Abstract
Background and Aim
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) account for a large and growing proportion of liver disease burden globally. The burden of NAFLD/NASH manifests in increasing levels of advanced liver disease and primary liver cancer in Australia. A Markov model was used to forecast NAFLD burden in Australia through 2030.
Methods
A model was used to estimate fibrosis progression, primary liver cancer, and liver deaths among the Australian NAFLD population, with changes in incident NAFLD cases based on long‐term trends for changes in the prevalence of obesity. Published estimates and surveillance data were applied to build and validate the model projections, including surveillance data for the incidence of liver cancer.
Results
Prevalent NAFLD cases were projected to increase 25% from the current burden (5 551 000 [4 748 000–6 306 000] cases in 2019) to 7 024 000 [5 838 000–7 886 000] cases in 2030. The projected increase in the number of NASH cases (40%) was greater than that of NAFLD cases. Incident cases of advanced liver disease are projected to increase up to 85% by 2030, and incident NAFLD liver deaths are estimated to increase 85% from 1900 (1100–3300) deaths in 2019 to 3500 (2100–6100) deaths in 2030.
Conclusions
Restraining growth of the obese and diabetic populations, along with potential therapeutic options, will be essential for mitigating disease burden.
Keywords: Fatty liver, Hepatocellular carcinoma, Liver cirrhosis, Liver neoplasms, Metabolic syndrome, Nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is recognized as a cause of advanced liver disease globally1, 2, 3 and particularly in Australia,4 where the increasing burden of metabolic syndrome is reflected in national survey data.5 NAFLD is defined as the presence of excessive liver fat in the absence of other causes such as excess alcohol.6 This analysis tracked NAFLD cases by fibrosis stage and categorized all cases as nonalcoholic steatohepatitis (NASH) or nonalcoholic fatty liver (NAFL) (simple steatosis). NASH is associated with liver fibrosis that may progress to advanced liver disease and related mortality.7, 8 Advanced age along with obesity, diabetes, and metabolic syndrome have been identified as predictors for progression to advanced fibrosis.9 Advanced liver disease and NAFLD‐related primary liver cancer typically develop after significant fibrosis has occurred; however, primary liver cancer may also occur among non‐cirrhotic NASH patients.10
Recent analyses have estimated the disease burden and economic costs related to NASH based on existing literature11 while dynamic modeling techniques can compensate for inherent limitations in estimates of NAFLD disease progression.12 There is an urgent need to understand the future burden of NAFLD‐related liver disease in Australia. Using a modeling framework to inform decision making may facilitate development of strategies to ameliorate further increases in disease burden.
Methods
Model
In order to estimate changes over time in NAFLD burden in Australia, a Markov model was constructed in Microsoft Excel to calculate the number of NAFLD cases by disease stage beginning in 1950.12 The total population by age group and gender was tracked over time, with new NAFLD cases entering the model based upon changes in the prevalence of adult obesity in Australia. Afflicted individuals were followed through each stage of the disease including fibrosis and advanced liver disease (Fig. S1) while also accounting for all‐cause mortality (including general background mortality and excess cardiovascular and non‐liver cancer mortality) as well as NAFLD‐related liver mortality. Fibrosis progression rates increased with advancing age and were varied by gender with male experiencing faster progression (Table S1). Model results were compared with data and studies that estimate the incidence of primary liver cancer attributable to NAFLD in Australia. A Delphi process was used to identify and incorporate key model inputs and review outputs with local experts against available estimates of disease burden (Table S2).
During initial model development, fibrosis progression and NASH status were adjusted to ensure distribution exceeded the number of NASH‐related primary liver cancer cases reported in the surveillance system,13 with adjustments based on relative rates of overweight (25 > body mass index [BMI] ≤ 30 kg/m2) and obesity (BMI ≥ 30 kg/m2)14 and published odds of disease progression to advanced fibrosis.15 Meta‐analysis of reported fibrosis progression rates among NAFLD and NASH cases7 has demonstrated a range so broad that modeling projections is not feasible. Therefore, ranges around fibrosis progression rates by age, gender, and fibrosis stage were back‐calculated as described in a previous analysis.16 Cohort data were used for the relative increase in progression by age and gender17 with rates further modified based on results of meta‐analysis18 and historical trends for hepatocellular carcinoma (HCC) incidence by age and gender.19 Progression rates to primary liver cancer, decompensated cirrhosis, and liver‐related death were based on reported estimates13 (Table S1).
Population and mortality
The annual Australian population (1950–2050) by age group and gender was based on population data from the Australia Bureau of Statistics.20, 21 To calculate annual mortality, estimated deaths by age group and gender from the United Nations were divided by population estimates.22 Background mortality rates were adjusted to account for incrementally increased mortality related to cardiovascular disease23 and non‐liver cancers.24 Excess non‐liver mortality varies by age; among a cohort of adults in the Sydney area with elevated liver enzymes, there was no significant increase in mortality reported for adults aged ≤ 49 years as compared with adults with normal enzyme results. Increased risk of mortality was observed among participants aged ≥ 50 years, and a significant mortality hazard ratio was observed for adults aged ≥ 80 years. Elevated non‐liver mortality may also vary significantly by fibrosis stage and NASH status,8, 25, 26 which are heavily correlated with age. Thus, a standard mortality ratio (SMR) of 1.15 [uncertainty range: 1.00–1.30] was applied to background mortality rates for ≥ F1 NAFLD cases and the portion of F0 cases that were classified as NASH. F0 cases with simple steatosis were assumed to have no elevated mortality (SMR = 1.0). Uncertainty analysis was used to account for the potential that NAFLD cases experience no excess mortality with SMR = 1.0 (low) or up to 30% excess background mortality with SMR = 1.3 (high) among non‐simple steatosis F0 cases. Model NAFLD‐related liver deaths were calculated as a progression rate among prevalent primary liver cancer and decompensated cirrhosis cases.1, 27, 28, 29
New nonalcoholic fatty liver disease cases
In order to estimate relative changes in the number of prevalent NAFLD cases, BMI data were used as a surrogate. While other factors are more strongly predictive of advanced NAFLD‐related disease, data for changes in the prevalence of adults in different BMI classes are available for long periods of time, allowing for the estimation of long‐term trends. The growth in NAFLD prevalence was assumed to occur simultaneous to changes in the prevalence of adults classified as obese. Because of variations in cutoff levels for obesity by race/ethnicity,30 the prevalence of obesity was calculated as a weighted average using a BMI cutoff of ≥ 25 kg/m2 for the population classified in the Australian Census as South‐East Asian, North‐East Asian, and Southern and Central Asian and a BMI ≥ 30 kg/m2 for the remaining population. The population with an obesity cutoff of ≥ 25 kg/m2 was estimated at 7.5% of the total Australian population in 2006, increasing to 12.9% in 2016. Extrapolating this trend linearly, the selected Asian populations would comprise 20.3% of the Australian population by 2030, meaning that the average BMI cutoff level for adult obesity will continue to decrease over time.
Temporal changes in adult obesity were estimated by trending prevalence data from both the Australian National Health Survey5 and NCD Risk Factor Collaboration meta‐analysis for Australia.31 Using the weighted average of adult obesity at different cutoff levels, the National Health Survey reported obesity prevalence at 21.6% in 1995, increasing to 31.8% prevalence in 2015. The NCD Risk Factor Collaboration adjusted obesity was estimated at 11.4% in 1975, increasing to 32.0% in 2014. Trends based on both data sources were considered for uncertainty analysis.5
Nonalcoholic fatty liver disease prevalence
Among individuals aged ≥ 15 years in 2015, there was an assumed NAFLD prevalence rate of 25% [uncertainty range: 20–30%]. A range of reported NAFLD prevalence exists for adults,32 and expert consensus was used to estimate the likeliest prevalence among adults and a conceivable high/low range of prevalence. Adjusting for lower prevalence among persons aged < 15 years, prevalence among all ages was estimated at 20.6% in 2015. The age and gender distribution of prevalent NAFLD cases was based on data from general population studies and is higher in men and those of advanced age.33, 34, 35 Prevalence among younger people (aged ≤ 18 years) is generally not estimated in large general population‐based studies33, 36 and was assumed to decline with decreasing age. Because of elevated competing mortality risk among NAFLD cases,8 it was also assumed that prevalence would naturally decline among the oldest age groups (≥ 80 years), with peak prevalence occurring in late middle age.
Nonalcoholic steatohepatitis prevalence
Prevalence of NASH was calculated based on the distribution of NAFLD cases by fibrosis stage given the total NAFLD population, with rates that varied by sex and age group. NASH‐related fibrosis can regress in NAFLD patients7; however, there is considerable uncertainty around the presence and staging of NASH due to limitations of liver biopsy results.37 The model assumed that 5% of NAFLD cases without NASH could have previously experienced NASH with subsequent regression, including a portion with fibrotic changes. Increasing fibrosis stage was assumed to result in lower probability of experiencing regressed NASH, with each increased fibrosis stage resulting in an exponential decrease in regressed NASH, and the overall number of regressed NASH cases limited to 5%. Overall, 2000 ≥ F2 cases were classified as non‐NASH NAFLD in 2019, or 0.47% of total NAFLD cases in 2019.
Liver transplants
Total annual liver transplants were reported by the Australia and New Zealand Organ Donation Registry.38 Based on expert input and analysis of diagnostic categories for transplant recipients, it was estimated that approximately 15% of current liver transplants are attributable to NAFLD/NASH. Analysis of liver transplant data from Australia and New Zealand has shown that NASH as an indicator grew from 2.0% in 2003 to 10.9% in 2017, making it the third leading indicator for liver transplants.39 Given the uncertainties around transplant demand and availability, it was assumed that the annual number of transplants would remain constant through 2030. However, this was a conservative estimate, as data already suggest that the proportion of NAFLD‐related transplants is increasing in Western countries and that some portion of transplants indicated for cryptogenic or idiopathic cirrhosis are likely related to NAFLD.40 In addition, there are overlapping indications for transplant, as alcoholic liver disease and chronic viral hepatitis can coexist with NAFLD.41
Model validation
Primary liver cancer surveillance data were used to validate the results of the model. The Australian Institute of Health and Welfare estimates that incident primary liver cancer increased from 1076 in 2005 to 2215 in 2018.42 These estimates were further adjusted for underreporting, cancer morphology, and the proportion of cancers that could be NAFLD related. For underreporting, it was assumed that 25% of cancers may not be reported to the registry, but based on a Melbourne study, underreporting could historically be as high as 50%.4 For cancer morphology, an estimated 90% of incident primary liver cancer were assumed to be classified as HCC. A study of incident HCC cases in the Victorian Cancer Registry from 2012 to 2013 reported risk factors of fatty liver disease (14%), other/unknown (6%), and more than one risk factor (27%).4 For modeling purposes, a range of 4.0% to 34.8% was considered for the proportion of HCC that could be NAFLD related.43, 44 This wide range was utilized because of uncertainty and changes over time in the etiology of liver cancer, with viral hepatitis expected to contribute relatively fewer cases in the future. Cholangiocarcinoma data were incorporated in the validation, with 5% of total primary liver cancers assumed to be classified as cholangiocarcinoma and an estimated 45% of cases potentially NAFLD related. The outcomes of this analysis were compared with model‐predicted incident primary liver cancer cases to ensure that the model was predictive based on surveillance data.
Results
Nonalcoholic fatty liver disease population
Between 2019 and 2030, NAFLD cases are expected to increase 25% from 5 556 000 (4 754 000–6 312 000) to 7 026 000 (5 842 000–7 890 000) (Fig. 1). Likewise, F0/F1 NAFLD cases are also projected to increase 25% from 5 124 000 (4 212 000–5 994 000) to 6 323 000 (4 946 000–7 393 000). F2 cases are expected to increase more (50%) during the same timespan, from 228 000 (142 000–345 000) cases in 2019 to 347 000 (218 000–524 000) cases in 2030. The F3 population is predicted to increase by 70% between 2019 and 2030, from 133 000 (79 100–193 000) to 223 000 (134 000–322 000) cases. Compensated cirrhotic cases were forecasted to increase 85% from 62 900 (37 500–105 000) cases in 2019 to 115 000 (68 700–190 000) cases in 2030. Prevalent cases of decompensated cirrhosis, primary liver cancer and liver transplants, are expected to increase concurrently from a combined 8500 (5600–14 500) to 16 000 (11 000–25 700) cases, an increase of 85% during 2019–2030. The prevalence of NAFLD in all ages is predicted to increase from 22.0% (18.8–25.0%) in 2019 to 23.6% (19.6–26.5%) by 2030. Prevalent NAFLD cases by age group and gender were compared with the distribution of the non‐NAFLD Australian population (Fig. 2). In 2019, the largest prevalent NAFLD age group was aged 55–59 years, with 595 000 NAFLD cases. By 2030, peak cases (687 000 cases) were observed in persons aged 60–64 years.
Figure 1.
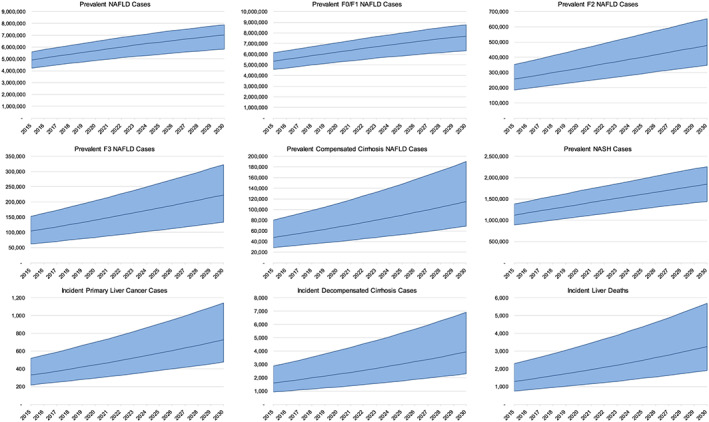
Nonalcoholic fatty liver disease (NAFLD)‐related disease burden with 95% uncertainty interval—Australia, 2015–2030.
Figure 2.
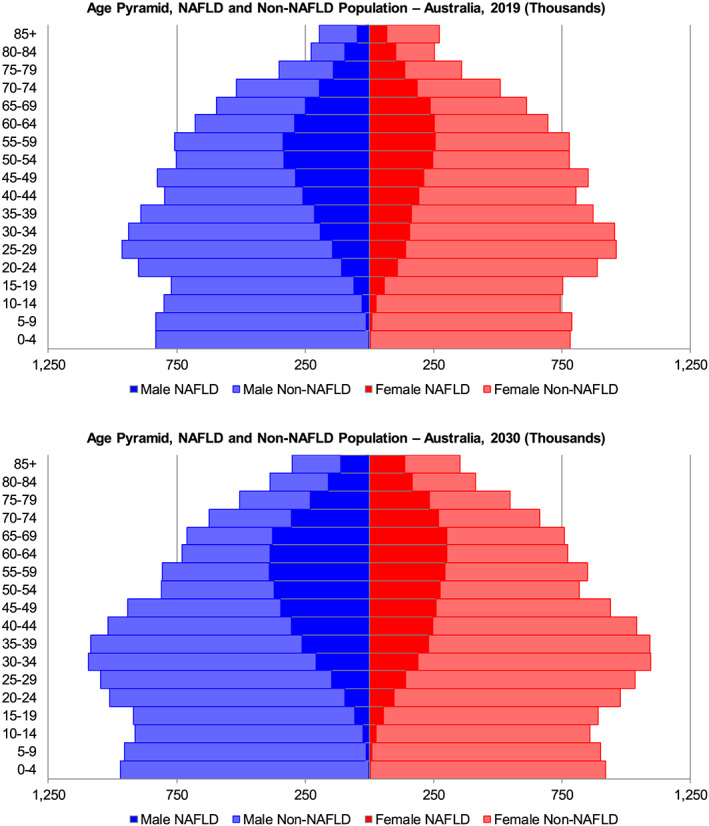
Age distribution of prevalent nonalcoholic liver disease (NAFLD) cases—Australia, 2019 and 2030.  , male NAFLD;
, male NAFLD;  , male non‐NAFLD;
, male non‐NAFLD;  , female NAFLD;
, female NAFLD;  , female non‐NAFLD.
, female non‐NAFLD.
Nonalcoholic fatty liver population
The NAFL population was assumed to be cases with simple steatosis that never progressed to NASH, with a relatively small number of cases that were formerly NASH and experienced disease regression. In 2019, the NAFL population is estimated to be 4 239 000 (3 714 000–4 690 000) cases, or 76.3% of all NAFLD cases. By 2030, the NAFL population is estimated to grow 20% to 5 178 000 (4 403 000–5 634 000) cases (73.7% of total). NAFL cases classified as ≥ F2 fibrosis were also estimated to increase from 2000 cases in 2019 to 3200 cases in 2030.
Nonalcoholic steatohepatitis population
The number of prevalent NASH cases was projected to increase 40% from 1 317 000 (1 040 000–1 622 000) to 1 848 000 (1 439 000–2 256 000) cases during the 2019–2030 timeframe (Fig. 1). NASH cases were projected to comprise 23.7% of all NAFLD cases in 2019, increasing to 26.3% of cases in 2030. NASH prevalence in the general population (all ages) was estimated as 5.2% (4.1–6.4%) in 2019 and is expected to increase to 6.2% (4.8–7.6%) in 2030 (Table 1).
Table 1.
Model estimates of NAFLD burden—Australia, 2015–2030
| Year | ||||
|---|---|---|---|---|
| 2015 | 2020 | 2025 | 2030 | |
| Country population | 23 816 000 | 25 710 000 | 27 794 000 | 29 747 000 |
| Prevalent cases | ||||
| NAFLD cases | 4 915 000 (4 220 000–5 605 000) | 5 710 000 (4 879 000–6 483 000) | 6 424 000 (5 387 000–7 253 000) | 7 026 000 (5 842 000–7 890 000) |
| NAFLD prevalence rate (all ages) | 20.6% (17.7–23.5%) | 22.2% (19.0–25.2%) | 23.1% (19.4–26.1%) | 23.6% (19.6–26.5%) |
| F0 | 4 211 000 (3 541 000–4 840 000) | 4 818 000 (4 009 000–5 515 000) | 5 337 000 (4 334 000–6 087 000) | 5 741 000 (4 557 000–6 581 000) |
| F1 | 360 000 (244 000–509 000) | 438 000 (295 000–619 000) | 513 000 (344 000–724 000) | 582 000 (389 000–812 000) |
| F2 | 186 000 (116 000–282 000) | 238 000 (149 000–361 000) | 293 000 (184 000–443 000) | 347 000 (218 000–524 000) |
| F3 | 105 000 (62 000–153 000) | 140 000 (83 600–204 000) | 180 000 (108 000–261 000) | 223 000 (134 000–322 000) |
| Compensated cirrhosis | 47 900 (28 600–80 200) | 67 000 (39 900–111 000) | 89 400 (53 200–148 000) | 115 000 (68 700–190 000) |
| Decompensated cirrhosis, HCC, and liver transplant | 6400 (4100–11 000) | 9100 (6000–15 500) | 12 200 (8300–20 100) | 16 000 (11 000–25 700) |
| NASH cases | 1 119 000 (886 000–1 380 000) | 1 366 000 (1 078 000–1 681 000) | 1 612 000 (1 264 000–1 974 000) | 1 848 000 (1 439 000–2 256 000) |
| NASH prevalence rate (all ages) | 4.7% (3.7–5.8%) | 5.3% (4.2–6.5%) | 5.8% (4.5–7.1%) | 6.2% (4.8–7.6%) |
| Incident cases | ||||
| Decompensated cirrhosis | 1600 (920–2900) | 2300 (1300–4000) | 3000 (1800–5400) | 3900 (2300–6900) |
| HCC | 330 (220–520) | 440 (300–700) | 580 (380–910) | 730 (480–1100) |
| Liver death | 1300 (760–2300) | 1800 (1100–3200) | 2500 (1500–4400) | 3200 (1900–5700) |
HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Among NASH cases in 2019, 204 000 were estimated to have F3/F4 fibrosis or advanced liver disease (decompensated cirrhosis, liver cancer, or liver transplant), encompassing 15.5% of all NASH cases and 0.8% of the total population (all ages). By 2030, this number was expected to increase 75% to 354 000 cases, accounting for 19.1% of all NASH cases and 1.2% of the total Australian population.
Nonalcoholic fatty liver disease‐related decompensated cirrhosis and primary liver cancer
Incident decompensated cirrhosis cases were projected to increase 85% from 2100 (1100–3800) cases in 2019 to 3900 (2300–6900) cases in 2030 (Fig. 1). Cumulative incidence of decompensated cirrhosis during the same time period was estimated to be 35 800 (20 800–63 200) cases. Cases of incident primary liver cancer were forecasted to increase by 75% from 420 (280–660) cases in 2019 to 730 (480–1100) cases in 2030; cumulative incident cases of primary liver cancer between 2019 and 2030 were estimated to be 6800 (4500–10 700). Modeled incident liver cancer was compared with reported estimates for 2005–2015, for which the most reliable data were available (Fig. 3). During this timespan, model incident liver cancer cases were within the expected range.
Figure 3.
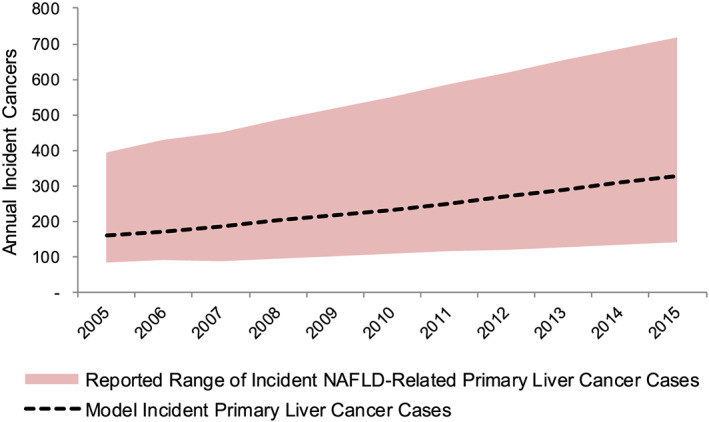
Reported range and model‐estimated incident nonalcoholic fatty liver disease (NAFLD)‐related primary liver cancer cases—Australia, 2005–2015.  , reported range of incident NAFLD‐related primary liver cancer cases;
, reported range of incident NAFLD‐related primary liver cancer cases;  , model incident primary liver cancer cases.
, model incident primary liver cancer cases.
Mortality
In the NAFLD population, annual liver‐related deaths are estimated to increase 90% from 1700 (1000–3000) to 3200 (1900–5700) cases during 2019–2030 (Fig. 1). Cumulative liver deaths during the same time period were estimated to be 29 300 (17 300–51 500). Total deaths in the NAFLD population in 2019 are estimated at 54 800 deaths increasing 55% to 85 500 deaths in 2030. In 2019, modeled liver mortality accounted for 3.1% of annual deaths in Australia's NAFLD population, increasing to 3.8% of deaths in 2030. Among the NASH population, annual deaths are estimated to be 19 800 in 2019, increasing 75% to 34 800 deaths in 2030. Modeled liver deaths in the NASH population were estimated to comprise 8.7% (1700 deaths) of total deaths in 2019, increasing to 9.3% (3200 deaths) in 2030.
Discussion
The results of NAFLD modeling suggest that NAFLD presents an expanding burden such that the Australian population22 is expected to experience substantial increases in NAFLD‐related disease burden in the coming decades. In the coming decade, NASH may become a leading indication for liver transplantation in Australia, in tandem with a reduced burden of viral hepatitis.
The current analysis utilized both literature review and expert interviews to design the model and validate model outputs. Longitudinal trends in adult obesity levels, including estimates from both the Australian National Health Survey and NCD Risk Factor Collaboration, were used.5, 31 Trending of obesity estimates from both data sources shows that the period of fastest growth in obesity has passed, while future growth rates in obesity remain uncertain.
Even if obesity prevalence in Australia stabilizes, NAFLD‐related morbidity and mortality are projected to rise. The Australian population aged ≥ 65 years is projected to increase by over 1.4 million persons between 2019 and 2030.22 With increasing average age, the population will experience greater risk for advanced liver disease.7, 15 The number of primary liver cancer cases identified as NAFLD‐related has already been increasing over time1; however, patients with NAFLD face high rates of non‐surveillance for liver cancer.45 Our results further confirm the continued growth in NAFLD‐related liver cancer in Australia and the pressing need to better identify NAFLD cases, especially persons with significant fibrosis. Persons with diabetes represent a substantial portion of the NASH population,6 and physicians should consider diabetics a high‐risk group. There were 1.2 million diagnosed diabetics in Australia in 2015,46 and approximately 20% of cases may be undiagnosed. By 2030, the diabetic population is projected to grow to 2.2–3.0 million cases.47
Modeling projected disease burden is subject to multiple limitations. Some limitations apply to all disease models, such as uncertainties around the future growth of the total Australian population21 and the impact of immigration. Other limitations are present when modeling the growth in chronic lifestyle conditions. Trends projecting growth in the prevalence of obesity, diabetes, and other facets of the metabolic syndrome are informed by current and historical data, but such trends may change in the future.
Rates of childhood/adolescent obesity are relatively high in Australia14 but may be stabilizing.48 In the future, a larger portion of NAFLD patients will have experienced a longer duration of obesity and a potentially earlier onset of NAFLD with disease progression occurring at younger ages.49 In contrast, another uncertainty is the potential availability of new therapies targeted at different facets of metabolic syndrome (e.g. NASH, diabetes, and cardiovascular disease) that would impact the natural history of disease by reducing the rate of disease progression.
This analysis differs from previous work,23 in that assumptions for model inputs, as well as low and high ranges for sensitivity analysis, varied. This analysis considered the impact of changing obesity at two different cutoff levels (≥ 25 and ≥ 30 kg/m2) to account for changes in demographics of the population and the impact on NAFLD prevalence. In addition, this analysis included assumptions for excess background mortality rates that were adjusted for the proportion of non‐NASH F0 cases, assuming that these cases did not experience elevated mortality. Given the long natural history of the metabolic syndrome and NASH, the impact of competing mortality will play a large role in future disease burden (Fig. S3).
The current analysis was calibrated and validated using surveillance data for advanced liver disease, primarily NAFLD‐related liver cancer. The historical incidence of liver cancer may be underreported to a greater degree than assumed in this analysis.4 The relative contribution of NAFLD to liver cancer changes over time as competing risk factors for liver cancer (e.g. viral hepatitis) vary greatly over time, and others such as alcoholic liver disease remain relatively constant.
Results of analyses demonstrate growing disease burden associated with NAFLD and NASH, following the trajectory of increasing obesity in Australia.5 Over one quarter of Australians aged 5–17 years are overweight or obese. This may translate to increasing rates of NAFLD in younger age groups and continued high levels of NAFLD‐related disease burden in the coming decades. Intervention is needed to slow the growth in obesity and metabolic syndrome. Both lifestyle modifications and other therapeutic options50 must be considered to avert the coming epidemic of NAFLD‐related liver disease.
Supporting information
Data S1. Supplementary Information.
Table S1. Fibrosis Transition Probabilities by Disease Stage, Sex and Age Group.
Table S2. Delphi Process.
Figure S1. NAFLD Disease Progression Model.
Figure S2. Reported Prevalence of Adult Obesity – Australia, 1975–2015.
Figure S3. Key Drivers of Uncertainty for Prevalent NAFLD and NASH Cases – Australia, 2030.
Adams, L. A. , Roberts, S. K. , Strasser, S. I. , Mahady, S. E. , Powell, E. , Estes, C. , Razavi, H. , and George, J. (2020) Nonalcoholic fatty liver disease burden: Australia, 2019–2030. Journal of Gastroenterology and Hepatology, 35: 1628–1635. 10.1111/jgh.15009.
Declaration of conflict of interest: Funding for this project was provided by Gilead Sciences. The funders had no role in the study design, data collection, analysis, interpretation of the data, or preparation of the manuscript.
Author contribution: H. R. and C. E. conceived and designed the analysis. L. A. A., S. K. R., S. I. S., S. E. M., E. P., C. E., H. R., and J. G. contributed the data and provided data analysis and interpretation. L. A. A., S. K. R., S. I. S., S. E. M., E. P., C. E., H. R., and J. G. provided critical revision of the work.
References
- 1. Younossi ZM, Otgonsuren M, Henry L et al Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015; 62: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 2. Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population‐based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010; 26: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 3. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013; 10: 686–690. [DOI] [PubMed] [Google Scholar]
- 4. Hong TP, Gow P, Fink M et al Novel population‐based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 2016; 63: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 5. Australian Institute of Health and Welfare . Overweight & obesity 2018 [Available from: https://www.aihw.gov.au/reports‐statistics/behaviours‐risk‐factors/overweight‐obesity/data].
- 6. Wong VW, Chan WK, Chitturi S et al Asia–Pacific Working Party on Non‐alcoholic Fatty Liver Disease guidelines 2017—part 1: definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018; 33: 70–85. [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta‐analysis of paired‐biopsy studies. Clin. Gastroenterol. Hepatol. 2015; 13: 643–654 e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dulai PS, Singh S, Patel J et al Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017; 65: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 2015; 62: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 10. White DL, Kanwal F, El‐Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012; 10: 1342–59.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non‐alcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence and outcomes. Hepatology. 2015. [DOI] [PubMed]
- 12. Estes C, Anstee QM, Arias‐Loste MT et al Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018; 69: 896–904. [DOI] [PubMed] [Google Scholar]
- 13. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2017. [DOI] [PMC free article] [PubMed]
- 14. Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017; 390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angulo P, Hui JM, Marchesini G et al The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45: 846–854. [DOI] [PubMed] [Google Scholar]
- 16. Blach S, Zeuzem S, Manns M et al Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2017; 2: 161–176. [DOI] [PubMed] [Google Scholar]
- 17. Harris RJ, Thomas B, Griffiths J et al Increased uptake and new therapies are needed to avert rising hepatitis C‐related end stage liver disease in England: modelling the predicted impact of treatment under different scenarios. J. Hepatol. 2014; 61: 530–537. [DOI] [PubMed] [Google Scholar]
- 18. Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV‐infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta‐analysis. AIDS 2008; 22: 1979–1991. [DOI] [PubMed] [Google Scholar]
- 19. Surveillance, Epidemiology, and End Results (SEER) Program research data (1973–2013) [Internet]. National Cancer Institute. 2016 [cited August 10th 2016]. Available from: www.seer.cancer.gov.
- 20. Australian Bureau of Statistics . 3105.0.65.001—Australian historical population statistics, 2016, 2019. [Available from: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3105.0.65.0012016?OpenDocument].
- 21. Australian Bureau of Statistics . 3222.0—population projections, Australia, 2017 (base)—2066, 2019. [Available from: https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3222.0Main+Features12017%20(base)%20‐%202066?OpenDocument].
- 22. United Nations, Department of Economic Social Affairs Population Division . World Population Prospects: The 2017 Revision. New York: United Nations, 2018. [Google Scholar]
- 23. Stepanova M, Rafiq N, Makhlouf H et al Predictors of all‐cause mortality and liver‐related mortality in patients with non‐alcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2013; 58: 3017–3023. [DOI] [PubMed] [Google Scholar]
- 24. Harding JL, Shaw JE, Anstey KJ et al Comparison of anthropometric measures as predictors of cancer incidence: a pooled collaborative analysis of 11 Australian cohorts. Int. J. Cancer 2015; 137: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 25. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V et al Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology 2018; 155: 443–57.e17. [DOI] [PubMed] [Google Scholar]
- 26. Hagstrom H, Nasr P, Ekstedt M et al Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J. Hepatol. 2017; 67: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 27. Sanyal AJ, Banas C, Sargeant C et al Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006; 43: 682–689. [DOI] [PubMed] [Google Scholar]
- 28. Rahman RN, Ibdah JA. Nonalcoholic fatty liver disease without cirrhosis is an emergent and independent risk factor of hepatocellular carcinoma: a population based study. Hepatology 2012; 56: 241A. [Google Scholar]
- 29. Ries L, Young G, Keel G, Eisner M, Lin Y, Horner M. SEER Survival Monograph: Cancer Survival among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program: Bethesda, MD, 2007. [Google Scholar]
- 30. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int. J. Obes. Relat. Metab. Disord. 1998; 22: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 31. Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Younossi Z, Anstee QM, Marietti M et al Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 33. Lazo M, Hernaez R, Eberhardt MS et al Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013; 178: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caballeria L, Pera G, Auladell MA et al Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur. J Gastroenterol. Hepatol. 2010; 22: 24–32. [DOI] [PubMed] [Google Scholar]
- 35. Fan JG, Farrell GC. Epidemiology of non‐alcoholic fatty liver disease in China. J. Hepatol. 2009; 50: 204–210. [DOI] [PubMed] [Google Scholar]
- 36. Choi YJ, Lee DH, Han KD et al Is nonalcoholic fatty liver disease associated with the development of prostate cancer? A nationwide study with 10,516,985 Korean men. PLoS ONE 2018; 13: e0201308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ratziu V, Charlotte F, Heurtier A et al Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 38. Australia and New Zealand Organ Donation (ANZOD) Registry . Annual report of the Australia and New Zealand Organ Donation Registry (ANZOD). 2018.
- 39. Calzadilla‐Bertot L, Jeffrey GP, Jacques B et al Increasing incidence of nonalcoholic steatohepatitis as an indication for liver transplantation in Australia and New Zealand. Liver Transpl. 2019; 25: 25–34. [DOI] [PubMed] [Google Scholar]
- 40. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014; 59: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 41. Thurnheer MC, Schulz TR, Nguyen T, MacLachlan J, Sasadeusz J. Regional challenges: evaluation of a hepatitis outreach programme using transient elastography (FibroScan) in Victoria. Intern. Med. J. 2016; 46: 273–281. [DOI] [PubMed] [Google Scholar]
- 42. Australian Institute of Health and Welfare . Cancer compendium: information and trends by cancer type 2018 [Available from: https://www.aihw.gov.au/reports/cancer/cancer‐compendium‐information‐trends‐by‐cancer/report‐contents/summary].
- 43. Weinmann A, Alt Y, Koch S et al Treatment and survival of non‐alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer 2015; 15: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dyson J, Jaques B, Chattopadyhay D et al Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014; 60: 110–117. [DOI] [PubMed] [Google Scholar]
- 45. Huang Y, Wallace MC, Adams LA et al Rate of nonsurveillance and advanced hepatocellular carcinoma at diagnosis in chronic liver disease. J. Clin. Gastroenterol. 2018; 52: 551–556. [DOI] [PubMed] [Google Scholar]
- 46. Australian Institute of Health and Welfare . Diabetes snapshot 2018 [Available from: https://www.aihw.gov.au/reports/diabetes/diabetes‐snapshot/contents/how‐many‐australians‐have‐diabetes].
- 47. Shaw J, Tanamas S. Diabetes: The Silent Pandemic and Its Impact on Australia. Diabetes Australia: Canberra, 2012. [Google Scholar]
- 48. Hardy LL, Mihrshahi S, Gale J, Drayton BA, Bauman A, Mitchell J. 30‐year trends in overweight, obesity and waist‐to‐height ratio by socioeconomic status in Australian children, 1985 to 2015. Int. J. Obes. (Lond) 2017; 41: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ratziu V, Marchesini G. When the journey from obesity to cirrhosis takes an early start. J. Hepatol. 2016; 65: 249–251. [DOI] [PubMed] [Google Scholar]
- 50. Chitturi S, Wong VW, Chan WK et al The Asia–Pacific Working Party on Non‐alcoholic Fatty Liver Disease guidelines 2017—part 2: management and special groups. J. Gastroenterol. Hepatol. 2018; 33: 86–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Information.
Table S1. Fibrosis Transition Probabilities by Disease Stage, Sex and Age Group.
Table S2. Delphi Process.
Figure S1. NAFLD Disease Progression Model.
Figure S2. Reported Prevalence of Adult Obesity – Australia, 1975–2015.
Figure S3. Key Drivers of Uncertainty for Prevalent NAFLD and NASH Cases – Australia, 2030.


