Abstract
We reported that inactivation of menin (the protein product of MEN1) increases activity of Dnmt1 and mediates DNA hypermethylation in the development of multiple endocrine neoplasia type 1 (MEN1) syndrome. We have developed a RCAS-TVA-based somatic gene transfer system that enables tissue-specific delivery of Dnmt1 to individual β-cells of the pancreas in a RIP-TVA mouse model. In the present study, we mediated Dnmt1 expression in islet β-cells in RIP-TVA mice by utilizing the RCAS-TVA system to test if the upregulation of Dnmt1 can promote β-cell proliferation. In vitro, we demonstrated that upregulation of Dnmt1 increased β-cell proliferation. In vivo, our results showed that the levels of serum insulin were increased in the RIP-TVA mice with RCASBP-Dnmt1 infection compared with wild-type control mice with RCASBP-Dnmt1 infection. Furthermore, we confirmed that mRNA and protein expression of Dnmt1 as well as Dnmt1 enzyme activity were upregulated in the RIP-TVA mice with RCASBP-Dnmt1 infection compared with wild-type control mice with RCASBP-Dnmt1 infection. Finally, we demonstrated that upregulation of Dnmt1 resulted in hyperplasia through β-cell proliferation. We conclude that the upregulation of Dnmt1 promotes islet β-cell proliferation and targeting Dnmt1 may be a promising therapy for patients suffering from pancreatic neuroendocrine tumors.
Introduction
MEN1 is a familial cancer syndrome characterized by tissue-specific tumorigenesis via largely unknown mechanisms. Patients with MEN1 mutations develop tissue-specific neoplasms in the parathyroid glands, anterior pituitary glands, and the endocrine pancreas [1–6]. Menin, encoded by MEN1, is a transcriptional modulator and a tumor suppressor. Menin is involved in the regulation of DNA repair, cell proliferation, and apoptosis [7–12].
Human cancer genomes are characterized by widespread aberrations in DNA methylation patterns [13–16]. Studies have shown that the loss of menin increases DNA hypermethylation at the promoter region of several genes [17, 18]. Using a novel genome-wide methylation analysis method, we recently demonstrated that inactivation of menin results in increased activity of DNA methyltransferase 1 (Dnmt1) by activating retinoblastoma-binding protein 5 (Rbbp5), a menin-associated protein [19]. Despite Men1 loss in all pancreas cell types, the increased Dnmt1 activity resulted in global DNA hypermethylation and tumorigenesis in endocrine tissues of the pancreas but not in exocrine tissues of the pancreas [19]. Therefore, the characterization of the role of Dnmt1 in endocrine tissues of the pancreas may provide an important insight into the possible role of epigenetic mechanisms in the pathogenesis of MEN1 tumor development.
Tumors develop through multiple stages, implicating the involvement of multiple effectors, but the tools to assess how candidate genes contribute to stepwise tumor progression have been limited due to a lack of a tissue-specific delivery system. We developed a Replication-Competent ASLV long terminal repeat (LTR) with a splice acceptor-tumor virus A (RCAS-TVA) expression system that enables tissue-specific delivery of genes to individual cells of the pancreas in adult mice [20, 21]. Using the tissue-specific rat insulin promoter (RIP) that drives the expression of the receptor for subgroup A avian leucosis virus (TVA), the RIP-TVA mice can be infected with avian viruses carrying genes of interest [20, 21]. In this study, we investigate the role of Dnmt1 in promoting β-cell proliferation by tissue-specific Dnmt1 upregulation in endocrine tissues of the pancreas using our established RCAS-TVA somatic gene transfer system.
Materials and methods
Cell culture
The N134 cell line was generated from an islet β-cell tumor in a RIP-Tag; RIP-TVA mouse [20]. DF1 is a chicken fibroblast cell line used to express RCASBP-Dnmt1 and RCASBP-Luciferase, respectively. The N134 cell line was infected with viruses carrying RCASBP-Dnmt1 following the established protocol [22]. The mouse DF1 and N134 cell lines were cultured in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin.
Animal experiments
Generation of RIP-TVA mice has been previously described [20]. All mice were housed in accordance with the institutional guideline. All procedures involving mice were approved by the institutional animal care and use committee of Weill Cornell Medicine. Viral propagation and titer determination were described previously [20, 22]. Two-day-old RIP-TVA mice (n = 4) and wild-type (WT, in C57BL/6 background, n = 3) control mice were injected with RCASBP-Dnmt1 at 1 × 108 IU/ml by intra-peritoneal (IP) injection. All mice were killed at 8 months post-infection.
Cloning of RCASBP-Dnmt1
RCASBP is a replication competent TVA with a splice acceptor and Bryan-RSV pol gene. RCASBP-Dnmt1 was generated by Biomatik (Biomatik, Wilmington, DE).
Viral propagation in DF1 cell line
Five-μg of viral RCASBP-Dnmt1 DNA and RCASBP-Luciferase (control) with DMEM (without serum, penicillin, or streptomycin) were diluted, respectively. The diluted RCASBP-Dnmt1 DNA and RCASBP-control were further transfected to DF1 cells as described [22], with the exception that lipofectamine 2000 (Life Technologies, Grand Island, NY) was used.
In vitro infection of TVA-expressing cells and In vivo infection of mice
Viral supernatant containing RCASBP-Dnmt1 or RCASBP-control was collected from confluent DF1 cells and the viral supernatant was passed through a 0.45 μm filter to obtain cell-free viruses as previously described [22]. The viral supernatants containing RCASBP-Dnmt1 or RCASBP-control were used to infect N134 cells, respectively. The infected N134 cells were harvested at 24 h and 48 h post-infection for the in vitro studies. A total of 0.5 million DF1 cells expressing RCASBP-Dnmt1 were injected into mice for the in vivo studies via IP injection.
Detection of viral integration in the RCASBP infected cells
Identification of viral integration was used to detect the presence of RCASBP in the infected cells. DNA was extracted from infected cells with RCASBP-Dnmt1 and RCASBP-control, respectively, using a DNeasy extraction kit (Qiagen, Valencia, CA). The viral integration was detected by PCR as described previously [22]. The primers for viral integration are listed in Supplementary Table 1.
Laser capture microdissection (LCM)
The pancreatic tissues of mice were harvested and embedded in OCT. The OCT-embedded tissue sections were stained with Arcturus Histogene Frozen Section Staining Kit (Applied Biosystems, Foster City, CA) and the islet tissues were further dissected using an Arcturus XT LCM system per manufacturer’s instructions.
Real-time RT-PCR analysis
Total RNA was extracted from infected cells using the RNeasy extraction kit (Qiagen, Valencia, CA) and from LCM islet tissues of RIP-TVA mice and WT control mice using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA). mRNA expression of the Dnmt1 gene was detected by quantitative RT-PCR. The primers for the Dnmt1 gene are listed in Supplementary Table 1.
Western analysis
Total protein was extracted from N134 cells infected with RCASBP-Dnmt1 or RCASBP-control using a RIPA lysis buffer (Pierce, Rockford, IL). The extracted proteins were quantified and equal amounts of protein were separated by SDS-PAGE for western blotting. The blots were incubated overnight at 4 °C with primary antibodies: mouse anti-GAPDH (1:1000 dilution) and mouse anti-Dnmt1 (1: 2000 dilution) (Abcam, Cambridge, MA), respectively. The secondary antibody used was: 1:5000 dilution of anti-mouse IgG-HRP (Thermo Fisher Scientific, Rockford, IL). The small differences in loading were corrected by comparison with the loading control, GAPDH.
MTS cell proliferation analysis
To detect β-cell proliferation with a MTS cell proliferation assay kit, 1 × 103 cells (N134) were seeded in a 96-well plate and the cells were infected with virus containing RCASBP-Dnmt1 and RCASBP-control, respectively. After 24 h and 48 h of incubation, cell proliferation was analyzed with a MTS cell proliferation assay kit (Abcam, Cambridge, MA) according to the manufacturer’s instruction.
Dnmt1 enzymatic activity assay
Dnmt1 enzyme activity was detected on nuclear complexes extracted from infected cells and mouse pancreatic tissue samples using a Dnmt1 enzyme assay kit (EpiGentek, Farmingdale, NY) per manufacturer’s instructions. The enzymatic activity of Dnmt1 was detected with a microplate reader at 450 nm [19].
Enzyme-linked immunosorbent assay (ELISA) analysis
Mouse blood was collected via a retro-orbital bleeding technique following an 18 h fast. Serum levels of insulin were measured by ELISA with the Ultrasensitive Mouse Insulin ELISA Kit (Mercodia, Winston-Salem, NC) as described previously [23].
Hematoxylin and Eosin (H&E) staining
Whole pancreata were frozen and sectioned (5-μm) on a rotating microtome (Microm, Walldorf, Germany). H&E staining was performed according to standard procedures. For morphometric analysis, three 100-μm sections were digitalized. Sections with maximal tumor diameter in the pancreatic cross-sectional area were determined by computerized pixel counting.
Immunofluorescence (IF) staining
IF staining was used to evaluate the expression levels of Dnmt1 and Ki67 in pancreatic tissues from treated mice. The sections were incubated overnight at 4 °C with primary antibodies: pig anti-insulin antibody (1:500 dilution; DAKO, CA, USA), mouse anti-Dnmt1 (1:200 dilution), and mouse anti-ki67 (1:1000 dilution) (Abcam, Cambridge, MA), respectively. After washing, the slides were incubated with goat anti-mouse Alexa Fluor 488 (Life Technologies, Grand Island, NY) and goat anti-guinea pig Alexa Fluor 647 (Life Technologies, Grand Island, NY) (both 1:200 dilutions) for 45 min in the dark. The slides were assembled in Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were obtained with an epifluorescence microscope with an attached camera.
Statistical analysis
The two-tailed student’s t-test was performed to assess the significance between experimental groups. GraphPad software (GraphPad Software, Inc, version 5.02) was used for all statistical analyses. Statistically significant P values are indicated in the figures.
Results
RCAS-TVA system enables delivery of the target gene to cells
In order to assess viral integration in the N134 TVA-expressing cells after RCASBP-Dnmt1 and RCASBP-control infection, DNA was extracted from infected N134 cells and PCR was performed. Virus integration was identified in N134 cells with both RCASBP-Dnmt1 and RCASBP-control infection at 24 h and 48 h, respectively (Fig. 1). These data indicate that the RCASBP-TVA system enables delivery of Dnmt1 to cells.
Fig. 1.
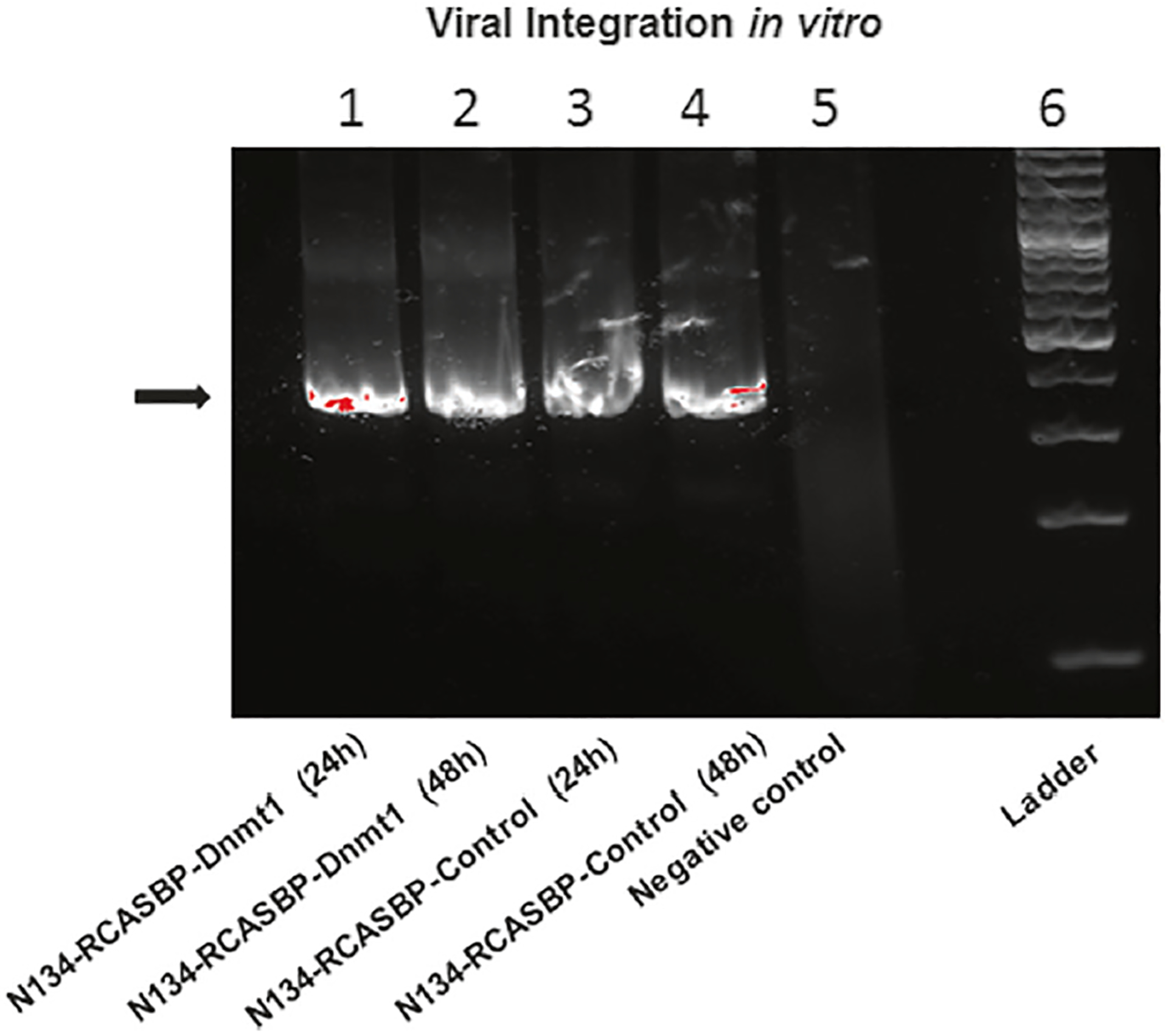
Detection of viral integration in RCASBP infected cells. Virus production was measured by PCR and the PCR products were run in 1% agarose. Viral integration was present in the infected N134 cells with both RCASBP-Dnmt1 (lane 1 and 2) and RCASBP-control (lane 3 and 4) at 24 h and 48 h, respectively
Islet β-cells with RCASBP-Dnmt1 infection show increased expression levels and enzyme activity of Dnmt1
To test if RCASBP-Dnmt1 infection can induce Dnmt1 expression in the N134 β-cell tumor cell line, we measured the expression levels and enzyme activity of Dnmt1 in N134 β-cell tumor cells. We confirmed that the mRNA and protein expression levels were significantly increased in the N134 cells with RCASBP-Dnmt1 infection when compared with the N134 cells with RCASBP-control infection, as measured by real-time PCR and western blot analysis (Fig. 2a, b). Furthermore, we found that Dnmt1 enzymatic activity was also significantly increased in the N134 cells with RCASBP-Dnmt1 infection when compared with the N134 cells with RCASBP-Luciferase control infection by a functional Dnmt1 enzymatic activity assay (Fig. 2c). These results indicate that the infection of N134 TVA-expressing cells with RCASBP-Dnmt1 can successfully deliver Dnmt1 to individual cells, upregulate Dnmt1 mRNA and protein expression and enhance Dnmt1 enzyme activity in vitro.
Fig. 2.
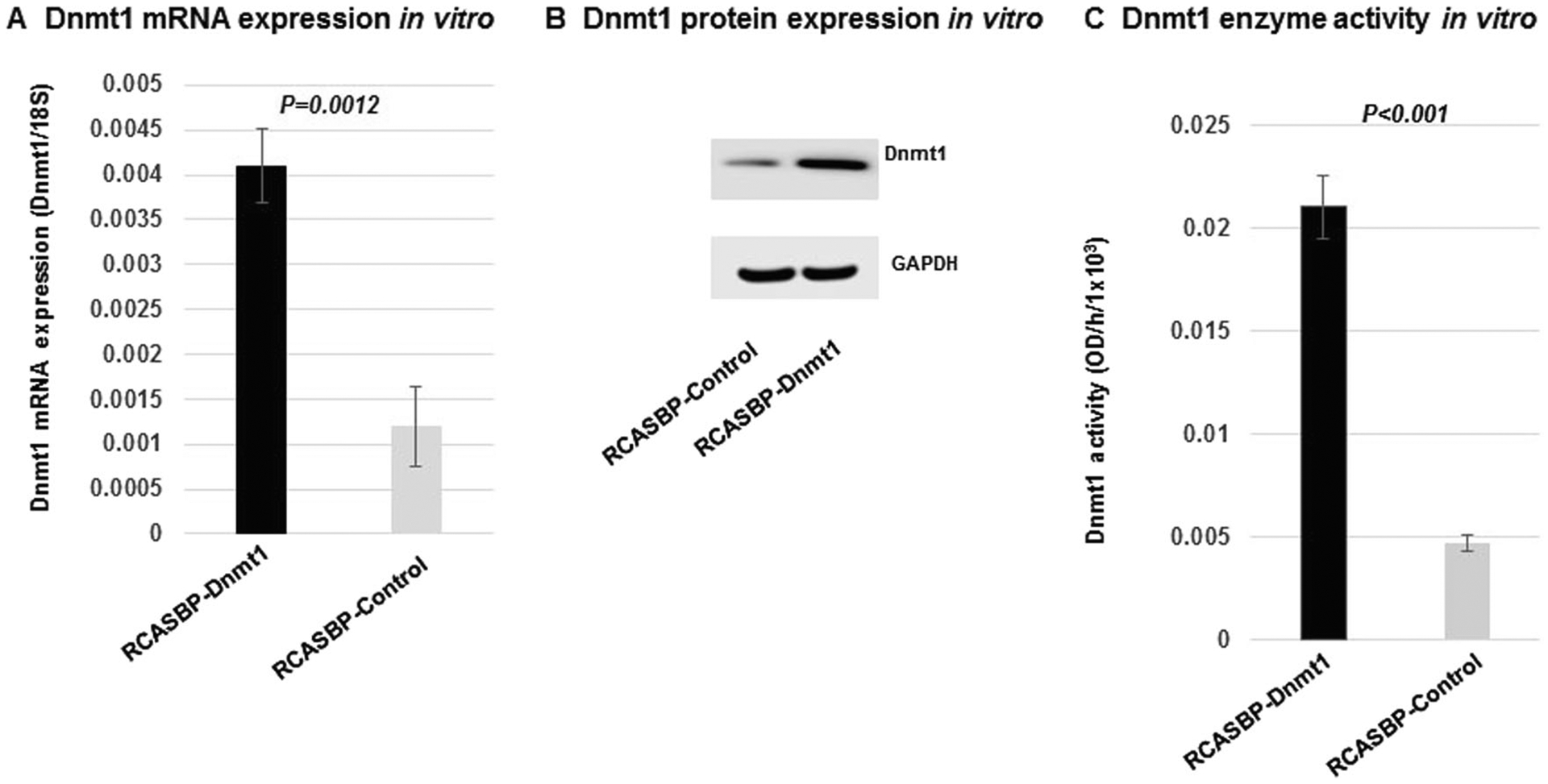
Upregulation of the expression and enzyme activity of Dnmt1 in the N134 β-cell tumor cell line with RCASBP-Dnmt1 infection. There was a significant increase in the expression levels of Dnmt1 RNA (a) (P = 0.0012) and protein (b) as well as Dnmt1 enzyme activity (c) (P < 0.001) in the RCASBP-Dnmt1 infected N134 cells when compared with those infected with RCASBP-control
Upregulation of Dnmt1 in N134 β-cell tumor cell line with RCASBP-Dnmt1 infection increased β-cell proliferation
Our previous study demonstrated that MEN1 tumors are associated with global DNA methylation and Dnmt1 overexpression [19]. Using a MTS cell proliferation assay, we demonstrated that upregulation of Dnmt1 by RCASBP-Dnmt1 infection promotes cell proliferation of islet cells as compared with RCASBP-control infected cells after 24 h (P = 0.001) and 48 h (P < 0.001) post-infection (Fig. 3). These results indicate that overexpression of Dnmt1 in β-cell can promote β-cells proliferation in vitro.
Fig. 3.
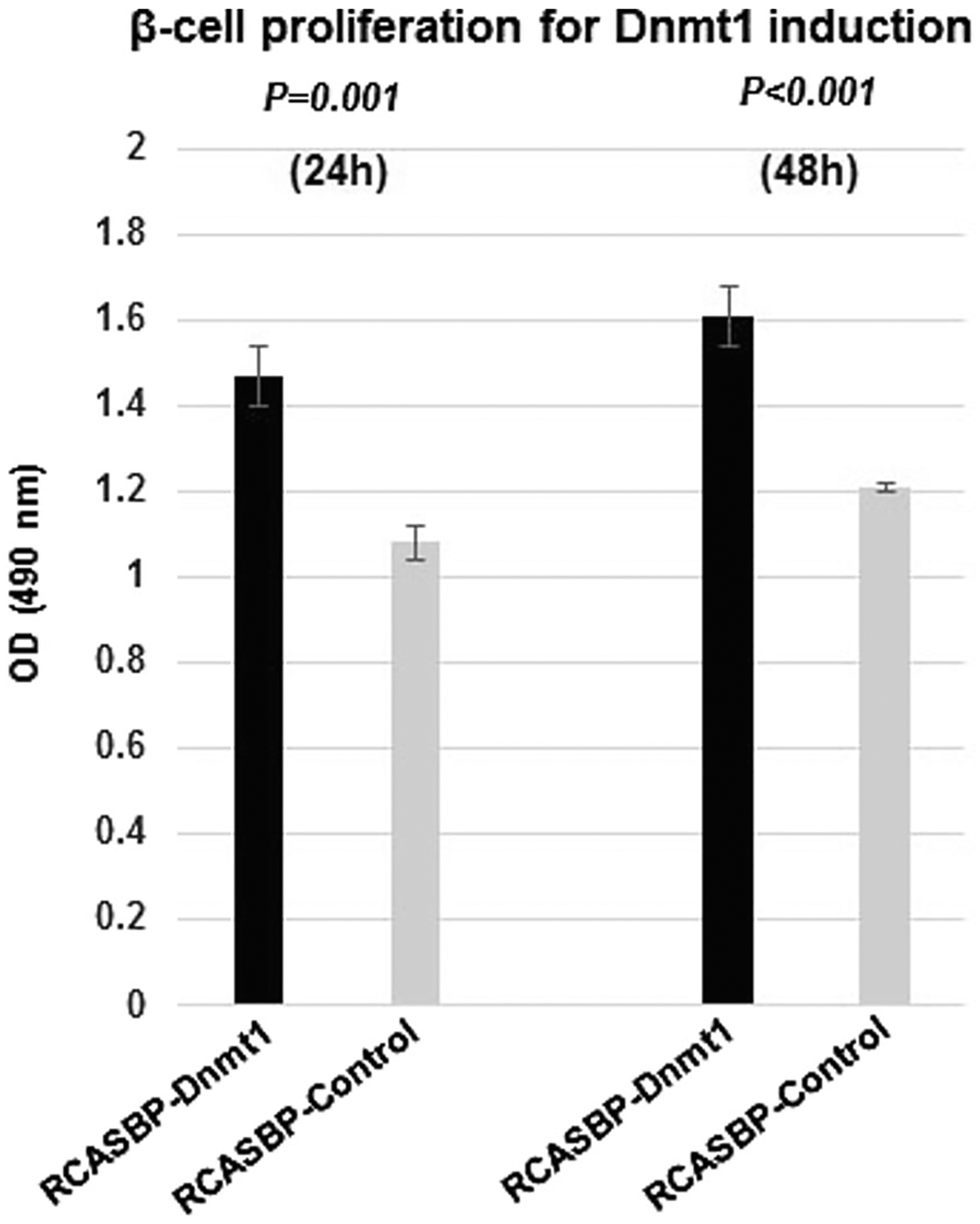
Increased β-cell proliferation after infection with RCASBP-Dnmt1 by a MTS assay. In the N134 β-cell tumor cell line, cell proliferation was measured after infection with RCASBP-Dnmt1 and RCASBP-control for 24 h and 48 h. The MTS assays showed that the cell proliferation was significantly increased in the N134 cells with RCASBP-Dnmt1 infection at 24 h (P = 0.001) and 48 h (P < 0.001) as compared with the N134 cells with RCASBP-control infection
Tissue-specific somatic Dnmt1 delivery in islet β-cells increased serum insulin levels in RIP-TVA mice
To determine the effect of Dnmt1 upregulation in islet β-cells in vivo, we injected DF1 cells expressing RCASBP-Dnmt1 into newborn RIP-TVA mice or WT control litter-mates (p1 or p2). Eight months later, we used serum insulin as a measurable surrogate for β-cell proliferation. The infected RIP-TVA mice (N = 4) had significantly higher serum insulin levels when compared with the infected WT mice (N = 3), as measured by an insulin ELISA assay (P = 0.0205) (Fig. 4).
Fig. 4.
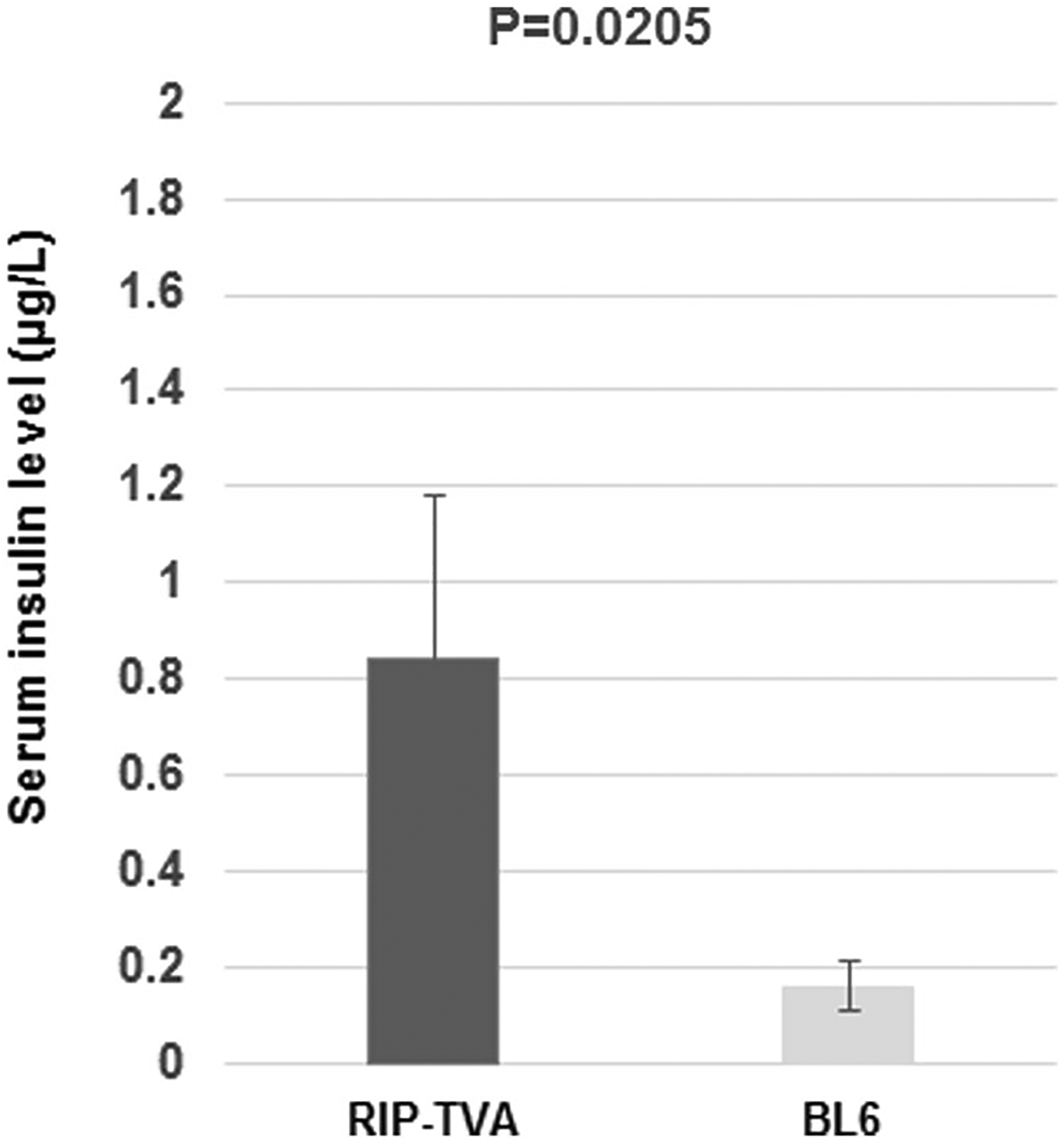
Serum insulin levels were increased in RIP-TVA mice with RCASBP-Dnmt1 infection. Mouse blood was collected following an 18 h fast and serum levels of insulin were measured by ELISA. There was a significant increase in serum insulin in the RIP-TVA mice (N = 4) compared to WT mice (N = 3) infected with RCASBP-Dnmt1 (P = 0.0205)
Tissue-specific somatic Dnmt1 delivery resulted in hyperplasia through β-cell proliferation in RIP-TVA mice
Using a RIP-TVA transgene to express the TVA receptor in β-cells of the pancreas, the candidate gene has been demonstrated to induce the somatic development of neo-plastic β-cell lesions in RIP-TVA transgenic mice by infection with the RCASBP vector [20, 21]. To identify the effects of tissue-specific upregulation of Dnmt1 in β-cells of the pancreas, we compared the islet size of the RIP-TVA mice to that of the WT control mice when treated with RCASBP-Dnmt1. Hyperplasia was observed in the islets of the pancreas and the islet size was significantly increased in the RIP-TVA mice compared with the WT control mice (2202 μm2 ± 708.6 vs. 441 μm2 ± 44.6; P = 0.0079) (Fig. 5). Furthermore, we demonstrated that β-cell proliferation was significantly increased in RIP-TVA mice infected with RCASBP-Dnmt1 when compared with the control mice, as measured by Ki67 staining (P = 0.0071) (Fig. 6). The results indicate that Dnmt1 overexpression in β-cells of the pancreas results in hyperplasia through β-cell proliferation.
Fig. 5.
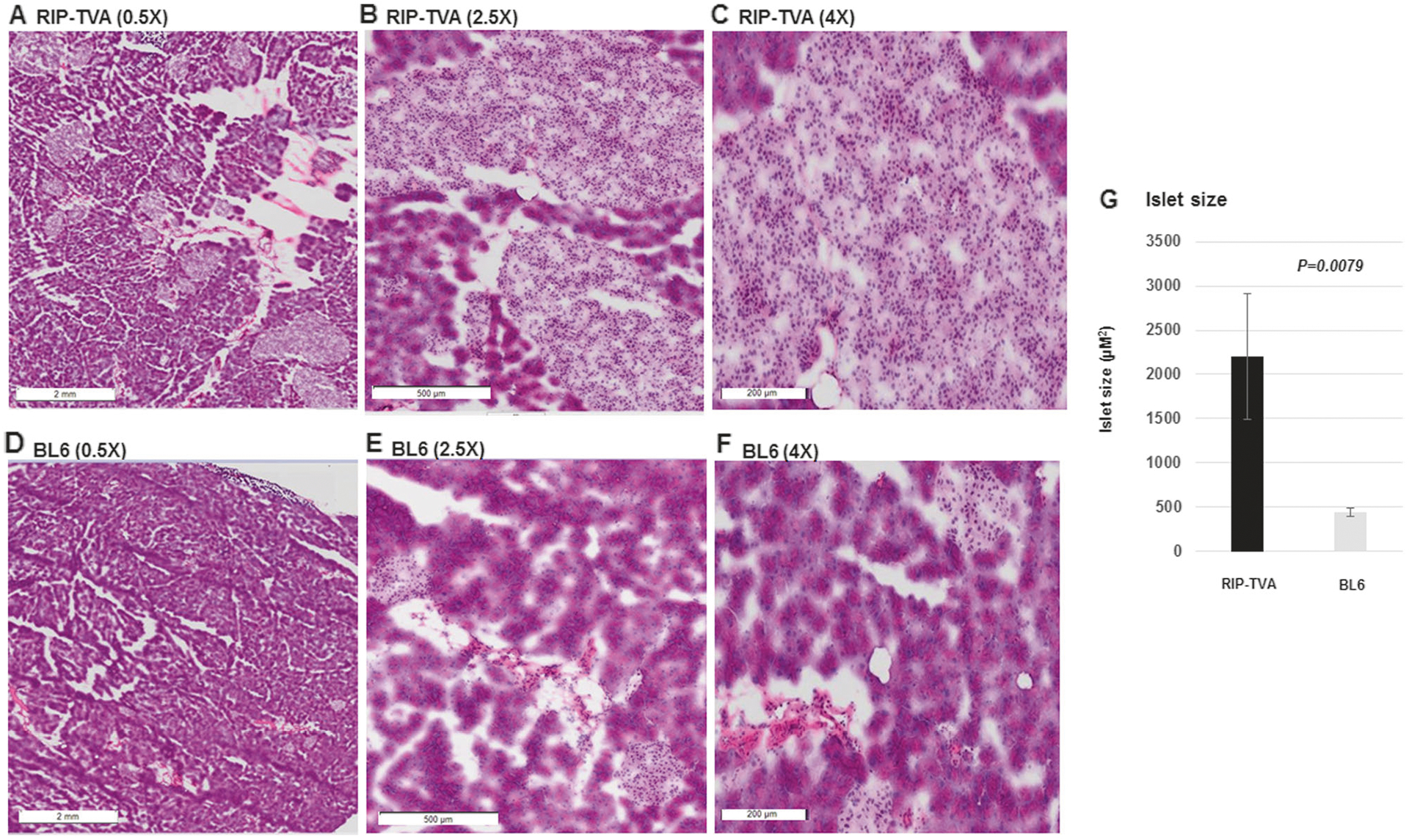
The islet size was significantly increased in the RIP-TVA mice with RCASBP-Dnmt1 injection. H&E staining showed that the RIP-TVA mice infected with RCASBP-Dnmt1 had a significant increase in islet size at magnification 0.5 × (a), magnification 2.5 × (b), and magnification 4.0 × (c) compared with the WT mice infected with RCASBP-Dnmt1 (d, e, and f). Quantification was achieved by measuring islet size (g) (P = 0.0079)
Fig. 6.
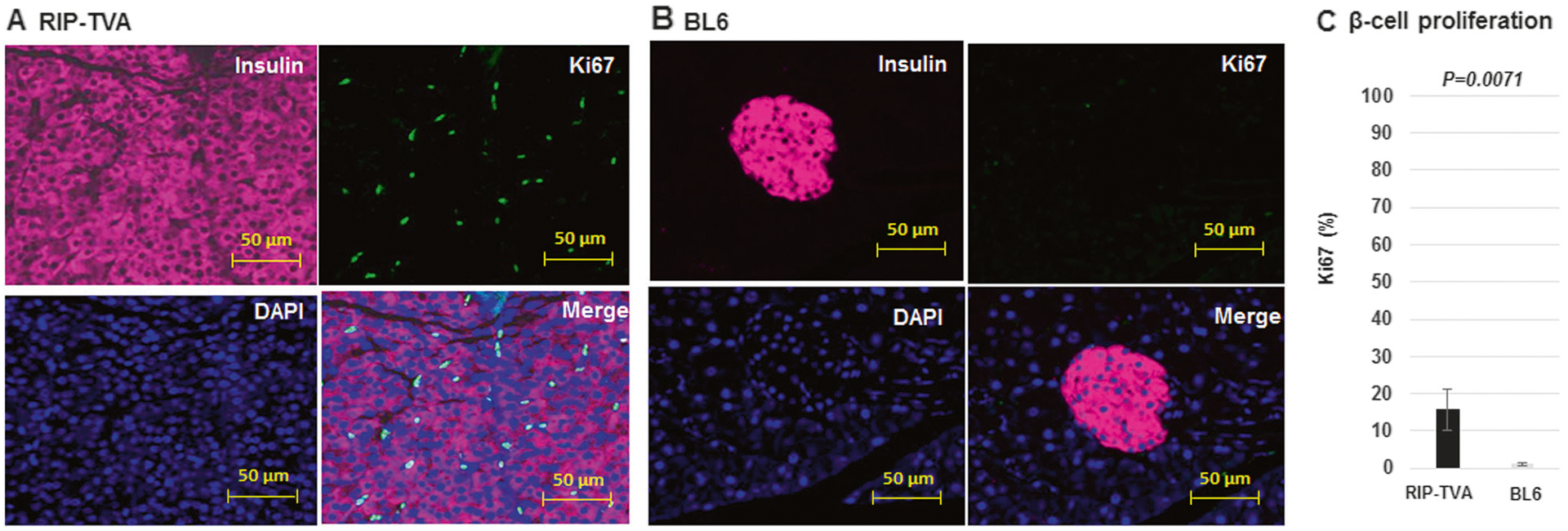
The islet β-cell proliferation is significantly increased in the RIP-TVA mice with RCASBP-Dnmt1. The islet β-cell proliferation was significantly increased by Ki67 analysis in the RIP-TVA mice with RCASBP-Dnmt1 injection (a) compared with the WT mice with RCASBP-Dnmt1 injection (b). Immunostaining of insulin (pink), Ki67 (green), and nuclei counterstained with DAPI (blue). Quantification was achieved by counting the fluorescent proliferating cells (c) (P = 0.0071) (magnification: 20×)
Tissue-specific somatic Dnmt1 delivery in islet β-cells increased the expression level and enzyme activity of Dnmt1 in RIP-TVA mice
We previously demonstrated the upregulation of Dnmt1 in insulinomas of Men1 conditional knockout mice [19]. In this present study, we examined the expression of Dnmt1 in pancreatic islet tissues using LCM real-time PCR and IF staining. We demonstrated a significant upregulation of Dnmt1 in the islets of RIP-TVA mice infected with RCASBP-Dnmt1 compared with infected WT control mice (P = 0.0115) (Fig. 7a, b). Furthermore, we found that Dnmt1 enzymatic activity was significantly increased in the islets of RIP-TVA mice infected with RCASBP-Dnmt1 compared with WT mice infected with RCASBP-Dnmt1 (P = 0.0361) (Fig. 7c). These results indicate that the RCAS-TVA expression system enables tissue-specific delivery of the Dnmt1 gene to individual dividing cells of the pancreas in adult mice.
Fig. 7.
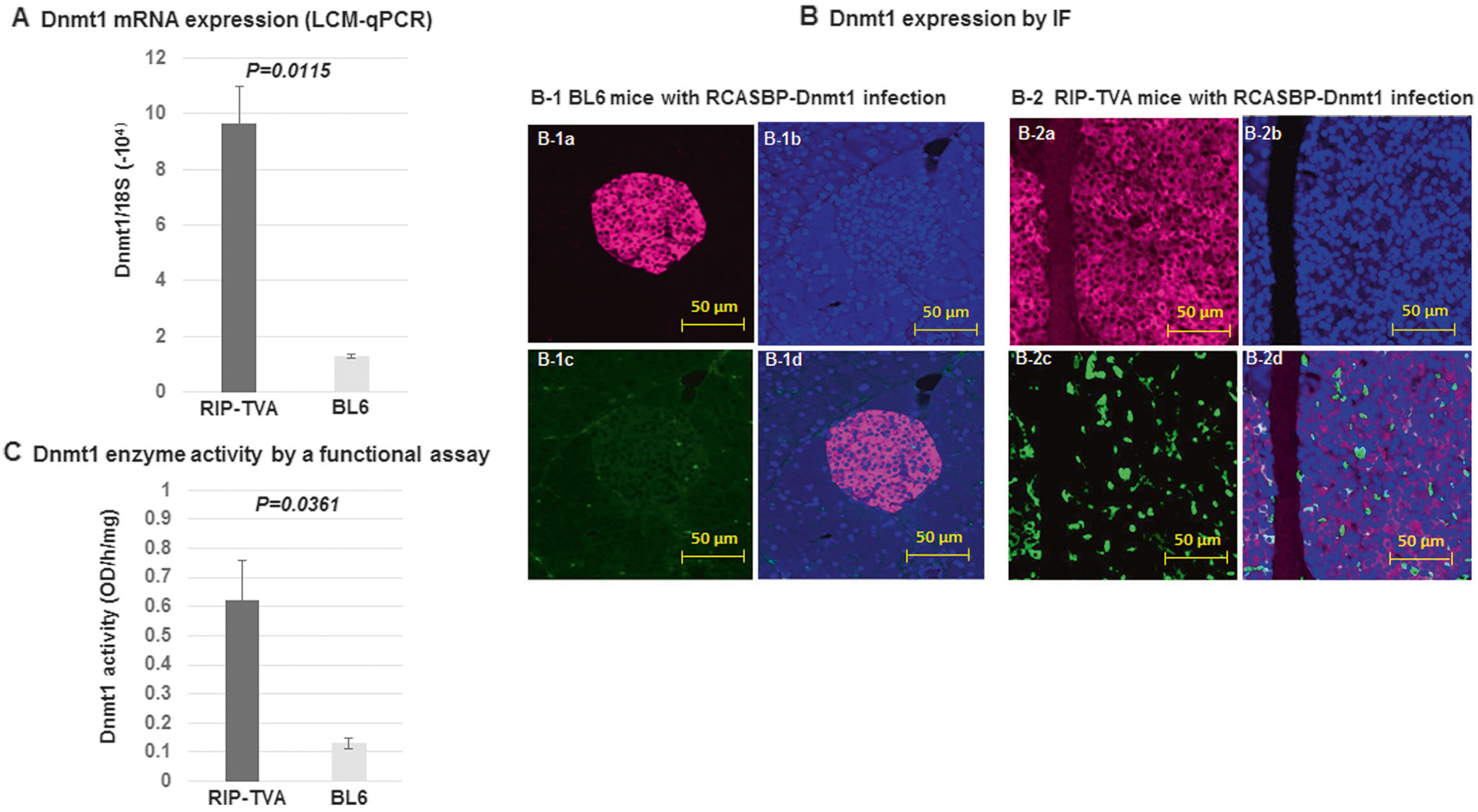
Increased expression and enzyme activity of Dnmt1 in the endocrine tissue of the pancreas from RIP-TVA mice with RCASBP-Dnmt1 infection. There was a significant increase of Dnmt1 mRNA (P = 0.0115) (a), Dnmt1 protein (b), and Dnmt1 enzyme activity (P = 0.0361) (c) in the endocrine tissue of the pancreas from RIP-TVA mice infected with RCASBP-Dnmt1 compared with the WT control mice infected with RCASBP-Dnmt1, as measured by LCM-PCR, IF staining, and Dnmt1 enzyme activity functional assay. Immunostaining of insulin (pink), Dnmt1 (green), and nuclei counterstained with DAPI (blue) (magnification: 20×)
Discussion
DNA methylation is the most common epigenetic modification in the mammalian genome, and aberrant methylation patterns can be found in human tumors [24–28]. DNA methylation is catalyzed by a family of DNA methyltransferases. Of these, Dnmt1 is a maintenance methylase, making it a key player in the important process of DNA methylation [29–32]. Overexpression of Dnmt1 has been detected in many types of human cancers, including neuroendocrine tumors [33–37]. Recently, we demonstrated that inactivation of menin increases activity of Dnmt1 and mediates global DNA hypermethylation in endocrine tissues of the pancreas and parathyroid glands resulting in MEN1 tumorigenesis [19]. Therefore, further characterization of the role of Dnmt1 upregulation in endocrine tissues of the pancreas appeared an attractive area of investigation. In this study, we tested the role of Dnmt1 in a mouse islet β-cell tumor cell line and islet β-cells of the pancreas in RIP-TVA mice by utilizing the RCAS-TVA somatic gene transfer system. The RCAS-TVA somatic gene transfer system enables tissue-specific delivery of genes to TVA-expressing cells of the pancreas in RIP-TVA mice [20, 21]. In the present study, we generated a RCASBP-Dnmt1 expression vector that expressed Dnmt1 and increased Dnmt1 enzyme activity in the N134 β-cell tumor cell line derived from a RIP-Tag; RIP-TVA mouse and in the islet tissues in RIP-TVA mice by RCASBP-Dnmt1 virus infection. In this well-characterized model, we demonstrated that Dnmt1 can be somatically delivered in a tissue-specific manner under the control of TVA-expressing β-cells of the pancreas. Our results demonstrated that this approach allows for the introduction of somatic genetic changes in a tissue-specific way by generating a vector carrying the gene-of-interest, which is advantageous to the extensive process of generating transgenic mouse models.
We previously showed that increased Dnmt1 activity resulted in global DNA methylation and tumorigenesis in endocrine tissue but not in exocrine tissue of the pancreas. In this current study, we performed phenotypic analysis and demonstrated that the upregulation of Dnmt1 in islet β-cells of the pancreas with RCASBP-Dnmt1 virus infection can increase serum insulin levels in the RIP-TVA mice. The islet hyperplasia was confirmed in RIP-TVA mice and β-cell proliferation was also increased in both in vitro and in vivo systems in RIP-TVA mice after RCASBP-Dnmt1 virus infection. Although the low efficiency of in vivo infection has been a limitation of using the RCAS-TVA system, with reports of islet β-cells gradually developing into tumors [20, 21], our present study demonstrated that islet hyperplasia in RIP-TVA mice was observed at 8 months post-infection with RCASBP-Dnmt1. These results support our observation that Dnmt1 may be an important driver in the initiation of pancreatic neuroendocrine tumors [19] and suggest that further testing of Dnmt1 blockade may be a strategy for treating MEN1 related pancreatic neuroendocrine tumors.
Previously, we demonstrated that the increased activity of DNMT1 mediates global DNA hypermethylation, which results in aberrant activation of the Wnt/β-catenin signaling pathway through inactivation of Sox regulatory genes in the development of MEN1 [19]. Several studies have linked decreased expression of the Sox family genes with tumorigenesis [38–43]. Therefore, a further investigation into the dysregulated signaling pathways relevant to MEN1 tumorigenesis and resulting from methylation changes by Dnmt1 activity is necessary.
In conclusion, we have assessed the effect of Dnmt1 in pancreatic neuroendocrine tumors using a RCAS-TVA somatic tissue-specific gene transfer mouse model without the need to generate a conditional transgenic mouse. Furthermore, we demonstrated that increasing Dnmt1 activity can promote β-cell proliferation. Our findings suggest that Dnmt1 may be an important driver in the promotion of pancreatic neuroendocrine tumors and therefore could be a potential therapeutic target for their treatment.
Supplementary Material
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41417-018-0046-x) contains supplementary material, which is available to authorized users.
References
- 1.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. [DOI] [PubMed] [Google Scholar]
- 2.Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–94. [DOI] [PubMed] [Google Scholar]
- 3.Marx SJ, Agarwal SK, Kester MB, Heppner C, Kim YS, Emmert-Buck MR, et al. Germline and somatic mutation of the gene for multiple endocrine neoplasia type 1 (MEN1). J Intern Med. 1998;243:447–53. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Lee Burns A, Sukhodolets KE, Kennedy PA, Obungu VH, Hickman AB, et al. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–98. [DOI] [PubMed] [Google Scholar]
- 5.Mutch MG, Dilley WG, Sanjurjo F, DeBenedetti MK, Doherty GM, Wells SA Jr, et al. Germline mutations in the multiple endocrine neoplasia type 1 gene: evidence for frequent splicing defects. Hum Mutat. 1999;13:175–85. [DOI] [PubMed] [Google Scholar]
- 6.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. [DOI] [PubMed] [Google Scholar]
- 7.Francis J, Lin W, Rozenblatt-Rosen O, Meyerson M. The menin tumor suppressor protein is phosphorylated in response to DNA damage. PLoS ONE. 2011;6:e16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busygina V, Kottemann MC, Scott KL, Plon SE, Bale AE. Multiple endocrine neoplasia type 1 interacts with fork head transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–403. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci Usa. 2003;100:10770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La P, Yang Y, Karnik SK, Silva AC, Schnepp RW, Kim SK, et al. Menin-mediated caspase 8 expression in suppressing multiple endocrine neoplasia type 1. J Biol Chem. 2007;282:31332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussein N, Casse H, Fontanière S, Morera AM, Asensio MJ, Bakeli S, et al. Reconstituted expression of menin in Men1-deficient mouse Leydig tumor cells induces cell cycle arrest and apoptosis. Eur J Cancer. 2007;43:402–14. [DOI] [PubMed] [Google Scholar]
- 12.Farnham PJ. Insights from genomic profiling of transcription factors. Nat Rev Genet. 2009;10:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Absher DM, Gulzar ZG, Young SR, McKenney JK, Peehl DM, et al. DNA methylation profiling reveals novel bio-markers and important roles for DNA methyltransferases in prostate cancer. Genome Res. 2011;21:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin B, Ernst J, Tiedemann RL, Xu H, Sureshchandra S, Kellis M, et al. Linking DNA methyltransferases to epigenetic marks and nucleosome structure genome-wide in human tumor cells. Cell Rep. 2012;2:1411–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–402. [DOI] [PubMed] [Google Scholar]
- 16.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg D, Akerström G, Westin G. Evaluation of CDKN2C/p18, CDKN1B/p27 and CDKN2B/p15 mRNA expression, and CpG methylation status in sporadic and MEN1-associated pancreatic endocrine tumours. Clin Endocrinol (Oxf). 2008;68:271–7. [DOI] [PubMed] [Google Scholar]
- 18.Juhlin CC, Kiss NB, Villablanca A, Haglund F, Nordenström J, Höög A, et al. Frequent promoter hypermethylation of the APC and RASSF1A tumour suppressors in parathyroid tumours. PLoS ONE. 2010;5:e9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Z, Claros CS, Suzuki M, Maggi EC, Kaner KD, Kinstlinger N, et al. Loss of MEN1 activates DNMT1 implicating DNA hypermethylation as a driver of MEN1 tumorigenesis. Oncotarget. 2016;7:12633–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du YC, Lewis BC, Hanahan D, Varmus H. Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion. PLoS Biol. 2007;5: e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du YC, Klimstra DS, Varmus H. Activation of PyMT in beta cells induces irreversible hyperplasia, but oncogene-dependent acinar cell carcinomas when activated in pancreatic progenitors. PLoS ONE. 2009;4:e6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Chi Y, Du YN. Identification and characterization of metastatic factors by gene transfer into the Novel RIP-Tag; RIP-tva Murine Model. J. Vis. Exp 2017;Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009;69:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–70. [DOI] [PubMed] [Google Scholar]
- 25.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird A DNA methylation patterns and epigenetic memory. Genes & Dev. 2002;16:6–21. [DOI] [PubMed] [Google Scholar]
- 27.Koh KP, Rao A. DNA methylation and methylcytosine oxidation in cell fate decisions. Curr Opin Cell Biol. 2013;25:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Li H, Sun M, Yuan Y, Sun LP. DNMT1 overexpression predicting gastric carcinogenesis, subsequent progression and prognosis: a meta and bioinformatic analysis. Oncotarget. 2017;8:96396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokul G, Khosla S. DNA methylation and cancer. Subcell Bio-chem. 2013;61:597–625. [DOI] [PubMed] [Google Scholar]
- 31.Ngollo M, Dagdemir A, Karsli-Ceppioglu S, Judes G, Pajon A, Penault-Llorca F, et al. Epigenetic modifications in prostate cancer. Epigenomics. 2014;6:415–26. [DOI] [PubMed] [Google Scholar]
- 32.Weisenberger DJ. Characterizing DNA methylation alterations from the cancer genome atlas. J Clin Invest. 2014;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etoh T, Kanai Y, Ushijima S, Nakagawa T, Nakanishi Y, Sasako M, et al. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol. 2004;164:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng DF, Kanai Y, Sawada M, Ushijima S, Hiraoka N, Kitazawa S, et al. DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis. 2006;27:1160–8. [DOI] [PubMed] [Google Scholar]
- 35.Qadir XV, Han C, Lu D, Zhang J, Wu T. miR-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am J Pathol. 2014;184:2355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Zhang LE, Guo S, Yuan T, Xia B, Zhang L, et al. Overexpression of DNA methyltransferase 1 as a negative independent prognostic factor in primary gastrointestinal diffuse large B-cell lymphoma treated with CHOP-like regimen and rituximab. Oncol Lett. 2015;9:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman MM, Qian ZR, Wang EL, Yoshimoto K, Nakasono M, Sultana R, et al. DNA methyltransferases 1, 3a, and 3b overexpression and clinical significance in gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2010;41:1069–78. [DOI] [PubMed] [Google Scholar]
- 38.Li B, Ge Z, Song S, Zhang S, Yan H, Huang B, Zhang Y. Decreased expression of SOX7 is correlated with poor prognosis in lung adenocarcinoma patients. Pathol Oncol Res. 2012;18:1039–45. [DOI] [PubMed] [Google Scholar]
- 39.Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Du L, Wen Z, Yang Y, Li J, Dong Z, et al. Sex determining region Y-box 2 inhibits the proliferation of colorectal adenocarcinoma cells through the mTOR signaling pathway. Int J Mol Med. 2013;32:59–66. [DOI] [PubMed] [Google Scholar]
- 41.Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet 2012. October;44:1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Han C, ZHENG L, GUO M. Epigenetic regulation of Wnt signaling pathway gene SRY-related HMG-box 17 in papillary thyroid carcinoma. Chin Med J (Engl). 2012;125:3526–31. [PubMed] [Google Scholar]
- 43.Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–56. 10.18632/oncotarget.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


