blockers, the expected mortality benefit of up‐titration is higher in HFrEF patients with eccentric hypertrophy compared to HFrEF patients with concentric hypertrophy. However, these results might be influenced by the relatively low number of patients with concentric hypertrophy. For ACEi/ARBs, we could not establish a statistically significant interaction between LV geometry and the effect of up‐titration on mortality. Nonetheless, the point estimates of hazard ratio are lower in successfully up‐titrated patients in eccentric hypertrophy compared to concentric hypertrophy. These observations are in line with an analysis of the Valsartan Heart Failure Trial (Val‐HeFT) echocardiographic data showing that patients with the largest LV internal diastolic diameters had the greatest relative and absolute risk reduction in response to valsartan.19 These findings cannot be explained by the assumption that patients with eccentric hypertrophy are more severe patients with a higher risk, since the Kaplan–Meier curves for the non‐up‐titrated patients showed similarly dismal prognosis in eccentric hypertrophy (Figure 4). We therefore hypothesize that, in addition to ejection fraction, geometry of the left ventricle might influence the response to up‐titration of beta‐blockers and ACEi/ARBs.
Unfortunately, our study has some limitations. First, the number of patients in the concentric hypertrophy group was smaller than in the eccentric group. Furthermore, the assessment of echocardiography was not performed in an echo core lab. Moreover, this is a post‐hoc analysis of a prospective study, introducing the risk of residual confounding. However, every effort was made to account for known potential confounders and results were consistent in both index and validation cohorts. Next, we studied protein–protein interactions using correlations only. Other methods of analysis, for instance incorporating existing knowledge on physical interactions in the networks, might offer additional information. Although we did not include patients with HFpEF in this analysis, a future question of particular interest would be whether a subset of HFpEF patients with eccentric hypertrophy would benefit from ACEi and beta‐blockers. Finally, since the majority of patients in this study are of Caucasian origin, it is not known if results are generalizable to other populations.
Conclusion
Patients with HFrEF with concentric LV hypertrophy have a clinical and biomarker phenotype that is different from HFrEF with eccentric LV hypertrophy. Patients with HFrEF and concentric LV hypertrophy may not experience similar benefit from up‐titration of ACEi/ARBs and beta‐blockers compared to HFrEF with eccentric LV hypertrophy.
Funding
This work was supported by a grant from the European Commission (FP7‐242209‐BIOSTAT‐CHF).
Conflict of interest: S.D.A. reports grants from Vifor and Abbott Vascular, and fees for consultancy from Vifor, Bayer, Boehringer Ingelheim, Brahms, Janssen, Novartis, Servier and Stealth Peptides. J.J.B. received speaker fees from Abbott Vascular. The Department of Cardiology of Leiden University Medical Centre received grants from Biotronik, Medtronic, Edwards Lifesciences, GE Healthcare, and Boston Scientific Corporation. All other authors report no conflict of interest.
Supporting information
Table S1. Baseline characteristics of the validation cohort.
Table S2. Measurements using Olink Proseek®.
Table S3. Baseline characteristics of the index cohort, including patients with normal geometry and concentric remodelling
Figure S1. Overall survival in the index cohort stratified by left ventricular geometry.
Figure S2. Overall survival in the validation cohort stratified by left ventricular geometry.
Figure S3. Unweighted Kaplan–Meier curves for <50% up‐titration vs. >50% up‐titration of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and beta‐blockers.
Figure S4. Heatmap of protein–protein correlations in concentric hypertrophy.
Figure S5. Heatmap of protein–protein correlations in eccentric hypertrophy.
Figure S6. Flow‐chart of patient selection.
References
- 1. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–271. [DOI] [PubMed] [Google Scholar]
- 2. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011;58:1733–1740. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 4. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot J‐S, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019;40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaasch WH, Delorey DE, St John Sutton MG, Zile MR. Patterns of structural and functional remodeling of the left ventricle in chronic heart failure. Am J Cardiol 2008;102:459–462. [DOI] [PubMed] [Google Scholar]
- 6. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 7. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Bucht Thorsen S, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S, eds. Homogenous 96‐Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 9. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw 2011;43:1–23. [Google Scholar]
- 11. Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 2005;111:2837–2849. [DOI] [PubMed] [Google Scholar]
- 12. Lindsey ML, Iyer RP, Jung M, DeLeon‐Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post‐myocardial infarction remodeling. J Mol Cell Cardiol 2016;91:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karshovska E, Zhao Z, Blanchet X, Schmitt MM, Bidzhekov K, Soehnlein O, von Hundelshausen P, Mattheij NJ, Cosemans JM, Megens RT, Koeppel TA, Schober A, Hackeng TM, Weber C, Koenen RR. Hyperreactivity of junctional adhesion molecule A‐deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circ Res 2015;116:587–599. [DOI] [PubMed] [Google Scholar]
- 14. Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2015;373:1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujisaka T, Fujita S, Maeda D, Shibata K, Takahashi H, Morita H, Takeda Y, Ito T, Sohmiya K, Hoshiga M, Ishizaka N. Association between suPAR and cardiac diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels 2017;32:1327–1336. [DOI] [PubMed] [Google Scholar]
- 16. Dibbs ZI, Diwan A, Nemoto S, DeFreitas G, Abdellatif M, Carabello BA, Spinale FG, Feuerstein G, Sivasubramanian N, Mann DL, Liu P. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation 2003;108:1002–1008. [DOI] [PubMed] [Google Scholar]
- 17. Putko BN, Wang Z, Lo J, Anderson T, Becher H, Dyck JR, Kassiri Z, Oudit GY, eds. Circulating levels of tumor necrosis factor‐alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS One 2014;9:e99495; Alberta HEART Investigators [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rom O, Aviram M. High‐density lipoprotein‐associated paraoxonase 1: a possible prognostic biomarker for heart failure? Eur J Heart Fail 2017;19:756–759. [DOI] [PubMed] [Google Scholar]
- 19. Wong M, Staszewsky L, Latini R, Barlera S, Glazer R, Aknay N, Hester A, Anand I, Cohn JN. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan Heart Failure Trial (Val‐HeFT) echocardiographic data. J Am Coll Cardiol 2004;43:2022–2027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of the validation cohort.
Table S2. Measurements using Olink Proseek®.
Table S3. Baseline characteristics of the index cohort, including patients with normal geometry and concentric remodelling
Figure S1. Overall survival in the index cohort stratified by left ventricular geometry.
Figure S2. Overall survival in the validation cohort stratified by left ventricular geometry.
Figure S3. Unweighted Kaplan–Meier curves for <50% up‐titration vs. >50% up‐titration of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and beta‐blockers.
Figure S4. Heatmap of protein–protein correlations in concentric hypertrophy.
Figure S5. Heatmap of protein–protein correlations in eccentric hypertrophy.
Figure S6. Flow‐chart of patient selection.


