To the Editor,
Thunderstorm asthma risk in geographic regions with temperate grasses is strongly correlated with the trifecta of ryegrass pollen (RGP) sensitization (serum RGP‐specific IgE), seasonal allergic rhinitis (SAR), and exposure to a thunderstorm during the pollen season. 1 , 2 Perennial ryegrass (Lolium perenne) is a wind‐pollinated pasture grass prevalent in southeastern Australia, North America, and Southern Europe. Importantly, RGP‐sensitized patients with SAR even without a previous doctor diagnosis of asthma may, in the presence of the trifecta, experience bronchoconstriction, known as epidemic thunderstorm asthma (ETSA). 1 , 3 , 4 In SAR, nasal and ocular symptoms and signs are typically elicited when intact RGP grains (≥30 microns) lodge in the upper airways. In contrast, it is suggested that ETSA is triggered when thunderstorm moisture ruptures RGP grains through osmotic shock to release respirable (<3 micron) allergen‐impregnated starch granules which then trigger bronchoconstriction. 5 Immunologically, the two most prevalent major allergens of Lolium perenne are Lol p 1 (30 kDa) and Lol p 5 (29‐31 kDa; formerly called Lol p IX), with each eliciting serum IgE reactivity in >90% of RGP‐sensitized individuals. 6 Lol p 1 is initially presented in the cytosol of pollen grains, from which it is subsequently secreted to coat their surface. It is then readily leached in soluble form from grains lodged in the upper airways, triggering SAR. In contrast, Lol p 5 resides in the starch granules (amyloplasts) that are released following pollen grain rupture and respired into the lower airways during thunderstorms. 7 Similarly, levels of airborne group 5 allergens for Phleum pratense (Phl p 5) in respirable‐sized particles are positively correlated to relative humidity. 8 Furthermore, while the majority of individuals sensitized to RGP might be expected to also be sensitized to both Lol p 1 and Lol p 5, the degree of sensitization to each major allergen may vary between individuals. The late Bruce Knox (Professor of Botany, University of Melbourne, Australia) hypothesized that RGP starch granules associated with Lol p 5 might be responsible for triggering an epidemic of thunderstorm asthma. 9
To test the Knox hypothesis, and to determine whether levels of sensitization to RGP generally, and Lol p 5 specifically, have diagnostic utility for predicting risk of ETSA, we quantitated the relevant serum specific (sp) IgE using enzyme‐linked immunosorbent assays (ELISA). Serum for ELISA was obtained from the blood of 60 patients who presented to the Alfred Hospital Emergency Department due to the catastrophic ETSA event of 21 November 2016. 2 For comparison, the same analysis was performed on serum drawn from 19 control individuals recruited from the Asthma & Allergy Clinic with symptoms of SAR, and who were present in Melbourne and outdoors on 21 November 2016, but who did not experience ETSA. The ETSA group had an age distribution similar to the control group but a greater proportion of male patients (Table 1). Detailed information about this study is available in this article's online repository.
Table 1.
Patient characteristics
| Thunderstorm asthma patients (n = 60) | Nonthunderstorm asthma controls (n = 19) | |
|---|---|---|
| Age, year (mean ± SD) | 39.8 ± 15.4 | 36.9 ± 12.0 |
| Female | 24 (40%) | 13 (68%) |
| Springtime allergic rhinitis symptoms | 60 (100%) | 19 (100%) |
| Current asthma before thunderstorm | 23 (38%) | 8 (37%) |
| Total IgE, kU/L (median, IQR) | 170 (93‐571) | 213 (88‐475) |
| Ryegrass pollen‐specific IgE, kU/L (median, IQR) | 51.5 (25.6‐100.0) | 16.7 (4.1‐49.5) |
| Lol p 1‐specific IgE, µg/mL (median, IQR) | 1.28 (0.55‐3.45) | 1.15 (0.29‐2.09) |
| Lol p 5‐specific IgE, µg/mL (median, IQR) | 2.61 (1.37‐4.05) | 1.70 (0.17‐2.64) |
For analysis of sp IgE, recombinant Lol p 1 (rLol p 1) was purchased (MyBiosource, San Diego, CA) and recombinant Lol p 5 (rLol p 5) 10 was produced in‐house in the insect cell line Sf21 using a baculovirus system. ETSA patients had a mean Lol p 1 sp IgE concentration similar to control participants, but higher mean RGP sp IgE and Lol p 5 sp IgE (Table 1, Figure 1A‐C). Using receiver operator characteristic (ROC) curve characteristics, both Lol p 5 sp IgE and RGP sp IgE levels distinguished ETSA‐affected patients from controls with an area under the curve (AUC) of 0.67 and 0.76, respectively, while Lol p 1 sp IgE had no such diagnostic utility (Figure 1D‐F).
Figure 1.
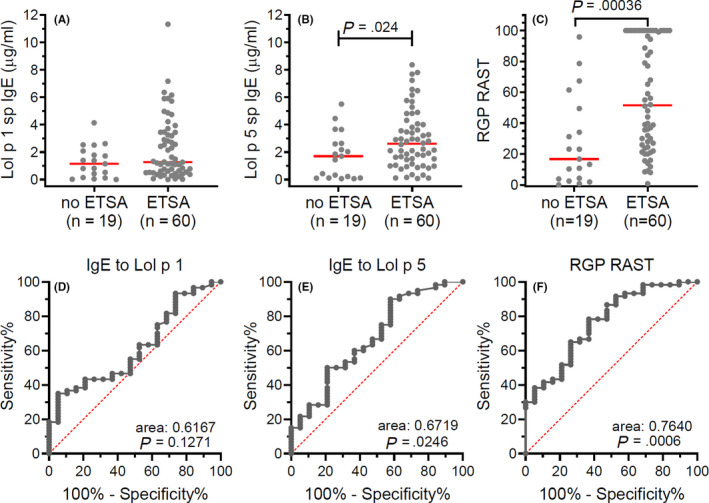
A‐C, Specific IgE to Lol p 1, Lol p 5, and RGP, respectively, in patients with and without ETSA. Individual measurements are depicted as gray dots with red bars representing medians. Statistics, nonparametric Mann‐Whitney U test. D‐F, Receiver operator characteristics of specific IgE to Lol p 1, Lol p 5, and ryegrass pollen, respectively, for ETSA. Statistics, Wilson/Brown method to test whether the confidence level of the outcome distribution is greater than 95%
Mechanistically, our results support the Knox hypothesis. 9 We show that susceptibility to ETSA is linked with greater sensitization to Lol p 5 found on the respirable starch granules, but not Lol p 1. These data have important ramifications for clinical practice. The absolute level of sp IgE to RGP, and to a lesser extent to Lol p 5, was features associated with those susceptible to ETSA. Given high rates of ryegrass pollen sensitization in ETSA areas (of up to 40% in Melbourne, Australia), 2 it is not feasible to implement protective strategies in all sensitized patients. However, our data suggest that the degree of sensitization can contribute to the assessment of absolute risk, to help focus attention on the patient subgroup most suitable for specific preventive measures. The future development of accurate risk prediction tools may benefit from combining immunological markers of risk with clinical risk factors such as concurrent asthma and past history of ETSA. Until Lol p 5 in vitro testing becomes routinely available, it may also be useful to explore the utility of specific IgE to Phl p 5 (as representative of highly cross‐reactive group 5 allergens). 8
Risk stratification is particularly relevant because at least two protective strategies are now indicated for ETSA. Case‐control data suggest inhaled corticosteroid use is beneficial, 11 while controlled trial results supported by evidence of immunological tolerance indicate RGP allergen immunotherapy protects from ETSA, 12 , 13 so both options should be considered in high‐risk individuals.
While every patient in the ETSA group was clearly susceptible to ETSA, we could not be completely certain that all individuals in the control group were protected, since allergen exposure during the 2016 thunderstorm epidemic may have varied between different outdoor locations. There is therefore a possibility that ETSA‐susceptible individuals may have been clinically misclassified as "protected." Such classification error, if present, would tend to dilute observable between‐group differences, so we may have underestimated the discriminatory utility of specific IgE to Lol p 5 and RGP. We acknowledge the sample size in this study was small, and the scientific community should prepare for a broader collection of clinical samples in the event of future ETSA events, in order to explore these and other immunological mechanisms.
To conclude, the utility of RGP and Lol p 5 sp IgE measurements should be explored further as potential indicators of RGP‐related ETSA risk. In particular, the development of risk prediction tools utilizing these immunological factors in combination with clinical features may enable more accurate risk prediction for RGP‐related ETSA among SAR patients, in order to offer appropriate protective strategies including allergen‐specific immunotherapy.
CONFLICT OF INTEREST
MH has received grants‐in‐aid, speaker fees, and fees for serving on the advisory boards of GlaxoSmithKline, AstraZeneca, Novartis, Teva, Sanofi, and Seqirus, all paid to his institutional employer Alfred Health. ROH is a minority shareholder of two early‐stage biotechnology companies—Aravax Pty Ltd and Paranta Bio Pty Ltd (in respect of both of which she is a named inventor of the IP assets)—and as such may benefit in the future if the respective experimental medicines are approved for use. All other authors declare no conflicts of interest.
Funding information
MH and ROH received grant funding from Num Pon Soon Charitable Trust. PMH and ROH were funded by a project grant (GNT1145303) and MCvZ by a senior research fellowship (GNT1117687) from the National Health and Medical Research Council (NHMRC), Australia.
Supporting information
Method S1
REFERENCES
- 1. D'Amato G, Annesi‐Maesano I, Cecchi L, D'Amato M. Latest news on relationship between thunderstorms and respiratory allergy, severe asthma, and deaths for asthma. Allergy. 2019;74(1):9‐11. [DOI] [PubMed] [Google Scholar]
- 2. Lee J, Kronborg C, O'Hehir RE, Hew M. Who's at risk of thunderstorm asthma? The ryegrass pollen trifecta and lessons learnt from the Melbourne thunderstorm epidemic. Respir Med. 2017;132:146‐148. [DOI] [PubMed] [Google Scholar]
- 3. Thien F, Beggs PJ, Csutoros D, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health. 2018;2(6):e255‐e263. [DOI] [PubMed] [Google Scholar]
- 4. Hew M, Lee J, Susanto NH, et al. The 2016 Melbourne thunderstorm asthma epidemic: Risk factors for severe attacks requiring hospital admission. Allergy. 2019;74(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 5. Suphioglu C, Singh MB, Taylor P, et al. Mechanism of grass‐pollen‐induced asthma. Lancet. 1992;339:569‐572. [DOI] [PubMed] [Google Scholar]
- 6. Singh MB, Hough T, Theerakulpisut T, et al. Isolation of cDNA encoding a newly identified major allergenic protein of rye‐grass pollen: Intracellular targeting to the amyloplast. Proc Natl Acad Sci USA. 1991;88:1384‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor PE, Staff IA, Singh MB, Knox RB. Localization of the two major allergens in rye‐grass pollen using specific monoclonal antibodies and quantitative analysis of immunogold labelling. Histochem J. 1994;26(5):392‐401. [DOI] [PubMed] [Google Scholar]
- 8. Buters J, Prank M, Sofiev M, et al. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season. J Allergy Clin Immunol. 2015;136(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 9. Knox RB. Grass pollen, thunderstorms and asthma. Clin Exp Allergy. 1993;23(5):354‐359. [DOI] [PubMed] [Google Scholar]
- 10. Chan SK, Pomés A, Hilger C, et al. Keeping Allergen Names Clear and Defined. Front Immunol. 2019;10:2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Girgis ST, Marks GB, Downs SH, et al. Thunderstorm‐associated asthma in an inland town in south‐eastern Australia. Who is at risk? Eur Respir J. 2000;16(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 12. O'Hehir RE, Varese NP, Deckert K, et al. Epidemic thunderstorm asthma protection with five‐grass pollen tablet sublingual immunotherapy: a clinical trial. Am J Respir Crit Care Med. 2018;198(1):126‐128. [DOI] [PubMed] [Google Scholar]
- 13. Heeringa JJ, McKenzie CI, Varese N, et al. Induction of IgG2 and IgG4 B‐cell memory following sublingual immunotherapy for ryegrass pollen allergy Allergy. 2020;75(5):1121‐1132. 10.1111/all.14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Method S1


