Abstract
Aims
To compare the pharmacokinetic (PK) and glucodynamic (GD) characteristics of ultra rapid lispro (URLi; Eli Lilly and Company, Indianapolis, Indiana), Fiasp® (Novo Nordisk, Bagsvaerd, Denmark), Humalog® (Eli Lilly and Company) and NovoRapid® (Novo Nordisk), in patients with type 1 diabetes (T1D).
Materials and Methods
This was a randomized, double‐blind, four‐period, crossover study, conducted in 68 patients with T1D. Patients received the same individualized subcutaneous dose of each study drug immediately prior to a liquid test meal. For comparison, 12 healthy subjects received the same test meal.
Results
URLi had a significantly faster insulin absorption compared to the other insulins tested. Early half‐maximal drug concentration was reached 13 minutes after administration of URLi, which was 6 minutes faster than Fiasp, 13 minutes faster than Humalog, and 14 minutes faster than NovoRapid (all P <0.0001). Early insulin exposure was significantly greater and late insulin exposure was reduced after URLi compared to the other insulins. URLi achieved the greatest numerical reduction in postprandial glucose (PPG) at 2 hours post‐meal (7 mg/dL vs Fiasp) and was significantly different from Humalog (21 mg/dL) and Novo Rapid (29 mg/dL). Additionally, glucose excursions over the first 3 hours post‐meal with URLi were comparable to those in healthy subjects.
Conclusions
URLi demonstrated the fastest insulin absorption and the greatest numeric PPG‐lowering effect compared to the other insulins tested. URLi more closely matched the early physiological glucose control observed in healthy subjects.
Keywords: glycaemic control, insulin analogues, pharmacodynamics, pharmacokinetics, randomized trial, type 1 diabetes
1. INTRODUCTION
Many patients with diabetes depend on insulin therapy to maintain their glycaemic control. An important part of glycaemic control is to address postprandial glucose (PPG). Rapid‐acting insulin analogues, such as insulin lispro, aspart and glulisine, were developed to be absorbed more rapidly and have a faster onset of insulin action compared with regular human insulin. Despite these improvements, the current formulations are not rapid enough to match carbohydrate absorption, limiting their efficacy in controlling PPG.
Ultrarapid lispro [URLi (LY900014); Eli Lilly and Company, Indianapolis, Indiana] is a novel insulin lispro formulation, developed to more closely match physiological insulin secretion and improve PPG control. The URLi formulation includes two key locally acting excipients, treprostinil and citrate, which accelerate the absorption of insulin lispro from the site of injection via independent mechanisms of action. Microdoses of treprostinil in URLi induce local vasodilation, while citrate increases vascular permeability. 1 , 18 , 19 In previous studies, URLi has demonstrated an accelerated insulin lispro absorption, resulting in significantly greater PPG‐lowering compared to Humalog® (Eli Lilly and Company). 2 , 3 Similarly, a fast‐acting insulin aspart formulation (Fiasp™; Novo Nordisk, Bagsvaerd, Denmark) was developed, which has shown a faster onset of insulin action compared to NovoRapid® (Novo Nordisk). 4 , 5 , 6 These ultra rapid insulin analogues have the potential to lead to improved PPG control for patients with diabetes.
The aim of the present study was to compare the pharmacokinetic (PK) and glucodynamic (GD) characteristics, safety and tolerability of URLi versus Fiasp, Humalog and NovoRapid during a standardized test meal in patients with type 1 diabetes (T1D). This is the first investigation that directly compares the PK and GD characteristics of these four meal‐time insulins under identical conditions. Additionally, the inclusion of a healthy subject cohort provided the “normal” insulin secretory and glucose response to the same test meal.
2. MATERIALS AND METHODS
2.1. Participants
Eligible participants included men or women who had been diagnosed with T1D for 1 year or longer, were treated with a prandial insulin and basal insulin therapy (total insulin dose demand of ≤1.5 U/kg/d), had fasting C‐peptide levels <0.30 nmol/L and glycated haemoglobin (HbA1c) levels ≤74.9 mmol/mol (9.0%), and had not experienced a severe hypoglycaemic episode in the past 6 months. Healthy subjects had fasting plasma glucose levels ≤100 mg/dL, normal glycaemic response to a 75‐g oral glucose tolerance test and HbA1c <38.8 mmol/mol (5.7%). All participants were aged 18 to 70 years and had a body mass index (BMI) of 18.5 to 30.0 kg/m2.
2.2. Study design
This was a phase 1, double‐blind, single‐site (Profil, Neuss, Germany), four‐treatment, four‐period, single‐dose, randomized, crossover study in patients with T1D. Healthy subjects participated in one period, without a dose of insulin. The study protocol was reviewed and approved by the local health authority and an independent ethics committee (Ärztekammer Nordrhein). The trial was conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines, and registered at ClinicalTrials.gov (NCT03449433). All patients provided written informed consent prior to participating. The study design is presented in Figure S1.
2.3. Procedure
For each patient with T1D, the study consisted of a screening visit, lead‐in, dose‐finding assessment, randomization, four treatment periods, and a follow‐up visit ≥14 days after the last treatment. Prior to the initiation of the test meal, patients were transitioned from their prescribed basal insulin to a once‐daily evening dose of insulin glargine during the 7‐ to 14‐day lead‐in period and maintained on insulin glargine throughout the study until the follow‐up visit.
Prior to randomization, the dose‐finding assessment was performed using Humalog to determine the individualized insulin dose for the test meal. A fasting blood glucose level of 70 to 80 mg/dL was required to conduct the dose‐finding assessment. Patients received a prandial insulin dose, deemed appropriate for the test meal, immediately prior to consuming the entire meal, within 15 minutes. Blood glucose levels were measured every 20 minutes for the 5‐hour period. If the blood glucose was maintained within the range of 70 to 240 mg/dL, this dose level was used for all four study drugs. If not within this range, the dose level was adjusted based on the investigator’s judgement.
Patients were then randomized to one of the four treatment sequences and received a single subcutaneous dose of either URLi, NovoRapid, Fiasp or Humalog per period. Injections were rotated among different injection sites on the anterior abdominal wall during the four study periods (left lower and upper quadrants, and right lower and upper quadrants).
Prior to dosing, a target blood glucose level of 7.4 mmol/L (135 mg/dL) ± 15% was achieved using variable intravenous infusion of either 20% dextrose solution or insulin glulisine (Apidra; Sanofi, Paris, France). Blood glucose was maintained at the target for the 30 minutes prior to study drug injection without infusion of glucose or Apidra. Study drugs were administered immediately (<1 minute) prior to the start of the test meal, which contained 100 g carbohydrates, 26 g protein and 22 g fat (16 fl/oz liquid Ensure Plus; Abbott Laboratories, Abbott Park, Illinois). Healthy subjects were fasted overnight and provided the same test meal. The test meal started between 7:00 and 11:00 am and had to be completed within 15 minutes; no further food intake was allowed for the duration of the assessment. Each patient with T1D completed the four assigned treatment periods within 6 weeks; at least a 21‐hour washout period between study drugs was required.
2.4. Bioanalytical methods
Blood glucose was measured at −30, −15, 0 minutes (pre‐meal), every 10 minutes up to 120 minutes post‐dose, and then every 15 minutes up to 300 minutes post‐dose. Glucose levels were measured using the Super GL glucose analyser (Dr Müller Gerätebau GmbH, Freital, Germany) that was readily available at the investigative site during the inpatient periods to provide real‐time glucose measurement.
Blood samples for measurement of insulin lispro and insulin aspart were collected immediately prior to dosing, and at 1, 2, 3 and 5 minutes, every 5 minutes up to 60 minutes, 70, 90, 120, 150, 180 minutes and every hour up to 7 hours post‐dose. Free insulin lispro serum concentrations were analysed using a validated enzyme‐linked immunosorbent method, specific for insulin lispro, conducted at Charles River Laboratories Montreal in Senneville, Quebec, Canada. The inter‐assay precision and accuracy of the insulin lispro assay was ≤11%. Serum concentrations of insulin aspart were analysed using a validated liquid chromatographic‐mass spectrometry/mass spectrometry (LC‐MS/MS) method, conducted at Algorithme Pharma located in Laval, Quebec, Canada. The inter‐assay precision and accuracy of the insulin aspart assay was ≤12%. The limit of quantitation for both assays was 8.6 pmol/L. Both assays were specific; endogenous insulin did not interfere with either assay. Insulin aspart did not cross‐react in the enzyme‐linked immunosorbent assay for insulin lispro, and insulin lispro did not cross‐react in the LC‐MS/MS method for insulin aspart.
Serum samples were collected from healthy subjects at the same time‐points as insulin lispro and insulin aspart, and analysed for insulin using a validated commercial kit at Covance Central Laboratory Services (CCLS) in Meyri, Switzerland. The CCLS Ultrasensitive Insulin® DXI 800 commercial assay, a simultaneous one‐step sandwich immunoassay, has no cross‐reactivity to proinsulin or C‐peptide.
2.5. PK analyses
Insulin lispro and insulin aspart PK parameters were calculated using non‐compartmental methods (Phoenix® version 8.0 and S‐PLUS® version 8.2). PK parameters included time to early or late half‐maximal drug concentration (early 50% tmax or late 50% tmax), maximum observed drug concentration (Cmax), time to maximum observed drug concentration (tmax), partial area under the curve (AUC) from time zero to time t, duration of exposure in the serum, defined as the time from study drug administration until the serum insulin lispro or insulin aspart concentrations reach the lower limit of quantification in the terminal phase, and AUC from time zero to infinity (AUC[0‐∞]). Additionally, normalized partial AUCs were calculated by taking the individual partial AUCs divided by the total exposure.
Five patients had biologically implausible insulin aspart concentrations during one of their two treatment periods (Fiasp or NovoRapid). The ratio of the total insulin aspart exposure, AUC(0‐∞), between both Fiasp and NovoRapid should be close to 1.0. 7 For these five patients, the AUC ratio between both Fiasp and NovoRapid was three standard deviations from the mean of all other patients in the study and the data were excluded.
2.6. GD analyses
The GD parameters were calculated using Phoenix® version 8.0 and S‐PLUS® version 8.2. A change from baseline (using the average of −30, −15 and 0 minutes to represent the 0‐hour time‐point) glucose was calculated for each patient and period. Incremental changes in baseline glucose AUC from time zero to time t (iAUCs), were calculated using the linear trapezoidal method, with negative areas included in the calculation. The glucose iAUCs were analysed: 1) by carrying the last observed glucose values prior to treatment intervention for a hypoglycaemic or hyperglycaemic event to the end of the glucose timing (LOCF) or 2) by using the glucose data prior to intervention. Given the similar outcome, the GD analysis using the LOCF is presented.
Additionally, the change from baseline glucose at 1 hour (∆BG1h) and at 2 hours (∆BG2h), and the maximum change from baseline glucose following the start of the meal (∆BGmax) were also calculated.
2.7. Tolerability
Safety assessment included adverse events, clinical laboratory variables, vital signs, and hypoglycaemia. Tolerability was evaluated via reported treatment‐emergent adverse events and hypoglycaemic events. Hypoglycaemic events [plasma glucose ≤3.89 mM (70 mg/dL) accompanied with symptoms] that required intervention during the test meal assessment were treated with either rapidly absorbable oral carbohydrates or intravenous glucose.
Hyperglycaemic events [blood glucose concentration ≥17 mM (306 mg/dL)] that lasted more than 1 hour during the test meal assessment were treated with intravenous Apidra.
2.8. Statistical analyses
Primary statistical analyses were conducted on patients who completed all treatment periods, had evaluable PK and GD parameters, completed the entire test meal, and maintained the same insulin dose for all treatments. Data analysis was performed using SAS® version 9.3 at a 5% significance level.
Log‐transformed AUCs, normalized partial AUCs, and Cmax were used to estimate geometric means, ratios of geometric means between treatments, and the corresponding 95% confidence intervals (CIs) of the ratios. A mixed‐effects model was used that included treatment, sequence and period as fixed effects and patient within sequence as a random effect. The same model without log transformation was used for the analysis of the PK time parameters and for GD parameters. Least squares (LS) means, treatment differences in LS means, and the corresponding 95% confidence intervals (CIs) for the treatment differences were estimated from the model. The two‐sided P value on the difference between LS means was used to determine statistical significance. The treatment ratios and 95% CIs for the ratios were calculated using Fieller's theorem. 7
Comparisons of PK and GD data between healthy subjects and patients were descriptive.
2.9. Sample size calculations
The study was designed to have 64 patients complete the study, which would provide at least 95% power to demonstrate a 35% reduction of early 50% tmax between URLi and Humalog or NovoRapid and detect a 20% reduction of early 50% tmax between URLi and Fiasp. With this sample size, there would be 95% power to detect a 40% reduction in PPG incremental area under the baseline‐subtracted glucose concentration versus time curve from time 0 to 1 hour (∆BGAUC[0–1 h]) between URLi and Humalog/NovoRapid and 80% power to demonstrate 25% reduction between URLi and Fiasp. The healthy subjects sample size was not intended to achieve any a priori statistical requirements.
3. RESULTS
3.1. Study population
Sixty‐eight patients with T1D participated in the study, and 67 patients completed it. One patient withdrew after Period 2 by their own decision. The mean age of the patients with T1D was 46.1 ± 13.3 years, mean BMI was 25.8 ± 2.4 kg/m2, mean HbA1c was 56.3 ± 9.8 mmol/mol (7.3 ± 0.9%), mean duration of diabetes was 21.5 ± 10.9 years, and 75% were men (Table S1). Twelve healthy men completed the study. The mean age of healthy subjects was 32.9 ± 7.8 years, the mean BMI was 25.4 ± 1.8 kg/m2, and the mean HbA1c was 33.3 ± 4.4 mmol/mol (5.2 ± 0.4%).
3.2. PK results
The insulin lispro concentration–time profile for URLi was shifted to the left compared to Humalog (Figure 1A, C). A similar leftward shift was observed for the insulin aspart concentration–time profile of Fiasp compared to NovoRapid (Figure 1B, D). In comparing the insulin concentration–time profile across all insulins tested, URLi demonstrated the greatest leftward shift (Figure 2A), indicating the fastest insulin absorption. Correspondingly, the early 50% tmax was reached 12.8 minutes after URLi administration, which was 5.9 minutes faster than Fiasp, 12.5 minutes faster than Humalog, and 13.9 minutes faster than NovoRapid (all P <0.0001; Figure 3A). This accelerated insulin absorption with URLi resulted in significantly greater early insulin exposure compared to the other study drugs (Figures 2B and 3B). URLi increased insulin exposure during the first 15 minutes [AUC(0–15 min)] by 1.5‐fold versus Fiasp, 5‐fold versus Humalog, and 5‐fold versus NovoRapid (all P < 0.002; Figure 3B).
FIGURE 1.

Mean insulin lispro concentration (±SE) versus time post injection (A) and for the first hour post injection (C), mean insulin aspart concentration (±SE) versus time post injection (B) and for the first hour post injection (D) by treatment. LLOQ, lower limit of quantification
FIGURE 2.
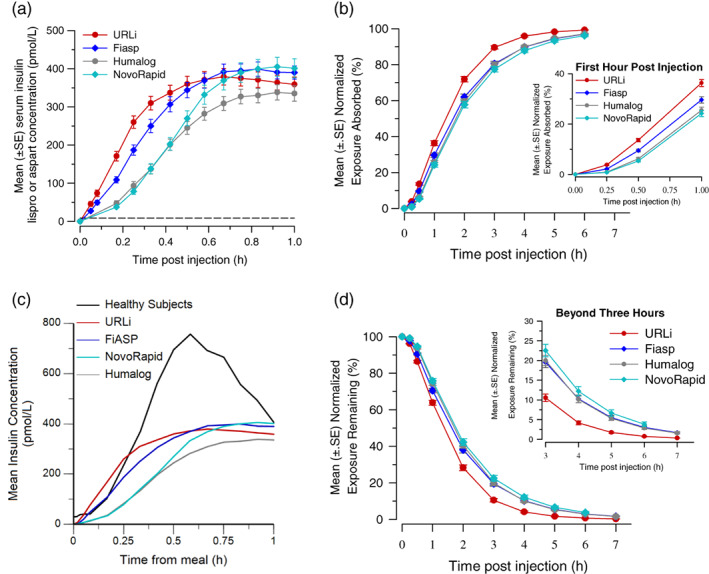
Mean insulin concentration (±SE) versus time for the first hour post injection by treatment (A). Mean normalized exposure absorbed (±SE) versus time (B) and for the first hour post‐injection (inset). Mean insulin concentration (±SE) by treatment compared to endogenous insulin (C). Mean normalized exposure remaining (±SE) versus time post‐meal (D) and from 3 to 7 hours post injection (inset)
FIGURE 3.
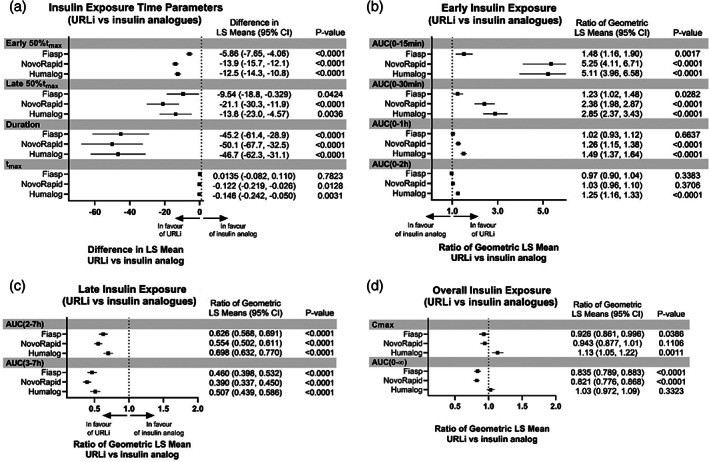
Forest plots of insulin exposure. AUC, area under the concentration versus time curve; AUC(0‐15 min), AUC from time 0 to 15 minutes post‐dose; AUC(0–30 min), AUC from time 0 to 30 minutes post‐dose; AUC(0–1 h): AUC from time 0 to 1 hour post‐dose; AUC(0–2 h), AUC from time 0 to 2 hours post‐dose; AUC(2–7 h), AUC from time 2 to 7 hours post‐dose; AUC(3–7 h), AUC from time 3 to 7 hours post‐dose; AUC(0–∞), AUC from time zero to infinity; CI, confidence interval; Cmax, maximum observed drug concentration; early 50% tmax, time to early half‐maximal drug concentration; late 50% tmax, time to late half‐maximal drug concentration; LS, least squares; tmax, time of maximum observed drug concentration. LS mean; Model: PK = period + treatment + sequence + patient (sequence) + random error, where patient (sequence) is fitted as a random effect. The CIs for the ratio were calculated using the Fieller's theorem. P value is for the test of the mean difference. LS mean calculated as pmol h/L. 'Duration' refers to time from study drug administration until the serum insulin lispro concentrations reached the lower limit of quantification
Late insulin exposure after URLi administration was significantly reduced compared to all insulins tested (Figures 2D and 3C). The insulin exposure beyond 3 hours [AUC(3–7 h)] after URLi administration was reduced by 54% compared to Fiasp, 49% compared to Humalog, and 61% compared to NovoRapid (all P <0.0001). The late 50% tmax occurred 9.5 minutes later with Fiasp, 13.8 minutes later with Humalog and 21.1 minutes later with NovoRapid compared to URLi (all P <0.05; Figure 3A). Additionally, the duration of insulin exposure in the serum after URLi administration was significantly shorter by approximately 45 minutes compared to Fiasp, 47 minutes compared to Humalog, and 50 minutes compared to NovoRapid (all P <0.001).
Following the same insulin unit dose, the total insulin lispro exposure, AUC(0–∞), was not significantly different between URLi and Humalog (Figure 3D); however, total insulin lispro exposure was significantly lower, approximately 16% to 18%, compared to the total insulin aspart exposure after Fiasp or NovoRapid administration (both P <0.0001). As this could potentially confound the PK analysis, the data were reanalysed by calculating the ratio of the partial AUCs to the total exposure within each study treatment. URLi still demonstrated the fastest insulin absorption, reduced late insulin, and shortest duration of exposure compared to all other insulins tested (Figure 2B‐D, Table S3).
3.3. GD results
URLi demonstrated the lowest mean glucose excursion during the test meal compared to Fiasp, Humalog and NovoRapid (Figure 4A). URLi had a numerically greater glucose‐lowering effect compared to all insulins tested, with a statistically significant improvement in PPG excursions over the first 5 hours compared to Humalog and NovoRapid (both P <0.02; Figure 4B, Figure S2A). The maximum PPG excursion (∆BGmax) and the excursions at 1 (∆BG1h) and 2 hours (∆BG2h) post‐meal were also significantly reduced with URLi compared to Humalog and NovoRapid (all P <0.05; Figure S2B).
FIGURE 4.
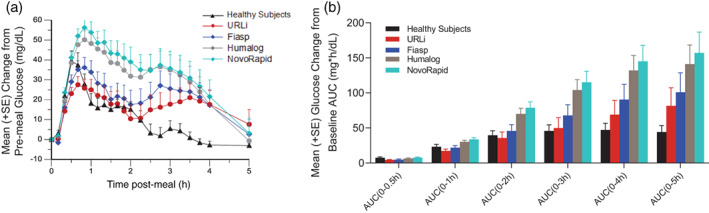
Mean (+SE) change from baseline glucose concentration versus time post‐meal by treatment (A) and partial glucose excursions (iAUC) over the 5 hours by treatment (B). AUC, area under the concentration versus time curve; iAUC, incremental area under the concentration versus time curve
3.4. Comparison of insulin analogue groups to healthy subjects
Endogenous insulin levels in healthy subjects increased at approximately 7 minutes post‐meal, and peak concentrations were observed 30 minutes post‐meal (Figure 2C). In comparing endogenous insulin concentrations to exogenous insulin concentration following subcutaneous injection of the insulins, peak insulin levels were approximately 50% lower with the insulin analogues than endogenous insulin. Notably, the insulin concentration rose the fastest after URLi administration in the first 15 minutes post‐meal and achieved higher insulin concentration than observed with endogenous insulin in healthy subjects (Figure 2C).
Similarly, when comparing the PPG profile between healthy subjects and the insulins tested, the PPG profile of URLi more closely matched the healthy subject profile over the first 2 hours (Figure 4A). Likewise, the PPG excursions over the first 3 hours [AUC(0–3 h)] were comparable between the URLi group and healthy subjects (Figure 4B). However, at later time‐points glucose levels were higher in all the tested insulin groups compared to those in healthy subjects (Figure 4A).
3.5. PK/GD relationship of insulin lispro compared to endogenous insulin in healthy subjects
In assessing the PK/PD relationship of insulin concentrations for PPG‐lowering, the faster rise in insulin lispro concentrations in the first 15 minutes after URLi administration compared to endogenous insulin levels (Figure 5A) resulted in numerically greater PPG‐lowering 1 hour after the meal with URLi compared with healthy subjects (Figure 5B). The PPG profiles were comparable between URLi and healthy subjects until 2 hours post‐meal, even though healthy subjects had higher insulin concentrations than those observed in the URLi group (Figure 5C). Interestingly, the insulin profiles from 2.5 hours and beyond of URLi, Humalog and endogenous insulin were similar (Figure 5C). However, the PPG profile from 2 to 5 hours post‐meal for URLi or Humalog did not provide the same glucose‐lowering as observed in healthy subjects (Figure 5D).
FIGURE 5.
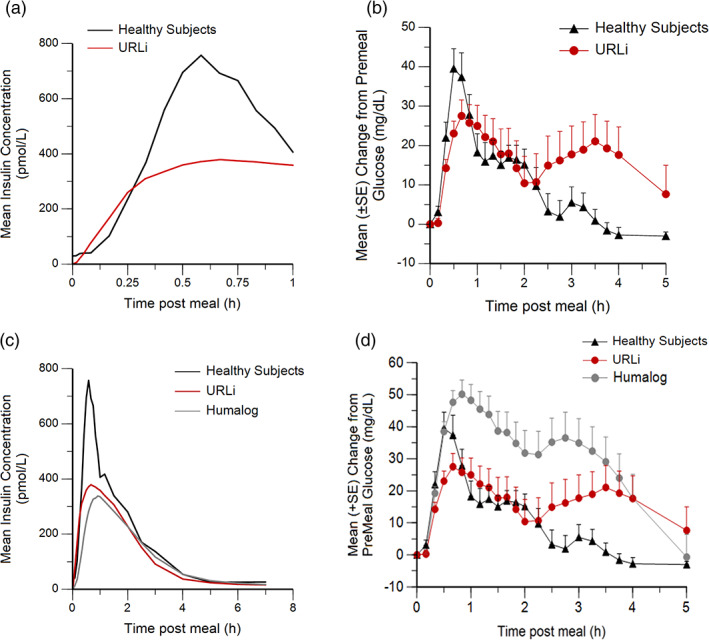
Mean insulin concentration versus time post‐meal (A) and change from baseline glucose concentration (+SE) versus time post‐meal (B) after URLi in patients with type 1 diabetes (T1D) and in healthy subjects and after Humalog or URLi in patients with T1D and in healthy subjects (C,D)
3.6. Hypoglycaemia during the test meal
No severe hypoglycaemic events were reported. During the test meals, the total number of hypoglycaemic events (≤70 mg/dL) was reported at similar frequencies for all study drugs (Table S4). URLi administration resulted in the lowest number of hypoglycaemic events during the test meal assessment: 12 for URLi, 19 for Fiasp, 14 for Humalog, and 13 for NovoRapid. The majority of these events occurred between 2 and 4 hours after the start of the test meal for all study drugs.
3.7. Tolerability and safety results
All insulins tested were well tolerated, with no clinically significant changes in clinical laboratory evaluations, vital signs or electrocardiograms. The study participants did not report any injection site reactions. The tolerability of URLi was comparable to the other insulins assessed.
4. DISCUSSION
This was the first study to compare the PK and GD characteristics of URLi, Humalog, NovoRapid and Fiasp following a standardized test meal under identical conditions. The inclusion of a healthy cohort provided the “normal” insulin secretory and glucose response to the same test meal. URLi demonstrated the fastest insulin absorption and the greatest numeric PPG‐lowering effect, resulting in the best glycaemic control compared to other insulins tested. The early PPG profile of URLi most closely matched the glucose profile of the healthy cohort.
As previously reported, URLi had a faster insulin lispro absorption, reduced late exposure, and an overall shorter exposure duration compared to Humalog. 8 , 9 Similarly, faster insulin aspart absorption was observed with Fiasp compared to NovoRapid. 3 , 4 , 5 , 6 , 10 This study found that URLi demonstrated the fastest insulin absorption of all the insulins tested, resulting in a significantly greater early insulin exposure in the circulation. Due to this, the lowest PPG profile was observed following URLi compared to Fiasp, Humalog and NovoRapid. The glucose excursion over the entire test meal (5 hours) was significantly reduced with URLi compared to Humalog and NovoRapid. Although the PPG excursions over the 4‐hour period were reduced with URLi compared to Fiasp, none of these reductions reached statistical significance, which may be reflective of the sampling size and powering of the study.
Administration of URLi resulted in less late insulin exposure and a shorter duration of insulin exposure in comparison with Fiasp, Humalog and NovoRapid. It is anticipated this would decrease the risk of late postprandial hypoglycaemia. Correspondingly, there were fewer hypoglycaemic events which needed intervention with URLi (six events) than the other insulins tested (10–12 events). However, further clinical studies would be needed to investigate the clinical impact of this finding.
In comparison to healthy subjects, the glucose excursions over the first 3 hours post‐meal were comparable with URLi, but the time‐course differed. The PPG excursion was slightly lower with URLi in the first hour after the meal, likely due to the faster rise in insulin concentrations in the first 15 minutes post‐meal. PPG levels were fairly similar to those of healthy subjects in the second hour post‐meal. However, the PPG excursion between 2 to 3 hours post‐meal increased with URLi, but declined in healthy subjects. Interestingly, the higher endogenous insulin concentrations in healthy subjects between 15 and 60 minutes post‐meal did not lead to substantial differences in the PPG excursion in the first 2 hours between healthy subjects and URLi. While peak insulin concentrations were lower with URLi, it seems that the faster rise in insulin concentrations resulted in a numerically improved PPG control in the first 2 hours after the meal. This observation is supported by a recent PK/PD analysis, which shows that PPG‐lowering is driven by insulin concentrations reaching an apparent threshold of ~200 pmol/L, similar to the human insulin receptor Type A dissociation constant (Leohr et al, manuscript in preparation). This threshold was reached faster with URLi than was observed with endogenous insulin.
Glucose levels were higher beyond 2 hours post‐meal after URLi compared to those in healthy subjects, despite the similar insulin tails of URLi and endogenous insulin. This suggests that the route of insulin administration may also play an important role. Subcutaneous administration of exogenous insulin initially bypasses the portal system, resulting in portal insulin concentrations which are similar to the peripheral levels, while endogenous insulin secretion leads to portal insulin concentrations approximately 2‐ to 3‐fold higher than what is detected in the periphery. 11 , 12 , 13 The insulin secretion in healthy subjects nearly completely suppresses endogenous glucose production over at least 3 hours postprandially, 14 whereas subcutaneously administered insulins primarily stimulate peripheral glucose uptake. 15 The effect of URLi on endogenous glucose production has not yet been investigated, but Humalog has been shown to suppress endogenous glucose production for only 90–120 minutes after glucose ingestion. 16 Overall, therefore, it may be difficult to improve PPG levels with subcutaneously injected insulins beyond the improvements seen with URLi. The rapid insulin absorption of URLi is able to control PPG to a similar degree as observed in healthy subjects over 2–3 hours, and a reduction of late PPG might require a greater effect on endogenous glucose production than is achievable with subcutaneously injected insulins. This is likely to be addressed with the advancement of closed‐loop pump systems with which insulin delivery would be adjusted to provide optimal glucose control. Given the more rapid insulin absorption and improved PPG‐lowering observed with URLi, it may be the optimal insulin to use in these closed‐loop systems.
This study was strengthened by the following: (a) it was conducted in patients with T1D, who lack endogenous insulin secretion to confound the PK and GD analyses; (b) the use of a crossover design allowing for a within‐subject comparison of PK and GD response across these insulins, using the same test meal and insulin dose; (c) a run‐in procedure facilitated the comparison in the PPG response and addressed inter‐occasion glucose variability by having a similar glucose concentration prior to the meal across the patients; and (d) the inclusion of the dose‐finding assessment, which ensured that the appropriate insulin dose was given for the test meal for each patient with T1D. A limitation of the study was the use of a liquid test meal, which is not a typical meal for patients. However, the PPG responses between URLi and Humalog have been assessed in both solid meals and liquid meals and showed similar responses to those observed in the present study. 17 Although these data are encouraging, the translation of the improvement in PPG response with URLi over other insulin analogues to a clinical benefit would warrant further evaluation in a larger, long‐term clinical study.
In conclusion, the results of this head‐to‐head study demonstrated that URLi has the fastest insulin absorption and greatest numerical reduction in PPG excursions compared to Humalog, Fiasp and NovoRapid. Thus, these results suggest that URLi may have the potential to improve glycaemic control over current rapid‐acting insulin analogues in patients with T1D. Furthermore, URLi more closely matched the early physiological glucose control observed in healthy subjects, and it may be difficult to see further improvements with subcutaneously injected insulin.
CONFLICTS OF INTEREST
H.L., D.E.C., E.L., J.B.‐V., Q.Z., M.A.D. and J.L. are employees and shareholders of Eli Lilly and Company. T.H., E.Z. and C.K. are employees of Profil. T.H. and C.K. are also shareholders of Profil. T.H. received speaker honoraria and travel grants from Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
All authors participated in the drafting, critical revision and approval of the final version of the manuscript. T.H., H.L., E.Z., J.B.V. and J.L. were involved in the study design, and T.H., E.Z. and C.K. were investigators in the study. H.L. was responsible for study monitoring. D.C., E.L. and J.L. conducted data analyses, and Q.Z. and M.A.D. conducted the statistical analyses. All authors were involved in interpretation of the study results.
DATA SHARING STATEMENT
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors would like to thank all study participants. Medical writing assistance was provided by Kristen Syring, PhD.
Heise T, Linnebjerg H, Coutant D, et al. Ultra rapid lispro lowers postprandial glucose and more closely matches normal physiological glucose response compared to other rapid insulin analogues: A phase 1 randomized, crossover study. Diabetes Obes Metab. 2020;22:1789–1798. 10.1111/dom.14094
Funding information This study was funded by Eli Lilly and Company.
REFERENCES
- 1. Pratt E. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin Lispro. Diabetes. 2017;66(Supplement 1):975. [Google Scholar]
- 2. Klaff L, Cao D, Dellva MA, et al. Ultra rapid Lispro (URLi) improves postprandial glucose (PPG) control vs. Humalog (Lispro) in T1D: PRONTO‐T1D study. Diabetes Obesity and Metabolism. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blevins, T , Zhang, Q , Frias, JP , Jinnouchi, H , Chang, AM . Randomized Double‐Blind Clinical Trial Comparing Ultra Rapid Lispro with Lispro in a Basal‐Bolus Regimen in Patients with Type 2 Diabetes: PRONTO‐T2D. Diabetes Care. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heise T, Hovelmann U, Brondsted L, Adrian CL, Nosek L, Haahr H. Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17(7):682‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heise T, Hovelmann U, Zijlstra E, Stender‐Petersen K, Jacobsen JB, Haahr H. A comparison of pharmacokinetic and pharmacodynamic properties between faster‐acting insulin aspart and insulin aspart in elderly subjects with type 1 diabetes mellitus. Drugs Aging. 2017;34(1):29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heise T, Stender‐Petersen K, Hovelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster‐acting insulin Aspart versus insulin Aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin Pharmacokinet. 2017;56(6):649‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast‐acting insulin Aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56(5):551‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chow S‐C, J‐p L. Design and Analysis of Bioavailability and Bioequivalence Studies. Boca Raton, FL: Chapman & Hall/CRC; 2009. [Google Scholar]
- 9. Leohr J, Dellva MA, Coutant DE et al. Pharmacokinetics and glucodynamics of ultra rapid lispro (URLi) accelerates insulin lispro absorption and insulin action vs. Humalog (lispro) in patients with type 2 diabetes. A phase 1 randomized, crossover study in T2D. Clin Pharmacokinet. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linnebjerg H, Zhang Q, LaBell E, et al. Pharmacokinetics and glucodynamics of ultra rapid lispro (URLi) versus Humalog® (lispro) in younger adults and elderly patients with type 1 diabetes: a randomised controlled trial. Clin Pharmacokinet. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fath M, Danne T, Biester T, Erichsen L, Kordonouri O, Haahr H. Faster‐acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18(8):903‐910. [DOI] [PubMed] [Google Scholar]
- 12. Magkos F, Fabbrini E, Patterson BW, Eagon JC, Klein S. Portal vein and systemic adiponectin concentrations are closely linked with hepatic glucose and lipoprotein kinetics in extremely obese subjects. Metabolism. 2011;60(11):1641‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C‐peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975;55(6):1278‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edgerton DS, Kraft G, Smith M, et al. Insulin's direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2(6):e91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowalski GM, Moore SM, Hamley S, Selathurai A, Bruce CR. The effect of ingested glucose dose on the suppression of endogenous glucose production in humans. Diabetes. 2017;66(9):2400‐2406. [DOI] [PubMed] [Google Scholar]
- 16. Senior P, Hramiak I. Fast‐acting insulin aspart and the need for new mealtime insulin analogues in adults with type 1 and type 2 diabetes: a Canadian perspective. Can J Diabetes. 2019;43(7):515‐523. [DOI] [PubMed] [Google Scholar]
- 17. Bruttomesso D, Alessandro Pianta AM, Valerio A, et al. Restoration of early rise in plasma insulin levels improves the glucose tolerance of type 2 diabetic patients. Diabetes. 1999;48(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 18. Plum‐Moerschel L, Leohr J, Liu R, et al. Ultra‐rapid Lispro (URLi) reduces postprandial glucose excursions vs. Humalog® in patients with T1D at multiple meal‐to‐dose timing intervals. Diabetes. 2018;67(Supplement 1):1010 ‐P. [Google Scholar]
- 19. Dodson MM, Zhang C, Siesky AM, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation. Diabetes. 2017;66(Suppl 1):A250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


