Abstract
Algal Oil Containing EPA and DHA (AOCED) at approximately 50% was developed as a sustainable n−3 fatty acid source. AOCED was incorporated in diets at dose levels of 0%, 0.75%, 1.5% and 3.0% (w/w) and administered to healthy domestic shorthair female cats starting two weeks before mating, then during mating, gestation, lactation and to their kittens until they reached 32 weeks of age. The diets were made isocaloric and met the Association of American Feed Control Officials (AAFCO) nutrient requirements of cats for growth and reproduction. Dietary AOCED treatment did not affect the overall health, physiological parameters, food consumption and body weights of the queens and their kittens. No AOCED‐related changes in haematology, coagulation or clinical chemistry parameters were observed in either generation when compared to control cats. Plasma levels of EPA and DHA were dose‐dependently increased in both generations, demonstrating bioavailability of the fatty acids. In this study, safety of AOCED at levels up to 3.0% in the diet was demonstrated in cats with administration starting in utero and until kittens reached 32 weeks of age. Bioavailability of EPA and DHA in cats supports use of AOCED as a source of EPA and DHA for feline growth and reproduction.
Keywords: Algal Oil, cats, DHA, EPA, growth, reproduction
1. INTRODUCTION
Long‐chain polyunsaturated n−3 fatty acids (LC‐PUFA), eicosapentaenoic acid (EPA, 20:5n−3) and docosahexaenoic acid (DHA, 22:6n−3), have multiple physiological roles related to cell membrane structure and optimal cell function and responses (Neuringer, Anderson, & Connor, 1988; Salem, Kim, & Yergey, 1986). In cats and dogs, n−3 LC‐PUFAs have been shown to be beneficial in the treatment of cardiovascular disorders, inflammatory skin disorders (allergic/atopic dermatitis) and osteoarthritis as reviewed by Bauer (2011).
Due to the low activities of some enzymes (e.g. ∆6‐desaturase), cats have limited capacity to synthesize LC‐PUFA from their precursors and, therefore, depend to a large extent on the preformed n−6 (arachidonic acid, ARA) and n−3 fatty acids (EPA and DHA) in their diets (Morris, 2007). Thus, EPA and DHA are essential nutrients for cats, especially in certain life stages such as growth and reproduction (AAFCO, 2019). In general, doses of EPA and DHA in commercial pet foods are relatively low (below 0.1% on dry matter basis) and the long‐term safety of the doses exceeding 1% in the diet has not been determined in healthy cats (Bauer, 2011; National Research Council, 2006). Thus, no safe upper level for these fatty acids has been established by NRC (National Research Council, 2006). High doses of EPA and DHA have been tested in feline species for alleviating symptoms of degenerative joint disease, osteoarthritis, renal diseases and dermatitis (Brown, 1999; Corbee, Barnier, Lest, & Hazewinkel, 2013; Lascelles et al., 2010; Lechowski, Sawosz, & Klucińskl, 1998). At high doses or with a long‐term consumption, EPA and DHA may cause disturbances in haemostasis by altering platelet function, fibrinolysis and bleeding time, although evidence in cats is contradictory (Bright, Sullivan, Melton, Schneider, & McDonald, 1994; Lenox & Bauer, 2013; Saker, Eddy, Thatcher, & Kalnitsky, 1998). Furthermore, increased intake of EPA and DHA fatty acid in combination with suboptimal antioxidant (vitamin E) status could result in increases in free radicals and lipid‐oxidative by‐products, followed by peroxidation of storage fat leading to fat necrosis. In cats with high intake of fish oil, steatitis caused by vitamin E deficiency has been reported (Hall, 1996). In addition, potential adverse effects of EPA and DHA in cats include gastrointestinal disturbance, increased energy intake resulting in weight gains, alterations in immune functions, and effects on glycemic control, as reviewed by Lenox and Bauer (2013).
The source of EPA and DHA in most commercial pet foods is primarily fish oil obtained from fatty fish such as menhaden, anchovies, herring and mackerel. However, due to the lack of key enzymes, the fish do not synthesize the fatty acids de novo, but obtain EPA and DHA from their diet (plankton/single cell algae) (Linder, Belhaj, Sautot, & Arab Tehrany, 2010). Thus, a high demand for fish oil for both human and animal nutrition increases the impact on ocean resources and endangers fish stocks (Naylor et al., 2000). A large‐scale fermentation technology allows by‐passing the marine food chain and producing EPA and DHA oil directly from microalgae. Algal Oil Containing EPA and DHA (AOCED) produced from Schizochytrium sp. has been developed as a sustainable source of these fatty acids free from a risk of environmental contaminants such as heavy metals and polychlorinated biphenyls. This technology enables the nutrition industry to produce sufficient amounts of EPA and DHA for the growing market's needs without endangering fish stocks.
Algal oil similar to AOCED and produced by Schizochytrium sp. (hereafter Algal Oil) is used in human nutrition as a food ingredient and dietary supplement (Fedorova‐Dahms, Marone, Bailey‐Hall, & Ryan, 2011). Comprehensive toxicity testing of Algal Oil including a 90‐day subchronic dietary study in rats and genotoxicity assays showed no effects on gene mutations, had no clastogenic or aneugenic potential and was, therefore, considered of no genotoxic concern. Algal Oil demonstrated safety even at high intake levels in the 90‐day dietary study in rats: no adverse effects were noted at any dose level. Therefore, the no observed adverse effect level was established at the highest tested dose of 5 wt% in the diet, corresponding to 3,250 mg/kg bw/day for male and female rats.
The aim of this study was to evaluate the safety and bioavailability of AOCED as a source of EPA and DHA when administered to male and female cats via diet starting at 2 weeks prior to mating, during mating, throughout gestation, lactation and growth, until the kittens reached 32 weeks of age. Algal Oil Containing EPA and DHA is a novel feed ingredient not yet commercially available. Results of the study intend to support AOCED regulatory approval.
2. MATERIALS AND METHODS
This study was conducted at Liberty Research (LRI) under LRI Standard Operating Procedures, in compliance with the Animal Welfare Act (9 CFR, Subchapter A), and the Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at LRI (study number 17.0705.002). The study was not conducted under GLP regulations but was well controlled and thoroughly documented ensuring that a non‐GLP status did not affect the quality of the collected data. Adherence to the study protocol and proper documentation process was monitored by a site quality control unit and sponsor' visits.
2.1. Study design
Algal Oil Containing EPA and DHA was administered to adult cats and their offspring in dry extruded cat foods ad libitum at three different dose groups: the dose intended for pet nutrition (1.5% of the diet on dry matter basis, DM), half of it (0.75% DM) as a low dose, and 3.0% (DM) as a high dose, as given in Table 1. Dose levels were selected based on data collected during the survey of labels of dog foods conducted by DSM in December 2016 (unpublished data). The mid‐dose (1.5%) was defined as the dose which provides the actual maximum dose of EPA and DHA currently available in non‐therapeutic dog and cat diets marketed worldwide. The low and high doses were selected to provide information on dose–response relations of test item‐related findings, if any. Control animals received extruded dry food, and all diets were made isocaloric by replacing 0.75%, 1.5% or 3% of the chicken fat in the control diet with AOCED to make the low‐dose, mid‐dose, or high‐dose test diets respectively. The males and females were let to acclimate for 2 weeks before the study start; the baseline data for females were recorded 1 week prior to the study beginning. Two weeks prior to mating (the study day 0), females were introduced to the experimental diets. Males were fed the control diet for 1 week prior to mating. Both males and females were treated with the experimental diets during mating (ca. 6 weeks). The queens continued on their designated diets during gestation and lactation until their kittens were weaned (end of lactation; 42 days after parturition). The kittens were fed their dams corresponding diets for another 26 weeks after weaning until they reached 32 weeks of age (244 days post‐partum).
Table 1.
Study design
| Study group | Dosage level (X of targeted dose) | Target AOCED dosage (% in the diet on DM basis) | Target AOCED dosage (% in the diet on As Is basis) | Target EPA + DHA content (% in the diet on DM basis) | Actual EPA + DHA contenta (% in the diet on DM basis) | Number of animals | |||
|---|---|---|---|---|---|---|---|---|---|
| Parental generation | Kittensb | ||||||||
| F | M | F | M | ||||||
| Control | 0 | 0 | 0 | ≥0.012% | 0.064% | 6 | 2 | 5 | 5 |
| AOCED Low‐dose | 1/2X | 0.75% | 0.72% | 0.413% | 0.414% | 6 | 2 | 5 | 5 |
| AOCED Mid‐dose | X | 1.50% | 1.45% | 0.825% | 0.819% | 6 | 2 | 5 | 5 |
| AOCED High‐dose | 2X | 3.00% | 2.89% | 1.650% | 1.617% | 6 (5)c | 2 | 5 | 5 |
Abbreviation: M, male; F, female, DM, dry matter.
Values presented as sum of EPA + DHA (% weight on dry matter basis) per Certificate of Analysis.
Five kittens per sex per group were selected randomly from all litters within a dietary group with considerations to select approximately equal number and sex of kittens from the litters.
Six females used for breeding, all females confirmed pregnant at the end of the mating period remained in the study.
2.2. Animals and housing
Twenty‐four female (1–7 years old and with body weight of 4.06 ± 0.53 kg) and eight male (1–11 years old, not weighed) domestic shorthair cats (LRI) were used for breeding. The male and female cats were non‐vaccinated during breeding, and females remained non‐vaccinated during gestation and lactation, as the animals were housed in a barrier (specific pathogen‐free) facility. All animals were proven breeders, and the queens had a history of at least one successful litter (weaning the majority of their kittens with no congenital abnormalities). Prior to mating, the females were individually housed in caging (ca. 2.5′ × 2.5′ × 2.5′). During mating, the animals were group housed (cage size ca. 7.6′ × 3.0′ × 2.0′) per dietary group in harems consisting of three females and one male. All queens confirmed pregnant by the end of breeding remained in the study and were moved to individual housing. The males were removed from the study and returned to the colony once the required number of females was confirmed pregnant. The individual caging with high‐density polyethylene surfaces included perches, stainless steel water/food bowls, litter pans and enrichment toys. Following parturition, kittens remained with their dams until weaning (approximately 6 weeks of age). At weaning, five kittens per sex per group were randomly selected from the litters within a dietary group (one from each) to continue on the study for a total of 40 kittens; the queens and remaining kittens were returned to the colony. All kittens were vaccinated at weaning following removal from the barrier facility. Kittens were housed in pairs (one male and one female to reduce variability in food consumption) in stainless steel cages (ca. 2.6′ × 3.0′ × 2.3′) until the age of approximately 13 weeks to avoid stress of single housing during the early development. Thereafter, the kittens were housed individually in stainless steel cages (ca. 2.6′ × 3.0′ × 2.3′) until the study termination at 32 weeks of age. Each animal was identified with a unique ear tattoo. Unweaned kittens were identified within a litter by sex and colour code. At 7 days of age, kittens were identified using a shave code, if necessary. At the end of the study, all animals were returned to the colony.
The study room environment was monitored for relative humidity and temperature to remain at ranges of 30%–70% humidity and 18–29°C. A light/dark cycle of 12/12 hr was maintained throughout the study. The animals were provided with municipal tap water (Waverly, NY) ad libitum. The municipal drinking water was monitored for contaminants periodically.
2.3. Animal assignment and blinding procedures
The queens were allocated to the treatment groups during the baseline period. Considerations to avoid common ancestry were taken. The females were ranked in ascending order by weight, assigned a random number and blocked to groups of four. The selection aimed to achieve groups as balanced as possible by weight at baseline. The animals were assigned into treatment groups by subsequent ranking of the random numbers within each block. At weaning, five kittens per sex per group were selected randomly from the litters within a dietary group with considerations to select one kitten of each sex from the litters. The person conducting the randomization was blinded to the treatment codes.
The diets including the control were coded using letters, numbers and a colour code. Raw data were collected using diet codes and animal identification numbers. The study personnel responsible for day‐to‐day care and management of the animals and persons doing the observations (e.g. clinical and physical examinations, food consumption) were blinded to the experimental treatment. Haematology, clinical chemistry, coagulation and plasma lipid analysis samples were identified by animal identification numbers. Diet samples for formulation analysis were identified by diet codes.
2.4. AOCED and experimental diets
Dosing of AOCED was confirmed with batch records and analytically, using combined EPA and DHA as a reference for AOCED addition level. A nominal content of EPA and DHA in AOCED is 50% but can vary depending on a production lot. The AOCED lot VY00015572 used for the test diets preparation contained DHA 400.3 mg/g oil (42.7 area% of total fatty acids) and EPA 124.7 mg/g oil (13.6 area% of total fatty acids), resulting in the total EPA and DHA content of 525 mg/g oil (56.3 area% of total fatty acids). Arachidonic acid was present in AOCED at 1.99 area% of total fatty acids. Mixed natural tocopherols were added to AOCED at 1,500 ppm during manufacturing. Peroxide value of AOCED was 0.30 meq/kg, and p‐anisidine value was 2.9. The content of heavy metals in AOCED was below 0.01 mg/kg (cadmium, mercury), below 0.05 mg/kg (lead), and below 0.02 mg/kg (arsenic).
The experimental diets were manufactured at the Kansas State University (Manhattan, KS, USA) in September 2017. The diets, including the control, were formulated by DSM Nutritional Products to meet or exceed the cat AAFCO nutrient profiles for growth and reproduction 2017. The experimental diets were made isocaloric by replacing 0.75%, 1.5% or 3.0% of the chicken fat in the control diet with AOCED to make the low‐dose, mid‐dose, or high‐dose test diets respectively. The AOCED and the chicken fat were coated on the dried kibbles after extrusion. No process loss of EPA and DHA in the experimental diets was observed. The diet compositions are provided in Table S1.
Diets were stored at a room temperature throughout the study. Duplicate samples from each experimental diet were collected on the first and last day of use and throughout the study at 2 months intervals. One of the samples was sent to Eurofins Nutrition Analysis Center for analysis of EPA and DHA contents, peroxide values and moisture content in the diets. The retention sample was kept frozen (−20°C) at LRI until finalization of the analysis. The acceptance criteria for the experimental diets were set to ±15% of nominal value for EPA and DHA content.
2.5. Clinical and physical examination
Clinical observations were conducted on queens once daily from the baseline until weaning of the kittens. The kittens were sexed at 7 days of age, and thereafter, clinical observations were collected once daily until the end of the study. The observations included, but were not limited to, changes in skin and fur, eyes and mucus membranes, ears, nose, overall body condition and behavioural pattern. Beside the clinical observations, all animals were observed throughout the study twice daily for morbidity, mortality, injuries and availability of food and water. Prior to 7 days of age, kittens were examined to determine litter size, number of stillborn/live kittens and for any gross visceral or skeletal abnormalities. Any animal in poor health was further monitored under the supervision of a veterinarian.
A physical examination of the queens was conducted at baseline and following the weaning of the kittens. Kittens were examined at ca. 7–10 days of age, at weaning (day 42 post‐partum), and at 16, 24 and 32 weeks of age (±3 days). The physical examination included assessment of general condition and behaviour, ocular, oral cavity, integument, musculoskeletal, gastrointestinal, body temperature, cardiovascular rate, respiratory rate, abdominal palpation and external genitalia.
2.6. Food consumption and body weights
Body weights of queens were recorded weekly from the study initiation (pre‐acclimatization) to weaning. Kittens' body weights were recorded weekly from 7 days of age until the study termination. Food consumption was recorded daily from the study initiation until the study termination approximately at the same time each morning. Food consumption was not recorded after overnight fasts preceding blood draws, or in case of significant spillage or adulteration (e.g. wet or containing faeces). For each study week, a mean daily food consumption was calculated from the recorded data. During mating, group food consumption of a harem was measured. For the pregnant queens, food consumption was recorded individually. After parturition, food consumption was recorded for queens and their kittens with records of number of animals in a cage. After weaning, two kittens (one male and female) were placed in one cage and the food consumption was recorded for both kittens together. Starting from approximately 13 weeks of age, when the kittens were placed in individual cages, individual food consumption was measured daily until the study end.
2.7. Haematology, clinical chemistry and plasma lipid analysis
Blood for haematology, clinical chemistry, coagulation and plasma fatty acid analysis was collected from queens at baseline and at weaning. From kittens, blood was collected for haematology and clinical chemistry at weeks 8, 16, 24 and 32. Coagulation parameters were analysed from kittens on weeks 16, 24 and 32. The animals were fasted overnight prior to sample collection, and the collected blood volume was limited to 1.0% of animal's body weight. The animals were sedated with ketamine hydrochloride, and acepromazine administrated intramuscularly prior to blood collection. Blood was collected from shaved and alcohol‐cleaned area in EDTA tubes (2 ml) for haematology, in sodium citrate tubes (2 ml) for coagulation, and in lithium heparin tubes (2 ml) for clinical chemistry. Samples were shipped to Cornell University for analysis. Plasma fatty acids (EPA, DHA, ARA) were evaluated from kittens at 8, 24 and 32 weeks of age. Samples were collected in EDTA tubes (2 ml), centrifuged and decanted for two aliquots. One aliquot was shipped on dry ice for fatty acids analysis to OmegaQuant LCC, while the other was retained at LRI at −20ºC until the study termination to ensure the availability of a duplicate sample for re‐analysis if needed.
2.8. Diet formulation analysis and peroxide value determination
Fatty acid concentrations in the dietary samples collected during the study were analysed at DSM Analytical Lab. Samples were lyophilized and then homogenized with a food grinder. The samples were dissolved in equal parts toluene and 1.5 N HCl in methanol. Tricosanoin was added as an internal standard (NuCheck Prep). Samples were heated for 2 hr at 80°C and neutralized with 6% sodium carbonate solution. Aliquots of the organic layer containing the FAMEs were analysed by using a gas chromatograph equipped with a FAMEWAX 30 m × 0.32 mm × 0.25 µm column (Restek). Fatty acids were identified by comparison with a standard mixture of fatty acids (GLC 714, NuCheck Prep). Fatty acid composition was expressed as a weight per cent (% w/w) of total identified fatty acids in a sample.
Recoveries of a total EPA and DHA content in diet samples collected throughout the study were calculated by dividing the measured EPA and DHA concentration at each time point by the EPA and DHA concentration reported by Eurofins in the initial Certificates of Analysis for each respective diet.
The FoodLABfat analyzer was used to analyse peroxide values in dog diet samples by a colorimetric assay to measure peroxide oxidized Fe2+ ions as a marker of lipid oxidation. Back‐up samples were analysed by Eurofins at the study completion.
2.9. Fatty acid analysis of plasma
Plasma samples were analysed using a validated assay (OMQ‐MTD‐003, Whole Plasma Fatty Acid Concentrations) for measurement of EPA and DHA plasma concentrations. The fatty acid analysis was done by gas chromatography with flame ionization detection (GC/FID). Plasma samples were directly trans‐esterified with 14% boron trifluoride, toluene and methanol, 35:30:35 v/v/v. Tricosanoin was added as an internal standard (NuCheck Prep). Samples were heated 45 min at 100°C. The fatty acid methyl esters (FAMEs) were extracted with hexane (EMD Chemicals) and washed with water. An aliquot of the hexane layer was analysed using a GC‐2010 Gas Chromatograph (Shimadzu Corporation) equipped with a SP‐2560, 100‐m fused silica capillary column (0.25 mm internal diameter, 0.2 μm film thickness; Supelco). Fatty acids were identified by comparison with a standard mixture of fatty acids (GLC‐782, NuCheck Prep) and expressed as concentrations, µg of fatty acid per mL of plasma.
2.10. Statistical analysis
Statistical analysis was performed according to the Center for Veterinary Medicine Guidance #185 (Target animal safety for veterinary pharmaceutical products 2009), #197 (Documenting statistical analysis programs and data files 2010) and #226 (Target animal safety data presentation and statistical analysis 2016).
Continuous outcomes with a pre‐treatment measurement and single measurement during treatment were subjected to an analysis of covariance (ANCOVA). The treatment group and the baseline measurement were set as fixed effects. The weight blocks used for the randomization were included as a random effect.
For outcomes with a pre‐treatment measurement and multiple ones during treatment, a repeated measures ANCOVA was employed. In addition to the above effects included in ANCOVA, it included time and the treatment‐by‐time two‐way interaction as fixed effects and an animal‐specific time‐varying random intercept with a structured covariance matrix. The structure of the covariance matrix was selected based on Akaike's information criterion and spacing of measurement interactions.
In case a pre‐treatment measurement was not available (e.g. in kittens) for an outcome, the above models were employed without the use of the baseline fixed effect covariate. This resulted to an analysis of variance (ANOVA) and repeated measured ANOVA.
For kittens, a fixed effect for sex and the three‐way interaction corresponding to the treatment‐by‐sex‐by‐time with its two‐way derivatives were also incorporated into the models. The three‐way interaction was considered significant if p < .05.
Evaluation of effects followed a hierarchical approach. Namely, three‐way interactions were first evaluated (α = .05), then two‐way interactions (α = .10) and lastly main effects (α = .10).
For the analysis of safety parameters, an unadjusted significance level of 0.10 was used to avoid false‐negative findings. This is recommended by CVM guidance #226 and is considered a statistically conservative approach. A significance level of 0.05 coupled with Tukey post hoc pairwise comparisons was used for the efficacy parameters that constituted of the plasma concentrations of DHA, EPA and ARA.
The main part of analysis was performed in SAS (SAS Institute, version 9.4). R (R Core Team, R: A Language and Environment for Statistical Computing, Vienna, Austria, version 3.5.2) was used for the analysis of food consumption data, overall derivation of descriptive statistics, and visualization.
3. RESULTS
3.1. Diets analysis
Eicosapentaenoic acid and DHA recoveries in the dietary samples collected throughout the study every 2 months were measured by dividing a found fatty acid concentration by its nominal value, as commonly used by the industry (Whitmire et al., 2010). The EPA and DHA recoveries were within the ±15% range from the values recorded in the certificates of analyses in all diets, except for the last day of the study, when the recoveries in the low‐ (82%), mid‐ (82%), and high‐dose (73%) diets were outside of the ±15% range. The EPA and DHA levels in the control diet were generally low compared to the nominal value (0.06%) and fluctuated over the study course from 0.04% (first day of use, months 2 and 8) to 0.05% (month 4) and then to 0.03% (month 6 and last day of use). This can be attributable to the analytical variation. In addition, the overall fat content in the diets decreased from 15.70% to 14.12% from the first to the last day of use respectively. Based on the analysis, the EPA and DHA content in the diets (as % in the diet on DM basis) was acceptable when compared to the targeted content (Table 1). The peroxide values of the AOCED diets were generally below 10 mEq/kg, except for the high‐dose diet in which the peroxide value remained below 15 mEq/kg up to and including month 8, and on last day of use was below 20 mEq/kg.
3.2. Reproductive parameters and kittens' viability
In the high‐dose group, one queen was not confirmed pregnant at the end of the mating period and was returned to the colony. All other queens (n = 6 in the control, low‐ and mid‐dose groups; n = 5 in the high‐dose group) were confirmed pregnant and successfully delivered litters. The litter size and viability of the kittens are presented in Table 2. Dose‐dependent increases in litter sizes in the AOCED‐treated groups compared to the control were observed (p < .05; ANOVA). The litter sizes in all groups were within the historical control range of the laboratory conducting the study. Two kittens were stillborn in the high‐dose group, and three kittens (one in the control group, one in the low‐dose group and one in the high‐dose group) were missing and presumed cannibalized within 24 hr post‐partum.
Table 2.
Litter size and viability of the kittens
| Treatment group | Number of littersa | Mean litter size at birth | Viability indexb DPP1 (%) | Survival indexc DPP42 (%) | Number of kittens weaned on DPP42 |
|---|---|---|---|---|---|
| Control | 6 | 3.8 | 95.7 | 100 | 22 |
| AOCED Low‐dose | 6 | 4.2 | 96.0 | 95.8 | 23 |
| AOCED Mid‐dose | 6 | 4.7 | 96.4 | 96.3 | 26 |
| AOCED High‐dose | 5 | 6.0 | 86.7 | 98.2 | 25 |
The centre's normal pregnancy rate is 75%–80%.
Percentage of live kittens on day 1 post‐partum.
Percentage of kittens alive on day 1 post‐partum that survived to day 42 post‐partum.
After the physical examination on days 7–10 post‐partum, two queens in the high‐dose group had their kittens fostered by other queens in the same treatment group because of overlicking. These queens remained in the study until the scheduled physical examination and blood collection on day 42 post‐partum. Collection of food consumption data for these queens was discontinued after the fostering of the kittens (during study week 17). All parental cats survived to the study termination.
Two kittens, one in the mid‐dose group and one in the high‐dose group, were found dead during the first 4 days post‐partum. These deaths were not considered related to dietary treatments as such accidents occur relatively often shortly after birth, especially in larger litters. Three kittens were euthanized during the study: one kitten in the low‐dose group on day 8 post‐partum and one kitten in the high‐dose group on day 9 due to the above‐mentioned overlicking by their queens, and the third kitten with an umbilical hernia on day 39 post‐parturition.
3.3. Clinical signs and physical examination
The clinical signs observed in the queens were incidental, consisted of isolated findings such as pinpoint spot on the cornea, sore on a neck or hair loss, and observed in all groups including the control. In kittens before weaning, the clinical observations (e.g. overlicked ears/shoulders, umbilical hernia or thin appearance) were also incidental and observed across all groups including the control. After weaning, clinical signs included thin appearance in some kittens shortly after weaning, sore paw (swollen toes with abrasions), swollen lip and chin‐suspected eosinophilic granuloma complex, or redness/scabs on whisker area. These were also isolated and observed in every group including the control. Dermal swelling and/or redness was treated with either Animax ointment or prednisolone depending on the severity of symptoms; sore paw observed in one kitten in the mid‐dose group was treated with Meloxidyl and Clavamox.
No statistically significant differences in physical examination parameters were observed in the queens in any treatment group when compared to the control. In kittens, there was a treatment effect on body temperatures and heart rate values based on the treatment‐by‐time statistical analysis, indicating that the AOCED diets affected different groups differently over time. Body temperatures in the high‐dose group were significantly lower compared to the control at weeks 16 and 32 (p ≤ .1) but unaffected at weeks 6 and 24. In the mid‐dose group, body temperatures were significantly lower than those of the control at the study completion, week 32, only (p < .1). Further, heart rate values in the mid‐ and high‐dose groups were significantly lower compared to those in the control if data were analysed over the whole time period rather than at certain time points (p ≤ .1). All these parameters were within a normal physiological range for kittens of this age and were not considered adverse by the veterinarian.
3.4. Food consumption and body weight
No AOCED‐related effects (overall treatment effects over all time points) were observed on the food consumption of the queens or their kittens compared to the control (Figures 1 and 2). A significant treatment by time interaction for the food consumption of the queens revealed a significantly higher food consumption in the high‐dose group compared to the control at the later stages of lactation (p = .003 for the study day 126) (Figure 1). This is thought to be attributable to the larger litter sizes in this group that consequently increased energy demands of the nursing dams. More kittens in a litter could have also contributed to the higher food consumption values at this period since kittens typically start consuming solid food shortly before weaning.
Figure 1.
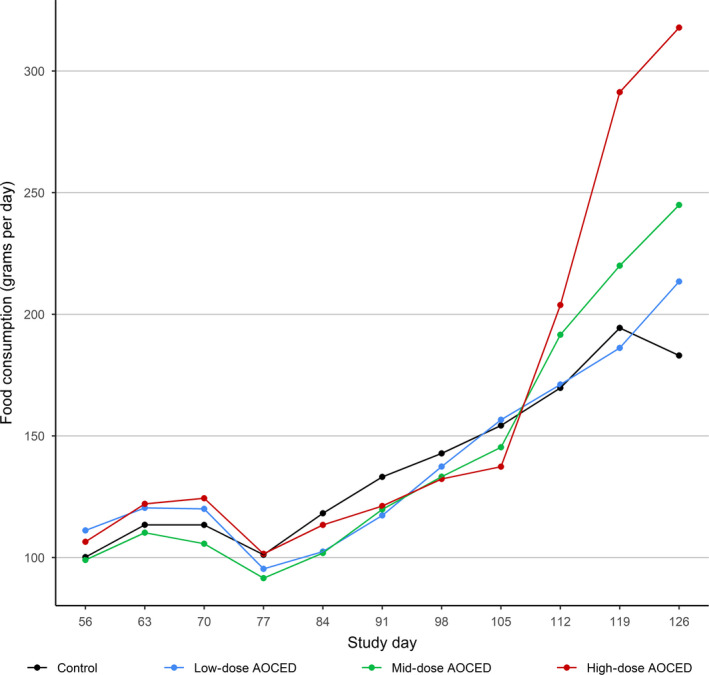
Food consumption of queens during confirmed pregnancy and lactation. Individual food consumption recording started at the end of mating after pregnancy was confirmed (study day 56). Following parturition between study days 77 and 84, kittens remained with their dams until weaning on day 126
Figure 2.
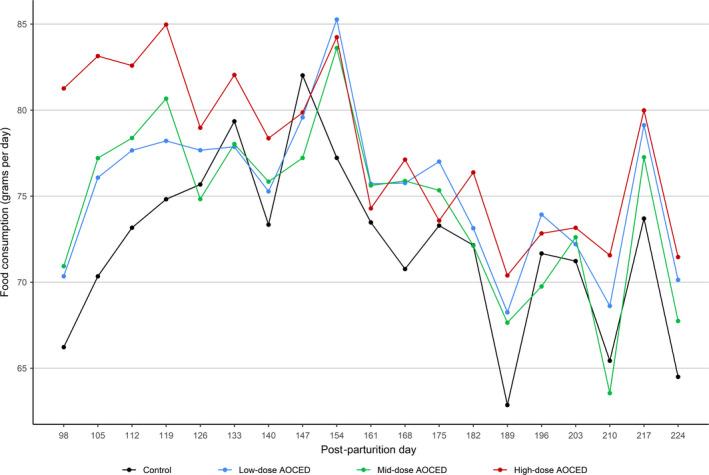
Food consumption of kittens (combined genders) after weaning until the study termination
No statistically significant differences were observed in the body weights of the queens when compared to the control in any dose group during pregnancy and lactation (Figure 3). Although the queens in the high‐dose group tended to be heavier during gestation and lighter during lactation due again to the larger litter sizes in this group, these differences did not reach statistical significance. In kittens, there were statistically significant differences in body weights before weaning when compared to the control: body weights were higher in the low‐dose group on days 7 and 14 post‐partum; lower in the mid‐dose group on days 21, 28, 35, and 42 post‐partum; and lower in the high‐dose group at all time points from day 14 to 42 post‐partum (Figure 4). During the growth phase (from weaning to the week 32), body weights of the kittens of either sex in any treatment group were not affected by the treatment (Figure 5). Towards the study end, males in the mid‐dose AOCED group and females in the high‐dose group appeared to be lighter than the controls; however, the differences did not reach statistical significance (p > .1).
Figure 3.
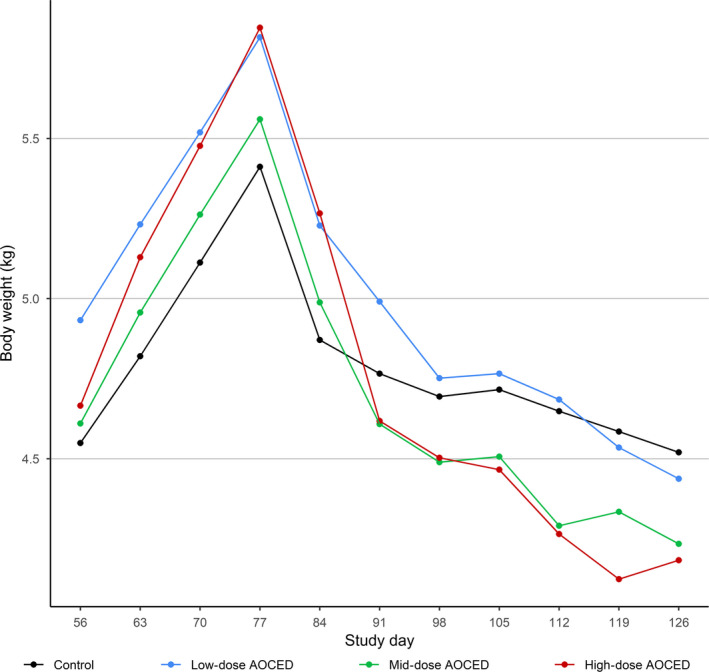
Body weights of queens during gestation and lactation. The kittens were born between study days 77 and 84
Figure 4.
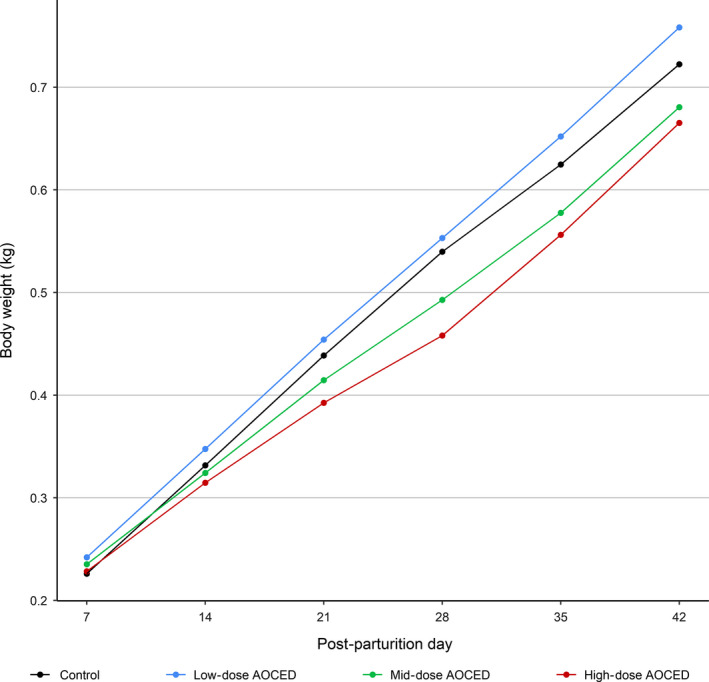
Body weights of kittens during lactation (birth to weaning at the age of 42 days)
Figure 5.
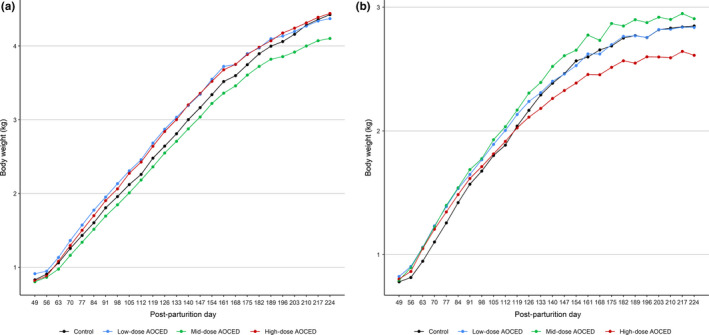
Body weights of kittens during the growth phase. (a) Males; (b) females
3.5. Haematology
In the queens, haematological and coagulation parameters were not affected by the AOCED treatment. Mean monocyte counts were statistically significantly increased in the mid‐ and high‐dose groups compared to the control at weaning (p < .1); however, the mean values were within the laboratory reference range, and therefore, this was not considered adverse. The haematological and coagulation data for the queens are provided in Table S2.
The AOCED treatment did not affect haematological and coagulation parameters of the kittens. The parameters remained within laboratory reference ranges at every time point; data for the last time point on week 32 are given in Table 3. No statistically significant treatment effects were observed on haemoglobin, MCH, MCHC, MCV, neutrophil count, platelet count, red blood cell count, large unstained cell counts or basophil counts. There were statistically significant treatment effects either by time or by sex on lymphocyte, monocyte, reticulocyte, white blood cell and eosinophil counts indicating that the AOCED diets affected one sex differently than the other or the effect changed over time. However, all the values remained within laboratory reference ranges, and changes were without an apparent dose–response relationship, small in magnitude and transient. Thus, they were not considered biologically relevant, or adverse.
Table 3.
Kittens' haematology and coagulation parameters at the study completion following AOCED exposure starting in utero until the 32 weeks of age
| Parameter | Sex | Control | Low‐dose AOCED | Mid‐dose AOCED | High‐dose AOCED |
|---|---|---|---|---|---|
| Haematocrit (%) | M | 40.20 ± 2.95 | 40.00 ± 2.12 | 40.40 ± 1.14 | 38.80 ± 3.70 |
| F | 37.25 ± 1.26 | 36.60 ± 2.51 | 35.00 ± 3.54 | 37.40 ± 3.05 | |
| Haemoglobin (mg/dl) | M | 13.16 ± 0.79 | 13.14 ± 0.50 | 13.00 ± 0.58 | 12.78 ± 1.43 |
| F | 12.38 ± 0.22 | 12.18 ± 0.94 | 11.38 ± 1.08 | 12.38 ± 0.98 | |
| RBC (red blood cell) count (×106/µl) | M | 9.66 ± 0.90 | 9.42 ± 1.17 | 9.72 ± 0.35 | 9.50 ± 1.33 |
| F | 9.28 ± 0.72 | 8.54 ± 0.62 | 8.66 ± 0.91 | 8.66 ± 0.92 | |
| Mean corpuscular volume (fL) | M | 41.60 ± 3.36 | 42.80 ± 3.96 | 41.80 ± 1.30 | 41.00 ± 1.87 |
| F | 41.40 ± 3.05 | 42.80 ± 1.64 | 40.80 ± 3.11 | 42.80 ± 1.64 | |
| Mean corpuscular haemoglobin, MCH (pg) | M | 13.80 ± 1.10 | 14.00 ± 1.41 | 13.40 ± 0.55 | 13.40 ± 0.89 |
| F | 13.50 ± 1.29 | 14.00 ± 0.00 | 13.20 ± 1.10 | 14.60 ± 0.55 | |
| MCH concentration (g/dl) | M | 32.60 ± 0.89 | 32.80 ± 0.84 | 32.20 ± 0.84 | 33.20 ± 0.84 |
| F | 33.25 ± 0.96 | 33.20 ± 0.84 | 32.40 ± 0.55 | 33.20 ± 0.45 | |
| WBC (white blood cell) count (×103/µl) | M | 7.58 ± 0.82 | 8.08 ± 0.59 | 7.68 ± 0.73 | 8.34 ± 2.00 |
| F | 9.13 ± 4.66 | 5.28 ± 0.89 | 7.34 ± 1.69 | 6.94 ± 1.73 | |
| Neutrophil counts (×103/µl) | M | 3.70 ± 1.19 | 4.76 ± 1.11 | 3.34 ± 0.56 | 3.78 ± 1.04 |
| F | 4.40 ± 3.90 | 1.98 ± 0.61 | 3.22 ± 1.57 | 3.02 ± 1.40 | |
| Lymphocyte count (×103/µl) | M | 3.16 ± 1.22 | 2.40 ± 0.68 | 3.62 ± 0.53 | 3.88 ± 1.46 |
| F | 3.43 ± 1.14 | 2.58 ± 0.79 | 2.70 ± 0.82 | 2.86 ± 0.29 | |
| Monocyte count (×103/µl) | M | 0.18 ± 0.08 | 0.18 ± 0.04 | 0.18 ± 0.04 | 0.16 ± 0.05 |
| F | 0.28 ± 0.10 | 0.18 ± 0.04 | 0.20 ± 0.10 | 0.22 ± 0.08 | |
| Eosinophil count (×103/µl) | M | 0.54 ± 0.29 | 0.74 ± 0.44 | 0.52 ± 0.24 | 0.52 ± 0.25 |
| F | 1.03 ± 0.30 | 0.54 ± 0.21 | 1.20 ± 0.62 | 0.84 ± 0.45 | |
| Basophil count (×103/µl) | M | n.d. | n.d. | n.d. | n.d. |
| F | n.d. | n.d. | n.d. | n.d. | |
| Large unstained cells (×103/µl) | M | n.d. | n.d. | n.d. | n.d. |
| F | n.d. | n.d. | n.d. | n.d. | |
| Platelet count (×103/µl) | M | 385.40 ± 89.40 | 339.80 ± 68.30 | 347.20 ± 70.47 | 316.20 ± 83.15 |
| F | 387.50 ± 60.97 | 380.60 ± 84.25 | 441.40 ± 87.15 | 339.00 ± 73.71 | |
| Reticulocyte count (×103/µl) | M | 0.26 ± 0.09 | 0.16 ± 0.09 | 0.14 ± 0.05 | 0.14 ± 0.11 |
| F | 0.25 ± 0.13 | 0.18 ± 0.11 | 0.14 ± 0.05 | 0.14 ± 0.05 | |
| APTT (s) | M | 14.96 ± 0.90 | 14.68 ± 1.29 | 14.36 ± 0.96 | 17.40 ± 3.18 |
| F | 14.08 ± 1.49 | 14.34 ± 2.05 | 13.98 ± 1.68 | 13.72 ± 0.68 | |
| Fibrinogen (mg/dl) | M | 182.80 ± 32.19 | 194.80 ± 18.09 | 176.20 ± 32.77 | 206.00 ± 51.54 |
| F | 144.60 ± 37.19 | 141.00 ± 12.83 | 133.20 ± 37.71 | 127.00 ± 7.52 | |
| PT (s) | M | 15.92 ± 0.36 | 15.92 ± 0.08 | 15.82 ± 0.51 | 16.08 ± 0.49 |
| F | 16.10 ± 0.40 | 16.10 ± 0.25 | 16.36 ± 0.60 | 16.06 ± 0.61 |
Values are given as mean ± SD (n = 5 in each group).
Abbreviation: n.d., not detected.
No statistically significant differences were observed in the kittens of either sex at any time point in prothrombin time, activated prothrombin time and fibrinogen when compared to the control (Table 3).
3.6. Clinical chemistry
In the queens, no statistically significant changes in any clinical chemistry parameter were observed in the AOCED‐treated animals compared to the control. The clinical chemistry values of the queens at the baseline and at the end of the lactation period are provided in Table S3.
In the kittens, no significant effect of the treatment either by sex or time was observed on ALT, AST, amylase, chloride, globulins, glucose, LDH, total protein, triglycerides, urea nitrogen, GGT, total bilirubin and creatinine. In other measured parameters, there were statistically significant treatment effects either by time or by sex indicating that the AOCED diets affected one sex differently than the other or the effect changed over time. However, there were no consistent or significant changes observed at any dose group compared to the control. Changes were small in magnitude, and/or observed only on isolated time points, and values remained within laboratory reference ranges. At the study completion, the kittens' clinical chemistry parameters did not differ from those of the control (Table 4).
Table 4.
Kittens' clinical chemistry parameters at the study completion following AOCED exposure starting in utero until the 32 weeks of age
| Parameter | Sex | Control | Low‐dose AOCED | Mid‐dose AOCED | High‐dose AOCED |
|---|---|---|---|---|---|
| A/G ratio | M | 1.56 ± 0.19 | 1.42 ± 0.11 | 1.48 ± 0.15 | 1.50 ± 0.16 |
| F | 1.46 ± 0.21 | 1.42 ± 0.08 | 1.36 ± 0.09 | 1.38 ± 0.16 | |
| ALP (U/L) | M | 76.00 ± 16.99 | 63.00 ± 7.35 | 73.80 ± 21.15 | 58.40 ± 16.89 |
| F | 58.40 ± 12.24 | 48.60 ± 7.77 | 57.00 ± 12.04 | 42.20 ± 5.36 | |
| ALT (U/L) | M | 90.60 ± 20.03 | 100.00 ± 25.78 | 88.60 ± 14.31 | 96.80 ± 30.38 |
| F | 71.80 ± 17.12 | 78.40 ± 22.19 | 86.20 ± 24.79 | 89.00 ± 13.58 | |
| AST (U/L) | M | 36.20 ± 19.87 | 30.40 ± 4.93 | 29.80 ± 6.76 | 32.00 ± 5.61 |
| F | 25.60 ± 5.03 | 27.20 ± 5.59 | 32.60 ± 6.50 | 28.20 ± 5.07 | |
| Albumin (g/dl) | M | 3.90 ± 0.16 | 3.64 ± 0.23 | 3.66 ± 0.18 | 3.70 ± 0.34 |
| F | 3.84 ± 0.09 | 3.76 ± 0.13 | 3.58 ± 0.18 | 3.74 ± 0.09 | |
| Amylase (U/L) | M | 766.80 ± 118.67 | 741.40 ± 86.25 | 710.60 ± 110.24 | 813.00 ± 112.25 |
| F | 676.00 ± 106.64 | 691.60 ± 86.56 | 684.60 ± 59.00 | 705.80 ± 97.91 | |
| Bile acids | M | 1.00 ± 1.00 | 0.40 ± 0.55 | 0.40 ± 0.55 | 0.20 ± 0.45 |
| F | 0.60 ± 0.55 | 0.40 ± 0.55 | 0.20 ± 0.45 | 0.20 ± 0.45 | |
| Calcium (mg/dl) | M | 10.60 ± 0.29 | 10.12 ± 0.41 | 10.16 ± 0.21 | 10.08 ± 0.18 |
| F | 10.20 ± 0.24 | 10.08 ± 0.28 | 10.08 ± 0.29 | 10.14 ± 0.11 | |
| Chloride (mEq/L) | M | 113.00 ± 1.58 | 113.20 ± 2.77 | 114.80 ± 2.17 | 113.40 ± 1.14 |
| F | 114.60 ± 1.67 | 115.00 ± 1.87 | 115.40 ± 1.82 | 115.80 ± 1.92 | |
| Cholesterol (mg/dl) | M | 176.00 ± 52.40 | 152.80 ± 11.95 | 146.80 ± 23.04 | 132.40 ± 18.51 |
| F | 158.20 ± 20.64 | 167.20 ± 17.92 | 151.40 ± 23.07 | 141.20 ± 23.00 | |
| Creatine kinase (U/L) | M | 305.20 ± 111.74 | 308.80 ± 145.05 | 368.20 ± 171.05 | 365.60 ± 162.75 |
| F | 381.60 ± 204.99 | 237.00 ± 80.16 | 409.80 ± 238.92 | 286.40 ± 179.24 | |
| Creatinine (mg/dl) | M | 0.98 ± 0.19 | 0.86 ± 0.11 | 1.02 ± 0.04 | 1.00 ± 0.19 |
| F | 0.92 ± 0.11 | 0.90 ± 0.07 | 1.02 ± 0.11 | 0.92 ± 0.08 | |
| GGT (U/L) | M | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| F | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Globulins (g/dl) | M | 2.50 ± 0.23 | 2.58 ± 0.19 | 2.50 ± 0.19 | 2.50 ± 0.14 |
| F | 2.66 ± 0.34 | 2.66 ± 0.21 | 2.66 ± 0.21 | 2.76 ± 0.36 | |
| Glucose (mg/dl) | M | 80.00 ± 6.82 | 82.60 ± 9.15 | 80.00 ± 4.47 | 88.40 ± 3.97 |
| F | 85.20 ± 9.31 | 88.60 ± 12.78 | 78.20 ± 7.46 | 79.00 ± 6.28 | |
| LDH (U/L) | M | 199.40 ± 30.92 | 116.80 ± 39.13 | 177.00 ± 42.94 | 150.40 ± 35.40 |
| F | 154.80 ± 18.35 | 112.40 ± 34.41 | 171.80 ± 40.03 | 134.80 ± 69.68 | |
| Magnesium (mEq/L) | M | 2.00 ± 0.07 | 1.84 ± 0.09 | 1.90 ± 0.00 | 1.94 ± 0.11 |
| F | 1.90 ± 0.10 | 1.84 ± 0.09 | 1.88 ± 0.08 | 1.90 ± 0.12 | |
| Phosphate (mg/dl) | M | 7.50 ± 0.86 | 7.06 ± 0.84 | 7.04 ± 0.18 | 6.92 ± 0.55 |
| F | 6.16 ± 0.51 | 6.06 ± 0.35 | 6.22 ± 0.33 | 5.82 ± 0.36 | |
| Potassium (mEq/L) | M | 4.68 ± 0.22 | 4.42 ± 0.28 | 4.46 ± 0.21 | 4.30 ± 0.20 |
| F | 4.44 ± 0.23 | 4.10 ± 0.29 | 4.32 ± 0.34 | 4.24 ± 0.35 | |
| Sodium (mEq/L) | M | 154.20 ± 1.10 | 153.40 ± 1.82 | 154.00 ± 1.87 | 152.60 ± 0.89 |
| F | 153.80 ± 1.10 | 153.00 ± 1.41 | 152.20 ± 1.10 | 152.40 ± 1.14 | |
| Taurine (nMol/ml) | M | 510.80 ± 85.15 | 420.20 ± 55.45 | 415.80 ± 67.87 | 635.50 ± 55.86 |
| F | 481.80 ± 101.80 | 362.60 ± 67.89 | 444.80 ± 101.12 | 351.00 ± 40.09 | |
| Total bilirubin (mg/dl) | M | 0 ± 0 | 0.02 ± 0.04 | 0 ± 0 | 0 ± 0 |
| F | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Total protein (g/dl) | M | 6.40 ± 0.31 | 6.22 ± 0.36 | 6.16 ± 0.23 | 6.20 ± 0.38 |
| F | 6.50 ± 0.22 | 6.42 ± 0.29 | 6.24 ± 0.32 | 6.50 ± 0.35 | |
| Triglycerides (mg/dl) | M | 35.40 ± 8.99 | 35.40 ± 5.55 | 27.60 ± 7.16 | 63.80 ± 77.47a |
| F | 29.20 ± 6.02 | 26.00 ± 5.48 | 25.20 ± 3.63 | 26.00 ± 5.92 | |
| Urea nitrogen (mg/dl) | M | 27.20 ± 3.63 | 23.60 ± 1.14 | 23.40 ± 2.30 | 24.60 ± 4.67 |
| F | 25.60 ± 3.97 | 28.00 ± 4.30 | 26.20 ± 1.30 | 25.80 ± 2.39 |
Values are given as mean ± SD (n = 5 in each group).
One individual with a high triglyceride level (220), increasing the mean and SD.
3.7. Plasma lipid analysis
At baseline, no differences in the queens' plasma levels of EPA, DHA, and ARA were observed between the groups (Figure 6, Figure S1). By the end of lactation, AOCED diets increased plasma EPA and DHA levels in all treatment groups compared to the control (Figure 6). The plasma EPA and DHA levels in the high‐dose group were statistically significantly higher than those in the low‐ and mid‐dose groups (Figure 6). By the end of lactation, no statistically significant differences in the queens' plasma ARA levels were observed between the groups (Figure S1).
Figure 6.
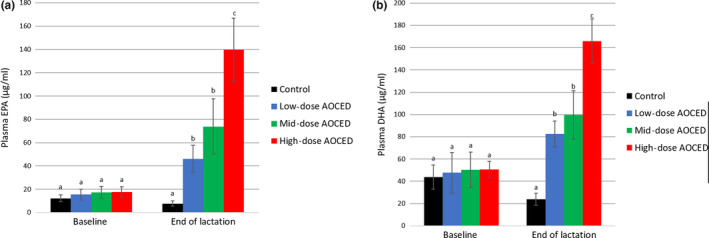
Mean plasma EPA (a) and DHA (b) levels of queens at baseline and at weaning. Values are shown as mean + SD (n = 6 in each group, except for the high‐dose AOCED group at weaning with n = 5). a, b and c indicate differences between groups at a time point (p < .05)
In the kittens, EPA plasma levels of the kittens were statistically significantly increased compared to the control in a dose‐dependent manner at every time point (Figure 7A). Over the growth period, the plasma EPA levels increased from week 8 to week 24 and plateaued after that as no increases were observed between weeks 24 and 32 in the AOCED groups (Figure 7A).
Figure 7.
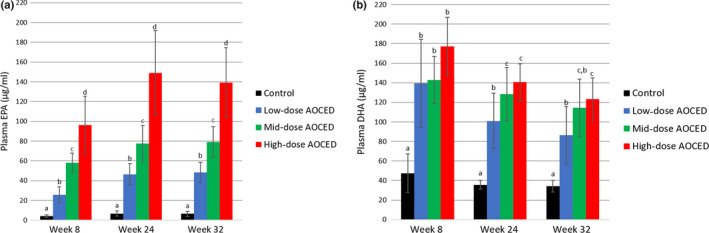
Mean plasma EPA (a) and DHA (b) levels in kittens (combined genders) at weeks 8, 24 and 32. Values are shown as mean + SD (n = 10 in each group, except for the control group at week 8 with n = 9). a, b, c and d indicate differences between groups at a time point (p < .05)
Docosahexaenoic acid plasma levels of the kittens in all AOCED groups were statistically significantly higher compared to the control at every measured time point (Figure 7B). The increases were generally dose‐dependent, although statistically significant differences between the dose groups were not observed at every time point. In all groups including the control, the mean DHA levels were at the highest on week 8, shortly after weaning, and then slightly decreased over time although not statistically significant in each dose group.
No statistically significant differences in the kittens' plasma ARA levels were observed between the control and AOCED groups at any time point (Figure S2).
4. DISCUSSION
The AOCED diets did not have any adverse effects on the reproduction of cats. Although one queen in the high‐dose group did not get pregnant, number of kittens weaned in this group was still higher compared to the control due to the increased litter sizes in the AOCED‐treated groups compared to the control (Table 2). A similar finding of increased litter sizes was reported in a recent AOCED dietary study in dogs (Dahms et al., 2019).
The survival of the queens and their kittens was not affected by the AOCED treatment. The survival index of kittens on post‐parturition day 42, at weaning, was comparable between the groups. The few deaths of kittens occurred in early lactation and were considered incidental and unrelated to the AOCED diets.
Algal Oil Containing EPA and DHA treatment did not affect food consumption of either generation except for higher food consumption in the high‐dose queens compared to the control at the later stages of lactation. The body weights of the queens were not affected by the AOCED treatment. During lactation, differences in the body weights of the kittens were observed: the low‐dose kittens were heavier than the control in the first 2 weeks after birth, whereas kittens in the mid‐ and high‐dose groups were lighter compared to the control from the second lactation week until weaning. This was likely caused by the larger litter sizes in these groups compared to the control. The differences in the body weights were transient as they were no longer present during the growth phase. Importantly, no body weight loss was observed in any treatment group at any time point. As no significant differences in the kittens' body weights were observed after weaning until the study completion, the lower body weights of the mid‐ and high‐dose kittens during lactation can be considered not related to the AOCED treatment, but attributable to the bigger litter size.
Algal Oil Containing EPA and DHA treatment did not influence any of the haematological, coagulation or clinical chemistry parameters in either generation. Occasional changes observed were not biologically relevant due to their transient nature and lack of the dose and time responses. These were attributed to the normal biological variation rather than related to the AOCED diets.
In the queens, the EPA and DHA plasma levels increased generally dose‐dependently following the AOCED dietary treatment during mating, gestation and lactation.
In the kittens, dose‐dependent increases in the plasma EPA levels were observed during the growth phase. Differences between AOCED groups in the plasma DHA levels were not as pronounced although generally dose‐dependent. Eicosapentaenoic acid levels increased from week 8 to week 24 and remained flat after that. The DHA levels decreased slightly between weeks 8 and 24 in the AOCED‐treated groups, suggesting that plasma DHA levels reached the saturation in the early development. In human and dog studies, it has been reported that plasma DHA concentrations typically plateau in about a month after the start of the DHA supplementation (Arterburn, Hall, & Oken, 2006; Dahms, Bailey‐Hall, & Salem, 2016). The queens' plasma DHA levels were measured after 126 days of exposure to the AOCED diets, and the kittens were exposed to DHA already in utero and subsequently during lactation.
Dietary EPA and DHA compete with ARA for incorporation into cell membrane phospholipids (Holub, Bakker, & Skeaff, 1987). Accordingly, supplemented EPA and DHA allow for the partial replacement of ARA in the lipids of blood cells and organ cells thereby reducing the ARA content and the amount of ARA available to form metabolites (Nakamura & Nara, 2004; Raatz et al., 2012). A competitive relationship between the long‐chain n−3 and n−6 fatty acids was reported in dogs as well (Bauer, 2011; Dahms et al., 2016). In the present study, the ARA levels in the AOCED‐treated groups were comparable to the control in both generations indicating that no ARA deficiency was observed. It has been reported previously that supplementation of feline diets with oils rich in EPA and DHA can cause ARA deficiency in cats due to the dietary dilution especially if the basal diet meets only the minimum ARA requirement (Angell, McClure, Bigley, & Bauer, 2012). In contrast, dietary supplementation with both fish oil and ARA did not lead to the plasma ARA reductions in cats (Angell et al., 2012). This was confirmed in the present study, when animals received 0.16%–0.22% ARA (w% as dry matter basis) in their diets either from the base diet or from AOCED (Food composition, Table S1).
The higher plasma EPA and DHA levels of the queens and their kittens compared to the control at every measured time point indicate that EPA and DHA from AOCED were bioavailable in cats during gestation, lactation and growth.
The rather unique design of the current study (dietary supplementation at different stages of reproduction and development in the two generations of cats) makes it difficult to compare bioavailability of AOCED to that of fish oil reported in the literature. In addition, we present plasma EPA and DHA data in µg/ml while most published studies expressed them as a percentage of total plasma lipids. To evaluate plasma responses in a more comparable way, we herein use data from an unpublished pilot study with AOCED which was conducted before the present study to confirm dose selection for the main study. In the pilot study, AOCED was incorporated into diets at 1.5% and 3.0% levels and fed to healthy young cats for 2 months. At the study completion, plasma EPA and DHA levels in the 3.0% AOCED group were 6.1% and 7.4% of total plasma lipids, respectively, (Table S4) and were comparable to that reported previously in cats (Bright et al., 1994). Cats in the Bright et al.'s study were supplemented daily with fish oil as a source of EPA (1,400 mg/day) and DHA (750 mg/day) for 8 weeks, and their mean EPA and DHA levels were shown to be 5.9% and 3.1% of total plasma lipids respectively (Bright et al., 1994; Lenox & Bauer, 2013; Saker et al., 1998). In the pilot study, average daily EPA and DHA intakes in the 3.0% AOCED group were 280 and 900 mg, respectively, based on the food consumption data. The findings are remarkable considering that the DHA dosage received by the 3.0% AOCED cats in the pilot study (900 mg/day) was slightly higher than that in the fish oil study (750 mg/day) while EPA dosage was substantially lower: 280 versus 1,400 mg/day.
5. CONCLUSIONS
In this study, safety of Algal Oil Containing EPA and DHA (AOCED) at levels up to 3.0% on dry matter basis in the diet was demonstrated in cats during gestation, lactation and growth until 32 weeks of age. EPA and DHA from AOCED were bioavailable supporting the use of AOCED in cats as a source of EPA and DHA for growth and reproduction.
ANIMAL WELFARE STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate Institutional Animal Care and Use Committee approval has been received. The authors confirm that they have followed the Animal Welfare Act (9 CFR, Subchapter A) and the Guide for the Care and Use of Laboratory Animals.
Supporting information
Vuorinen A, Bailey‐Hall E, Karagiannis A, et al. Safety of Algal Oil Containing EPA and DHA in cats during gestation, lactation and growth. J Anim Physiol Anim Nutr. 2020;104:1509–1523. 10.1111/jpn.13324
REFERENCES
- Angell, R. J. , McClure, M. K. , Bigley, K. E. , & Bauer, J. E. (2012). Fish oil supplementation maintains adequate plasma arachidonate in cats, but similar amounts of vegetable oils lead to dietary arachidonate deficiency from nutrient dilution. Nutrition Research, 32(5), 381–389. 10.1016/j.nutres.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Arterburn, L. M. , Hall, E. B. , & Oken, H. (2006). Distribution, interconversion, and dose response of n−3 fatty acids in humans. The American Journal of Clinical Nutrition, 83(6), 1467S–1476S. 10.1093/ajcn/83.6.1467S [DOI] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO) (2019). Official publication (pp. 166–170). Association of American Feed Control Officials (AAFCO). [Google Scholar]
- Bauer, J. E. (2011). Therapeutic use of fish oils in companion animals. Journal of American Veterinary Medical Association, 239(11), 1441–1451. 10.2460/javma.239.11.1441 [DOI] [PubMed] [Google Scholar]
- Bright, J. M. , Sullivan, P. S. , Melton, S. L. , Schneider, J. F. , & McDonald, T. P. (1994). The effects of n−3 fatty acid supplementation on bleeding time, plasma fatty acid composition, and in vitro platelet aggregation in cats. Journal of Veterinary Internal Medicine, 8(4), 247–252. 10.1111/j.1939-1676.1994.tb03227.x [DOI] [PubMed] [Google Scholar]
- Brown, S. A. (1999). Effects of dietary lipids on renal function in dogs and cats. Compendium on Continuing Education for the Practising Veterinarian, 21(Suppl K), 11–14. [Google Scholar]
- Corbee, R. J. , Barnier, M. M. C. , van de Lest, C. H. A. , & Hazewinkel, H. A. W. (2013). The effect of dietary long‐chain omega‐3 fatty acid supplementation on owner's perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. Journal of Animal Physiology and Animal Nutrition, 97(5), 846–853. 10.1111/j.1439-0396.2012.01329.x [DOI] [PubMed] [Google Scholar]
- Dahms, I. , Bailey‐Hall, E. , & Salem, N. Jr (2016). Kinetics of docosahexaenoic acid ethyl ester accumulation in dog plasma and brain. Prostaglandins, Leukotrienes and Essential Fatty Acids, 113, 1–8. 10.1016/j.plefa.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Dahms, I. , Bailey‐Hall, E. , Sylvester, E. , Parenteau, A. , Yu, S. , Karagiannis, A. , & Wilson, J. (2019). Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation‐lactation‐growth feeding study in Beagle dogs. PLoS ONE, 14(6), e0217794 10.1371/journal.pone.0217794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova‐Dahms, I. , Marone, P. A. , Bailey‐Hall, E. , & Ryan, A. S. (2011). Safety evaluation of Algal Oil from Schizochytrium sp. Food and Chemical Toxicology, 49(1), 70–77. 10.1016/j.fct.2010.09.033 [DOI] [PubMed] [Google Scholar]
- Hall, J. A. (1996). Potential adverse effects of long‐term consumption of (n−3) fatty acids. Compendium on Continuing Education for the Practicing Veterinarian, 18(8), 879–895. [Google Scholar]
- Holub, B. J. , Bakker, D. J. , & Skeaff, C. M. (1987). Alterations in molecular species of cholesterol esters formed via plasma lecithin‐cholesterol acyltransferase in human subjects consuming fish oil. Atherosclerosis, 66(1), 11–18. 10.1016/0021-9150(87)90174-2 [DOI] [PubMed] [Google Scholar]
- Lascelles, B. D. X. , DePuy, V. , Thomson, A. , Hansen, B. , Marcellin‐Little, D. J. , Biourge, V. , & Bauer, J. E. (2010). Evaluation of a therapeutic diet for feline degenerative joint disease. Journal of Veterinary Internal Medicine, 24(3), 487–495. 10.1111/j.1939-1676.2010.0495.x [DOI] [PubMed] [Google Scholar]
- Lechowski, R. , Sawosz, E. , & Klucińskl, W. (1998). The effect of the addition of oil preparation with increased content of n−3 fatty acids on serum lipid profile and clinical condition of cats with miliary dermatitis. Journal of Veterinary Medicine Series A, 45(1–10), 417–424. 10.1111/j.1439-0442.1998.tb00844.x [DOI] [PubMed] [Google Scholar]
- Lenox, C. E. , & Bauer, J. E. (2013). Potential adverse effects of omega‐3 fatty acids in dogs and cats. Journal of Veterinary Internal Medicine, 27(2), 217–226. 10.1111/jvim.12033 [DOI] [PubMed] [Google Scholar]
- Linder, M. , Belhaj, N. , Sautot, P. , & Arab Tehrany, E. (2010). From Krill to Whale: An overview of marine fatty acids and lipid compositions. OCL, 17(4), 194–204. 10.1051/ocl.2010.0328 [DOI] [Google Scholar]
- Morris, J. G. (2007). Idiosyncratic nutrient requirements of cats appear to be diet‐induced evolutionary adaptations. Nutrition Research Reviews, 15(1), 153–168. 10.1079/NRR200238 [DOI] [PubMed] [Google Scholar]
- Nakamura, M. T. , & Nara, T. Y. (2004). Structure, function, and dietary regulation of δ6, δ5, and δ9 desaturases. Annual Review of Nutrition, 24(1), 345–376. 10.1146/annurev.nutr.24.121803.063211 [DOI] [PubMed] [Google Scholar]
- National Research Council (2006). Nutrient requirements of dogs and cats. Washington, DC: The National Academies Press. [Google Scholar]
- Naylor, R. L. , Goldburg, R. J. , Primavera, J. H. , Kautsky, N. , Beveridge, M. C. M. , Clay, J. , … Troell, M. (2000). Effect of aquaculture on world fish supplies. Nature, 405(6790), 1017–1024. 10.1038/35016500 [DOI] [PubMed] [Google Scholar]
- Neuringer, M. , Anderson, G. J. , & Connor, W. E. (1988). The essentiality of n−3 fatty acids for the development and function of retina and brain. Annual Review of Nutrition, 8, 517–541. [DOI] [PubMed] [Google Scholar]
- Raatz, S. K. , Young, L. R. , Picklo, M. J. , Sauter, E. R. , Qin, W. , & Kurzer, M. S. (2012). Total dietary fat and fatty acid content modifies plasma phospholipid fatty acids, desaturase activity indices, and urinary prostaglandin E in women. Nutrition Research, 32(1), 1–7. 10.1016/j.nutres.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Saker, K. E. , Eddy, A. L. , Thatcher, C. D. , & Kalnitsky, J. (1998). Manipulation of dietary (n−6) and (n−3) fatty acids alters platelet function in cats. The Journal of Nutrition, 128(12), 2645S–2647S. 10.1093/jn/128.12.2645S [DOI] [PubMed] [Google Scholar]
- Salem, N. , Kim, H.‐Y. , & Yergey, J. A. (1986). Chapter 15 – Docosahexaenoic acid: Membrane function and metabolism In Simopoulos A. P., Kifer R. R., & Martin R. E. (Eds.), Health effects of polyunsaturated fatty acids in seafoods (pp. 263–317). New York, NY: Academic Press. [Google Scholar]
- Whitmire, M. L. , Bryan, P. , Henry, T. R. , Holbrook, J. , Lehmann, P. , Mollitor, T. , … Wietgrefe, H. D. (2010). Nonclinical dose formulation analysis method validation and sample analysis. The AAPS Journal, 12(4), 628–634. 10.1208/s12248-010-9226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


