Abstract
Presence of bacteria in wounds can delay healing. Addition of a regularly instilled topical solution over the wound during negative‐pressure wound therapy (NPWT) may reduce bioburden levels compared with standard NPWT alone. We performed a prospective, randomised, multi‐centre, post‐market trial to compare effects of NPWT with instillation and dwell of polyhexamethylene biguanide solution vs NPWT without instillation therapy in wounds requiring operative debridement. Results showed a significantly greater mean decrease in total bacterial counts from time of initial surgical debridement to first dressing change in NPWT plus instillation (n = 69) subjects compared with standard NPWT (n = 63) subjects (−0.18 vs 0.6 log10 CFU/g, respectively). There was no significant difference between the groups in the primary endpoint of required inpatient operating room debridements after initial debridement. Time to readiness for wound closure/coverage, proportion of wounds closed, and incidence of wound complications were similar. NPWT subjects had 3.1 times the risk of re‐hospitalisation compared with NPWT plus instillation subjects. This study provides a basis for exploring research options to understand the impact of NPWT with instillation on wound healing.
Keywords: bacterial load, negative‐pressure wound therapy, topical negative‐pressure therapy, wound cleansing, wound healing
1. INTRODUCTION
Although the clinical benefits of negative‐pressure wound therapy (NPWT) have been well established, the effect of standard NPWT in reducing bacterial counts in wounds is unclear. 1 , 2 , 3 While a decrease in bacteria bioburden was initially reported during use of standard NPWT, 4 other authors have since reported increased bacterial levels over time in wounds culturing gram‐positive cocci (eg, Staphylococcus aureus). 1 , 5 For this reason, use of NPWT in infected wounds has been limited. However, the addition of a regular and automatically instilled topical solution over the wound during NPWT has been reported to reduce bioburden levels compared with standard NPWT alone in multiple clinical and scientific studies. 6 , 7 , 8 , 9 , 10
The presence of bacteria in wounds is known as a significant factor that can delay healing. While infections of the deep and surrounding compartments require systemic treatment, wound‐related bacterial damage occurs on the surface and can be treated topically. 11 Wound cleansing has long been recognised as a foundation of wound management by helping to remove cellular debris and surface pathogens contained in wound exudates. 12 The steady flow of a solution across an open wound surface is meant to maintain a moist wound environment and remove superficial and deeper debris. 13 Combined with debridement, cleansing is a critical step in facilitating progression from the inflammatory to the proliferative phase of wound healing by removing debris and bacteria that can inhibit the healing process.
Unlike standard NPWT, during which wounds receive negative pressure for up to 2 to 3 days in a sealed moist environment, NPWT with cyclical instillation and a period of dwell (NPWTi‐d) delivers a topical solution to cleanse the wound bed while the dressing remains in place. Regularly instilling topical solutions with NPWTi‐d may assist with wound cleansing and lowering wound fluid viscosity to facilitate more efficient removal of exudates and infectious material during the negative‐pressure phases. 14
The objective of this study was to compare the effects of NPWTi‐d with instillation of polyhexamethylene biguanide (PHMB) solution vs the effects of using the same system without instillation therapy in wounds that required operative debridement. At the time this study protocol was developed (2011), the decision to instill PHMB vs other appropriate antimicrobial topical solutions including hypochlorous‐ and hypochlorite‐based solutions was primarily based on wide availability, broad spectrum of activity, and favourable preliminary results with NPWTi‐d (subsequently published in 2014). 15 Difference in bacterial counts between time points, number of required OR debridements, time to closure/coverage, closure rate, and incidence of complications were the collected endpoints for analysis.
2. METHODS
2.1. Study design and evaluation criteria
This study was a prospective, randomised, multi‐centre, post‐market human subject trial. The study was registered at ClinicalTrials.gov (NCT01867580) and took place between 5 December 2012 and 16 November 2015. The study protocol was conducted in accordance with “Good Clinical Practice” as defined by the International Conference on Harmonisation guidelines, and applicable regulations, including, where applicable, principles outlined in the Declaration of Helsinki. All investigators were required to submit protocols and receive approval from their appropriate Institutional Review Board to proceed with the study. Written informed consent was obtained from all participants in the study.
Inclusion and exclusion criteria are listed in Table 1. Table 2 displays the effectiveness endpoints.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Wound prior to informed consent | Pregnant (determined by a positive serum or urine pregnancy test at screening) | Received NPWT on the study wound within the last 30 days |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: NPWT, negative‐pressure wound therapy; PHMB, polyhexamethylene biguanide.
Once necrotic tissue or eschar was removed from the wound, the subject could be included.
TABLE 2.
Effectiveness endpoints
|
2.2. Randomisation
Stratified randomisation by investigative site was used. For each investigative site (stratum), permuted blocks were used to achieve equal numbers of subjects assigned to NPWTi‐d and NPWT to generate a randomisation schedule. Envelopes were prepared corresponding to each row in the randomisation schedule. Opening of the randomisation envelope occurred intra‐operatively at the conclusion of the initial surgical debridement of the wound and after confirmation that patient met inclusion and no exclusion criteria.
2.3. Patient and wound assessments
Wound assessments were performed by the investigator at the screening visit (day −10 through day 0), on the day of initial surgery (day 0), and during treatment and follow‐up as outlined in the following. At baseline, study‐wound aetiology, wound type, and wound history were assessed. A wound was considered “chronic” if it was present for >30 days prior to study admission. Wound area and wound volume were measured at scheduled visits, including baseline. Wound volume was recorded in cubic centimetres by standardised measurement of length, width and depth using a ruler.
Blood specimen tests (white blood cell count with differential; HbA1c; pre‐albumin/albumin/total protein; glucose; and BUN/Creatinine) were collected through phlebotomy. All female subjects of child‐bearing potential received a urine pregnancy test. Blood and urine testing were performed at the investigative site laboratory where the subject was being treated. Each investigator determined if abnormal laboratory test results were clinically significant. If an abnormal result from a lab test was worse than the result obtained at consent prior to NPWTi‐d application and the clinician deemed it clinically significant, it was captured as a complication.
Pain was self‐evaluated and estimated using a visual analogue scale (VAS).
Determination of a wound's appropriateness for closure or coverage was viewed as a clinically subjective event at most institutions participating in this trial. Readiness for closure/coverage was established when the investigator determined that the wound had been adequately debrided, cleaned, and a definitive procedure could be performed. With respect to the number of wounds that were closed/covered, the proportion was determined from wounds that had an initial definitive closure or coverage as defined by complete re‐epithelialisation, coverage with a biological tissue matrix, such as GRAFTJACKET regenerative tissue matrix (Wright Medical Technology, Inc., licensed by KCI, an Acelity company, San Antonio, Texas) or Integra (Integra LifeSciences, Plainsboro, New Jersey), a free or local tissue transfer (flap), or autologous skin graft. Coverage did not include temporary coverage with cell‐based skin substitute products such as Apligraf (Organogenesis, Canton, Massachusetts), Dermagraft (Advanced BioHealing, Westport, Connecticut), or cadaveric skin.
2.4. Debridement
All subjects were hospitalised patients with a wound. Wounds were considered infected if they showed clinical signs of infection (eg, erythema, oedema, and drainage), positive swab cultures, laboratory markers (eg, elevated white blood cell count), and/or radiographic signs (eg, cortical erosion and subcutaneous gas).
All wounds were surgically (ie, excisionally) debrided prior to placement of either NPWT system. Surgical debridement included excision of the wound with removal of all non‐viable tissue (eg, liquified fat and necrotic tissue) until bleeding, healthy appearing tissue was identified. Sharp surgical instrumentation included the use of scissors, scalpel, rongeur, and/or hydrosurgical debridement device. All wounds were irrigated with normal saline prior to application of either NPWT system.
The necessity for repeat surgical debridements was made at the discretion of each investigator. However, every site was required to perform a minimum of 1 dressing change during hospitalisation. The final procedure, at time of hospitalisation, to close/cover (eg, split thickness, skin graft, and biologic dressing) or leave open, was at the discretion of the investigator.
2.5. Quantitative and qualitative cultures
The difference in total bacterial counts was measured in colony forming units (CFUs) as determined by quantitative polymerase chain reaction (PCR) analysis. The bacterial counts were log‐transformed and the difference in bacterial count was calculated by:
Differences in bacterial count (Log10CFU/g) = [Log10CFUat first dressing change] − [Log10CFUafter initial surgical debridement].
Culture specimens were collected through tissue scrapings of the wound bed before and after the initial debridement, at the first dressing change, before and after each additional debridement, and when the wound was deemed ready for closure. The investigational sites were provided with instructions on proper preservation and shipping of specimens to the designated processing facility. The samples were analysed for bacterial speciation and quantification through PCR techniques.
In addition to specimens collected for PCR analysis, culture specimens were also collected through swab cultures at the deepest point in the wound (excluding tunnels) before and after the initial debridement, at the first dressing change, before and after each additional debridement, and when the wound was deemed ready for closure. Swab specimens were obtained using the Z‐technique. 16 A swab was used to contact the deepest portion of the wound surface avoiding contact with the wound edges, and the swab was moved across the wound surface in a zigzag motion while rotating the swab between the fingers to ensure complete coverage of the tip of the swab. Slight downward pressure to release fluid from the wound surface was applied if appropriate.
Culture swabs were placed in an appropriate transport media and sent to the institutional laboratory for qualitative aerobic and anaerobic analysis. The investigational sites used the qualitative aerobic and anaerobic culture results as the indicator of wound infection post‐debridement for the purposes of guiding treatment. The qPCR results were provided to the statistician for analysis, but not to the investigational sites, because they did not normally have access to this information.
2.6. Application of NPWT
In patients randomised to the Treatment arm, NPWTi‐d (V.A.C.ULTA Therapy Unit with V.A.C.VERAFLO Dressing, KCI, an Acelity company, San Antonio, Texas) was applied in the OR immediately following surgical debridement. The clinician selected the volume of topical solution to be delivered to the wound bed based on wound volume, and this volume was saved in the device for each patient. Ongoing treatment cycles were programmed to instill PHMB (Prontosan Wound Cleansing Solution, B. Braun, Bethlehem, Pennsylvania) and dwell for 20 minutes, followed by 2.0 hours of NPWT, based on protocol used in a comparative study of outcomes during NPWTi‐d with saline vs NPWTi‐d with PHMB. 17 The programmed therapy unit was initiated to administer −125 mm Hg of continuous negative pressure with medium pressure intensity. Dressings were changed every 3 days.
In patients randomised to the control arm, continuous NPWT (V.A.C.ULTA Therapy Unit with V.A.C. GRANUFOAM Dressing, KCI, an Acelity company) was applied in the OR immediately following surgical debridement. The pre‐programmed therapy unit was initiated to administer −125 mm Hg of continuous negative pressure with medium pressure intensity. Dressing changes occurred every 3 days.
2.7. Treatment visits and follow‐up
After the initial surgical debridement procedure, study treatment visits were conducted every 3 days at each dressing change. Treatment study visits occurred for 56 (± 8) days of study participation from the initial debridement procedure or until the wound was deemed ready for closure or coverage, whichever occurred first. The following assessments were performed at each study treatment visit: (a) wound assessments (volume measurement, characteristics and appearance scale, ASEPSIS); (b) digital photographs; (c) wound culture swab and PCR scraping); (d) VAS pain scale; (e) dressing change/removal; (f) laboratory analytes; (g) debridement (when indicated); (h) concomitant medications; and (i) complications reporting.
Once the wound was deemed ready for closure or coverage, study treatment ended, and post‐treatment follow‐up visits were conducted every 14 (±2) days until wound closure or end of the study. A wound closure follow‐up visit was required after a subject's wound had closed at 30 (±5) days. If the subject's wound remained open, the final post‐treatment follow‐up visit was scheduled to occur on day 56 (± 8), or between days 48 and 64.
2.8. Statistical methods
An adaptive design was used to monitor the study to determine the target number of Subjects required to achieve significance at the alpha (α) = .05 level. In the study protocol, one interim analysis was assumed when about half of the initial estimated subjects had completed the 56 (±8) day assessment or completed the study.
The sample size estimation was based on the endpoint “number of operative debridements.” The mean and SD were 3.6 and 5.7 for the Control group queried from a managed care claims database. Assumed mean and SD were 1.6 and 1.6, respectively, for the treatment (NPWTi‐d) arm, and with a total of 164 subjects (82 Treatment and 82 Control), the study has an approximately 80% power to detect the difference using the two‐sided Wilcoxon rank‐sum test with a 95% confidence interval (by adjusting one interim, α = .048 in the final analysis). To verify the assumptions used for sample size calculation, the planned interim analysis was performed on 82 subjects from the intent‐to‐treat population (ITT) population (42 subjects in Treatment therapy and 40 subjects in Control) who completed the study or completed the 56 (±8) day assessment after initial debridement.
Baseline was defined as the most recent assessment prior to the time of initial OR wound debridement, or prior to the opening of the randomisation envelope, or the time of initial application of either Control or Treatment therapy. Categorical parameters were summarised as a proportion and compared between Control and Treatment therapy using either a Chi‐square test or Fischer's exact test, as appropriate.
Continuous parameters were summarised using descriptive statistics (N, mean, SD, and median). The log‐transformed total bacterial counts at the initial post‐surgical debridement, at first dressing change, and the differences were computed and displayed by treatment group and overall. The difference between Control and Treatment therapy was compared using a two‐sample t‐test or Wilcoxon rank‐sum test based on the normality of the distribution. It is important to note here that even if a statistically significant difference in bioburden reduction has been achieved, a ≥ 3 log10 (99.9%) reduction in bacteria and a change in speciation are required in most cases for a therapy to prove clinical efficacy in wound bacteria reduction. 18 , 19 The number of debridements was modelled and compared between Control and Treatment therapy using an ordinal multinomial logistic regression model.
2.9. Populations
The procedures for obtaining subject informed consent complied with all applicable regulatory requirements. Seven study sites provided subjects for participation in this study. Definitions of subject population and number of subjects in each group are listed in Table 3.
TABLE 3.
Definitions of subject populations in study
| Population name | Number analysed (n) | Definition |
|---|---|---|
| Safety population (SP) | 181 | All subjects who were randomised and received either control or treatment therapy regardless of its duration. |
| Intent‐to‐treat population (ITT) | 181 | All subjects who were randomised and received either control or treatment therapy regardless of its duration and without a major medical event after enrolment unrelated to the study treatment that significantly altered the treatment course or would affect the subject's ability to participate in the study. |
| Per‐protocol population (PP) | 137 | All subjects who were randomised and received either control or treatment therapy until the wound was deemed ready for closure or coverage, completed the protocol's required visits and evaluations, and did not have any major protocol deviations. The effectiveness analyses for this study were based on the PP population. |
| Modified per‐protocol population | 157 | All subjects who were randomised and received either control or treatment therapy until the wound was deemed ready for closure/coverage or completed treatment up to 56 (±8) days after the initial operating room (OR) visit, in addition to completing the protocol's required visits and evaluations in which assessment for any of the endpoints was required. |
3. RESULTS
Figure 1 provides the disposition of study subjects from informed consent and screening through the end of the study. There was no statistically significant demographic difference between the NPWTi‐d and NPWT subjects at baseline (Table 4). Most of the wounds were classified as chronic, and most chronic wounds were diabetic ulcers (78/181; 43.1%). Baseline wound characteristics are listed in Table 5.
FIGURE 1.
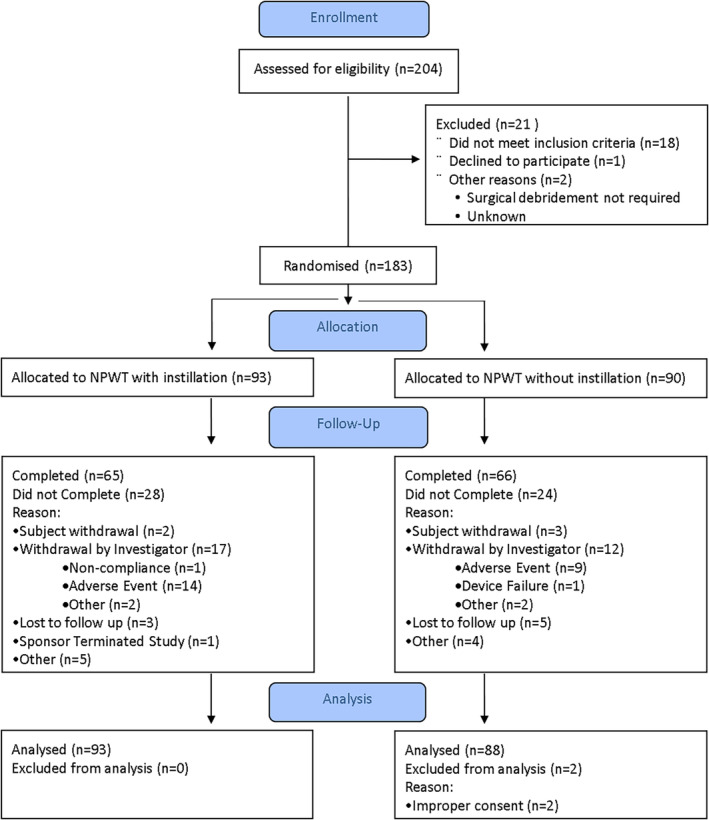
CONSORT statement
TABLE 4.
Demographic characteristics and comorbidities in intent‐to‐treat population
| NPWT (n = 88) | NPWTi‐d (n = 93) | P‐value | |||
|---|---|---|---|---|---|
| Age | Years | mean (SD) | 52.4 (14.3) | 52.8 (13.9) | .83 |
| Gender | Male | n (%) | 56 (64%) | 62 (67%) | .76 |
| Race |
Black White Unknown |
n (%) n (%) n (%) |
34 (39%) 53 (60%) 1 (1%) |
34 (37%) 59 (63%) 0 (0%) |
.76 |
| Ethnicity | Hispanic | n (%) | 14 (16%) | 20 (22%) | .35 |
| Body mass index | kg/m2 | mean (SD) | 31.7 (8.3) | 30.7 (7.5) | .46 |
| Smoking history |
Current Past user Never used |
n (%) n (%) n (%) |
23 (26%) 29 (33%) 36 (41%) |
15 (16%) 32 (34%) 46 (50%) |
.24 |
| Alcohol use history |
Current Past user Never used |
n (%) n (%) n (%) |
40 (45%) 22 (25%) 26 (30%) |
40 (43%) 31 (33%) 22 (24%) |
.42 |
| Substance use history |
Current Past user Never used |
n (%) n (%) n (%) |
2 (2%) 15 (17%) 71 (81%) |
7 (7%) 10 (11%) 76 (82%) |
.15 |
| Diabetes | Yes | n (%) | 55 (63%) | 58 (62%) | 1.00 |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with instillation and dwell time.
TABLE 5.
Wound type, wound aetiology, and wound size at initial operating room (OR) post‐debridement (intention‐to‐treat population)
| Parameter | Treatment group | ||||||
|---|---|---|---|---|---|---|---|
| NPWTi‐d (n = 93) | NPWT (n = 88) | Overall (N = 181) | |||||
| n | % | n | % | n | % | ||
| Wound type | Acute | 28 | 30.1 | 23 | 26.1 | 51 | 28.2 |
| Chronic | 65 | 69.9 | 65 | 73.9 | 130 | 71.8 | |
| Aetiology | Arterial ulcers | 2 | 2.2 | 3 | 3.4 | 5 | 2.8 |
| Burn | 1 | 1.1 | 0 | 0.0 | 1 | 0.6 | |
| Diabetic ulcers | 39 | 41.9 | 39 | 44.3 | 78 | 43.1 | |
| Necrotizing fasciitis | 1 | 1.1 | 0 | 0.0 | 1 | 0.6 | |
| Other | 1 | 1.1 | 1 | 1.1 | 2 | 1.1 | |
| Pressure ulcers | 19 | 20.4 | 12 | 13.6 | 31 | 17.1 | |
| Radiation ulcer | 2 | 2.2 | 1 | 1.1 | 3 | 1.7 | |
| Surgical dehisced | 13 | 14.0 | 10 | 11.4 | 23 | 12.7 | |
| Surgical non‐dehisced | 10 | 10.8 | 14 | 15.9 | 24 | 13.3 | |
| Traumatic | 3 | 3.2 | 5 | 5.7 | 8 | 4.4 | |
| Venous ulcers | 2 | 2.2 | 3 | 3.4 | 5 | 2.8 | |
| Anatomic location | Abdomen | 1 | 1.1 | 0 | 0.0 | 1 | 0.6 |
| Back | 2 | 2.2 | 0 | 0.0 | 2 | 1.1 | |
| Buttock | 13 | 14.0 | 7 | 8.0 | 20 | 11.0 | |
| Chest | 6 | 6.5 | 9 | 10.2 | 15 | 8.3 | |
| Forearm | 1 | 1.1 | 0 | 0.0 | 1 | 0.6 | |
| Head/neck | 0 | 0.0 | 1 | 1.1 | 1 | 0.6 | |
| Lower extremity | 67 | 72.0 | 70 | 79.5 | 137 | 75.7 | |
| Pelvic/perineal | 3 | 3.2 | 1 | 1.1 | 4 | 2.2 | |
| NPWTi‐d | NPWT | Overall | |||||
| Wound area (cm2) | n | 93 | 88 | 181 | |||
| Mean | 75.0 | 72.9 | 73.9 | ||||
| Median | 31.5 | 48.1 | 36.0 | ||||
| SD | 183.27 | 96.92 | 147.34 | ||||
| Wound volume (cm3) | n | 92 | 88 | 180 | |||
| Mean | 183.8 | 209.1 | 196.1 | ||||
| Median | 64.0 | 74.8 | 70.0 | ||||
| SD | 405.56 | 409.95 | 406.77 | ||||
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi, NPWT with cyclical instillation.
3.1. Effectiveness results
There was no statistically significant difference in the primary endpoint: mean number of inpatient OR debridements required during the inpatient stay after the initial debridement until the wound was deemed ready for closure/coverage between NPWTi‐d and NPWT subjects (1.1 vs 1.0, respectively; P = .68) (Table 6). Microbiological evaluation of results showed a mathematically significant mean decrease in total bacterial counts between time of initial surgical debridement and first dressing change in NPWTi‐d (n = 69) subjects compared with NPWT (n = 63) subjects (−0.18 Log10 CFU/g vs 0.6 Log10 CFU/g, respectively; P = .02) (Figure 2). Of subjects with high bacterial count after initial OR debridement, the NPWTi‐d group had a bacterial count decrease while the NPWT group had a bacterial count increase at the first dressing change (−1.5*106 vs 3.1*105 P = .09). Similarly, of subjects who had a high bacterial count after the initial OR debridement, a lower percentage of the NPWTi‐d group vs the NPWT group had an increase in bacterial count at the first dressing change (0/7 vs 8/12, P = .25), but the difference was not significant (Table 7).
TABLE 6.
Summary of the number of inpatient operating room (OR) debridements required during inpatient stay after the initial debridement (by treatment group)
| Number of inpatient OR debridements | Treatment group | ||||
|---|---|---|---|---|---|
| NPWTi‐d (n = 71) | NPWT (n = 66) | Overall (n = 137) | P‐Value of ordinal logistic regression | P‐Value from Wilcoxon rank‐sum test | |
| 0 (%) | 9 (12.7%) | 13 (19.7%) | 22 (16.1%) | ||
| 1 (%) | 52 (73.2%) | 41 (62.1%) | 93 (67.9%) | ||
| 2 (%) | 5 (7.0%) | 10 (15.2%) | 15 (10.9%) | ||
| 3 (%) | 3 (4.2%) | 2 (3.0%) | 5 (3.6%) | ||
| 4 (%) | 2 (2.8%) | 0 (0.0%) | 2 (1.5%) | ||
| Mean (SD) | 1.1 (0.78) | 1.0 (0.69) | 1.1 (0.74) | 0.68 | 0.68 |
| 95% confidence interval | 0.93‐1.30 | 0.85‐1.18 | 0.94‐1.19 | ||
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell.
FIGURE 2.
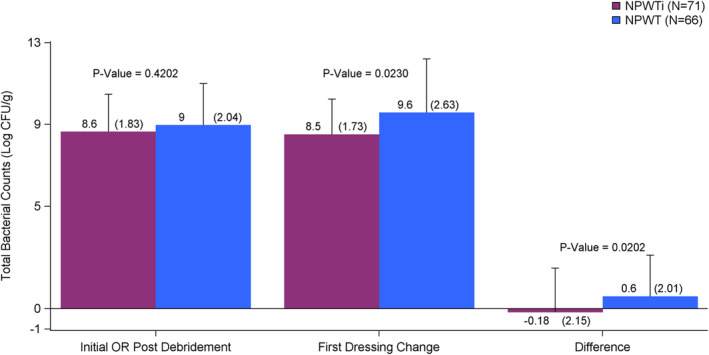
Difference in total bacterial counts (LOG‐Data) from time of initial operating room (OR) post‐debridement to first dressing change and time wound deemed ready for closure
TABLE 7.
High vs low bacteria count at first dressing change in subjects with high bacterial count at start of study
| Treatment group | |||
|---|---|---|---|
| NPWTi‐d | NPWT | P‐Value of Wilcoxon or Fisher's exact tests | |
| Subjects with high bacteria (HB) count (≥105 CFU/mL) at start of study prior to initial operating room (OR) debridement (n) | 23 | 24 | |
| Subjects with high bacteria count (≥105 CFU/mL) at start of study after initial OR debridement (n) | 9 | 13 | |
| Mean change in bacterial count between initial OR post‐debridement and first dressing change | −1.5*106 | 3.1*105 | .09 |
| Subjects who had HB count after initial debridement and had HB count at first dressing change | 0/7 | 8/12 | .25 |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell.
There were no statistically significant differences in the mean time until wound was deemed ready for closure/coverage between NPWTi‐d (n = 71) and NPWT (n = 66) subjects (mean 6.8 vs 6.3 days, P = .71) (Table 8). Time to readiness for closure was shorter in the NPWTi‐d group vs NPWT group for patients with higher bacteria counts among all subjects and subjects who received at least one debridement, but this was not significant (5.3 days vs 7.9 days, P = .18; 4.8 days vs 6.5 days, P = .16). There was no statistical difference in proportion of wound closure/coverage by day 56 (± 8 days) between the two groups (68/71 [95.8%] vs 64/66 [97.0%], P = 1.00) (Table 8). Table 9 displays the methods of closure for all wounds that were closed during the study period.
TABLE 8.
Proportion of patients with closed wounds and time to readiness for closure/coverage in subjects with high vs low bacteria count
| Treatment group | |||
|---|---|---|---|
| NPWTi‐d | NPWT | P‐Value of Log‐rank test | |
| Proportion of subjects with wound deemed ready for closure or coverage (n; %) | 68/71 (95.8) | 64/66 (97.0) | 1.00 |
| Mean (days) to readiness for wound closure/coverage for all subjects | 6.8 | 6.3 | .71 |
| Mean (days) to readiness for wound closure/coverage for all subjects with low bacteria count (<105 CFU/mL) | 8.6 | 7.8 | .53 |
| Mean (days) to readiness for wound closure/coverage for all subjects with high bacteria count (≥105 CFU/mL) | 5.3 | 7.9 | .18 |
| Mean (days) to readiness for wound closure/coverage for all subjects with low bacteria count (<105 CFU/mL) and ≥ 1 debridement | 8.4 | 7.9 | .69 |
| Mean (days) to readiness for wound closure/coverage for all subjects with high bacteria count (≥105 CFU/mL) and ≥1 debridement | 4.8 | 6.5 | .16 |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with instillation and dwell time.
TABLE 9.
Type of wound closure performed (intent‐to‐treat population population)
| Treatment group | |||
|---|---|---|---|
| NPWTi‐d | NPWT | Overall | |
| N = 67 | N = 69 | N = 136 | |
| Autologous skin graft, n (%) | 11 (16.4) | 24 (34.8) | 35 (25.7) |
| Flap coverage, n(%) | 20 (29.9) | 9 (13.0) | 29 (21.3) |
| Delayed primary closure, n (%) | 35 (52.2) | 36 (52.2) | 71 (52.2) |
| Cadaver skin allograft, n (%) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell.
There was no significant difference in incidence rate of subjects experiencing wound complications between NPWTi‐d (n = 71) and NPWT (n = 66) subjects (28 vs 21, respectively; P = .38) in the per‐protocol population (Table 10 ). Fewer patients were re‐hospitalised in the NPWTi‐d group vs NPWT group for the ITT population after initial hospital discharge, but the difference wasn't statistically significant (3 vs 9, P = .07) (Table 11). However, an ad‐hoc analysis to determine relative risk based on original categorial parameters did show that NPWT subjects had 3.1 times the risk of re‐hospitalisation compared with NPWTi‐d subjects.
TABLE 10.
Incidence rates of subjects experiencing wound complications
| Wound complications in per‐protocol population | Treatment group | ||
|---|---|---|---|
| NPWTi‐d (n = 71) | NPWT (n = 66) | P‐value Fisher's exact test | |
| Yes | 28 (39.4%) | 21 (31.8%) | |
| No | 43 (60.6%) | 45 (68.2%) | .38 |
| MedDRAa System Organ Class | Treatment group | ||
| Treatment‐related complications by system organ class for safety population | NPWTi‐d (n = 93) | NPWT (n = 88) | |
| Subjects experiencing at least 1 treatment‐related complication | 20 (21.5%) | 11 (12.5%) | |
| Skin and subcutaneous tissue disorders (skin maceration, rash, and dermatitis) | 18 (19.4%) | 9 (10.2%) | |
| General disorders and administration site conditions (pain and necrosis) | 3 (3.2%) | 1 (1.1%) | |
| Injury, poisoning, and procedural complications (blister, wound complication, and wound dehiscence) | 1 (1.1%) | 2 (2.3%) | |
| Infections and infestations (purulent discharge) | 1 (1.1%) | 0 (0.0%) | |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell.
MedDRA version 13.0.
TABLE 11.
Rate of patients who were re‐hospitalised after initial discharge
| Re‐hospitalisation | Treatment group | |||
|---|---|---|---|---|
| NPWTi‐d (n = 73) | NPWT (n = 70) | Overall (n = 143) | P‐Value from Fisher's exact test | |
| Yes | 3 (4.1%) | 9 (12.9%) | 12 (08.4%) | |
| No | 70 (95.9%) | 61 (87.1%) | 131 (91.6%) | .07 |
| Reason for re‐hospitalisation | ||||
| Infection, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.4) | |
| Osteomyelitis, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.4) | |
| Cellulitis, n (%) | 0 (0.0) | 1 (1.4) | 1 (0.7) | |
| Wound closure, n (%) | 3 (4.1) | 1 (1.4) | 4 (2.8) | |
| Debridement, n (%) | 0 (0.0) | 1 (1.4) | 1 (0.7) | |
| Dehiscence, n (%) | 0 (0.0) | 1 (1.4) | 1 (0.7) | |
| Open wound, n (%) | 0 (0.0) | 1 (1.4) | 1 (0.7) | |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell.
3.2. Safety
There were four deaths (three NPWTi‐d subjects and one NPWT subject), none of which were treatment‐related. A total of 20/93 (21.5%) of the NPWTi‐d subjects and 11/88 (12.5%) of the NPWT subjects experienced at least one treatment‐related complication. Classification of treatment‐related AEs is listed in Table 10.
3.3. Surgical dehisced wounds subgroup analysis
Further subgroup analysis of wounds classified by aetiology showed that for surgical dehisced wounds (n = 23), there was a significant decrease in mean bacterial count (Log10 CFU/g) in the NPWTi‐d vs NPWT group at first dressing change (−0.6 vs +0.5, P < .01) as well as at the point the wound was deemed ready for closure (−0.8 vs +0.6, P < .01). There was also a significantly lower mean number of debridements in the NPWTi‐d group vs NPWT group (0.7 vs 1.8, P = .01). Hospital length‐of‐stay was marginally significantly shorter (9.3 days vs 21.8 days, P = .05), and a significantly lower pain score (52.0 vs 79.0, P = .03) was recorded in the NPWTi‐d group. Table 12 displays dehisced wound subgroup analysis results.
TABLE 12.
Endpoint analysis for dehisced wounds subgroup
| Treatment group | |||
|---|---|---|---|
| NPWTi‐d (n = 13) | NPWT (n = 10) | P‐Value | |
| Mean difference in bacterial count from initial debridement to first dressing change (Log10 CFU/g) | −0.6 | +0.5 | <.01 |
| Mean difference in bacterial count from initial debridement to ready for closure (Log10 CFU/g) | −0.8 | +0.6 | <.01 |
| Mean debridements | 0.7 | 1.8 | .01 |
| Time to readiness for closure (days) | 6.0 | 11.0 | .10 |
| Maximum VAS pain score | 52.0 | 79.0 | .03 |
| Hospital length‐of‐stay (days) | 9.3 | 21.8 | .05 |
Abbreviations: NPWT, negative‐pressure wound therapy; NPWTi‐d, NPWT with cyclical instillation and a period of dwell; VAS, visual analogue scale.
4. DISCUSSION
This is the first multi‐centre RCT that compares the effects of using NPWT with instillation vs NPWT without instillation of a topical solution on bacterial bioburden in acute and chronic wounds. The original intent of this study was exploratory, and because of this and the small population size for subgroup analysis, this study is classified as a “pilot” study. Results showed a significant decrease in total bacteria counts demonstrated in wounds of NPWTi‐d subjects compared with traditional NPWT subjects at the first dressing change, prior to debridement, in this study population. Bioburden reduction is consistent with past studies that have shown reduced bacterial levels with NPWTi‐d with a variety of topical solutions in animal and clinical models. 6 , 9 , 20
The data showed no significant difference in the number of required OR debridements during inpatient use of NPWTi‐d vs NPWT. This is a somewhat unexpected finding based on these authors' clinical observations as well as reports of reduced debridement requirements with NPWTi‐d in previous studies. 15 , 21 , 22 These endpoint results suggest that there was not a strong association between a mean reduction in bioburden and a mean reduction in debridement for the wounds included in this study. Many factors could have contributed to this result, including a wide range of clinical practice patterns among the investigators at the different institutions, particularly with respect to how aggressively and frequently excisional debridement is performed, varying levels of access to the OR, and differences in criteria that determine the rate of patient discharge.
Prior to and throughout the study, there was a failure to harmonise certain aspects of the protocol, such as uniformity in debridement techniques and criteria for determining the necessity for successive debridement. Additionally, determination of a wound's appropriateness for closure or coverage was left to the discretion of the investigator and viewed as a clinically subjective event at most institutions participating in this trial. Detailed criteria to help guide investigators in determining the need for additional debridements and timing for wound closure may have reduced bias. A subgroup analysis of wound type outcomes showed a significantly faster time to readiness for closure for pressure ulcers in the NPWTi‐d group vs NPWT group (P = .05), which may be worthy of further analysis.
The protocol did not require the use of antibiotics and investigators followed their own standard of care. While patient treatment with systemic antibiotics was not collected as a data point, the name of each concomitant medication was collected for each patient. Data showed that most patients were treated with at least one antibiotic, but no additional analyses were performed to determine possible effects of antibiotics on outcomes, such as number of required debridements, wound conditioning to readiness for closure, or bacterial load.
For the protocol, the decision to instill PHMB (0.1% polyhexanide plus 0.1% betaine), vs other possible topical solutions, including saline, was primarily based on the predominance of earlier published studies that described instillation of an antimicrobial solution (hypochlorite, silver nitrate, polyhexanide, etc.) in bacterial colonised wounds treated with NPWTi‐d. 15 , 17 , 23 , 24 , 25 , 26 It was assumed that most of the wounds in the study would have a history of acute or chronic infection and that PHMB would be an appropriate topical cleanser. Extensive research has demonstrated that it has a microbicidal effect for a broad spectrum of bacteria, yeast and viruses, and is well tolerated in solution and gel formulations. 27 , 28 , 29
However, PHMB is a potent antiseptic with a higher log kill rate vs other approved topical wound cleansers. 7 , 20 The combination of PHMB with NPWTi‐d has been shown to significantly slow granulation tissue formation in wounds compared with NPWT alone in two porcine pre‐clinical studies of healthy wounds. 7 In this current study, the majority of patients in both arms, pre and post‐debridement, had a bacterial count less than 105 CFU/mL; thus, we might expect potential slowing of granulation and limited benefit of NPWTi‐d with PHMB in this population.
Since the start of this study, several studies have been published regarding successful use of saline with NPWTi‐d. 30 , 31 , 32 , 33 , 34 An independent RCT 31 was published examining the outcomes of NPWTi‐d with two different solutions: saline vs PHMB. Eighty‐three patients with an infected wound requiring surgical debridement were analysed. The results showed a statistically significant lower number of days to the time of final surgical procedure in the normal saline vs PHMB cohorts (5.57 days vs 7.46 days, respectively, P = .035), and no significant differences between the two groups with respect to number of OR visits, length of hospital stay, proportion of wounds closed/covered, and proportion of wounds that remained closed at the 30‐day follow‐up. Based on these prior results and this current RCT, normal saline is now routinely used for most patients requiring NPWTi‐d, with PHMB used sparingly for cases involving orthopaedic fixation hardware at the lead author's institution. A clinical trial to understand the benefit of saline with NPWTi‐d on wounds with bacterial colonisation less than 105 CFU/mL is warranted.
These study results are limited by many factors, including high heterogeneity in the wound population and the potential for inconsistent documentation. Classifying wound types was left to the discretion of each investigator, which could have led to inconsistencies in wound classification. Additionally, wide variability of patient length‐of‐stay at the various institution sites and relatively small populations in the study reduced the possibility of determining statistically significant differences between the two groups. Likewise, the positive trend towards fewer hospital re‐admissions reported in the NPWTi‐d group suggests use of NPWTi‐d could potentially impact re‐hospitalisation rates, but its financial impact related to this quality metric endpoint needs to be evaluated in future well‐controlled studies.
Literature supports the use of NPWTi‐d as a helpful tool in preparing the wound bed for definitive closure. Results of this and other studies suggest that NPWTi‐d technology, along with debridement and appropriate antibiotics, should be primarily focused on wounds with high bacterial bioburden (≥105 CFU/mL). Results from other authors have shown benefit with use of NPWTi‐d in large wounds with a high bacterial count and a definitive closure strategy. 35 , 36 , 37 , 38 Discontinuation of NPWTi‐d is recommended when the wound is deemed ready for surgical closure and/or the next stage of treatment. 39 Following application of NPWTi‐d, wounds may be closed with an autograft, flap, or other definitive closure strategy.
Although it is difficult to draw strong conclusions from this study, the study provides a basis for exploring other clinical trial options to understand and demonstrate the impact of NPWTi‐d on wound healing, particularly in wounds with high bacterial bioburden. Future studies that measure changes in bacteria count should include a detailed analysis of bacteria type and changes in bacteria type spectrum over time. It would also be interesting in future studies that explore the biomechanical effects of instillation therapy to compare the incremental effects of instillation on the expression of growth factors, inflammatory cytokines, and matrix metalloproteinases and their enzymatic inhibitors during use of NPWTi‐d in chronic and acute wounds.
CONFLICT OF INTEREST
The authors have the following to disclose. Each of the authors received funding from KCI to conduct the study described in this manuscript. P. J. K. is a Consultant for KCI. B. H. B. is a Consultant for KCI. L. A. L. is a Consultant for KCI. C. E. A. is a Consultant for KCI.
ACKNOWLEDGEMENTS
The authors thank Karen Beach and Rico Martinez (KCI, San Antonio, Texas) for their assistance with manuscript preparation.
Kim PJ, Lavery LA, Galiano RD, et al. The impact of negative‐pressure wound therapy with instillation on wounds requiring operative debridement: Pilot randomised, controlled trial. Int Wound J. 2020;17:1194–1208. 10.1111/iwj.13424
REFERENCES
- 1. Moues CM, Vos MC, Van Den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11‐17. [DOI] [PubMed] [Google Scholar]
- 2. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390‐397. [DOI] [PubMed] [Google Scholar]
- 3. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276‐279. [DOI] [PubMed] [Google Scholar]
- 4. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553‐562. [DOI] [PubMed] [Google Scholar]
- 5. Stinner DJ, Waterman SM, Masini BD, Wenke JC. Silver dressings augment the ability of negative pressure wound therapy to reduce bacteria in a contaminated open fracture model. J Trauma. 2011;71:S147‐S150. [DOI] [PubMed] [Google Scholar]
- 6. Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC II. Negative pressure wound therapy with instillation (NPWTi) better reduces postdebridement bioburden in chronically infected lower extremity wounds than NPWT alone. J Am Col Clin Wound Specialists. 2014;4:74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tahir S, Malone M, Hu H, Deva A, Vickery K. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials. 2018;11:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uoya Y, Ishii N, Kishi K. Comparing the therapeutic value of negative pressure wound therapy and negative pressure wound therapy with instillation and dwell time in bilateral leg ulcers: a case report. Wounds. 2019;31:E61‐E64. [PubMed] [Google Scholar]
- 9. Yang C, Goss SG, Alcantara S, Schultz G, Lantis Ii JC. Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds. 2017;29:240‐246. [PubMed] [Google Scholar]
- 10. Hodson T, West JM, Poteet SJ, Lee PH, Valerio IL. Instillation negative pressure wound therapy: a role for infected LVAD salvage. Adv Wound Care. 2019;8:118‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sibbald RG, Elliott JA, Verma L, Brandon A, Persaud R, Ayello EA. Update: topical antimicrobial agents for chronic wounds. Adv Skin Wound Care. 2017;30:438‐450. [DOI] [PubMed] [Google Scholar]
- 12. Wheeler CB, Rodeheaver GT, Thacker JG, Edgerton MT, Edlich RF. Side‐effects of high pressure irrigation. Surg Gynecol Obstet. 1976;143:775‐778. [PubMed] [Google Scholar]
- 13. Kim PJ, Applewhite A, Dardano AN, et al. Use of a novel foam dressing with negative pressure wound therapy and instillation: recommendations and clinical experience. Wounds. 2018;30:S1‐S17. [PubMed] [Google Scholar]
- 14. Horch RE, Braumann C, Dissemond J, et al. Use of negative pressure wound therapy with instillation and dwell time for wound treatment—results of an expert consensus conference. Zentralbl Chir. 2018;143:609‐616. [DOI] [PubMed] [Google Scholar]
- 15. Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative‐pressure wound therapy with instillation compared with standard negative‐pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133:709‐716. [DOI] [PubMed] [Google Scholar]
- 16. Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14:548‐557. [DOI] [PubMed] [Google Scholar]
- 17. Back DA, Scheuermann‐Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions—when, where and how to use: what does the evidence show? Int Wound J. 2013;10:32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schedler K, Assadian O, Brautferger U, et al. Proposed phase 2/step 2 in‐vitro test on basis of EN 14561 for standardised testing of the wound antiseptics PVP‐iodine, chlorhexidine digluconate, polihexanide and octenidine dihydrochloride. BMC Infect Dis. 2017;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitten FA, Werner HP, Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect. 2003;55:108‐115. [DOI] [PubMed] [Google Scholar]
- 20. Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013;10:48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabriel A, Kahn K, Karmy‐Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost‐effectiveness. Eplasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 22. Chowdhry SA, Wilhelmi BJ. Comparing negative pressure wound therapy with instillation and conventional dressings for sternal wound reconstructions. Plast Reconstr Surg Glob Open. 2019;7:e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: a case series. Wounds. 2005;17:37‐48. [Google Scholar]
- 24. Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5:399‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raad W, Lantis JC II, Tyrie L, Gendics C, Todd G. Vacuum‐assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schintler MV, Prandl EC, Kreuzwirt G, Grohmann MR, Spendel S, Scharnagl E. The impact of V.A.C. instill in severe soft tissue infections and necrotizing fasciitis. Infection. 2009;37:31‐32. [Google Scholar]
- 27. Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281‐1287. [DOI] [PubMed] [Google Scholar]
- 28. Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Adv Skin Wound Care. 2011;24:78‐84. [DOI] [PubMed] [Google Scholar]
- 29. Romanelli M, Dini V, Barbanera S, Bertone MS. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polihexanide for wound irrigation. Skin Pharmacol Physiol. 2010;23:41‐44. [DOI] [PubMed] [Google Scholar]
- 30. Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10:56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim PJ, Attinger CE, Oliver N, et al. Comparison of outcomes for normal saline and an antiseptic solution for negative‐pressure wound therapy with instillation. Plast Reconstr Surg. 2015;136:657e‐564e. [DOI] [PubMed] [Google Scholar]
- 32. Muller CS, Burgard B, Zimmerman M, Vogt T, Pfohler C. On the significance of negative‐pressure wound therapy with instillation in dermatology. J Dtsch Dermatol Ges. 2016;14:786‐795. [DOI] [PubMed] [Google Scholar]
- 33. Fernandez L, Ellman C, Jackson P. Use of negative pressure wound therapy with instillation in the management of complex wounds in critically ill patients. Wounds. 2019;31:E1‐E4. [PubMed] [Google Scholar]
- 34. Milcheski DA, Portocarrero ML, Alvarez DM, Mazuca LGMP, Monteiro AA Jr, Gemperli R. Initial experience with negative‐pressure wound therapy with instillation in complex wounds. Rev Colegio Brasileiro Cirurgioes. 2017;44:348‐353. [DOI] [PubMed] [Google Scholar]
- 35. Yang CK, Alcantara S, Goss S, Lantis JC II. Cost analysis of negative‐pressure wound therapy with instillation for wound bed preparation preceding split‐thickness skin grafts for massive (>100 cm(2)) chronic venous leg ulcers. J Vasc Surg. 2015;61:995‐999. [DOI] [PubMed] [Google Scholar]
- 36. Fluieraru S, Bekara F, Naud M, et al. Sterile‐water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care. 2013;22:293‐299. [DOI] [PubMed] [Google Scholar]
- 37. Reider K, McElroy E, Lemay S. The use of negative pressure with instillation and dwell for the treatment of necrotizing fasciitis. Cureus. 2018;10:e3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall KD, Patterson JS. Three cases describing outcomes of negative‐pressure wound therapy with instillation for complex wound healing. J Wound Ostomy Continence Nurs. 2019;46:251‐255. [DOI] [PubMed] [Google Scholar]
- 39. Kim PJ, Attinger CE, Constantine T, et al. Negative pressure wound therapy with instillation: international consensus guidelines update. Int Wound J. 2020;17:174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]


