Abstract
Low blood pressure is common in patients with heart failure and reduced ejection fraction (HFrEF). While spontaneous hypotension predicts risk in HFrEF, there is only limited evidence regarding the relationship between hypotension observed during heart failure (HF) drug titration and outcome. Nevertheless, hypotension (especially orthostatic hypotension) is an important factor limiting the titration of HFrEF treatments in routine practice. In patients with signs of shock and/or severe congestion, hospitalization is advised. However, in the very frequent cases of non‐severe and asymptomatic hypotension observed while taking drugs with a class I indication in HFrEF, European and US guidelines recommend maintaining the same drug dosage. In instances of symptomatic or severe persistent hypotension (systolic blood pressure < 90 mmHg), it is recommended to first decrease blood pressure reducing drugs not indicated in HFrEF as well as the loop diuretic dose in the absence of associated signs of congestion. Unless the management of hypotension appears urgent, a HF specialist should then be sought rather than stopping or decreasing drugs with a class I indication in HFrEF. If symptoms or severe hypotension persist, no recommendations exist. Our HF group reviewed available evidence and proposes certain steps to follow in such situations in order to improve the pharmacological management of these patients.
Keywords: Hypotension, Heart failure, Beta‐blocker, Angiotensin‐converting enzyme inhibitor, Angiotensin receptor blocker, Angiotensin receptor–neprilysin inhibitor, Mineralocorticoid receptor antagonist, Diuretics
Introduction
Low blood pressure (BP) is reported in 10–15% of patients with heart failure (HF) in clinical trials, although this proportion is much more frequent in routine clinical practice.1, 2, 3, 4, 5, 6, 7 Orthostatic hypotension is similarly frequent (more than 10%), especially in elderly subjects.8
Blood pressure has two main components: a pulsatile component related to arterial stiffness mostly associated with pulse pressure or systolic BP (SBP), and a steady component related to systemic vascular resistance mostly associated with mean BP and diastolic BP (DBP). In patients with impaired left ventricular ejection fraction (LVEF), both SBP and pulse pressure are primarily dependent on left ventricular stroke volume,9 while mean BP and DBP are highly dependent on total blood volume and the degree of peripheral vasodilatation. Both these components can be influenced by treatments used in chronic HF with reduced LVEF (HFrEF).10, 11, 12
Low BP in HFrEF may have multiple origins such as low cardiac function, hypovolaemia (usually due to diuretics), treatment‐related vasodilatation and altered vasoreactivity related to comorbidities such as diabetes.
On the basis of landmark clinical trials, an established armamentarium of therapies for HFrEF exists, which reduces morbidity and mortality; clinical practice guidelines strongly articulate recommendations for initiation and titration of these therapies to target doses. In daily clinical practice, dose adjustment of HFrEF drugs relies on signs and symptoms of HF, BP, heart rate, biological parameters (mainly creatinine, serum potassium, haemoglobin, natriuretic peptides), or imaging parameters.10, 11, 13, 14, 15 Target doses of therapies for HFrEF may be challenging due to dose‐related reduction in BP, which results in clinician hesitancy to further titrate therapies, as the meaning of low BP in patients with HF is established: low BP (<90 mmHg) has been repeatedly emphasized as a marker of poor outcome in acute HF.10, 16 In contrast, the prognostic value of low BP in ambulatory chronic HF appears attenuated (especially when adjusting for other clinical variables) as SBP was used in some clinical scores (i.e. the Heart Failure Survival Score17 and the Seattle Heart Failure Model18) but not retained as a relevant variable in the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score.19 In addition, SBP association with outcome in ambulatory patients does not necessarily imply that lower SBP is causally related to poorer outcome. The attenuated association between BP and outcome in ambulatory patients compared to acute settings and the uncertainties regarding causality advocates for maintaining HFrEF lifesaving drugs despite low BP. However, practical guidance is lacking.
Despite the evidence from clinical trials (usually including younger and less sick patients than registries), and a class I level A indication in current clinical practice guidelines,10, 11 registry data from various regions of the world consistently show a lower use of angiotensin‐converting enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARB), angiotensin receptor–neprilysin inhibitors (ARNi), beta‐blockers or mineralocorticoid receptor antagonists (MRA) than their observed use in clinical trials.20 Low BP is a factor often found to limit the use and up‐titration of class I life‐saving drugs in HFrEF20, 21 and can prompt stopping (at best temporarily) these drugs. This should be a concern since HFrEF patients with the lowest BP may obtain a similar benefit from these therapies when compared to patients with higher BP.22, 23
The European Society of Cardiology guidelines recommend that a HF specialist should be sought rather than stopping or decreasing drugs with class I indication in HFrEF in patients with persisting low BP or symptoms of orthostatic hypotension. However, despite representing a frequent dilemma in clinical practice, there is currently no management/algorithm recommended in this clinical setting. We consequently deemed it important to propose a pharmacological management algorithm, based on a comprehensive review of available evidence aimed at helping physicians treat HFrEF patients with low BP.
Available evidence related to hypotension in patients treated with heart failure with reduced ejection fraction drugs
Angiotensin‐converting enzyme inhibitors
Angiotensin‐converting enzyme inhibitors were the first pharmacological class to demonstrate a significant decrease in clinical events in HFrEF and currently have a class I indication in this setting.10, 11
In the CONSENSUS trial conducted in patients with HFrEF treated with loop diuretics (98%), spironolactone (50%), cardiac glycosides (92%) and nitrates (47%), hypotension necessitating treatment discontinuation was by far more common in the ACEi group (5.5%) than in the placebo group (no discontinuation for hypotension). In this trial, factors associated with a higher risk of hypotension were serum sodium <130 mmol/L, serum creatinine 150–300 μmol/L, an increase in diuretic dose in the previous week and concomitant treatment with potassium‐sparing agents.1
In the SOLVD trial, hypotension was also twice more common in the ACEi group (14.8% vs. 7.1%, P < 0.001). Factors associated with hypotension risk were hyponatraemia, New York Heart Association (NYHA) class IV and persistent signs of congestion requiring high doses of diuretics or vasodilators.24 In the CHARM‐Alternative study, hypotension was the reason for ACEi intolerance justifying its replacement by ARB in 14.1% of cases.6
The ATLAS trial compared high‐dose vs. low‐dose ACEi. In this trial, the high‐dose group more frequently developed hypotension (11% vs. 7%), although the latter rarely led to drug discontinuation (0.8% vs. 0.6%).4 Importantly, as in the CONSENSUS trial, no pre‐defined numerical threshold was used to define hypotension.4 Importantly, available evidence regarding high‐ vs. low‐dose ACEi therapy in chronic HF suggest a lower risk of hospitalization in high‐dose ACEi compared to low‐dose ACEi,25 mostly based on the results of ATLAS (P = 0.002 for a reduction in the risk of death or hospitalization) and CHIPS (P = 0.06 for a reduction in the risk of hospitalization for HF).26
In all of these aforementioned trials, clinical judgment rather than a pre‐defined BP threshold was used to characterize hypotension. The management of low BP was left to the discretion of the treating physicians and investigators. The strategy used by these physicians was usually a temporary reduction or suspension (rather than a withdrawal) of the ACEi. It should be emphasized that it is this very pharmacological management strategy that led to a significant and substantial decrease in HF‐related events.
Angiotensin II receptor blockers
Angiotensin II receptor blockers are recommended when ACEi are poorly tolerated (grade IB).10 In a subgroup analysis of the CHARM‐Alternative trial, candesartan was well tolerated by 9 in 10 patients with prior hypotension treated with ACEi, although to a slightly lesser degree than in the total study population (96%).6
In the Val‐HeFT study, the drop in SBP at 4 months after valsartan introduction was associated with an increased risk of clinical events regardless of baseline SBP level. Importantly, unlike in the higher SBP groups, valsartan did not further reduce SBP in those with lowest baseline pressures. The effect of valsartan on morbidity and mortality risk was similar across all baseline SBP categories (with an absolute effect seemingly even greater in case of low SBP).27
In the HEAAL trial,28 losartan 150 mg daily reduced the rate of death or admission for HF in patients with HFrEF compared with losartan 50 mg daily despite higher rates of hypotension. These findings strengthen the value of up‐titrating ARB doses, with this strategy notably resulting in markedly lower BP.28
Angiotensin receptor–neprilysin inhibitors
The ARNi class is recommended in symptomatic HFrEF (NYHA class II to IV) treated with ACEi/ARB, beta‐blockers and MRAs. The switch from ACEi to ARNi yields a significant beneficial effect on symptoms, morbidity and mortality.10, 11
The PARADIGM‐HF study tested the efficacy of sacubitril/valsartan (ARNi) compared to enalapril in patients who previously tolerated a run‐in to full dose of enalapril and ARNi prior to starting the assessment phase.7, 29 Despite the run‐in, hypotension was the second leading cause for non‐completion of the study during this short run‐in phase, just behind the occurrence of worsening kidney function.30, 31 The risk of hypotension after randomization was 13.4% with ARNi; however, only 2.7% had a SBP <90 mmHg associated with symptoms.7, 32 Investigators either reduced or temporarily stopped ARNi treatment (54.1% of cases), simply waited for a spontaneous improvement (34.3%), or changed concomitant treatments (12.8%). A permanent discontinuation of the treatment was observed in only 2.2% of cases.32 In patients who developed hypotension with ARNi, the SBP recorded during the event was 106 ± 18 mmHg, suggesting symptomatic hypotension without necessarily a sharp drop in SBP.32 Baseline factors independently associated with the occurrence of hypotension on ARNi were age, low SBP, no history of hypertension, high creatinine and presence of an implantable automatic defibrillator.32 Notably, patients with lower BP (usually receiving low‐dose ARNi) experienced a comparable benefit from ARNi as patients with higher BP.22, 33
In the TITRATION trial, condensed (19 days) or conservative (40 days) ARNi initiation strategies were associated with a similar risk of hypotension (9.8% vs. 8.7%). SBP <95 mmHg was however more frequent in the initially naïve or low‐dose group of ACEi/ARB when receiving a condensed dosage regimen of ARNi.31 In addition, rates of treatment success (maintained target dose of sacubitril/valsartan without down‐titration/dose interruption over 12 weeks) did not differ significantly according to baseline BP.34 However, a higher percentage of patients with lower SBP (100–110 mmHg) achieved treatment success with gradual up‐titration (6 weeks) (∼80%) than with rapid up‐titration (∼69%).34
Even if performed in patients with acutely decompensated HF, the PIONEER‐HF and TRANSITION trials provide insightful evidence regarding the BP tolerance of ARNi. The PIONEER‐HF trial showed that the proportion of patients experiencing symptomatic hypotension was not significantly higher when treated with ARNi than when treated with enalapril (15% vs. 12.7%)35 in this context at higher‐risk for adverse effects. The TRANSITION trial showed that the introduction of ARNi was feasible even prior to patient discharge following worsening HF and that symptomatic hypotension was infrequent and not significantly different in the pre‐discharge vs. post‐discharge initiation (12.7% vs. 9.5%).36 Importantly, as both the PIONEER‐HF and TRANSITION trials included patients with SBP ≥ 100 mmHg, current evidence does not support the initiation of ARNi in patients with SBP < 100 mmHg.
Beta‐blockers
Beta‐blockers have a class I indication in patients with HFrEF.10, 11 The trials that have assessed their effects in HFrEF were mostly conducted in patients already treated with ACEi.
Hypotension was more frequently observed in patients treated with carvedilol2 than in those receiving placebo (9% vs. 4%); however, discontinuation of treatment was nonetheless exceptional in both groups (0.3% vs. 0.3%). Similar results were reported with metoprolol in MERIT‐HF with a 0.6% discontinuation for hypotension vs. 0.3% for placebo.3
A dose–response curve reported in the MOCHA trial revealed that carvedilol produced dose‐related improvements in left ventricular function and dose‐related reductions in both mortality and hospitalization rate.37
Of noted importance, the COPERNICUS trial showed that subjects with the lowest BP were also those who experienced the greatest benefit.23 In this analysis, the additional moderate (2 mmHg) drop in BP observed in patients randomized to beta‐blockers persisted for approximately 4 months, but was no longer apparent after 8 months suggesting a transient BP‐lowering effect.
Despite carvedilol having multi‐receptor effects potentially causing greater decrease in BP, the proportion of target dose achieved does not appear to differ in patients treated with either bisoprolol or carvedilol.38
Mineralocorticoid receptor antagonists
Mineralocorticoid receptor antagonists are recommended (class IA) in symptomatic (NYHA class II to IV) HFrEF on top of ACEi and beta‐blockers.10
While serum potassium and creatinine are closely monitored when taking MRAs, BP changes is not emphasized as a restriction of MRA use. In the RALES trial assessing spironolactone vs. placebo,39 no difference in BP was reported in the two groups. However, the proportion of beta‐blocker prescriptions was low (11%) in comparison with current standards, which may have decreased the incidence of hypotension.
In the EPHESUS40 and EMPHASIS‐HF41 trials, most patients (>75%) were on beta‐blockers. Patients treated with MRAs experienced marginally and non‐significant lower BP. The treatment effect of MRAs was reported not to be affected by baseline BP in a joint analysis of RALES and EMPHASIS‐HF, and hypotension was infrequent and not more common with MRA therapy than with placebo overall (4.6% vs. 3.9%) regardless of baseline BP.42
If channel inhibitors
Since the inhibition of If channels by ivabradine has a purely heart rate‐lowering action, no hypotensive effect was expected and none was reported in the SHIFT study. Hypotension was the primary reason (other than bronchospastic lung disease in 45% of cases) for not prescribing a beta‐blocker.43 In addition, the introduction of ivabradine also allowed the introduction of a beta‐blocker secondarily and/or enabled increasing the beta‐blocker dose more easily compared with introducing a beta‐blocker directly.44
Loop diuretics
The use of a loop diuretic reduces the signs and symptoms of HF and as such reduce risk for hospitalization; nonetheless, risk for hazard is directly linear to the dose of loop diuretic, in part due to association with disease severity but also related to risks of electrolyte abnormalities and kidney dysfunction.10, 11 Accordingly, loop diuretic dosage should therefore be strictly adjusted to the congestion status. More concerningly, diuretics can be associated with hypovolaemia, which can in turn be associated with low BP and hence limit the initiation or up‐titration of HFrEF drugs; this is particularly the case when adding and titrating ARNi. For these reasons, a decrease in diuretic dose may be considered in patients without peripheral oedema or significant HF symptoms.45 Importantly, inferior vena cava measurements can be performed to assess the patient's intravascular volume, which can be particularly helpful for tailoring diuretic doses.
Sodium–glucose co‐transporter 2 inhibitors
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors have been shown to reduce morbi‐mortality in patients with HFrEF. Importantly, in the DAPA‐HF trial, the mean SBP drop observed in patients treated with dapagliflozin was only 1.3 mmHg.46 In addition, older patients included in this trial derived similar benefits from dapagliflozin and experienced similar BP drop (P for interaction 0.97),47 suggesting a favourable safety profile in elderly patients despite their inherent higher likelihood for postural hypotension. Yet, as SBP < 95 mmHg was an exclusion criteria of DAPA‐HF, we do not have sufficient evidence to support the use of SGLT2 inhibitors in patients with initially low SBP.
There is however currently little information regarding the management of low BP in patients treated concomitantly with SGLT2 inhibitors, ARNi/ACEi/ARB, MRAs and diuretics.
Expert opinion
After a careful review of available literature in ambulatory chronic HF, it appears that:
Most of available evidence regarding low BP in HF is derived from trials data. As most trials have included patients without significant hypotension at baseline, younger than real‐life population and with a limited comorbidity burden, a cautious evaluation of individual benefit to risk ratio should be performed when managing HF drugs in the sickest patients with low SBP.
In trials, clinical judgment rather than a pre‐defined BP threshold was generally used to characterize hypotension. There is consequently no strong consensus regarding the definition of severe hypotension as well as the pharmacological management to apply in this setting.
In trials, low BP was mostly considered as relevant when associated with symptoms. Symptoms should consequently guide the management of HFrEF treatment in patients with low BP.
Most severe patients often benefited the most from the prescribed treatments. Specifically, patients with the lowest baseline BP drew similar or greater treatment benefits22, 42 despite being most likely to experience hypotension following treatment initiation. Higher BP drops under HF treatment do not appear to hamper the benefit from drugs,22, 23, 42 suggesting that the (often short‐term) BP‐lowering effects of these drugs are largely compensated by other (long‐term) beneficial systemic effects.
Dose–response curves have been reported for most class I HFrEF drugs.4, 28, 37
Given the lack of specific management guidelines, our group proposes an algorithm for the management of hypotension in HFrEF patients based on a five‐step process. After each step, BP must be re‐assessed prior to making any additional changes. In addition, as a general rule, we favour maintaining a low dose of all HFrEF classes of drugs rather than using a high‐dose of a single drug.4, 48, 49
There is no current consensus on the algorithm for modifying HFrEF treatments in the presence of hypotension. The assumption is to focus on treatments shown to have the greatest benefit on morbidity and mortality. For example, it has been established that beta‐blockers and MRAs improve overall mortality by more than 30% when compared to placebo, ACEi by around 20% when compared to placebo, and sacubitril/valsartan by around 16% when compared to ACEi.7, 24, 29, 41 The background setting also plays a crucial role, with all patients being unique. Importantly, these drugs have demonstrated a prognostic effect in addition to previously established treatments; all drugs should be used whenever possible, even if lower doses are used.
From a pragmatic standpoint, the impact of hypotension must be assessed according to the vintage of treatment introduction: if hypotension occurs a few days after increasing the treatment dose, it is likely that treatment is the cause of hypotension. In contrast, when doses have been stable over a long period, other causes of hypotension should always be investigated (fever, diarrhoea, other new drugs, dehydration, etc.) rather than systematically and permanently decreasing treatment dose.
Proposed five‐step algorithm for the management of hypotension in heart failure with reduced ejection fraction patients
The prerequisite is to identify settings in which hypotension is related to cardiogenic shock and reflect a drop in stroke volume. In this case, although patients may have signs or symptoms of hypotension, they will also show significant congestion and/or signs of hypoperfusion (possibly accompanied by severe worsening renal function). As specified in Figure 1, in such circumstances, an urgent admission should be considered and if cardiogenic shock is confirmed, inotropes and/or mechanical support should be used. In the next steps, we will solely focus on stable HFrEF patients.
Figure 1.
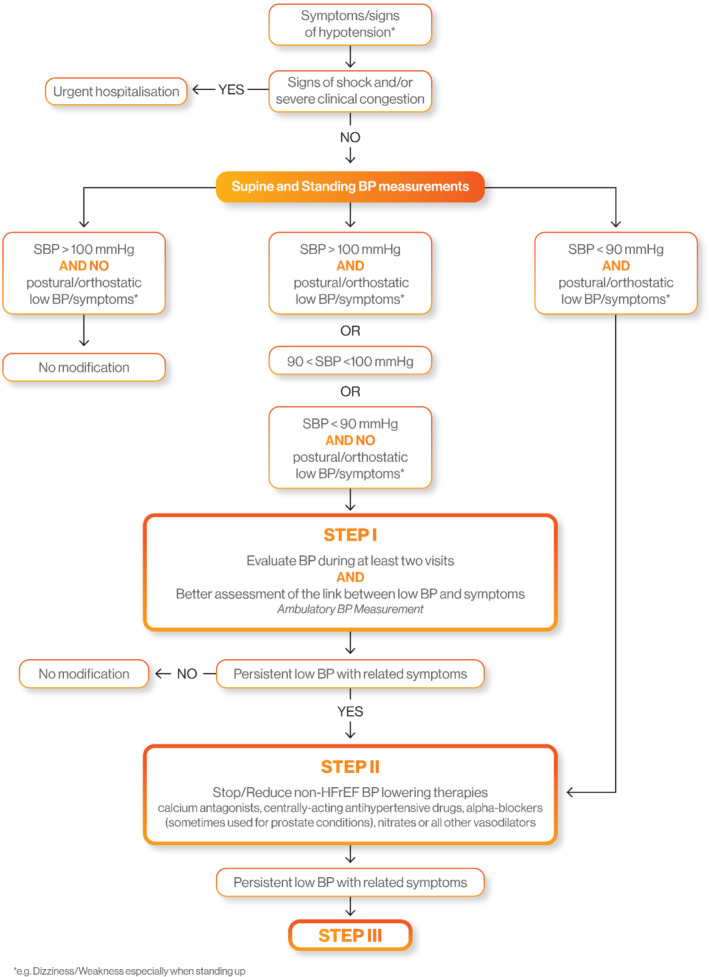
Decision tree for a heart failure with reduced ejection fraction patient with low blood pressure (Step I and Step II). BP, blood pressure; HFrEF, heart failure with reduced ejection fraction; SBP, systolic blood pressure.
Step I: Confirm low blood pressure and assess its link with symptoms
Obviously, haemodynamic emergencies should lead to hospitalization. Given the importance of HFrEF drugs for prognosis, the link between symptoms suggestive of hypotension (dizziness, fatigue, especially when standing up and in upright position) and low BP must be established. The measurement of supine BP should be supplemented by a standing BP evaluation. A decrease of 20 mmHg in SBP and/or 10 mmHg in DBP within the first 3 min after standing up suggests that symptoms are BP‐related. In the absence of orthostatic hypotension, ambulatory BP monitoring (ABPM) may be considered to detect hypotensive episodes related to symptoms (which would have been reported by the patient during ABPM recordings).
When SBP is ≥ 100 mmHg with no postural or orthostatic symptom of hypotension, HF medication should not be lowered/stopped. On the other hand, when SBP is < 90 mmHg with clear symptoms of postural or orthostatic hypotension, we recommend going to step II. Importantly, stockings should be systematically considered in patients with postural hypotension.
In other instances, e.g. when SBP levels do not appear to agree with (postural or orthostatic) hypotensive symptoms, particular care should be taken for an early re‐assessment in consultation and by using ABPM and standing BP evaluation as mentioned above. Following this additional evaluation, when symptoms are clearly related to measured hypotension, we recommend going to step II (Figure 1).
Step II: Identify hypotensive factors unrelated to heart failure with reduced ejection fraction and stop/reduce non‐heart failure with reduced ejection fraction blood pressure‐lowering therapies
Non‐drug related causes of hypotensive episodes should be investigated, such as diarrhoea, fever, dehydration, etc. In such cases, the cause of hypotension should be corrected, and chronic HF treatment should not be changed over the long term. However, transient discontinuation of HF therapies may be considered (especially for diuretics when dehydration is observed) until resolution of the acute event. Early re‐introduction should be attempted whenever possible.
In the absence of the aforementioned causes of hypotension, BP‐lowering treatments without evidence of morbidity‐mortality reduction in HFrEF patients should be decreased or stopped. Cardiovascular treatments not indicated in HFrEF, such as calcium channel blockers, centrally‐acting antihypertensive drugs or alpha‐blockers, should be reduced or discontinued, regardless of the form of administration. It is also critical to identify ‘hidden’ hypotensive drugs (such as alpha‐blockers in the context of prostate disease or intraocular beta‐blockers in case of glaucoma) and replace the latter with another drug class (Figure 2).
Figure 2.
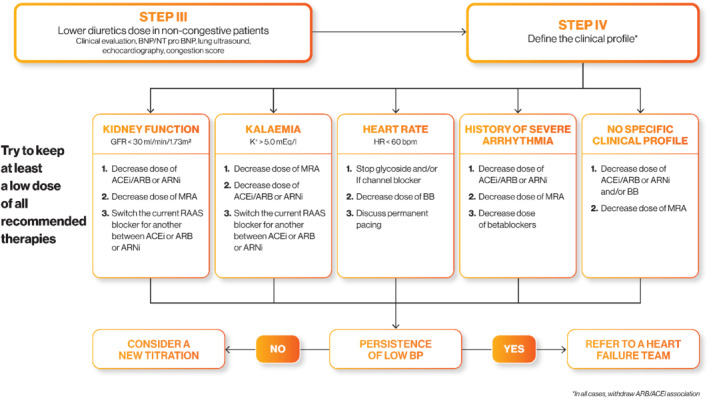
Decision tree for a heart failure with reduced ejection fraction patient with low blood pressure (Step III and Step IV). ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; BNP, B‐type natriuretic peptide; BP, blood pressure; GFR, glomerular filtration rate; HR, heart rate; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal B‐type natriuretic peptide; RAAS, renin–angiotensin–aldosterone system.
Step III: Adjust diuretic doses
If the above first measures are not sufficient, HFrEF treatment should then be adjusted.
The first step is to assess the total extracellular volume in order to determine whether it is possible to lower the dose of diuretics. Clinical, biological, or ultrasound (lung and/or cardiac) signs of congestion should be identified, or congestion scores used13 to attain this goal.
In the absence of congestive signs,13 diuretics should be carefully decreased and their replacement by an MRA (or MRA up‐titration) can even be considered in some cases. Only a few trials have targeted the decrease in diuretics.45 A controlled randomized trial has recently been reported and confirmed the feasibility of this approach in stable HFrEF patients.50, 51 Serial monitoring of stable natriuretic peptide concentrations may be useful during diuretic titration to provide reassurance that congestion is not worsening significantly.
Salt intake should also be assessed. Indeed, low salt intake could participate in low extracellular volume, such that, in some cases, salt intake could be increased cautiously. If significant congestive signs and severe hypotension are present, this should be considered as a state of acute cardiogenic pre‐shock or terminal heart disease. The patient should then be referred promptly to a specialized HF management team (HF Team).
In case of failure or inability to adjust the diuretics, and if hypotension or hypotensive symptoms persist, an adjustment of other HFrEF therapeutic classes should be discussed (Step IV).
Step IV: Adjust heart failure with reduced ejection fraction treatments according to clinical profile
Spreading the medication dose throughout the day (e.g. half dose in the morning and half dose in the evening) may be useful. Other measures such as increased physical activity, cardiovascular rehabilitation, etc., may also help countering the hypotension.
The clinical phenotype of the patient also plays an important role. We propose adjusting the patient's medication depending on the clinical profile as follows:
Reduced estimated glomerular filtration rate < 30 mL/min/1.73 m2 will preferably prompt a decrease in ACEi/ARB or ARNi rather than a decrease in beta‐blockers as the latter is less associated with worsening renal function. MRA discontinuation is considered only as a second step since it only has a limited impact on BP.
Hyperkalaemia (> 5.0 mmol/L) will preferably prompt a decrease in MRAs as they are the medication associated with the most substantial increase in serum potassium.
Heart rate < 60 bpm, glycoside and/or If channel inhibitor should be discontinued as a first step. As a second step, reduction in beta‐blocker dosage, or in the most severe cases, their discontinuation, should be considered.
In patients with a history of severe arrhythmia, beta‐blockers should be maintained at the highest possible dose; a decrease in ACEi/ARB or ARNi should be preferably considered.
In patients lacking a specific profile, ACEi/ARB, ARNi or beta‐blocker doses can be decreased.
Overall, although MRAs exert the least hypotensive effect in chronic HF patients, they nonetheless exhibit a favourable effect on mortality; hence, their reduction should be considered as a last resort. This is highly important given the confusion with their use as antihypertensive drugs. With regard to HF, MRAs can be used at low ‘hormonal’ doses with mild hypotensive effect.41
If several dose adjustments are necessary, it is still preferable to retain low doses of each therapeutic class to maintain a beneficial effect on prognosis by using all neuro‐hormonal blocking pathways.4, 48, 49 This is currently mainly justified by a pragmatic pharmacological approach as it appears wiser to target several pharmacological mechanisms using several lower‐dose drugs rather than obliterating only one pharmacological mechanism using one high‐dose drug. This has been shown to be true in the field of hypertension52 but is yet to be formally tested in HFrEF.
Irrespective of the therapeutic class, when the drug has been decreased or discontinued during the patient's hypotensive period, re‐introduction or dose escalation should always be considered, particularly when a triggering hypotensive factor has been identified and is deemed resolved. If BP does not improve, low BP is likely to be a sign of chronically impaired cardiac output, and it is preferable to refer the patient promptly to a HF Team to discuss other treatment options.
Finally, it is important to remember that even in the case of global clinical improvement or increase in LVEF or decrease in natriuretic peptide under treatment, all therapeutic classes must be maintained.
In addition to the clinical scenarios reported above, the presence of comorbidities should be considered when performing treatment modifications. A number of features can favour low BP such as complicated diabetes, anaemia, and neurological conditions. The accumulation of comorbidities and/or the presence of frailty or cognitive troubles should generally encourage a cautious use of high‐dose HF medications. In this specific clinical setting, the decrease in HF medication even in the absence of symptoms can be envisaged as the risk of adverse effects of low BP appears higher.
Conclusion
There is no strong consensus regarding the definition of severe hypotension as well as the pharmacological management to apply in this setting. In the absence of direct evidence, we propose a detailed algorithm to manage HFrEF drugs according to clinical setting. Dissemination of this algorithm will favour using higher doses of HFrEF life‐saving drugs. This treatment optimization could have a significant impact since patients with the lowest baseline BP incur the greatest benefit22 despite being the most likely to experience hypotension following treatment initiation.
Conflict of interest: J.C. reports personal fees from Novartis, Janssen, BMS, and Leo pharma. J.C.T. reports honoraria from Novartis. A.C.S. received grants and honoraria from Novartis, Servier, Astra Zeneca, MSD, Bayer, Vifor, Impulse, CVRX, Sanofi, and Menarini. J.J. reports grants or personal fees from Novartis, Roche Diagnostics, Abbott, and Janssen. F.R. reports grants, personal fees and non‐financial support from Novartis, Servier, Abbott, MSD, Sanofi, Bayer, BMS‐Pfizer, Air Liguide, Abiomed, Resmed, Medtronic. N.G. received honoraria from Novartis and Boehringer. The authors acknowledge technical support from Novartis for illustrations.
References
- 1. CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 3. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT‐HF). MERIT‐HF Study Group. JAMA 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Poole‐Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin‐converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 5. Cohn JN, Tognoni G; Valsartan Heart Failure Trial I. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 8. Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol 2015;66:848–660. [DOI] [PubMed] [Google Scholar]
- 9. Tartiere JM, Logeart D, Safar ME, Cohen‐Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens 2006;20:213–219. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, PE MB, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GY, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 13. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, Fillieux L, Abraham WT, Januzzi J Jr, Sebbag L, Zannad F, Mebazaa A, Rossignol P; INI‐CRCT, Great Network, and the EF‐HF Group . Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail 2018;6:273–285. [DOI] [PubMed] [Google Scholar]
- 14. Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol 2004;93:785–788. [DOI] [PubMed] [Google Scholar]
- 15. Jackson CE, Castagno D, Maggioni AP, Kober L, Squire IB, Swedberg K, Andersson B, Richards AM, Bayes‐Genis A, Tribouilloy C, Dobson J, Ariti CA, Poppe KK, Earle N, Whalley G, Pocock SJ, Doughty RN, McMurray JJ; Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC). Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta‐analysis. Eur Heart J 2015;36:1106–1114. [DOI] [PubMed] [Google Scholar]
- 16. Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subacius H, Konstam MA, Maggioni AP, Swedberg K, Gheorghiade M. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J 2013;165:216–225. [DOI] [PubMed] [Google Scholar]
- 17. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997;95:2660–2667. [DOI] [PubMed] [Google Scholar]
- 18. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 19. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN; Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 20. Komajda M, Schope J, Wagenpfeil S, Tavazzi L, Bohm M, Ponikowski P, Anker SD, Filippatos GS, Cowie MR; QUALIFY Investigators . Physicians' guideline adherence is associated with long‐term heart failure mortality in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2019;21:921–929.30933403 [Google Scholar]
- 21. Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L; QUALIFY Investigators . Physicians' adherence to guideline‐recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail 2016;18:514–522. [DOI] [PubMed] [Google Scholar]
- 22. Bohm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJ. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J 2017;38:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rouleau JL, Roecker EB, Tendera M, Mohacsi P, Krum H, Katus HA, Fowler MB, Coats AJ, Castaigne A, Scherhag A, Holcslaw TL, Packer M; Carvedilol Prospective Randomized Cumulative Survival Study Group . Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004;43:1423–1429. [DOI] [PubMed] [Google Scholar]
- 24. Kostis JB, Shelton B, Gosselin G, Goulet C, Hood WB Jr, Kohn RM, Kubo SH, Schron E, Weiss MB, Willis PW III, Young JB, Probstfield J. Adverse effects of enalapril in the Studies of Left Ventricular Dysfunction (SOLVD). SOLVD Investigators. Am Heart J 1996;131:350–355. [DOI] [PubMed] [Google Scholar]
- 25. Roffman DS. High‐versus low‐dose ACE inhibitor therapy in chronic heart failure. Ann Pharmacother 2004;38:831–838. [DOI] [PubMed] [Google Scholar]
- 26. Clement DL, De Buyzere M, Tomas M, Vanavermaete G. Long‐term effects of clinical outcome with low and high dose in the Captopril in Heart Insufficient Patients Study (CHIPS). Acta Cardiol 2000;55:1–7. [DOI] [PubMed] [Google Scholar]
- 27. Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, Benza RL, Gottlieb SO, Kleemann TD, Rosconi F, Vandervoort PM, Cohn JN; Val‐HeFT Heart Failure Trial Investigators . Valsartan benefits left ventricular structure and function in heart failure: Val‐HeFT echocardiographic study. J Am Coll Cardiol 2002;40:970–975. [DOI] [PubMed] [Google Scholar]
- 28. Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GAJ, Malbecq W, Smith RD, Guptha S, Poole‐Wilson PA. Effects of high‐dose versus low‐dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double‐blind trial. Lancet 2009;374:1840–1848. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013;15:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desai AS, Solomon S, Claggett B, McMurray JJ, Rouleau J, Swedberg K, Zile M, Lefkowitz M, Shi V, Packer M. Factors associated with noncompletion during the run‐in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM‐HF trial. Circ Heart Fail 2016;9:e002735. [DOI] [PubMed] [Google Scholar]
- 31. Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, Andreka P, Shehova‐Yankova N, Anand I, Yilmaz MB, Gogia H, Martinez‐Selles M, Fischer S, Zilahi Z, Cosmi F, Gelev V, Galve E, Gómez‐Doblas JJ, Nociar J, Radomska M, Sokolova B, Volterrani M, Sarkar A, Reimund B, Chen F, Charney A. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double‐blind, randomized comparison of two uptitration regimens. Eur J Heart Fail 2016;18:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vardeny O, Claggett B, Kachadourian J, Pearson SM, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray J, Solomon SD. Incidence, predictors, and outcomes associated with hypotensive episodes among heart failure patients receiving sacubitril/valsartan or enalapril: the PARADIGM‐HF trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail 2018;11:e004745. [DOI] [PubMed] [Google Scholar]
- 33. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJV, Solomon SD; Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) Investigators . Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Senni M, McMurray JJ, Wachter R, McIntyre HF, Anand IS, Duino V, Sarkar A, Shi V, Charney A. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018;20:491–500. [DOI] [PubMed] [Google Scholar]
- 35. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E; PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 36. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, Buraiki JA, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D; TRANSITION Investigators . Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019;21:998–1007. [DOI] [PubMed] [Google Scholar]
- 37. Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807–2816. [DOI] [PubMed] [Google Scholar]
- 38. Edelmann F, Musial‐Bright L, Gelbrich G, Trippel T, Radenovic S, Wachter R, Inkrot S, Loncar G, Tahirovic E, Celic V, Veskovic J, Zdravkovic M, Lainscak M, Apostolović S, Neskovic AN, Pieske B, Düngen HD; CIBIS‐ELD Investigators and Project Multicenter Trials in the Competence Network Heart Failure . Tolerability and feasibility of beta‐blocker titration in HFpEF versus HFrEF: insights from the CIBIS‐ELD trial. JACC Heart Fail 2016;4:140–149. [DOI] [PubMed] [Google Scholar]
- 39. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 40. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 41. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 42. Serenelli M, Jackson A, Dewan P, Jhund PS, Petrie MC, Rossignol P, Campo G, Pitt B, Zannad F, Ferreira JP, McMurray JJ. Mineralocorticoid receptor antagonists, blood pressure, and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2020;8:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swedberg K, Komajda M, Bohm M, Borer J, Robertson M, Tavazzi L, Ford I; SHIFT Investigators . Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta‐blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol 2012;59:1938–1945. [DOI] [PubMed] [Google Scholar]
- 44. Bagriy AE, Schukina EV, Samoilova OV, Pricolota OA, Malovichko SI, Pricolota AV, Bagriy EA. Addition of ivabradine to beta‐blocker improves exercise capacity in systolic heart failure patients in a prospective, open‐label study. Adv Ther 2015;32:108–119. [DOI] [PubMed] [Google Scholar]
- 45. Verbrugge FH, Martens P, Boonen L, Nijst P, Verhaert D, Noyens P, de Vusser P, Dupont M, Tang WH, Mullens W. Loop diuretic down‐titration in stable chronic heart failure is often achievable, especially when urinary chloride concentration is low. Acta Cardiol 2018;73:335–341. [DOI] [PubMed] [Google Scholar]
- 46. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction N Engl J Med 2019;381:1995–2008.31535829 [Google Scholar]
- 47. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz‐Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJ. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation 2020;141:100–111. [DOI] [PubMed] [Google Scholar]
- 48. Ajam T, Ajam S, Devaraj S, Fudim M, Kamalesh M. Effect on mortality of higher versus lower beta‐blocker (metoprolol succinate or carvedilol) dose in patients with heart failure. Am J Cardiol 2018;122:994–998. [DOI] [PubMed] [Google Scholar]
- 49. Ruwald AC, Gislason GH, Vinther M, Johansen JB, Nielsen JC, Philbert BT, Torp‐Pedersen C, Riahi S, Jøns C. Importance of beta‐blocker dose in prevention of ventricular tachyarrhythmias, heart failure hospitalizations, and death in primary prevention implantable cardioverter‐defibrillator recipients: a Danish nationwide cohort study. Europace 2018;20:f217–f224. [DOI] [PubMed] [Google Scholar]
- 50. da Rosa PR, Rohde LE, Doebber M, Ribeiro AL, Prado DP, Bertoldi EG, Figueiredo Neto JA, Kohler I, Beck‐da‐Silva L, Danzmann LC, Moura LZ, Rover M, Simões MV, Sant'Anna RT, Biolo A. Rational and design of a randomized, double‐blind, multicenter trial to evaluate the safety and tolerability of furosemide withdrawal in stable chronic outpatients with heart failure: the ReBIC‐1 trial. Am Heart J 2017;194:125–131. [DOI] [PubMed] [Google Scholar]
- 51. Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simões MV, Silvestre OM, Ribeiro ALP, Moura LZ, Beck‐da‐Silva L, Prado D, Sant'Anna RT, Bridi LH, Zimerman A, Raupp da Rosa P, Biolo A. Short‐term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double‐blind, multicentre, randomized trial. Eur Heart J 2019;40:3605–3612. [DOI] [PubMed] [Google Scholar]
- 52. Webster R, Salam A, de Silva HA, Selak V, Stepien S, Rajapakse S, Amarasekara S, Amarasena N, Billot L, van der Silva AP, Fernando M, Guggilla R, Jan S, Jayawardena J, Maulik PK, Mendis S, Mendis S, Munasinghe J, Naik N, Prabhakaran D, Ranasinghe G, Thom S, Tisserra N, Senaratne V, Wijekoon S, Wijeyasingam S, Rodgers A, Patel A; TRIUMPH Study Group . Fixed low‐dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA 2018;320:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]


