Abstract
The aim of this paper was to explain the insurance coverage status of therapeutic apheresis (excluding CHDF) in Japan, alongside the social system of medical reimbursement and concerns regarding the future sustainability of the healthcare system. Insurance schemes and premiums differed for individuals at different levels in the society (eg, municipal residents, employees, and public servants). Insurance premiums and their rates varied depending on the total household income, the number of people living together, age, and the place of residence. In addition, the medical expense subsidies for children through public expenditure were also described. Japan's generous insurance system and multiple medical expense subsidies provide financial support for patients. With Japan's history of medical expense subsidies based on the policy of supporting intractable diseases, we have established an environment where all citizens can receive therapeutic apheresis when needed if they are affected by a disease for which insurance coverage is indicated.
Keywords: apheresis, health expenditures, major medical insurance, out of pocket payments, reimbursement mechanisms
1. INTRODUCTION
This paper will review the types of therapeutic apheresis available in Japan (except continuous hemodiafiltration [CHDF]), and discuss the insurance coverage of medical devices used for this treatment in Japan and the medical expenses subject to insurance reimbursement. First, it is necessary to explain the current health insurance system in Japan.
In Japan, all citizens are usually required to sign up for some kind of medical insurance, called the Universal Health Insurance coverage. This system reduces the cost of medical care for Japanese citizens receiving medical services such as treatment, medication, medical care, and rehabilitation in a medical institution due to illness or injury or receiving prescribed medicines at pharmacies. The insurance covers all services other than cosmetic surgery and medical treatment due to work‐related accidents. Patients may choose to go to any medical institution at any time irrespective of their income or place of residence. Japan's health insurance system is highly regarded and has been ranked first among the medical systems of countries around the world published by the World Health Organization (WHO). 1 There are concerns about the future sustainability of the healthcare system in Japan, due to the various problems the system is now facing. These include the increasing trend of national medical expenses paired with the increasing necessity of medical services as Japan's aging society progresses, the increased need for nursing care support, low birth rate, shortage of healthcare workers, and disparities in the convenience of medical consultations between rural and urban healthcare. 2 However, to date, this medical insurance system has enabled many patients to benefit from advanced medical services. The Japanese Ministry of Health, Labour and Welfare believes that universal health insurance coverage has enabled Japan to achieve the world's highest life expectancy and level of healthcare. The Ministry feels that it will be possible to guarantee safe and secure living for the nation by maintaining universal health insurance coverage based on the current social insurance system.
2. MEDICAL INSURANCE SYSTEM IN JAPAN, SUBSIDIES FOR MEDICAL EXPENSES
The types of medical insurance include the National Health Insurance, which covers general municipal residents; the Japan Health Insurance Association's health insurance, to which the majority of employees belong; Association/union's health insurance, mainly covering the employees of large and group companies; and the Mutual Aid Association for public servants and private school teachers. The Advanced Elderly Medical Service System covers senior citizens aged 75 years or older and persons between 65 and 75 years who have been certified as having certain disabilities. The system is designed to cover all Japanese citizens with some kind of medical insurance wherein the dependents of the insured can also receive the benefits of their respective health insurance policies.
Municipal residents, employees and public servants, each pay insurance premiums based on their income and in line with each type of medical insurance, which means that people are only required to pay 10%‐30% of the medical costs when they are seen at medical institutions as patients. Calculation of insurance premiums is complicated, and their rates vary depending on the total household income, the number of people living together, age, and place of residence. However, in a summary of the Comprehensive Survey of Living Conditions issued by the Ministry of Health, Labour and Welfare in 2018, 3 the annual premium for National Health Insurance was approximately JPY300 000‐400 000 based on a household of three people, comprising a couple in their 40s with a median annual income of JPY4 200 000 per year and a child under 18 years of age, as a model case.
The specific amount of medical expenses to be paid by the patient is 20% of the percentage of copayment against the incurred medical expenses for patients younger than 6 years old, 30% for patients aged 6‐70 years, 20% for patients aged 70 years or older (30% for people with incomes comparable to the current workforce) and 10% for those over 75 years (30% for people with incomes comparable to the current workforce; Table 1). In addition, the medical expense subsidies for children through public expenditure, which is described later, and the high cost medical treatment expenses system based on the health insurance system, further reduces the medical expenses paid by the patient.
TABLE 1.
Fixed fee medical expenses
| Age | Preschool (up to 6 years old) | 6‐69 years | 70‐74 years | Advanced Elderly Medical Service System (75 years and older or 65 years and older for people with disability certification) |
|---|---|---|---|---|
| Copayment percentage | 20% | 30% | Generally 20% a | 10% |
| People with income comparable to the current workforce 30% | ||||
Note: People with income comparable to the current workforce: Annual income of approx. JPY3 700 000 or more.
Generally: Annual income of less than approx. JPY3 700 000.
The high cost medical treatment expenses system is a mechanism put in place to reduce the burden if copayments are still high even when the medical expenses are reduced to about 10%‐30% of the total cost (Figure 1, Tables 2 and 3). For example, for households like the model case mentioned above, the insured person will be required to pay 30% of their own expenses, but if the patient incurs JPY1 million in a certain month, they will pay JPY300 000 initially, but the copayment limit will be calculated as JPY87 430 due to reduction measures. Hence, JPY212 570 will be reimbursed to households at a later date as part of the high cost medical treatment expenses system (Figure 1).
FIGURE 1.
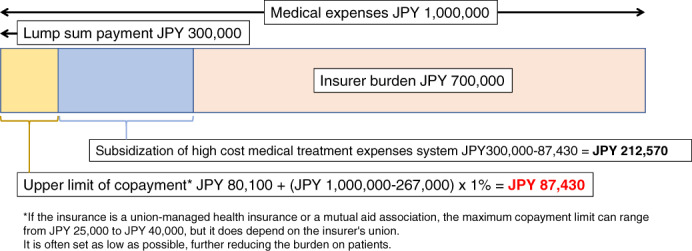
A high cost medical treatment expenses system. Model case: National Health Insurance policy, annual income JPY 4 200 000. A scenario wherein the payment at the place of treatment is JPY300 000 (30% copayment) with the medical fees of 1 million yen per month
TABLE 2.
The upper limit of copayment in relation to the annual income of people under 70 years old
| Applicable to cases with at least 4 high‐cost medical treatment expenses in the past year | ||
|---|---|---|
| Income | Copayment limit per month | Limit per month after 4th expense |
| Annual income approx. JPY11 600 000 or more | JPY252 600 + (total medical expense − JPY842 000) × 1% | JPY140 100 |
| Annual income approx. JPY7 700 000‐11 600 000 | JPY167 400 + (total medical expense − JPY558 000) × 1% | JPY93 000 |
| Annual income approx. JPY3 700 000‐7 700 000 | JPY80 100 + (total medical expense − JPY267 000) × 1% | JPY44 400 |
| Annual income less than JPY3 700 000 | JPY57 600 | JPY44 400 |
| Exempt from residence tax | JPY35 400 | JPY24 600 |
TABLE 3.
The upper limit of copayment in relation to the annual income of people 70 years and older
| Copayment limit per month | |||
|---|---|---|---|
| Income | Outpatient (per person) | Outpatient + inpatient (household) up to 3 times | Outpatient + inpatient (household) 4 times and more |
| Annual income approx. JPY11 600 000 or more | JPY252 600 + (total medical expense − JPY842 000) × 1% | JPY140 100 | |
| Annual income approx. JPY7 700 000‐11 600 000 | JPY167 400 + (total medical expense − JPY558 000) × 1% | JPY93 000 | |
| Annual income approx. JPY3 700 000‐7 700 000 | JPY80 100 + (total medical expense − JPY267 000) × 1% | JPY44 400 | |
| Annual income approx. JPY1 560 000‐3 700 000 | JPY18 000 (JPY144 000/year) | JPY57 600 | JPY44 400 |
| Exempted from residence tax | JPY8000 | JPY24 600 | JPY24 600 |
| Exempted from residence tax (income is below a certain level) | JPY8000 | JPY15 000 | JPY15 000 |
Furthermore, if a patient has insurance policies in schemes such as union‐managed health insurance or mutual aid union, they can also set up an independent supplementary benefit system, which can reduce the final copayment to around JPY25 000‐JPY40 000 in some cases.
There is also a medical expense subsidy system provided by the national and local government's public funding, which has been expanded mainly from the viewpoint of welfare and public health, thereby further reducing the burden of medical expenses on patients.
The main publicly‐funded health care is largely categorized and designed as follows: (Table 4).
-
Subsidies for patients with intractable diseases and disease control.
This system promotes research on treatment and efficient collection of data on patients with intractable diseases, to establish treatment methods for patients suffering from a designated intractable disease, set in accordance with the Act on “Medical Care for Patients with Intractable Diseases.” The system also provides support for medical expenses to reduce their economic burden due to the long‐term medical treatment of each patient with an intractable disease, until efficient treatment methods are established. There are now 333 types of designated intractable diseases in various fields, including neurology, endocrinology, genetic diseases, cardiovascular, gastrointestinal, respiratory, renal, hematology, collagen diseases, and congenital pediatric diseases (as of May 2020). An application form is created by the designated physician and submitted by the patient with the intractable disease, which is then reviewed by the local governments of each prefecture and designated city. If approved, a Medical Care Certificate is issued. The Medical Care Certificate must be renewed every year, and if approved, the monthly maximum copayment limit for patients with an intractable disease is reduced to JPY2500‐JPY30 000 per month, depending on their income.
There are also medical subsidies for specific chronic pediatric diseases stipulated by the “Ministry of Health, Labor and Welfare under the Child Welfare Act.” These include malignant tumors, hematological diseases, chronic kidney diseases, heart diseases, endocrine diseases, metabolic diseases, collagen diseases, autoimmune diseases, neurological diseases, chronic gastrointestinal diseases, genetic mutation‐related diseases, skin diseases, and systemic bone diseases. There are also subsidies for specific disease treatment research projects for patients with subacute myelo‐optic neuropathy (SMON), prion disease, fulminant hepatitis and severe acute pancreatitis, patients with HIV infection caused by congenital blood coagulation factor deficiency, and blood coagulation products.
-
Support for handicapped children, disabled persons.
This support includes welfare and medical care for persons with disabilities aged 18 and over based on the “Services and Support for Persons with Disabilities Act.” Medical services and financial support is provided for persons with disabilities under the age of 18, and subsidies for mental health outpatient treatment for persons with mental disorders based on the Act for “Mental Health and Welfare for persons with mental disorders.”
-
Support for maternal and child health and improved child welfare.
This support includes subsidies for children with disabilities under the “Child Welfare Act” and medical and infant care services for low birth‐weight children under the “Maternal and Child Health Act.” In addition, as part of local government measures to counter the low birth rate, all municipalities are currently implementing some form of medical expense subsidies program for children, including infants. Many municipalities have set zero copayments for medical expenses for children under the age of 15, although there are differences in the amount of expenses covered and target age depending on the local government. Local governments also provide medical expense subsidies for parents and children in single‐parent families such as fatherless families.
-
Subsidies for patients with infectious diseases, disease control and public health.
There are subsidies for patients with infectious diseases such as tuberculosis, ebola, plague, diphtheria, severe acute respiratory syndrome (SARS), Middle‐East respiratory syndrome (MERS), and novel coronavirus (COVID‐19) based on the “Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases Act,” as well as a subsidy system for treatment of new infectious diseases that have a significant impact on the public. There are also subsidies for treatment costs associated with hepatitis B and C as part of the Special Promotion Project for the Treatment of Hepatitis.
-
State reparations related to war and relief systems for injury to health from pollution.
There is a subsidy based on the Act for “Special Aid to the Wounded and Sick Retired Soldiers and Atomic Bomb Survivors Relief Law,” and medical expense subsidization for treatment of pollution‐related diseases, such as the Minamata disease.
-
Public assistance and relief for economically vulnerable persons.
Medical expenses are subsidized for impoverished persons under the “Public Assistance Act.” In addition, households receiving social welfare are withdrawn from the National Health Insurance scheme and are exempt from paying premiums, to be applicable for this medical aid program.
TABLE 4.
Examples of publicly‐funded medical expenses
| 1. Subsidies for patients with intractable diseases and disease control |
| • Subsidies for treatment of 333 types of certified designated intractable diseases (as of January 2020) |
| • Subsidies for specified diseases, including specific chronic childhood diseases, SMON, prion diseases |
| 2. Support for handicapped children and disabled persons |
| • Assistance based on the “Services and Support for Persons with Disabilities Act” and the Act for the “Mental Health and Welfare of the Persons with Mental Disorders” |
| 3. Support for maternal and child health and improved child welfare |
| • Medical expense subsidization for children, including infants |
| 4. Subsidies for patients with infectious diseases, disease control, and public health |
| • Subsidies for treatment of infections expected to have a major impact on the public based on the Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases |
| • Medical expense subsidization of treatment for hepatitis B and hepatitis C with interferon, direct acting antivirals (DAAs) and nucleic acid analog preparations |
| 5. State reparations related to war and relief systems for injury to health from pollution |
| • Subsidies based on the Act for “Special Aid to the Wounded and Sick Retired Soldiers and Atomic Bomb Survivors Relief Law” and medical expense subsidization for treatment of Minamata disease |
| 6. Public assistance and relief for economically vulnerable persons |
| • Medical expense subsidization for impoverished persons based on the “Public Assistance Act” |
By utilizing these social resources, each patient can receive multiple medical expense subsidies if they meet the conditions, which will reduce the out‐of‐pocket expenses that still occur even with the medical insurance system. Of the total medical expenses of JPY42 364.4 billion in Japan in 2017, the copayments paid by patients to medical institutions was 12.3%, and the total premium paid by citizens to insurers was 48.8%. The remaining 38.9% was public expenditure paid by the national treasury or local government.
Please refer to the English material posted on the Ministry of Health, Labour and Welfare website for detailed information. 4
Although introduction of this treatment took a long time, the cost burden for patients undergoing apheresis in Japan ranges from JPY2500 to a maximum of JPY30 000 per month for the monthly fee based on the above‐mentioned upper limit of copayment, if an application for a designated intractable disease has been submitted. If the disease treated with apheresis is not a designated intractable disease, the high cost medical treatment expenses system or the child medical expenses subsidy will be applied to the medical expenses paid according to their income and age. If the patient has authorization of disability, the grading will further reduce or exempt the medical expenses.
3. BUNDLED PAYMENT SYSTEM FOR INPATIENT TREATMENT: EXPLANATION OF DPC/PDPS
Another feature of the Japanese medical expenses reimbursement system is the flat‐rate system based on the Diagnosis Procedure Combination / Per‐Diem Payment System (DPC/PDPS) for inpatient treatment applied to some hospitals. This is a Japanese version of the Diagnosis‐Related Group (DRG) medical system, a comprehensive medical reimbursement system where in the case of surgery, treatment, medication, etc. for a disease with a predetermined name, within a specified comprehensive range; daily medical expenses would be reimbursed for any drug or treatment prescribed in advance. For medical institutions included in this system, the insurance reimbursement paid for medical expenses will be the total amount calculated by multiplying the fixed amount prescribed for each disease and each medical activity by the number of hospitalization days and multiplying by the hospital coefficient. The adjustment coefficient differs at each hospital, including hospitals adopting DPC/PDPS. The amount reimbursed by the hospitals will differ even if a similar medical treatment is performed.
Although the proportion of hospitals adopting DPC/PDPS accounts for about 1% of the total number of hospitals in Japan, this system tends to be incorporated by the core and university hospitals. Hence, based on the number of beds, the system covers approximately 30% of the all of hospitals beds in the entire country. 5 , 6 The majority of hospitals that do not implement DPC/PDPS, reimburse the cost of inpatient treatment on a volume basis in the same way as outpatient treatment.
4. INSURANCE REIMBURSEMENT FOR APHERESIS IN JAPAN
Insurance reimbursement for therapeutic apheresis is currently applied to 35 diseases in Japan. A diverse range of diseases are targeted, including neuropathies, cutaneous diseases, kidney diseases, gastrointestinal diseases, hematological diseases, cardiovascular diseases, collagen diseases, drug poisoning, and severe blood type incompatibility pregnancies. The following provides an estimate of the standard total reimbursed for one session of therapeutic apheresis for each disease, including the estimates for the insurance application conditions, the maximum number of treatments, the material costs, treatment fee, anticoagulant, and replacement fluid applicable for insurance reimbursement, for each treatment type and used modality performed for each target disease (Tables 5 and 6). The estimations of reimbursed medical expenses are based on both, the scenario of volume calculations where apheresis is performed on an outpatient basis and with admission to a hospital not subject to DPC/PDPS, and the scenario in which bundled payment is used with admission to a hospital that is eligible for DPC/PDPS. The prescription volume of the replacement fluid used as a model case here is assumed to be used when a standard treatment is performed for each patient at our institution. This does not mean that it is a standardized volume of replacement fluid for which all institutions that perform apheresis throughout Japan have reached a consensus. It should also be noted that the detailed calculation results may differ slightly depending on the institution. Hence, the calculation of treatment costs is only an estimate.
TABLE 5.
Medical expenses reimbursed for therapeutic apheresis
| Membrane separation method | Centrifugal separation method | |||
|---|---|---|---|---|
| Transaction | DPC/PDPS a | Transaction | DPC/PDPS a | |
| PE | Approx. JPY120 000 b ~218 000 c | Fixed amount + JPY42 000 | Approx. JPY90 000 b ~188 000 c | Fixed fee + JPY42 000 |
| DFPP | Approx. JPY108 000 d ~123 000 e | Fixed amount + JPY42 000 | – | – |
| PA | Approx. JPY146 000 f ~159 000 g | Fixed amount + JPY42 000 | – | – |
| LCAP or GCAP | Approx. JPY147 000~150 000 | Fixed amount + JPY42 000 | Approx. JPY21 000 | Fixed fee + JPY42 000 |
| HA (for hepatic coma) | Approx. JPY155 000 | Fixed amount + JPY42 000 | – | – |
| HA (for endotoxin adsorption) | Approx. JPY384 000 | Fixed amount + JPY42 000 | – | – |
Abbreviations: DFPP, double filtration plasmapheresis; GCAP, granulocyte apheresis; HA, hemoadsorption; PA, plasma adsorption; PE, plasma exchange; LCAP, leukocytapheresis.
The amount reimbursed for DPC/PDPS fixed amount comprehensive medical reimbursement has complicated variations depending on the disease, medical activity performed, and hospital coefficient.
For plasma exchange using albumin preparation as replacement fluid.
For plasma exchange using fresh frozen plasma as replacement fluid.
When using 1 × 250 mL 5% albumin preparation and 1 × 50 mL 25% albumin preparation as replacement fluid.
When using 1 × 250 mL 5% albumin preparation and 4 × 50 mL 25% albumin preparation as replacement fluid.
For plasma component adsorber for fulminant hepatitis.
For plasma component adsorbers other than those for fulminant hepatitis (eg, LDL adsorption, immunoadsorption, etc.).
TABLE 6.
Insurance application of apheresis by disease and maximum number of treatments
| Disease | Type of treatment | Number of times the treatment can be used | Application conditions |
|---|---|---|---|
| Hematologic diseases | |||
| Multiple myeloma | PE, DFPP | Once a week, up to 3 months | None |
| Thrombotic thrombocytopenic purpura | PE, DFPP | Generally, up to 2 days after the platelet count has reached 150 000/μL. One month after starting is the upper limit. | None |
| Hemolytic‐uremic syndrome | PE, DFPP | Up to 21 times | None |
| Macroglobulinemia | PE, DFPP | Once a week, up to 3 months | None |
| Hemophilia with inhibitors | PE, DFPP | Not specified | Inhibitor titer is ≥5 Bethesda units |
| Gastrointestinal diseases | |||
| Fulminant hepatitis | PE, PA | About 10 times | To remove bilirubin and bile acids |
| Postoperative liver failure | PE, DFPP | About 7 times | Indicated for postoperative liver dysfunction that satisfies the following conditions: |
| 1. Total bilirubin ≥5 mg/dL and continuously elevated | |||
| 2. Hepaplastin test (HPT) ≤40% or have ≥2 conditions of Coma Grade II or higher | |||
| Acute liver failure | PE, DFPP | About 7 times | Only when it can be determined that the severity is the same as fulminant hepatitis or postoperative liver failure based on findings such as prothrombin time, coma grade, total bilirubin and hepaplastin test |
| Chronic hepatitis C | PE, DFPP | Up to 5 times | Genotype lb where the blood HVC‐RNA load is ≥100K IU/mL (5 log IU/mL) even after interferon treatment |
| Hepatic coma | HA | Not specified | Not specified |
| Ulcerative colitis | c‐LCAP, f‐LCAP, GCAP | Up to 10 times, but up to 11 times for patients with fulminant conditions | Calculated only for improving the active disease phase and inducing remission for patients with severe, fulminant and refractory conditions (diagnostic criteria from the MHLW Grant‐in‐Aid for Intractable Disease Policy Research Project “Research on Intractable Inflammatory Bowel Disease”) |
| Crohn's disease | c‐LCAP, GCAP | Up to 10 times | For inducing remission in patients with moderate to severe Crohn's disease in the active phase of the disease, which is unresponsive to nutritional therapy and existing pharmacotherapy or such treatment is not indicated, and there are clear clinical symptoms caused by residual colon lesions |
| Transplantation‐related conditions | |||
| Allogenic liver transplantation with ABO incompatibility or antilymphocyte antibody positive | DFPP | Up to 4 times preoperatively and 2 times postoperatively | For allogenic liver transplantation with ABO incompatibility or antilymphocyte antibody positive allogenic liver transplantation |
| Allogenic kidney transplantation with ABO incompatibility or antilymphocyte antibody positive | DFPP | Up to 4 times preoperatively and 2 times postoperatively | For allogenic kidney transplantation with ABO incompatibility or antilymphocyte antibody positive allogenic kidney transplantation |
| Neuropathies | |||
| Myasthenia gravis | PE, DFPP, PA | 7 times a month, up to 3 months | Serious symptoms tending to worsen within 5 years after onset or insufficient response to thymectomy or corticosteroids |
| Multiple sclerosis | PE, DFPP, PA | 7 times a month, up to 3 months | None |
| Chronic inflammatory demyelinating polyneuropathy | PE, DFPP, PA | 7 times a month, up to 3 months | None |
| Guillain‐Barré syndrome | PE, DFPP, PA | 7 times a month, up to 3 months | ≥4 on Hughes Functional Grading Scale |
| Kidney diseases | |||
| Focal glomerulosclerosis | PE, DFPP, PA | Up to 12 times within 3 months | Non‐responsive to conventional pharmacotherapy, with persistent nephrotic condition, and serum cholesterol does not fall below 250 mg/dL |
| Anti‐glomerular basement membrane disease type rapidly progressive glomerulonephritis | PE, DFPP | Treatment up to 7 times in 2 weeks is one treatment course, up to 2 courses | Patients diagnosed with rapidly progressive glomerulonephritis (RPGN) positive for antineutrophil cytoplasmic antibodies (ANCA) |
| Collagen diseases | |||
| Rheumatoid arthritis | f‐LCAP | Once a week, up to 5 weeks | Patients with highly active, drug‐resistant disease or rapidly progressive drug‐resistant rheumatoid arthritis with systemic symptoms such as fever and severe synovitis in multiple joints who satisfy the following two conditions: |
| 1. ≥6 swollen joints | |||
| 2. ESR ≥50 mm/h OR CRP ≥3 mg/dL | |||
| Malignant rheumatoid arthritis | PE, DFPP, PA | Once a week | Patients recognized by the prefectural governor as a recipient of specific disease treatment, presenting with high‐grade extraarticular symptoms due to vasculitis (refractory lower leg ulcers, polyneuritis and melena due to mesenteric artery thrombosis), who is non‐responsive to conventional therapy |
| Systemic lupus erythematosus | PE, DFPP, PA | 4 times a month | Patients for whom any of the following are applicable: |
| 1. Recognized by the prefectural governor as a recipient of specific disease treatment | |||
| 2. Serum complement level (CH50) is ≤20 units, complement protein (C3) is ≤40 mg/dL and anti‐DNA antibodies are markedly elevated, and patient is unresponsive to steroid therapy or treatment is clinically inappropriate | |||
| 3. Patient diagnosed with rapidly progressive glomerulonephritis (RPGN) or central nervous system lupus (CNS lupus) | |||
| Cutaneous diseases | |||
| Pemphigus | PE, DFPP | Up to twice a week, for a maximum of 3 months. However, for patients with moderate or worse severity (based on pemphigus score proposed by the MHLW Specific Disease Research Group) after 3 months' treatment, insurance may be calculated for a further 3 months only | Limited to patients with a definitive diagnosis whose condition is refractory to other treatment or who cannot be treated with high‐dose steroids due to complications or adverse drug reactions |
| Pemphigoid conditions | PE, DFPP | Up to twice a week, for a maximum of 3 months. | Limited to patients whose condition is refractory to other treatment or who cannot be treated with high‐dose steroids due to complications or adverse drug reactions |
| Toxic epidermal necrosis | PE, DFPP | Up to 8 times | None |
| Stevens‐Johnson syndrome | PE, DFPP | Up to 8 times | None |
| Psoriasis vulgaris | c‐LCAP, GCAP | Treatment once a week, up to 5 weeks is one treatment course, with a maximum of 1 course | To improve clinical symptoms in patients with moderate or worse condition who are non‐responsive to or cannot be treated with pharmacotherapy (diagnostic criteria from the MHLW Grant‐in‐Aid for Intractable Disease Policy Research Project on Rare and Intractable Skin Diseases) |
| Psoriatic arthritis | c‐LCAP, GCAP | Treatment once a week, up to 5 weeks is one treatment course, with a maximum of 2 courses However, treatment should be stopped after one course if it is determined that the patient is non‐responsive to treatment | To improve clinical symptoms in patients who are non‐responsive to or cannot be treated with existing pharmacotherapy in accordance with guidelines issued by related associations |
| Cardiovascular diseases | |||
| Arteriosclerosis obliterans | PE, DFPP, PA | Up to 10 times for a maximum of 3 months | Calculated only for patients with arteriosclerosis obliterans applicable to all the following conditions: |
| 1. Patients presenting with symptoms at or higher than Fontaine classification II | |||
| 2. Patients with hypercholesterolemia whose total cholesterol is 220 mg/dL or LDL cholesterol will not fall below 140 mg/dL with pharmacotherapy | |||
| 3. Patients with obstruction below the popliteal artery or an extensive occlusion site for whom surgery is difficult and who are not sufficiently responsive to conventional pharmacotherapy | |||
| Familial hypercholesterolemia | PE, DFPP, PA | Once a week | Patients applicable to any of the following conditions with xanthoma and coronary arteriosclerosis is apparent on load ECG and angiography: |
| 1. Homozygous patients with fasting steady state serum total cholesterol exceeding 500 mg/dL | |||
| 2. Heterozygous patients with steady state (state where weight and plasma albumin can be maintained) serum cholesterol exceeding 400 mg/dL with diet therapy and whose serum cholesterol will not fall below 250 mg/dL with pharmacotherapy | |||
| Other | |||
| Drug poisoning | PE, HA | PE: up to 8 times, HA: not specified | None |
| Severe blood type incompatibility pregnancy | PE, DFPP | None | Blood type incompatibility pregnancies with a history of intrauterine fetal distress or neonatal jaundice due to Rh blood type incompatibility pregnancy and indirect Coombs test is 64‐times or higher at less than 20 weeks' gestation or 128‐times or higher at more than 20 weeks' gestation |
| Kawasaki disease | PE, DFPP | Up to 6 times | When immunoglobulin therapy, steroid pulse therapy or neutrophil elastase inhibitors are either ineffective or not indicated |
| Sepsis | HA | Up to 2 times | In patients older than 18 years, calculated for patients applicable to all the conditions listed from 1) to 2) below: |
| 1. Simultaneously satisfies one or more of the items listed in (a) to (c) below | |||
| a. Positive blood cultures for gram‐negative bacilli | |||
| b. Gram‐negative bacillary infection was suspected at another hospital and antibiotics were administered | |||
| c. Septic shock due to gram‐negative bacilli is strongly suspected and the criteria for acute phase DIC scores are ≥4 or equivalent state | |||
| 2. Meeting the definition of septic shock in the Japanese Guidelines for the Management of Sepsis 2016 | |||
| In patients younger than 18 years, Patients suspected endotoxin sepsis or gram‐negative bacterial infection who meet the criteria for pediatric SIRS in the Japanese Guidelines for the Management of Sepsis 2016 | |||
Abbreviations: DFPP, double filtration plasmapheresis; GCAP, granulocyte apheresis; HA, hemoadsorption; PA, plasma adsorption; PE, plasma exchange; LCAP, leukocytapheresis.
The reimbursement costs shown here (as of May 2020) were revised from 1 October 2019, due to the revision of the consumption tax rate. These costs may change in the future depending on the social conditions.
5. ESTIMATED REIMBURSED MEDICAL EXPENSES FOR EACH TYPE OF APHERESIS TREATMENT
The medical expenses reimbursed for each apheresis treatment type are listed by modality below (Table 5).
As described above, the reimbursed medical expenses differ for apheresis performed on an outpatient basis and during hospitalization at non‐DPC/PDPS hospitals (volume calculation). They also differ for apheresis performed in a DPC/PDPS hospital during hospitalization (bundled calculation).
For volume calculations, the breakdown of reimbursed medical expenses includes the material cost of the therapeutic membrane as a medical device used for membrane separation apheresis, the treatment fee for performing the apheresis, the cost of anticoagulants used in the treatment, and the physiological saline used to clean the circuit, as well as the price of albumin, fresh frozen plasma, etc., when using fluid replacement. The price of the circuit cannot be charged. With centrifugal apheresis, the breakdown of reimbursed medical expenses includes the treatment fee, the anticoagulants used in the treatment, the cost of the physiological saline used to clean the circuit, and the price of fluid replacement, such as albumin and fresh frozen plasma.
In bundled calculations, of the above‐mentioned costs incurred by performing an apheresis, the cost of materials for therapeutic membranes, treatment fees, anticoagulants used in the treatment, the cost of saline used to clean the circuit, and price of fluid replacement, such as albumin, fresh frozen plasma, etc. is included in the fixed reimbursement amount. In DPC/PDPS hospitals, the reimbursement cost is added each time only when the apheresis treatment fee is implemented as a volume calculation. Under the DPC/PDPS system, when apheresis is performed for the same disease name with the same hospitalization period, the rank of the bundled fixed amount increases compared to cases without apheresis. Therefore, even if the cost of the apheresis performed under the DPC/PDPS system is absorbed in the bundled fixed reimbursement amount, the reimbursement cost received by the medical institution will often not be lower than in the non‐DPC/PDPS institutions. However, because the results of calculations differ depending on the complicated calculations based on (a) the number of days the patient was hospitalized for with a particular disease, (b) whether other procedures such as surgery were performed during hospitalization, and (c) the kind of tests performed and medications provided, especially when apheresis was performed multiple times during the same hospitalization. In some cases, the reimbursed expenses may be less than the cost required to administer a therapeutic apheresis.
5.1. Plasma exchange (PE)
With volume calculations, a reimbursement claim can be submitted for the price of the material for the plasma separation membrane at JPY29 500, and the treatment fee at JPY42 000. The cost of the replacement fluid depends on the amount of plasma processed. If the plasma exchange was performed at a processing volume equivalent to one plasma volume, the cost would be approximately JPY144 000 when replenishing an equal volume of fresh frozen plasma as the replacement fluid (JPY24 054 per pack of fresh frozen plasma x 6 packs = JPY144 324), and approximately JPY46 000‐JPY51 000 when using albumin and Ringer's lactate solution (using 9‐10 50 mL bottles of 25% albumin preparation, at about JPY5000 per bottle). When PE is performed using the membrane separation method, the total reimbursement would be JPY29 500 + JPY42 000 + replacement fluid cost + anticoagulant cost = approximately JPY120 000 (for albumin replacement)‐JPY218 000 (for fresh frozen plasma replacement). The total reimbursement for PE using the centrifugation method would approximately be JPY90 000‐JPY188 000, corresponding to JPY42 000 + replacement fluid cost + anticoagulant cost.
With bundled calculation where the patient was treated in a DPC/PDPS hospital as an inpatient, the treatment fee of JPY42 000 would be paid as an insurance reimbursement as a volume calculation in addition to the bundled medical expenses. Therefore, approximately JPY78 000‐JPY176 000 would be included in the total bundled reimbursement of medical expenses per treatment for treatments using the membrane separation method and approximately JPY48 000‐JPY146 000 for treatments using the centrifugal separation method.
In the case of selective plasma exchange (sPE), which uses a plasma fractionation membrane as the primary membrane, a reimbursement claim can be submitted for the price of the material for the plasma separation membrane at JPY24 100, and the treatment fee at JPY42 000. The cost of the replacement fluid depends on the amount of plasma processed and the albumin concentration of the replacement fluid. If sPE was performed at a processing volume equivalent to one plasma volume, the cost would be approximately JPY31 000‐JPY41 000 when albumin and Ringer's lactate were used as the replacement fluid (using 6‐8 bottles of 25% albumin preparation (50 mL), at about JPY5000 per bottle), the total reimbursement would be JPY24 100 + JPY42 000 + replacement fluid cost + anticoagulant cost = approximately JPY100 000‐JPY110 000 with volume calculations.
In addition, plasma filtration with dialysis (PDF), which is a modified method of sPE, is a treatment that uses plasma fractionation membranes as the primary membrane and circulates dialysate solution outside the hollow fiber while performing plasma exchange with FFP or albumin solution. With volume calculations, a reimbursement claim can be submitted for the price of the material for the plasma fractionation membrane at JPY24 100, and the treatment fee at JPY42 000. Two to four packs of fresh frozen plasma (JPY24 054 per pack of fresh frozen plasma × 2‐4 packs = JPY48 108‐JPY96 216) and one to two 50 mL bottles of 25% albumin (approximately JPY5000/bottle × 1‐2 = JPY5000‐JPY10 000) will be used. These FFP, albumin solutions and anticoagulants would be reimbursed by insurance, and the total reimbursement would be JPY24 100 + JPY42 000 + replacement fluid cost + anticoagulant cost = approximately JPY126 000‐JPY182 000. However, the dialysate solution (approximately 8400 mL, approximately JPY3500‐4800) used during the PDF is not included in the reimbursement coverage.
5.2. Double filtration plasmapheresis (DFPP)
In Japan, DFPP is performed only using the membrane separation method. With volume calculations, the material prices of the plasma separation membrane and the plasma fractionation membrane are JPY29 500 and JPY24 100, respectively, and the procedure fee for plasma exchange is JPY42 000. When albumin is used as the replacement fluid, for example, our institution uses 1 × 250 mL bottles of 5% albumin preparation (approximately JPY4600/bottle) and one 4 × 50 mL bottles of 25% albumin preparation (approximately JPY5000/bottle). However, in this case, in addition to the replacement fluid used, the anticoagulant, the saline used for cleaning, etc., are combined and the reimbursement amount for treatment will approximately be JPY108 000‐JPY123 000. With bundled calculation where the patient was treated in a DPC/PDPS hospital as an inpatient, the treatment fee of JPY42 000 would be paid as an insurance reimbursement as a volume calculation in addition to the bundled medical expenses. Therefore, approximately JPY66 000‐JPY81 000 would be included in the total bundled reimbursement of medical expenses per treatment using the membrane separation method.
Cryofitration is a therapy that can be considered a modification of DFPP, in which the separated plasma is cooled to 4°C to precipitate cryoglobulin. With volume calculations, the material prices of the plasma separation membrane and the plasma fractionation membrane are JPY29 500 and JPY24 100, respectively, and the procedure fee for plasma exchange is JPY42 000. Two to three 50 mL bottles of 25% albumin (approximately JPY5000/bottle are used as a replacement solution. With volume calculations, the total reimbursement would be JPY29 500 + JPY24 100 + JPY42 000 + replacement fluid cost + anticoagulant cost = approximately JPY109 000‐JPY114 000. However, the cost of the devices used to cool the separated plasma is not included in the reimbursement payment.
5.3. Plasma adsorption (PA)
In Japan, only the membrane separation method is used for PA that returns the separated plasma to the plasma adsorber. The insurance reimbursement amount for PA differs depending on the disease to be treated. The reimbursement is JPY70 800 when using a selective plasma component adsorber that adsorbs bilirubin and bile acids when treating fulminant hepatitis or postoperative liver failure. However, when used for other diseases, namely when using an LDL adsorber for refractory familial hypercholesterolemia, focal glomerulosclerosis or arteriosclerosis obliterans, or when using immunoadsorbent membrane for myasthenia gravis, malignant rheumatoid arthritis, systemic lupus erythematosus, Guillain‐Barré syndrome, multiple sclerosis or chronic inflammatory demyelinating polyneuropathy; the insurance reimbursement is JPY83 600, as material costs. In other words, with volume calculation, a total of approximately JPY146 000 would be reimbursed for treatment of fulminant hepatitis and postoperative hepatic failure, comprising JPY29 500 for the plasma separation membrane + JPY70 800 for the plasma component adsorber + JPY42 000 for the treatment fee + anticoagulant. When PA treatment is used for other diseases, a total of approximately JPY159 000 would be reimbursed, comprising JPY29 500 for the plasma separation membrane + JPY83 600 for the plasma component adsorber + JPY42 000 for the treatment procedure + anticoagulant. With bundled calculation where the patient was treated in a DPC/PDPS hospital as an inpatient, the treatment fee of JPY42 000 would be paid as an insurance reimbursement (as a volume calculation) in addition to the bundled medical expenses. Therefore, approximately JPY102 000‐JPY117 000 would be included in the total bundled reimbursement of medical expenses per treatment.
5.4. Leukocytapheresis (LCAP) or granulocytapheresis (GCAP)
With LCAP and GCAP, the type of therapeutic apheresis used, differs depending on the target disease. All treatment types using leukocytapheresis membrane (Cellsorber), granulocytapheresis membrane (Adacolumn), and leukocytapheresis by centrifugation are indicated for insurance reimbursement for ulcerative colitis. However, only the leukocytapheresis membrane is covered by insurance for rheumatoid arthritis, and only the granulocytapheresis membrane and centrifugation method are covered by insurance for Crohn's disease, psoriasis vulgaris, and psoriatic arthritis. When leukocytapheresis is performed using the leukocyte adsorption material, the insurance reimbursement is JPY128 000 when using a membrane with low priming volume for low body weight patients and children (as of May 2020, this product is only available as a leukocytapheresis membrane). JPY125 000 is reimbursed as medical material cost for other commonly used leukocyte removal membranes. In other words, when LCAP is performed for rheumatoid arthritis using a leukocytapheresis membrane, the material cost is JPY125 000 or JPY128 000, and the procedure fee is JPY20 000 (unlike other therapeutic apheresis, the treatment fee for hemoadsorption and cytapheresis is set at JPY20 000). Therefore, with volume calculation, the insurance reimbursement cost per treatment, including an anticoagulant etc., would be approximately JPY147 000‐JPY150 000. When leukocytapheresis or granulocytapheresis is performed for ulcerative colitis, approximately JPY147 000‐JPY150 000 would be reimbursed with the same volume calculation, and a treatment fee of JPY20 000 + approximately JPY21 000 for the anticoagulant would be reimbursed when the treatment uses centrifugation. Similarly, when granulocytapheresis or centrifugation is performed for Crohn's disease, psoriasis vulgaris, or psoriatic arthritis, approximately JPY147 000 and JPY22 000 would be reimbursed including volume calculation. With bundled calculation where the patient was treated in a DPC/PDPS hospital as an inpatient, the treatment fee of JPY20 000 would be paid as an insurance reimbursement as a volume calculation in addition to the bundled medical expenses. Therefore, approximately JPY127 000‐JPY130 000 would be included in the total bundled reimbursement of medical expenses per treatment when using membrane separation, and approximately JPY2000 would be included when using centrifugation.
5.5. Hemoadsorption (HA)
With volume calculation, the insurance reimbursement for an adsorption blood purifier such as activated carbon for hepatic coma or drug poisoning is JPY133 000, while the insurance reimbursement for an adsorption blood purifier for removing endotoxins when treating endotoxemia is JPY362 000. It should be noted that reimbursement can be claimed only up to two adsorptive blood purifiers for endotoxin removal. In this case also, the total insurance reimbursement will vary depending on the target disease treated with HA. With volume calculation, the insurance reimbursement for hepatic coma or drug poisoning would be a total of approximately JPY155 000, comprising JPY133 000 for the adsorption blood purifier + JPY20 000 for the treatment fee (unlike other apheresis therapies, the treatment fee for HA and cytapheresis is set at JPY20 000) + anticoagulant, etc. While for endotoxemia, the insurance reimbursement would be a total of approximately JPY384 000, comprising JPY362 000 for the adsorption blood purifier + JPY20 000 for the treatment procedure fee + anticoagulant, etc. With bundled calculation where the patient was treated in a DPC/PDPS hospital as an inpatient, the treatment fee of JPY20 000 would be paid as an insurance reimbursement as a volume calculation in addition to the bundled medical expenses. Therefore, approximately JPY135 000‐JPY364 000 would be included in the total bundled reimbursement of medical expenses per treatment.
The following information outlines the indication conditions and the maximum number of times treatment is possible under insurance for each disease for which apheresis is eligible for a reimbursement (Table 6).
5.6. Hematologic diseases
5.6.1. Multiple myeloma
Applicable treatments are PE or DFPP. There are no clearly stipulated conditions for insurance coverage for apheresis indications. The maximum number of treatments allowed for insurance reimbursement is limited to once a week per series for a maximum of 3 months.
5.6.2. Thrombotic thrombocytopenic purpura
Applicable treatments are PE or DFPP. There are no clearly stipulated conditions for insurance coverage for apheresis indications. The maximum length of treatment is 1 month after starting it and is generally calculated as up to 2 days after the platelet count reaches 150 000/μL or more. If thrombotic thrombocytopenic purpura relapses within 1 month after the platelet count reaches 150 000/μL or more, additional treatment is possible by describing the medical necessity in the medical record and the summary section of the medical fee statement.
5.6.3. Hemolytic‐uremic syndrome
Applicable treatments are PE or DFPP. There are no clearly stipulated conditions for insurance coverage for apheresis indications. Can be calculated for up to 21 times per series.
5.6.4. Macroglobulinemia
Applicable treatments are PE or DFPP. There are no clearly stipulated conditions for insurance coverage for apheresis indications. Can be calculated up to once a week for one series, for a maximum of 3 months.
5.6.5. Hemophilia with inhibitors
Applicable treatments are PE or DFPP. Can only be calculated for cases where the inhibitor titer is 5 Bethesda units or more. The maximum number of treatments is not stated.
5.7. Gastrointestinal diseases
5.7.1. Fulminant hepatitis
Applicable treatments are PE or PA. Calculated for removing bilirubin and bile acids, and generally the maximum number of treatments is 10 times per series.
5.7.2. Postoperative liver failure
Applicable treatments are PE or DFPP. Can be calculated for postoperative liver failure that satisfies the conditions stipulated in the table. Generally, the maximum number of treatments is seven times per series.
5.7.3. Acute liver failure
Applicable treatments are PE or DFPP. Only when it can be determined that the severity is the same as fulminant hepatitis or postoperative liver failure, based on findings such as prothrombin time, coma grade, total bilirubin and a hepaplastin test. Generally, the maximum number of treatments is seven times per series.
5.7.4. Chronic hepatitis C
Applicable treatments are PE or DFPP. Insurance is indicated for genotype Ib where the blood HVC‐RNA load is ≥100 KIU/mL (5 log IU/mL) even after interferon treatment. The maximum number of treatments is five times.
5.7.5. Hepatic coma
Treatment type is limited to hemoadsorption purification method only. Hemoadsorption purification method can only be calculated for hepatic coma and drug poisoning. The number of treatments is not set.
5.7.6. Ulcerative colitis
LCAP using membrane separation apheresis, GCAP using membrane separation apheresis, and leukocytapheresis using centrifugation apheresis may all be used as cytapheresis. This treatment can only be calculated for improving the active disease phase and for inducing remission for patients with severe, fulminant and refractory conditions (diagnostic criteria from the MHLW Intractable Disease Policy Research Project on Intractable Inflammatory Bowel Disease). Treatment can be taken up to 10 times per series, but up to 11 times for patients with fulminant conditions.
5.7.7. Crohn's disease
Only cytapheresis with GCAP or centrifugation apheresis may be used. Calculations are limited to treatment for inducing remission in patients with a moderate to severe condition in the active phase of the disease, which is unresponsive to nutritional therapy and existing pharmacotherapy or if such a treatment is not indicated and there are clear clinical symptoms caused by residual colon lesions. The maximum number of treatments is 10 times per series.
5.8. Transplantation‐related diseases
-
Allogenic liver transplantation with an ABO incompatibility or antilymphocyte antibody (positive).
Applicable treatment is DFPP. Calculations are limited to treatment for allogenic kidney transplantation or allogenic liver transplantation with ABO incompatibility or allogenic kidney transplantation or allogenic liver transplantation in patients positive for antilymphocyte antibodies. A maximum of four times preoperatively and two times postoperatively per series.
-
Allogenic kidney transplantation with ABO incompatibility or antilymphocyte antibody (positive).
Applicable treatment is DFPP. Calculations are limited to treatment for allogenic kidney transplantation or allogenic liver transplantation with ABO incompatibility or allogenic kidney transplantation or allogenic liver transplantation in patients positive for antilymphocyte antibodies. A maximum of four times preoperatively and two times postoperatively per series.
6. NEUROPATHIES
6.1.1. Myasthenia gravis
Applicable treatments are PE, DFPP or PA. Indicated for cases where serious symptoms tend to worsen within 5 years after its onset or cases with insufficient response to thymectomy or corticosteroids. Calculations are limited up to 7 treatments per month, for a maximum of 3 months.
6.1.2. Multiple sclerosis
Applicable treatments are PE, DFPP or PA. There are no conditions indicated for insurance calculations, and insurance is only calculated for up to seven treatments per month, for a maximum of 3 months.
6.1.3. Chronic inflammatory demyelinating polyneuropathy
Applicable treatments are PE, DFPP or PA. There are no conditions indicated for insurance calculations, and insurance is only calculated for up to seven treatments per month, for a maximum of 3 months.
6.1.4. Guillain‐Barré syndrome
Applicable treatments are PE, DFPP or PA. Insurance is calculated only for apheresis implemented for cases that are Grade 4 or higher on the Hughes Functional Grading Scale. Limited to 7 treatments per month, for a maximum of 3 months.
6.2. Kidney diseases
6.2.1. Focal glomerulosclerosis
Applicable treatments are PE, DFPP or PA. Calculated for cases that are non‐responsive to conventional pharmacotherapy, with a persistent nephrotic condition, and serum cholesterol that does not fall below 250 mg/dL. Insurance is indicated for up to 12 treatments per series, for a maximum of 3 months.
6.2.2. Anti‐glomerular basement membrane disease (anti‐GBM‐antibodies) type rapidly progressive glomerulonephritis
Applicable treatments are PE or DFPP. Insurance is calculated for patients diagnosed with rapidly progressive glomerulonephritis (RPGN) positive for anti‐glomerular basement membrane antibodies (anti‐GBM‐antibodies). Treatment up to seven times in 2 weeks comprising one treatment course, with a maximum of two courses per series.
6.2.3. Anti‐neutrophil cytoplasmic antibodies (ANCA) type rapidly progressive glomerulonephritis
Applicable treatments are PE or DFPP. Insurance is calculated for patients diagnosed with rapidly progressive glomerulonephritis (RPGN) positive for anti‐neutrophil cytoplasmic antibodies (ANCA). Treatment up to seven times in 2 weeks comprising one treatment course, with a maximum of two courses per series.
6.3. Collagen diseases
6.3.1. Rheumatoid arthritis
Cytapheresis with LCAP is the only applicable treatment. Insurance is calculated for patients with highly active, drug‐resistant rheumatoid arthritis or rapidly progressive drug‐resistant rheumatoid arthritis with systemic symptoms such as fever and severe synovitis in multiple joints who satisfy the following two conditions: (a) six or more swollen joints and (b) ESR ≥50 mm/h OR CRP ≥3 mg/dL. Calculations are limited to one course of treatment per series to improve clinical symptoms, limited to once a week per course for a maximum of 5 weeks.
However, the manufacturer of Cellsorba, which was the only product approved as an adsorptive leukocytapheresis membrane in Japan, decided to discontinue its sale in March 2020.
6.3.2. Malignant rheumatoid arthritis
Vasculitis, which is broadly classified into systemic arteritis or peripheral arteritis, known as rheumatoid vasculitis, alongside associated disease types that present with extra‐articular symptoms in the lungs, kidneys and skin, and refractory clinical symptoms such as non‐vasculitis interstitial pneumonia; are called malignant rheumatoid arthritis (malignant RA: MRA) in Japan.
Applicable treatments are PE, DFPP or PA. Calculations are limited to patients who are recognized by the prefectural governor as a recipient of specific disease treatment, and present with high‐grade extraarticular symptoms due to vasculitis (refractory lower leg ulcers, polyneuritis and melena due to mesenteric artery thrombosis), and are non‐responsive to conventional therapy. Calculations are limited to one treatment a week.
6.3.3. Systemic lupus erythematosus
Applicable treatments are PE, DFPP or PA. Patients qualify if any of the following are applicable to them: (a) recognized by the prefectural governor as a recipient of specific disease treatment, (b) serum complement titer (CH50) is ≤20 units, complement protein (C3) is ≤40 mg/dL and anti‐DNA antibodies are markedly elevated, and patient is unresponsive to steroid therapy or treatment is clinically inappropriate and, (c) diagnosed with rapidly progressive glomerulonephritis (RPGN) or central nervous system lupus (CNS lupus). Calculations are limited to four treatments a month. Insurance coverage specifies that the measured serum complement titer, complement protein value, or anti‐DNA antibody value is recorded in the patient's medical record.
6.4. Cutaneous diseases
6.4.1. Pemphigus
Applicable treatments are PE or DFPP. Insurance calculations are limited to patients with a definitive diagnosis as a result of medical examination and tests, whose condition is refractory to other treatment or who cannot be treated with high‐dose steroids due to complications or adverse drug reactions. Treatment is limited to twice a week per series, for a maximum of 3 months. However, for patients with moderate or worse severity (based on pemphigus score proposed by the MHLW Specific Disease Research Group) after 3 months' treatment, insurance may be calculated for an additional 3 months only.
6.4.2. Pemphigoid conditions
Applicable treatments are PE or DFPP. Insurance calculations are limited to patients with a definitive diagnosis as a result of medical examination and tests, whose condition is refractory to other treatments or who cannot be treated with high‐dose steroids due to complications or adverse drug reactions. Treatment is limited to twice a week per series, for a maximum of 3 months. Unlike pemphigus, there are no calculation standards for extension of treatment based on severity.
6.4.3. Toxic epidermal necrosis
Applicable treatments are PE or DFPP. There are no stipulated conditions for insurance coverage. Calculations are limited to eight treatments per series.
6.4.4. Stevens‐Johnson syndrome
Applicable treatments are PE or DFPP. There are no stipulated conditions for insurance coverage. Calculations are limited to eight treatments per series.
6.4.5. Psoriasis vulgaris
Applicable treatment is cytapheresis with GCAP and leukocytapheresis with centrifugation apheresis. Insurance calculations are limited to treatment implemented to improve clinical symptoms in patients with moderate or worse psoriasis vulgaris who are non‐responsive to or cannot be treated with pharmacotherapy (diagnostic criteria from the MHLW Grant‐in‐Aid for Intractable Disease Policy Research Project on Rare and Intractable Skin Diseases). Insurance can only be calculated for one treatment course, limited to once a week for one course, for a maximum of 5 weeks.
6.4.6. Psoriatic arthritis
Applicable treatment is cytapheresis with GCAP and leukocytapheresis with centrifugation apheresis. Insurance calculations are limited to treatment implemented to improve clinical symptoms in patients with psoriatic arthritis who are non‐responsive to or cannot be treated with existing pharmacotherapy in accordance with guidelines issued by related associations. Insurance can only be calculated for a maximum of two courses. One course is limited to treatment once a week, for a maximum of 5 weeks.
However, treatment should be stopped after one course if it is determined that the patient is non‐responsive to it.
6.5. Cardiovascular diseases
6.5.1. Arteriosclerosis obliterans
Applicable treatments are PE, DFPP or PA. Insurance is calculated only for patients with arteriosclerosis obliterans applicable to all the following conditions: (a) patients presenting with symptoms at or higher than Fontaine classification II, (b) patients with hypercholesterolemia whose total cholesterol is 220 mg/dL or whose LDL cholesterol does not fall below 140 mg/dL with pharmacotherapy and, (c) patients with obstruction below the popliteal artery or an extensive occlusion site for whom surgery is difficult and they are not sufficiently responsive to conventional pharmacotherapy. Insurance is only calculated for up to 10 times for a maximum of 3 months.
6.5.2. Familial hypercholesterolemia
Applicable treatments are PE, DFPP or PA. Patients with familial hypercholesterolemia that can be treated with apheresis, to whom any of the following conditions can be applied. Patients with xanthoma and apparent coronary arteriosclerosis on load ECG and angiography who are (a) homozygous patients with fasting steady state serum total cholesterol exceeding 500 mg/dL or (b) heterozygous patients with steady state (where weight and plasma albumin can be maintained) serum cholesterol exceeding 400 mg/dL with diet therapy and whose serum cholesterol will not fall below 250 mg/dL with pharmacotherapy. Insurance calculations are limited to treatment once a week as maintenance therapy.
6.6. Other
6.6.1. Drug poisoning
Applicable treatments are PE or HA. There are no stipulated conditions for insurance coverage. Calculations are limited to eight times for PE, with no specified limitations on number of treatments for HA.
6.6.2. Severe blood type incompatibility pregnancy
Applicable treatments are PE or DFPP. The calculation criteria determine severe blood type incompatibility pregnancies as blood type incompatibility pregnancies with a history of intrauterine fetal distress or neonatal jaundice due to Rh blood type incompatibility pregnancy and indirect Coombs test is 64‐times or higher at less than 20 weeks' gestation or 128‐times or higher at more than 20 weeks' gestation. There are no conditions set for the number of treatments.
6.6.3. Kawasaki disease
Applicable treatments are PE or DFPP. Insurance calculations are limited to cases when immunoglobulin therapy, steroid pulse therapy or neutrophil elastase inhibitors are either ineffective or not indicated. The maximum number of treatments is six times per series.
6.6.4. Sepsis
The applicable treatment is HA. In patients older than 18 years, insurance is calculated for patients applicable to all of the conditions listed under (1)‐(2): (1) he/she simultaneously satisfies one or more of the items listed in (a)‐(c). (a) Positive blood cultures for gram‐negative bacilli, (b) if gram‐negative bacillary infection was suspected at another medical facility and antibiotics were administered, (c) septic shock due to gram‐negative bacilli is strongly suspected and the criteria for acute phase DIC scores are ≥4 or equivalent state, (2) those meeting the definition of septic shock in the Japanese Guidelines for the Management of Sepsis 2016.
In patients younger than 18 years, patients suspected endotoxin sepsis or gram‐negative bacterial infection who meet the criteria for pediatric SIRS in the Japanese Guidelines for the Management of Sepsis 2016.The maximum number of treatments is up to two adsorption blood purifiers (for endotoxin removal).
7. CONCLUSION
This paper aimed to explain the insurance coverage status of apheresis (excluding CHDF) in Japan and the social system of medical reimbursement. The first half of the paper explained the universal health insurance coverage system in Japan and the available medical expense subsidies for its people. It also explained the insurance reimbursement system of medical expenses that medical institutions can receive when apheresis is implemented in Japan. The second half estimated specific insurance reimbursement costs when apheresis was performed for various diseases, and outlined the insurance indications for each disease, stating the maximum number of times apheresis could be performed.
Medical costs for items such as materials and replacement fluids tend to be high for therapeutic apheresis, but in Japan, the generous insurance system and multiple medical expense subsidies provide financial support for patients. Even if a patient ends up with a high copayment, using the high cost medical treatment expenses system can partially reduce the patient's expenses. With Japan's history of medical expense subsidies based on the policy of supporting intractable diseases, we have established an environment where all citizens can receive therapeutic apheresis when needed if they are affected by a disease for which insurance coverage is indicated.
The reimbursement costs for medical expenses listed as examples in this paper (as of May 2020) were revised from October 1, 2019, due to an increase of the consumption tax rate. Please note these costs may change in the future depending on social conditions.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article.
Kusaoi M, Murayama G, Tamura N, Yamaji K. Reimbursement for therapeutic apheresis devices and procedures for using the healthcare insurance system in Japan. Ther Apher Dial. 2020;24:530–547. 10.1111/1744-9987.13550
REFERENCES
- 1. Hatanaka T, Eguchi N, Deguchi M, Yazawa M, Ishii M. Study of Global Health strategy based on international trends: promoting universal health coverage globally and ensuring the sustainability of Japan's universal coverage of health insurance system: Problems and proposals. Japan Med Assoc J. 2015;58(3):78–101. [PMC free article] [PubMed] [Google Scholar]
- 2. Mackey T, Bekki H, Matsuzaki T, Mizushima H. Examining the potential of Blockchain technology to meet the needs of 21st‐century Japanese health care: Viewpoint on use cases and policy. J Med Internet Res. 2020;22(1):e13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ministry of Health, Labour and Welfare . Summary of the comprehensive survey of living conditions [homepage on the Internet]. Tokyo: Ministry of Health, Labour and Welfare, 2018 [updated 7 Dec 19; cited 29 May 2020]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/k‐tyosa/k‐tyosa18/index.html.
- 4. Ministry of Health, Labor and Welfare . An Outline of the Japanese Medical System [homepage on the Internet]. Tokyo: Ministry of Health, Labour and Welfare, 2019 [updated 2 Jun 18; cited 29 May 2020]. Available from: https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf.
- 5. Ministry of Health, Labour and Welfare . Current state of DPC/PDPS at a one hospital scale [homepage on the Internet]. Tokyo: Ministry of Health, Labour and Welfare, 2018 [updated 26 Jun 18; cited 29 May 2020]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000212583.pdf.
- 6. Ministry of Health, Labour and Welfare . Outline of medical facility (dynamic) surveys and hospital reports [homepage on the Internet]. Tokyo: Ministry of Health, Labour and Welfare, 2018 [updated 25 Sep 19; cited 29 May 2020]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/iryosd/18/dl/02sisetu30.pdf.


