Abstract
To cope with the reduced availability of O2 at high altitude, air-breathing vertebrates have evolved myriad adjustments in the cardiorespiratory system to match tissue O2 delivery with metabolic O2 demand. We explain how changes at interacting steps of the O2 transport pathway contribute to plastic and evolved changes in whole-animal aerobic performance under hypoxia. In vertebrates native to high altitude, enhancements of aerobic performance under hypoxia are attributable to a combination of environmentally induced and evolved changes in multiple steps of the pathway. Additionally, evidence suggests that many high-altitude natives have evolved mechanisms for attenuating maladaptive acclimatization responses to hypoxia, resulting in counter-gradient patterns of altitudinal variation for key physiological phenotypes. For traits that exhibit counteracting environmental and genetic effects, evolved changes in phenotype may be cryptic under field conditions and can only be revealed by rearing representatives of high-and low-altitude populations under standardized environmental conditions to control for plasticity.
Keywords: adaptation, high elevation, hypoxia, oxygen cascade, phenotypic plasticity, physiology
INTRODUCTION
A primary challenge for vertebrate life at high altitude is that the reduced partial pressure of O2 (PO2) limits rates of aerobic metabolism, thereby compromising physiological performance capacities that affect survival and reproduction. To maintain an adequate level of energy turnover in the form of aerobic ATP (adenosine triphosphate) synthesis, the O2 transport system needs to match O2 supply (delivery of O2 to the tissue mitochondria) with metabolic O2 demand (chemical utilization of O2 by oxidative phosphorylation in the electron transport chain). Thus, a reduction in the PO2 of inspired air (hypoxia) requires respiratory and cardiovascular adjustments to minimize reductions in O2 flux to the cells of respiring tissues (Ivy & Scott 2015; McClelland & Scott 2019; Scott 2011; Storz et al. 2010b, 2019) and/or metabolic adjustments to reduce O2 demand (Ramirez et al. 2007). For endotherms living at high altitude, reducing systemic O2 demand via metabolic suppression is not a viable long-term strategy for coping with chronic hypoxia owing to the energetic demands of thermoregulation, reproduction (including lactation in female mammals), and other exigencies of daily life (foraging, digging burrows, defending territories, escaping from predators, etc.). As high-altitude environments are also characterized by low ambient temperatures, endotherms are forced to increase metabolic heat production in spite of the reduced availability of O2 to power aerobic thermogenesis. Thus, metabolic O2 demands may be undiminished or even increased at high altitude. These considerations suggest a high premium on physiological adjustments that help sustain adequate flux through the O2 transport system.
Mammals and birds that are native to high altitude exhibit a characteristic suite of differences in respiratory, cardiovascular, and metabolic traits relative to their lowland counterparts (Ivy & Scott 2015; McClelland & Scott 2019; Monge & León-Velarde 1991; Scott 2011; Scott et al. 2015b; Storz et al. 2010b, 2019). Insights into the nature of these phenotypic differences are important for understanding physiological mechanisms of acclimatization and adaptation to hypoxia. Observed phenotypic differences between high- and low-altitude populations also prompt a number of questions about evolutionary mechanisms. For example: What fraction of observed trait differences is attributable to evolved (genetically based) changes in phenotype, and what fraction is attributable to plasticity, during either adulthood (acclimatization) or development? Follow-up questions about the adaptive significance of observed trait differences are applicable to both genetic and environmentally induced changes in phenotype. In the case of genetically based trait differences, we want to know: Are evolved changes in phenotype attributable to a history of directional selection that favored different trait values at different altitudes? In the case of environmentally induced trait differences, we want to know: Is the plasticity adaptive (consistent with the beneficial acclimation hypothesis)? That is, did plasticity evolve via selection that favored the capacity to express different phenotypes in response to different environmental stimuli? Insights into the relative contributions of genetic and environmentally induced changes in hypoxia-responsive phenotypes—and the potential synergy or antagonism between them—are central to understanding mechanisms of physiological adaptation and the process by which adaptive phenotypes evolve (Storz et al. 2015).
Here, we present a synthesis of our current understanding of physiological mechanisms of hypoxia adaptation in high-altitude vertebrates. We start by presenting a conceptual framework for interpreting altitudinal patterns of variation in hypoxia-responsive physiological traits and for testing hypotheses about the causes of observed patterns. We explain why altitude-related patterns of trait differentiation can sometimes mislead inferences about the particular phenotypes favored by selection for hypoxia tolerance because genetic and environmental influences on the expression of hypoxia-responsive phenotypes may covary with altitude in opposite directions. We then describe empirical evidence for such patterns in natural populations of mammals (including humans) and birds that are native to different elevational zones. We document a number of cases in which genetic and environmental effects on hypoxia-responsive physiological traits clearly work at cross-purposes, indicating that some acclimatization responses to environmental hypoxia are nonadaptive.
PLASTICITY AND GENETIC ADAPTATION
In principle, physiological adjustments that contribute to hypoxia tolerance could represent environmentally induced changes (reversible or irreversible plasticity) and/or evolved, genetically based changes. Plasticity is adaptive if the phenotype expressed in response to a particular environmental change is in the same direction as the environment-specific optimum favored by selection. Depending on how closely the plastic response matches the phenotypic optimum, plasticity may even eliminate the opportunity for selection on genetically based trait variation.
When members of a species colonize a novel environment, plastic responses to newly encountered conditions are more likely to be adaptive if those conditions fall within the range of conditions experienced in the recent ancestry of the species. Conversely, plastic responses to novel environmental stimuli may be miscued and misdirected if the new conditions fall outside that range. In the context of high-altitude adaptation, the important point is that some plastic responses to environmental hypoxia may be nonadaptive in species with strictly lowland ancestries.
ALTITUDINAL PATTERNING OF GENETIC AND ENVIRONMENTAL VARIATION IN HYPOXIA-RESPONSIVE PHENOTYPES
Consider the new selection pressures encountered by members of a low-altitude species that colonize a high-altitude environment. Due to the reduced PO2 at high altitude, the optimal value of some physiological phenotypes (e.g., pulmonary ventilation, heart rate, etc.) may be shifted relative to the value that is optimal at low altitude. Consequently, a hypoxia-induced change in phenotype in the high-altitude environment would be adaptive if it moved the mean trait value closer to the new optimum (Figure 1a). However, some physiological phenotypes may be characterized by a single global optimum that is independent of prevailing environmental conditions. In such cases, maintaining the physiological status quo may confer the greatest benefit upon ascent to high altitude, and hypoxia-induced changes in phenotype would be detrimental. When environmentally induced deviations from the global phenotypic optimum reduce fitness, selection on genetically based trait variation would be expected to counteract the plastic change, thereby restoring the ancestral phenotype (Figure 1b). This form of genetic compensation may occur whenever environmentally induced changes in phenotype shift the mean trait value away from the optimum in the new environment (Ghalambor et al. 2007, Grether 2005).
Figure 1.
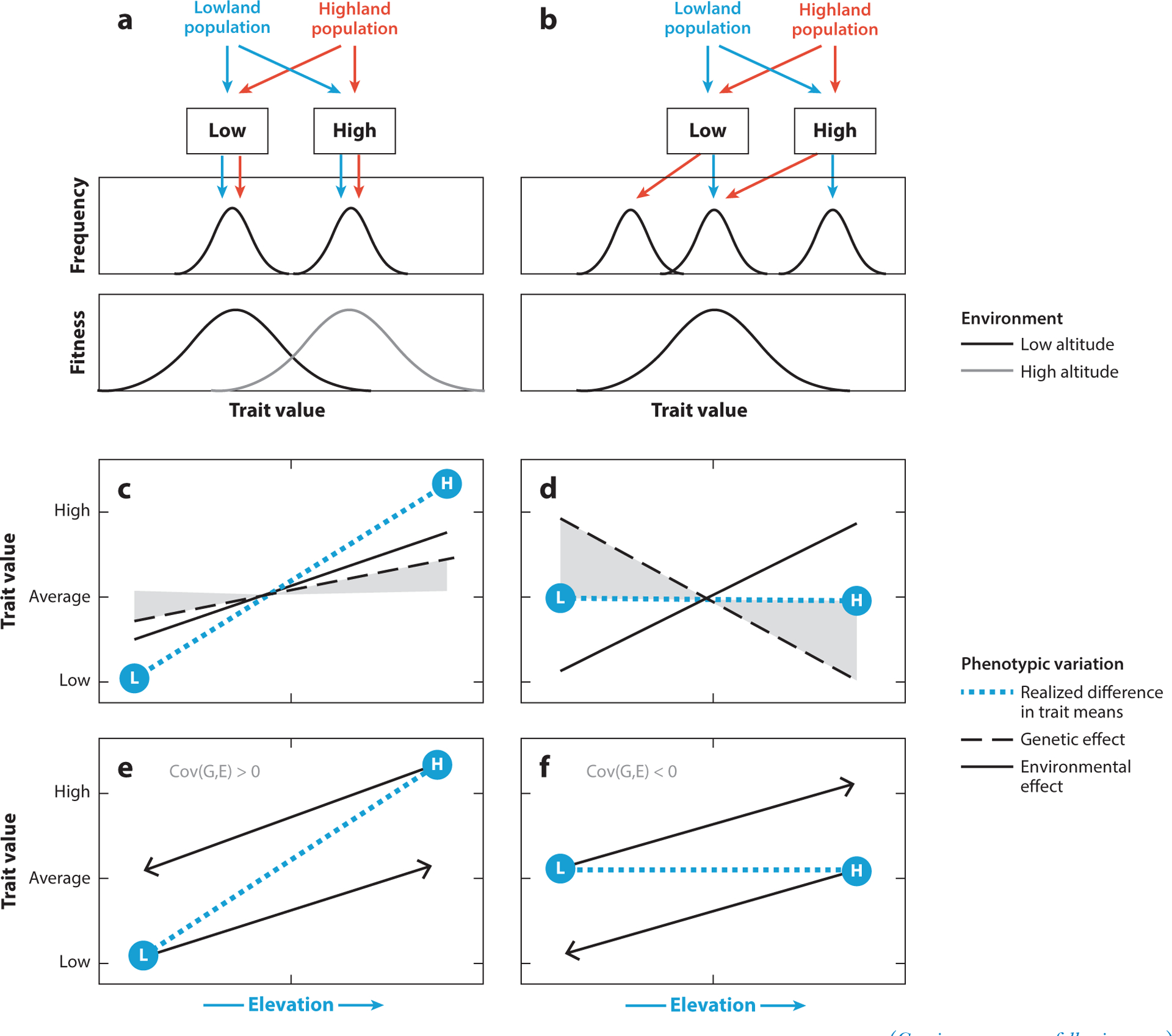
Effects of plasticity and genetic compensation on patterns of phenotypic variation across environmental gradients. (a,b) Hypothetical relationships between environmentally induced phenotypes and fitness in different environments. Panel a shows an example of how adaptive plasticity matches the population mean trait value to the phenotypic optima of high- and low-altitude environments. The top graph shows the distribution of trait values expressed in each environment, and the bottom graph shows the relationship between phenotype and fitness. In this example, optimal trait values are different for each environment (fitness functions for the low- and high-altitude environments are depicted by black and gray curves, respectively). The arrow paths show the phenotypes that are expressed in the native and non-native environments of each population. Panel b shows an example of genetic compensation. When low-altitude natives are exposed to the high-altitude environment, an environmentally induced change in phenotype shifts the mean trait value away from the global optimum. In high-altitude natives, selection on genetically based trait variation has counteracted the plastic change, thereby restoring the ancestral phenotype (i.e., the same phenotype expressed by low-altitude natives in the ancestral low-altitude environment). In this particular example, genetic compensation shifts the trait mean back to the ancestral value without increasing canalization (the Y-intercept of the reaction norm is shifted, but the slope is unaltered). (c,d) Hypothetical examples of how the covariance of genetic and environmental effects on a trait influences the spatial patterning of phenotypic variation across an altitudinal gradient. In each panel, the blue dotted line depicts the change in trait value as a function of altitude. Environmental and genetic contributions to the altitudinal pattern of trait variation are depicted by solid and dashed lines, respectively. The shaded region depicts the magnitude of the evolved, genetically based change in phenotype. Panel c shows an example of cogradient variation in which a heritable trait exhibits adaptive plasticity (as in panel a), such that genetic and environmental effects are aligned in the same direction [Cov(G,E), the covariance between genetic and environmental components of phenotypic variance, is positive] and their joint influence accentuates the change in trait mean across the altitudinal gradient. Panel d shows an example of counter-gradient variation (resulting from genetic compensation, as in panel b) in which genetic and environmental effects counteract each other [Cov(G,E) is negative], so that identical phenotypes are expressed across the gradient in spite of underlying genotypic differentiation. The L and H symbols denote mean trait values for representative low- and high-altitude genotypes, respectively, measured in their native environments at opposite ends of the gradient. (e,f) Hypothetical outcomes of reciprocal transplant experiments showing the expression of L and H phenotypes in different environments (reaction norms). The end points of the lines show outcomes of reciprocal transplants (phenotypes expressed in the non-native environment). Panel e shows the reaction norms that would result in cogradient variation (as in panel c): Cov(G,E) is positive, so the genetic and environmental effects reinforce each other. Panel f shows the reaction norms that would result in counter-gradient variation (as in panel d): Cov(G,E) is negative, so the genetic and environmental effects counteract each other. These examples assume linear reaction norms and no G × E interaction (i.e., no variation in slopes of the reaction norms).
In species that are distributed across altitudinal gradients, insights into the causes of spatial patterns of trait differentiation require information about the genetic and environmental components of phenotypic variation. This is because genetic and environmental influences on a selected trait can covary across the gradient in a way that either accentuates or attenuates altitudinal differentiation in phenotype. Components of variance in values of a quantitative trait (VP) can be partitioned as:
where VG and VE represent the variance in trait values attributable to genetic and environmental effects, respectively; VG×E is the nonadditive interaction between genetic and environmental effects; and Cov(G,E) is the covariance between genetic and environmental components of phenotypic variance (Falconer & Mackay 1996). In nonhuman animals, genetic and environmental components of trait variation can be disentangled by conducting genetic crosses or by standardizing rearing conditions, as in common garden or reciprocal transplant experiments. If variation in a given trait is exclusively genetic (VE = 0), plasticity makes no contribution to local adaptation, and the observed pattern of altitudinal variation reflects past evolutionary change via drift and/or selection. If variation in a trait is exclusively environmental (VG = 0), the observed pattern of altitudinal variation reflects the outcome of plastic responses to systematically varying environmental stimuli (e.g., altitudinal changes in PO2). The G×E interaction term is nonzero if genotypes differ in their plastic response to a given environmental stimulus. The Cov(G,E) term is nonzero if genotypes that express different phenotypes are nonrandomly distributed across environments that influence the expression of those phenotypes (Falconer & Mackay 1996). For example, due to a history of spatially varying selection, genotypes that differ in sensitivity to a reduction in PO2 may be nonrandomly distributed with respect to altitude if hypoxia-induced phenotypes have different fitnesses in different altitudinal zones. The covariance term can be positive if the genetic and environmental effects reinforce each other or negative if they oppose each other (Falconer 1990).
Plasticity in a given trait can be considered adaptive if the environmentally induced change in phenotype is in the same direction as the genetically based changes in phenotype caused by selection. This adaptive plasticity would be reflected by a positive covariance between G and E across the altitudinal gradient. In this case, environmental and genetic influences on phenotype reinforce one another and accentuate the altitude-related change in mean trait value. The synergistic effects of genetic and environmental influences results in a cogradient pattern of altitudinal variation (Figure 1c). Conversely, plasticity can be considered maladaptive if the environmentally induced change in phenotype moves the trait mean farther from the optimum. In this case, environmental and genetic effects counteract each other (negative covariance between G and E), which would attenuate the altitude-related change in mean trait value. The antagonistic effects of genetic and environmental influences results in a counter-gradient pattern of altitudinal variation (Conover & Schultz 1995, Levins 1969) (Figure 1d).
In the scenario in which there exists a single phenotypic optimum and the plastic response to hypoxia produces a deviation from that optimum, selection will favor genotypes at high altitude that counteract the plastic response and express the ancestral phenotype (illustrated in Figure 1b). Thus, as a result of genetic compensation, high-altitude populations will be enriched for genotypes that exhibit a reduced sensitivity to the phenotype-altering environmental stimulus that is specific to the high-altitude environment (e.g., reduced PO2), or they will exhibit a blunted response to the stimulus, so that the mean value for the plastic trait remains similar to that observed in the low-altitude population in the absence of the stimulus. The nonintuitive result is that spatially varying selection impedes phenotypic differentiation across the gradient even though different genotypes are favored in different elevational zones. This outcome is nonintuitive because we are accustomed to thinking that observed population differences in mean trait values provide a reliable guide as to which phenotypes are favored by selection in different environments.
For traits that exhibit a counter-gradient pattern of altitudinal variation, evolved changes are cryptic under field conditions and can only be revealed by rearing representatives of high- and low-altitude populations under standardized environmental conditions to control for plasticity. In the case of traits exhibiting a cogradient pattern of altitudinal variation (Figure 1c), a reciprocal transplant experiment involving representatives from high- and low-altitude populations would reveal altitude-related differences in mean phenotype consistent with what would be predicted by plasticity alone (Figure 1e). However, the magnitude of the altitudinal difference in trait values would exceed that produced by the plastic response owing to the additional genetic effect. In the case of a trait exhibiting a counter-gradient pattern of altitudinal variation (Figure 1d), the same reciprocal transplant would reveal genetically based differences in phenotype between high- and low-altitude natives that are not observed in the field owing to offsetting genetic and environmental effects (Figure 1f). Other patterns of partial genetic compensation or overcompensation are also possible (Conover & Schultz 1995, Conover et al. 2009). In such cases, the important point is that—owing to the negative covariance between genetic and environmental effects—the underlying genetic differentiation is greater than what would be predicted by the observed difference in phenotype. Moreover, in the case of partial compensation, the residual difference in phenotype revealed by reciprocal transplants may be in the opposite direction of what is locally favored by selection (Conover & Schultz 1995, Conover et al. 2009, Levins 1969), illustrating how a negative covariance between genetic and environmental effects can mislead inferences about the adaptive significance of trait differences observed in the field. For example, if members of a highland population have a higher mean value for a particular trait than lowland conspecifics, one possibility is that the observed difference is attributable to a history of directional selection that shifted the trait mean in the highlanders toward the local, habitat-specific optimum. However, if a hypoxia-responsive trait has the same global optimum at high and low altitudes, the higher observed value in highlanders may simply reflect the effects of selection that only partially offset the effects of environmentally induced deviations away from the global optimum. Without performing the appropriate common garden or reciprocal transplant experiments, the observed altitudinal difference in trait values could be misinterpreted as evidence for local selection that favored higher trait values in the high-altitude population when, in fact, it could be exactly the opposite, with selection favoring lower trait values to mitigate the maladaptive acclimatization response.
As discussed below, there are examples of physiological differences between high- and low-altitude vertebrates that appear to conform to both cogradient and counter-gradient patterns of trait variation, suggesting that we need to account for the interplay between genetic and environmental effects to make correct inferences about hypoxia adaptation. Before exploring these specific examples, we first review general principles of physiological adaptation to high-altitude hypoxia.
PHYSIOLOGY OF HIGH-ALTITUDE ADAPTATION
Whole-Animal Aerobic Performance and Metabolism
Measures of whole-animal aerobic performance represent an obvious point of focus in studies of high-altitude adaptation (McClelland & Scott 2019, Storz et al. 2019). In endotherms, many ecologically relevant measures of physiological performance, such as the capacity for sustained exercise and the ability to be active in the cold, are directly related to the rate of aerobic metabolism, which can be measured as the rate of O2 consumption . The maximum metabolic rate (MMR) (measured by the maximal rate of O2 consumption, ) can be elicited by challenging an animal’s aerobic capacity via forced exercise or acute cold exposure. In response to cold exposure, homeothermic endotherms maintain a constant body temperature by increasing metabolic heat production (thermogenesis) fueled by aerobic metabolism. If the cold stress is sufficiently severe, it will elicit the maximal rate of heat production, which—because it is fueled by aerobic metabolism—is reflected by the maximal rate of O2 consumption (also referred to as summit metabolism). In endotherms living at high altitude, performance measures such as exercise- or cold-induced can be expected to provide strong, causal links between integrated physiological capacities and Darwinian fitness (Hayes & O’Connor 1999, Storz et al. 2019).
Whereas MMR sets the ceiling for aerobic metabolism, and thus the capacity for many high-intensity aerobic performance traits, routine metabolism reflects the energy demands of an animal over longer periods of submaximal activity. Average daily metabolic rates (also called field metabolic rates in free-ranging animals) can be measured using various techniques (e.g., isotopic tracers such as doubly labeled water or heart rate telemetry) to reveal routine energy requirements under natural conditions. Such requirements may be significant in small endotherms living at high altitude due to the elevated metabolic costs of thermoregulation (Hayes 1989) and may therefore impose severe constraints on energy allocation for growth and reproduction.
O2 Transport and Utilization Pathways
The aerobic metabolism of an organism is determined by the rate at which tissue mitochondria consume O2 in the process of oxidative phosphorylation, which requires that O2 be transported to mitochondria via several interacting transport steps in the cardiorespiratory system (Figure 2). O2 from inspired air diffuses from the lungs into arterial blood and is transported convectively by the blood to the tissues, where it then diffuses into the cells of metabolizing tissues and is used for respiration by mitochondria. The O2 transport pathway involves five transfer steps that are arranged in series from inspired air to the tissue mitochondria (Figure 2a): (a) pulmonary ventilation, the convective movement of inspired air into the lungs; (b) pulmonary O2 diffusion, the diffusion of O2 across the air–blood interface; (c) circulatory O2 delivery, the convective movement of arterial blood to the capillaries of perfused tissues [with nearly all O2 chemically bound to hemoglobin (Hb) in red blood cells]; (d) tissue O2 diffusion, the diffusion of O2 from capillary blood to the mitochondria of tissue cells; and (e) mitochondrial O2 utilization, the consumption of O2 that generates ATP via oxidative phosphorylation. Differences in PO2 provide the driving force for O2 movement across each of the diffusive steps and account for the overall pressure gradient between the ambient PO2 of the outside air and the PO2 in the mitochondria (Figure 2b). A reverse multistep pathway exists for the transport of CO2 from the cells, where it is produced as a byproduct of oxidation, to the lungs, where it is expired (Figure 2b). In principle, plasticity or adaptation to hypoxia could involve modifications to the flux capacity of multiple convective or diffusive steps in the O2 transport pathway, or changes in the dynamic regulation of the cardiorespiratory system in response to changes in metabolism or blood O2 levels (Ivy & Scott 2015, McClelland & Scott 2019, Storz et al. 2010b).
Figure 2.
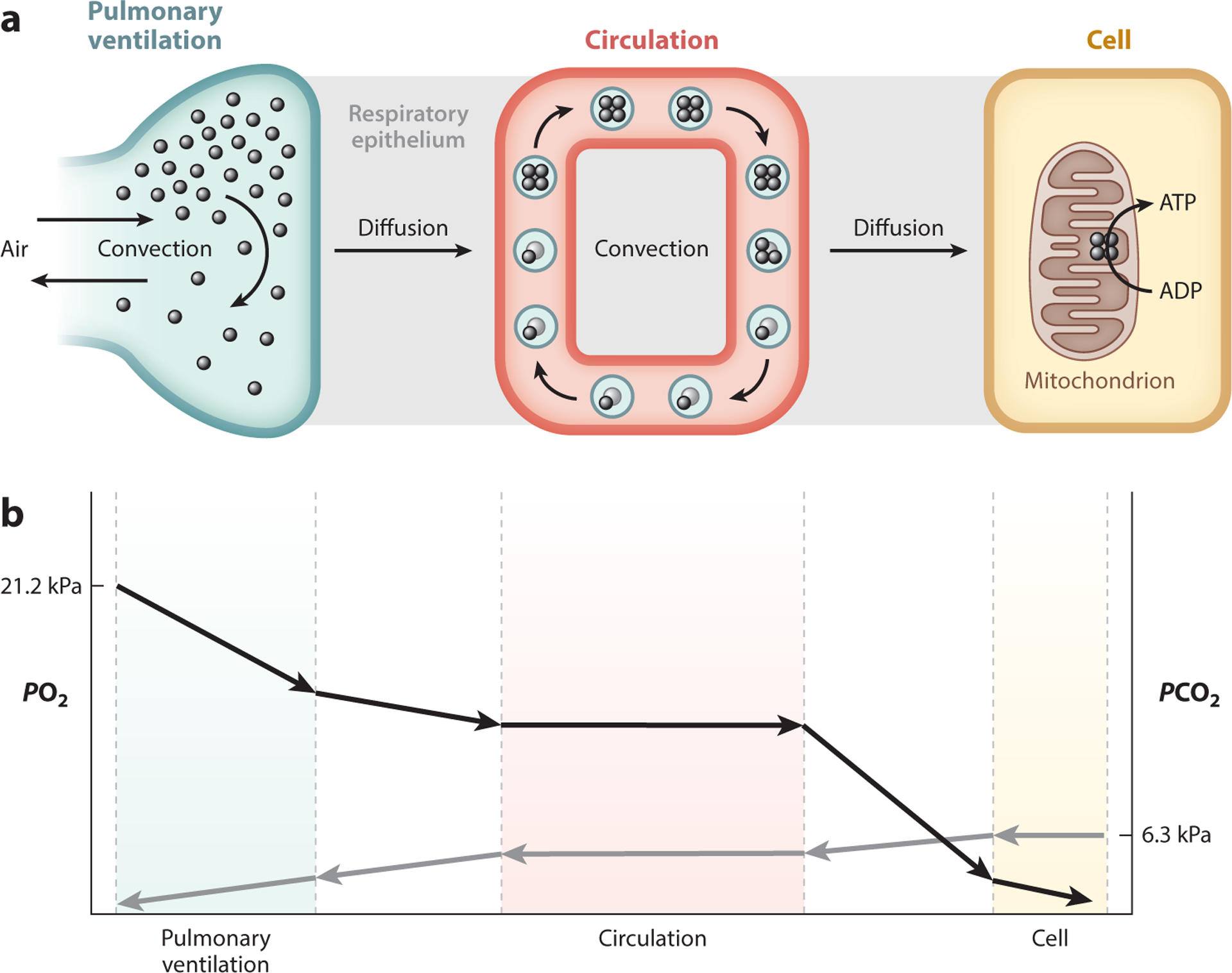
The O2 and CO2 transport cascades. (a) Diagrammatic representation of compartments of gas transport in the mammalian cardiorespiratory system. O2 is transported from atmospheric air to the tissue mitochondria along a pathway with several diffusive and convective steps: pulmonary ventilation, pulmonary O2 diffusion, circulatory O2 delivery, tissue O2 diffusion, and mitochondrial O2 utilization. Hb is schematically depicted as a tetrameric (four-subunit) protein that reversibly binds up to four O2 molecules. (b) Diffusive O2 flux is driven by gradients in PO2, resulting in declines in PO2 from one compartment to the next. Blood PO2 declines from the afferent (arterial) inlet of the tissue capillary bed to the efferent (venous) outlet as Hb unloads O2 to the perfused tissue. PO2 also declines with distance from the capillaries, so a gradient of cellular PO2 should reflect variation in capillary PO2 and diffusion distance. An equivalent multistep pathway exists for the transport of CO2 in the reverse direction. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; Hb, hemoglobin; PO2, partial pressure of O2.
Experimental analysis of adaptive changes in the O2 transport pathway can be used to address a number of key evolutionary questions: To what extent do interacting and integrated traits (e.g., steps in the pathway) evolve in concert? Are changes in some traits more likely to constitute the first steps in hypoxia adaptation? Are some traits particularly influential, acting as a limiting factor in adaptation? Do evolved changes in some traits alter the adaptive value of possible changes in other traits? Such questions could be profitably addressed using artificial selection experiments or comparative analyses of closely related taxa that have different durations of residency at high altitude.
RELATIVE CONTRIBUTIONS AND PHYSIOLOGICAL UNDERPINNINGS OF PLASTIC AND EVOLVED CHANGES IN AEROBIC PERFORMANCE AND METABOLISM
Decades of research on the physiology of hypoxia tolerance in vertebrates has revealed altitude-related changes in aerobic performance and in the underlying subordinate traits that govern flux through the O2 transport pathway. Here, we discuss evidence that both plastic and evolved changes in these various traits contribute to the unique phenotype of high-altitude natives. We do not discuss altitude-related changes in the transport and utilization of metabolic substrates (carbohydrates, lipids, etc.); instead, we direct the reader to other recent reviews on the subject (McClelland & Scott 2019).
Cogradient Patterns of Altitudinal Variation in
Several high-altitude taxa have a higher capacity for aerobic performance under hypoxia than their low-altitude counterparts. In humans (Brutsaert 2016, Brutsaert et al. 2003), deer mice (Peromyscus maniculatus) (Cheviron et al. 2012, 2013, 2014; Lui et al. 2015; Tate et al. 2017), and leaf-eared mice (Phyllotis xanthopygus) (Schippers et al. 2012), high-altitude natives exhibit a higher than low-altitude natives under hypoxia, but the performance differences are not necessarily manifest at the PO2 that is typical at sea level (normoxia). These findings are consistent with the expectation that it is beneficial to have a higher to support aerobic thermogenesis and/or exercise in environments that are characterized by low PO2 and low ambient temperature. In many cases, it is not clear whether such high-altitude phenotypes represent evolved, genetically based adaptations or the outcome of acclimatization to hypoxia (Brutsaert 2016). However, our recent research using common garden experiments with deer mice from high and low altitudes has revealed the importance of both plastic and evolved increases in aerobic thermogenic capacity and exercise capacity under hypoxia (Cheviron et al. 2012, 2013, 2014; Lui et al. 2015; Tate et al. 2017) (Figure 3).
Figure 3.

High-altitude deer mice (Peromyscus maniculatus) have higher aerobic capacities under hypoxia in comparison with lowland conspecifics. Performance capacities under hypoxia were measured as the maximal rate of O2 consumption during thermogenesis or exercise. The absolute data (in units of mL/min) were corrected for body mass using a residual approach (see Lui et al. 2015 and Tate et al. 2017 for details) and are here divided by the body mass of an average-sized mouse (data represent means ± SE). Significant main effects of native elevation and hypoxia acclimation are denoted by * and †, respectively, in two-way analysis of variance (P < 0.05).
What are the contributions of plastic and evolved changes in the O2 transport pathway to altitudinal variation in ? In both humans and nonhuman animals, a long history of research has investigated the determinants of under normoxia. Some have argued that convective transport of O2 in the circulatory system sets limits on normoxic because it dictates the rate of O2 delivery to metabolizing tissues (Bassett & Howley 2000). Others have argued that the capacities of all steps in the O2 transport pathway are matched, such that each step exerts a similar degree of control over normoxic (Weibel et al. 1991). Theoretical principles of gas exchange have been used to model the cardiorespiratory system in several species, and this work has suggested that increases in the capacity of individual steps in the O2 transport pathway can increase (Scott & Milsom 2006, 2009; Wagner 1996). However, steps can differ in their relative influence over , and the capacity for O2 diffusion from capillary blood to the mitochondria has the greatest influence in both normoxia and hypoxia (Scott & Milsom 2006, 2009; Wagner 1996). Consistent with this theoretical expectation, artificial selection for increased running endurance in rats revealed that increases in after 7 generations could be entirely explained by increases in the capacity of the locomotory muscle for O2 diffusion and mitochondrial O2 utilization, without any changes in circulatory O2 delivery, pulmonary function, or ventilation (Henderson et al. 2002, Howlett et al. 2009). Continued increases in after 15 generations of selection was attributable to further changes in skeletal muscle, in addition to increases in the convective pumping of blood by the heart (i.e., increases in cardiac output) that increased circulatory O2 delivery to the tissues and to increases in pulmonary ventilation and pulmonary O2 diffusing capacity (Gonzalez et al. 2006, Howlett et al. 2009, Kirkton et al. 2009). These results indicate that modest initial improvements in can arise from changes at a single step in the O2 transport pathway, but continued enhancement of requires additional changes in other steps.
Results of these theoretical and empirical studies suggest that increases in in high-altitude natives should result from increases in the capacity for O2 diffusion and mitochondrial utilization in the tissues that are active during locomotion and/or thermogenesis (i.e., skeletal muscles and brown adipose tissue) and may also be associated with increases in the capacities for circulatory and pulmonary O2 transport. However, the theoretical studies also suggest that the relative influence of different steps can change in hypoxic versus normoxic conditions, with pulmonary O2 transport exerting a greater influence over under hypoxia (Scott & Milsom 2006, Wagner 1996). Thus, the physiological mechanisms that are responsible for enhancements of hypoxic in high-altitude natives may be somewhat distinct from those that are responsible for enhancements of under normoxia. The remainder of this section addresses how changes in the capacity of each step of the O2 transport pathway may contribute to plastic and evolved changes in in high-altitude natives. The findings suggest that cogradient variation in arises from both environmentally induced changes and genetically fixed, evolved changes in multiple steps of the pathway. There does not appear to be a single limiting factor in high-altitude adaptation, but rather a series of integrated physiological changes that jointly contribute to enhancements of O2 transport.
The capacity for mitochondrial O2 utilization in locomotory muscles is elevated in many species that sustain intense metabolic activity at high altitude. For example, high-altitude deer mice have evolved a greater mitochondrial respiratory capacity in the gastrocnemius muscle (Mahalingam et al. 2017)—a large hindlimb muscle used for both locomotion and shivering thermogenesis (Günther et al. 1983, Pearson et al. 2005)—and this difference is largely attributable to increases in the proportional abundance of oxidative (mitochondrion-rich) fiber types (Lui et al. 2015, Scott et al. 2015a) and to increases in mitochondrial abundance within these oxidative fibers (Mahalingam et al. 2017). Similarly, bar-headed geese (Anser indicus), a species that flies at high altitude during its biannual migration across the Himalayas, and high-altitude torrent ducks (Merganetta armata) have greater mitochondrial respiratory capacity in locomotory muscles (Dawson et al. 2016; Scott et al. 2009a,b). In deer mice, the various determinants of mitochondrial respiratory capacity in the gastrocnemius do not change in response to chronic hypoxia, either during adulthood or during early development (Lui et al. 2015, Mahalingam et al. 2017, Nikel et al. 2017), suggesting that the muscle’s enhanced capacity for mitochondrial O2 utilization is attributable to genetically fixed, evolved changes. However, human high-altitude natives (who, unlike other highland mammals and birds, do not sustain especially high metabolic rates at high altitude) have not evolved a greater capacity for mitochondrial O2 utilization in locomotory muscles (Horscroft et al. 2017; Kayser et al. 1991, 1996). Instead, changes at other steps in the O2 transport pathway appear to contribute to the increased under hypoxia (Gilbert-Kawai et al. 2014).
The capacity for O2 diffusion into skeletal muscles is also augmented in many high-altitude natives. Studies of mammals and birds suggest that native highlanders tend to have greater capillarity in the skeletal muscle than lowlanders when compared in their native environments (Dawson et al. 2018, Kayser et al. 1991, León-Velarde et al. 1993, Mathieu-Costello et al. 1998, Scott et al. 2015a). In some cases, these differences in muscle capillarity persist in comparisons between highlanders and lowlanders reared in the same environments (Lui et al. 2015, Scott et al. 2009a), suggesting that the differences have an evolved genetic basis. Some high-altitude natives have also evolved higher muscle myoglobin content (Lechner 1977), and the mitochondria in their muscle cells are situated closer to capillaries, thereby reducing intracellular O2 diffusion distances (Mahalingam et al. 2017, Scott et al. 2009a). In deer mice, increases in capillarity (Figure 4) and O2 transport in the gastrocnemius muscle are insensitive to chronic hypoxia exposure (Lui et al. 2015, Mahalingam et al. 2017, Nikel et al. 2017) and may thus represent another example of an evolved change in the O2 transport pathway that helps augment aerobic capacity. The mechanisms underlying enhancements of O2 diffusion capacity and mitochondrial O2 utilization in skeletal muscle are not entirely clear, but in high-altitude deer mice, they are associated with differences in the expression of genes involved in energy metabolism, muscle development, and vascular development (Cheviron et al. 2012, 2014; Scott et al. 2015a).
Figure 4.
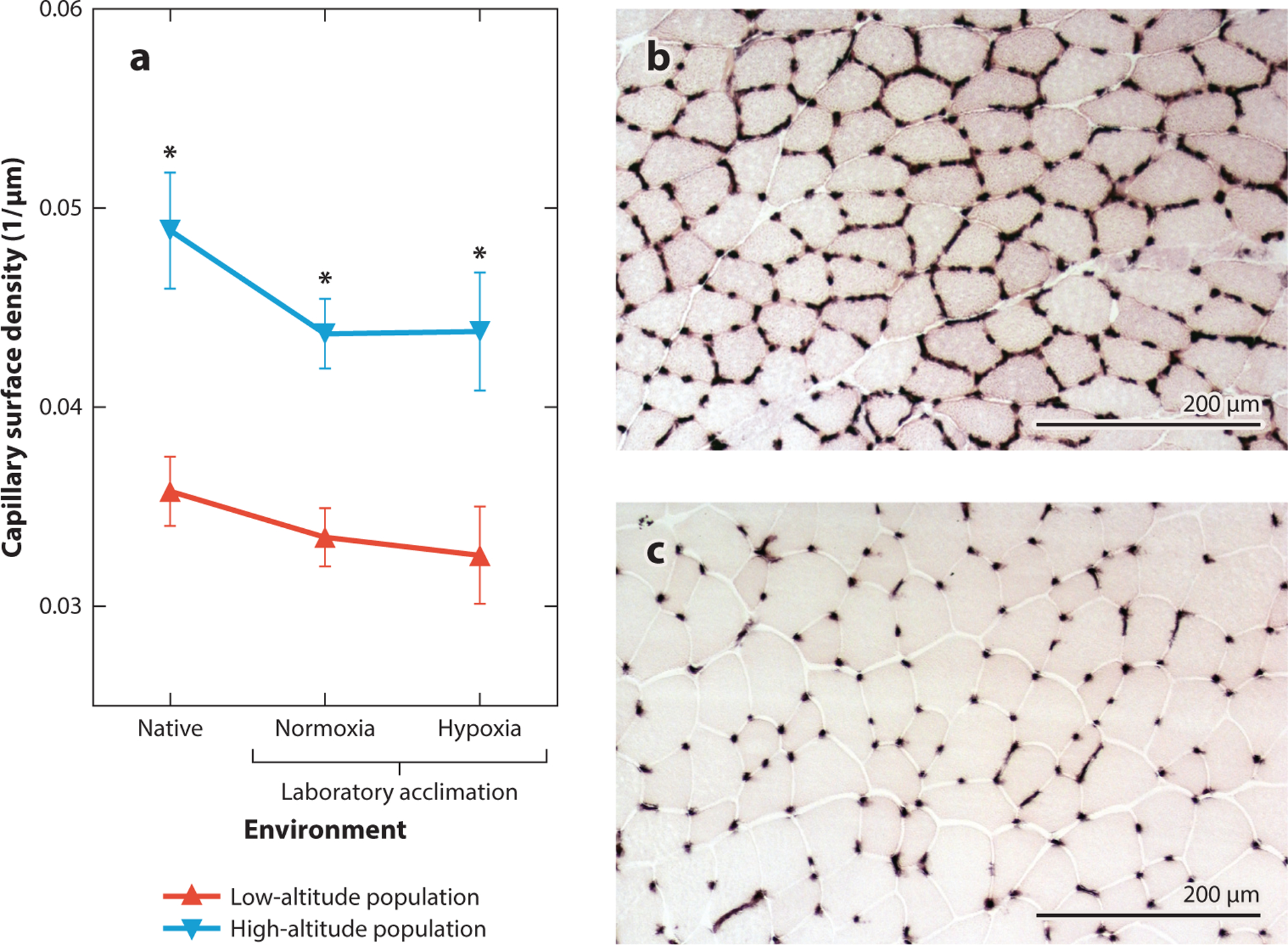
High-altitude deer mice have evolved a fixed increase in the capillarity of the gastrocnemius muscle. (a) Experimental tests revealed a significant main effect of population (F1,70 = 41.15, P < 0.0001) on capillary surface density (μm of capillary surface per μm2 of transverse muscle area) but no significant main effect of environment (F2,70 = 1.97, P = 0.147) or population × environment interaction (F2,70 = 0.24, P = 0.789). Data from Lui et al. (2015) and Scott et al. (2015a). Two-factor analysis of variance with Bonferroni posttests were used for statistical comparisons, and * represents a significant pairwise difference between populations within an environment (P < 0.05). (b,c) Representative images of capillary staining using alkaline phosphatase activity in wild-caught highland (b) and lowland (c) deer mice, each shown at the same scale.
The capacity for circulatory O2 delivery also appears to be enhanced in high-altitude natives. Circulatory O2 delivery is determined by cardiac output (the flow rate of blood that is pumped by the heart) and the O2 content of arterial blood. The latter is determined by both the quantity of Hb (its concentration in the blood) and the quality of Hb (its oxygenation properties). Some evidence suggests that high-altitude natives can sustain higher cardiac outputs in hypoxia than their low-altitude counterparts. For example, Tibetan humans achieve higher maximal cardiac outputs during exercise in hypoxia than Han Chinese lowlanders, even after prolonged acclimatization at high altitude (Chen et al. 1997, Ge et al. 1995). High-altitude deer mice may also be capable of higher cardiac output during hypoxic (Tate et al. 2017). In contrast, the Hb content of the blood is often lower in highlanders than in lowlanders after exposure to chronic hypoxia, such that a counter-gradient pattern of altitudinal variation in blood Hb content frequently occurs (discussed in the next section).
The capacity for circulatory O2 delivery also appears to be enhanced in high-altitude natives by fine-tuned shifts in Hb-O2 affinity that increase arterial O2 saturation, thereby compensating for the reduced arterial PO2. Such shifts can be mediated by changes in intrinsic Hb-O2 affinity, changes in the responsiveness of Hb to the effects of allosteric cofactors in the red cell (such as H+, CO2, Cl− ions, and organic phosphates), or changes in the cellular concentrations of such cofactors (Storz 2016, 2019). Comparative studies have provided strong evidence that evolved increases in Hb-O2 affinity have contributed to hypoxia adaptation in a diverse range of mammals and birds (Galen et al. 2015; Natarajan et al. 2015b, 2016, 2018; Projecto-Garcia et al. 2013; Signore et al. 2019; Storz et al. 2010a; Tufts et al. 2015; Zhu et al. 2018). For example, high-altitude deer mice have evolved an increased Hb-O2 affinity, via both increased intrinsic O2 affinity and suppressed sensitivity to allosteric cofactors (Jensen et al. 2016; Natarajan et al. 2013, 2015a; Storz et al. 2009, 2010a), and arterial O2 saturation in hypoxia is also higher than in low-altitude conspecifics (Tate et al. 2017). Overall, increased capacities for circulatory O2 delivery in high-altitude natives are attributable to both plastic and evolved increases in maximal cardiac output as well as evolved increases in Hb-O2 affinity that enhance arterial O2 saturation.
The capacity for O2 diffusion across the air–blood interface in the lungs is enhanced in many high-altitude taxa. By augmenting O2 uptake into the blood, increases in the O2 diffusing capacity of the lungs can also help increase arterial O2 saturation under hypoxia. The morphological diffusing capacity of the lungs appears to be augmented in high-altitude mammals and birds as a result of increases in total lung volume and/or in alveolar surface area (Brutsaert 2016, Maina et al. 2017, Pearson & Pearson 1976). Some of these differences can be explained by plasticity, because hypoxia exposure in early life (Burri & Weibel 1971; Hsia et al. 2005, 2007) and during adulthood (Dane et al. 2018) can induce lung growth and/or remodeling of existing lung structure. Whether evolved changes in lung structure also help augment O2 diffusing capacity in high-altitude natives is unclear, with some studies showing evidence for genetically based changes in the lungs (Scott et al. 2011, York et al. 2018) and others showing that altitudinal variation can be completely explained by environmental effects (Brutsaert 2016, Lechner 1977). Therefore, hypoxia-induced plasticity can augment O2 diffusing capacity of the lungs, but controlled common garden experiments are still needed to determine the relative importance of evolved changes in lung structure and gas exchange efficiency in high-altitude natives.
In contrast to other steps in the O2 transport pathway, little evidence exists for increases in pulmonary ventilation at in high-altitude humans or other vertebrates. For example, in high-altitude Tibetan and Andean natives, total ventilation is lower during exercise than in representatives of low-altitude groups, even when the lowlanders are well acclimatized to high altitude (Wagner et al. 2002, Zhuang et al. 1996). These studies revealed that the lungs of high-altitude natives have much greater gas exchange efficiencies due to their higher O2 diffusing capacities, off-setting the effects of lower ventilation rates to help maintain (Wagner et al. 2002, Zhuang et al. 1996). Many additional studies of high-altitude natives report lower absolute ventilation and a lower air convection requirement (quotient of total ventilation and ) during exercise (Brutsaert 2016), for which there appear to be contributions of both developmental plasticity and genetically based changes unique to highland natives (Brutsaert et al. 2000, 2005). It is likely that pulmonary uptake of O2 from air into the blood is sufficiently enhanced by other changes (e.g., increases in lung O2 diffusing capacity) so that highlanders can breathe less and thus divert some of the O2 that would have been needed to support the metabolic activity of breathing muscles to other tissues. Lower rates of pulmonary ventilation may also reduce respiratory water loss and minimize perturbations of blood CO2/pH homeostasis (Powell 2007, Zhuang et al. 1996). Nevertheless, apart from these findings in high-altitude humans, we know very little about whether increases in pulmonary ventilation contribute to enhancements of in other high-altitude taxa.
The above findings suggest that cogradient variation in across altitudinal gradients arises from both plastic and evolved changes in individual steps of the O2 transport pathway. Our work on high-altitude deer mice suggests that evolved increases in the capacity for O2 diffusion (Figure 4) and O2 utilization in locomotory muscle—a major site of O2 demand during thermogenesis and exercise—contribute to increases in . By contrast, it is less clear whether plasticity leads to any significant changes in these muscle phenotypes. Both plastic and evolved increases in the capacities for circulatory O2 delivery and for pulmonary O2 diffusion also appear to contribute to increasing in high-altitude natives. These findings are largely consistent with predictions of theoretical models of the O2 transport pathway (Scott & Milsom 2006, 2009; Wagner 1996), with the exception that increases in pulmonary ventilation do not contribute to increases in in the limited number of studies that have addressed the issue in high-altitude humans. There may be trade-offs associated with increasing pulmonary ventilation that limit its adaptive value or otherwise limit its potential for evolutionary change.
Counter-Gradient Patterns of Altitudinal Variation and the Attenuation of Maladaptive Plasticity
Many of the physiological adjustments to chronic hypoxia described above are thought to represent examples of adaptive plasticity because they contribute to enhanced physiological performance and fitness at high altitude (Swenson & Bärtsch 2014). However, some plastic responses to high-altitude hypoxia appear to be nonadaptive or even maladaptive. For example, some common responses to chronic hypoxia are clearly pathological and contribute to high-altitude diseases such as chronic mountain sickness in humans and brisket disease in cattle (Dempsey & Morgan 2015, Ivy & Scott 2015, Monge et al. 1992, Rhodes 2005, Storz et al. 2010b). High-altitude natives appear to have evolved mechanisms that attenuate many of the maladaptive responses to high-altitude hypoxia, leading to counter-gradient patterns of altitudinal variation for key phenotypes. Below we describe two well-documented examples of maladaptive plasticity in the O2 transport system.
Hypoxic pulmonary hypertension (HPH), an especially serious malady at high altitude (Dempsey & Morgan 2015, Shimoda & Laurie 2014, Sylvester et al. 2012), occurs when pulmonary arteries constrict in response to hypoxia and then remodel, thicken, and become less distensible over time (Shimoda & Laurie 2014). The constriction of pulmonary vessels is beneficial at sea level for matching regional variation in ventilation and blood flow. Modest variation in ventilation and O2 levels throughout the lungs is normal, and the constriction or dilation of vessels in relation to O2 supply (ventilation–perfusion matching) enhances the efficacy of gas exchange across the air– blood interface. However, this constriction is counterproductive at high altitude because hypoxia occurs throughout the entirety of the lungs, and the global constriction of pulmonary arterial vessels initiates vascular changes that ultimately culminate in HPH (Dempsey & Morgan 2015, Shimoda & Laurie 2014, Sylvester et al. 2012). HPH can become life-threatening by impairing O2 uptake and causing pulmonary edema, right-ventricle hypertrophy, and heart failure (Sylvester et al. 2012). However, several high-altitude species have been shown to exhibit little to no HPH (Ge et al. 1998, Groves et al. 1993). High-altitude deer mice do not experience right-ventricle hypertrophy under chronic hypoxia and—in comparison with lowland mice subjected to the same conditions—they differentially express a number of genes that are known to be involved in cardiac hypertrophy (Velotta et al. 2018). The attenuation of HPH in high-altitude natives should prevent hypoxia-induced increases in blood pressure in pulmonary arteries, thereby minimizing the incidence of HPH and its pathological effects.
In humans and some other lowland mammals, the acclimatization response to severe hypoxia involves an increase in blood Hb concentration. This increase is initially caused by a reduction in plasma volume and is later followed by increased erythropoiesis (red blood cell production), stimulated by increased circulating levels of the hormone erythropoietin (Siebenmann et al. 2017). At face value, an augmentation of blood Hb concentration seems like a beneficial response to hypoxia because it increases the O2 carrying capacity of the blood. However, the associated increase in blood viscosity produces a higher peripheral vascular resistance that can compromise cardiac output and, hence, . Among Tibetans at high altitude, exercise-induced is negatively associated with blood Hb concentration (Simonson et al. 2015b, Wagner et al. 2015). Likewise, during human pregnancy at high altitude, increased blood Hb concentration is associated with increased risks of stillbirth, preterm birth, and reduced birth weight, all of which appear to stem from reduced blood flow in the uteroplacental circulation (Moore et al. 2011). Hypoxia-induced polycythemia also plays a well-documented role in the pathogenesis of chronic mountain sickness. It is therefore not surprising that many high-altitude natives do not exhibit constitutively elevated blood Hb concentrations (Beall & Reichsman 1984, Black & Tenney 1980, Lui et al. 2015, Storz et al. 2010b), owing to a blunted erythropoietic response to chronic hypoxia or a compensatory increase in plasma volume, thereby yielding a counter-gradient pattern of altitudinal variation. For example, when subject to chronic hypoxia, high-altitude deer mice exhibit a much less pronounced increase in Hb concentration in comparison with white-footed mice (Peromyscus leucopus), a closely related lowland species (Figure 5). Likewise, among Tibetan humans living at elevations of ~4,000 m, blood Hb concentrations are unexpectedly low and generally fall within the range that would be expected for people living at sea level. Evidence suggests that this blunted increase in Hb concentration in Tibetans is associated with a hyporesponsive hypoxia-inducible factor (HIF) pathway (Simonson et al. 2015a). Noncoding changes that alter the expression of genes in the HIF pathway such as EPAS1 and EGLN may have recalibrated the homeostatic set point for the erythropoietic response to hypoxia. Alternatively, the reduced Hb concentration may represent a secondary consequence of genetically based changes in other traits related to ventilation, cardiovascular function, or muscle diffusion capacity that were brought about by regulatory changes in the HIF cascade (Storz 2010, Storz et al. 2010b, Wagner et al. 2015).
Figure 5.

When subjected to the same degree of chronic hypoxia, high-altitude deer mice (Peromyscus maniculatus) exhibit a less-pronounced increase in blood hemoglobin (Hb) concentration relative to low-altitude white-footed mice (Peromyscus leucopus) reared under common conditions. Experimental tests revealed a significant main effect of acclimation environment (F1,64 = 26.9, P < 0.0001) and a significant population × environment interaction (F1 64 = 6.53 , P = 0.013) on blood Hb concentration but no overall main effect of population (F1,64 = 0.85, P = 0.360) (C.M. Ivy & G.R. Scott, unpublished data). This empirical pattern approximates the theoretical pattern depicted in Figure 1f. Two-factor analysis of variance was used for statistical comparisons.
Evolution of Plasticity in the Hypoxic Chemoreflex: Disentangling of a Physiological Control System at High Altitude
The physiological control systems that contribute to homeostasis are often complex, adjusting a range of flexible phenotypes to promote the dynamic stability of homeostatically regulated variables. For example, chemoreflexes are a class of reflexes that are initiated by chemoreceptors and that detect chemical stimuli, and the hypoxic chemoreflex is critical for regulating pulmonary O2 uptake and circulatory O2 transport in response to decreases in blood O2 levels (Ivy & Scott 2015, Kumar & Prabhakar 2012, Lindsey et al. 2018) (Figure 6). This reflex is initiated by reductions in the PO2 of arterial blood that are sensed by the carotid bodies, the body’s key O2 chemoreceptors, which are located on the carotid arteries (Lopez-Barneo et al. 2016, Prabhakar & Peng 2017). The carotid bodies are innervated, and when stimulated, they send afferent information to the brain that triggers a recruitment of respiratory muscles and a corresponding increase in pulmonary ventilation termed the hypoxic ventilatory response (HVR) (Ivy & Scott 2015, Powell et al. 1998). Carotid body stimulation also activates the sympathetic nervous system (SNS) and leads to the release of catecholamines (epinephrine, norepinephrine, etc.) into the circulation from the adrenal medulla. This sympathoadrenal response to hypoxia has a significant effect on the circulation—increasing cardiac output, inducing vasoconstriction in many tissues, and increasing systemic vascular resistance—with the net effect being that blood flow is preferentially redistributed toward core hypoxia-sensitive organs such as the brain and the heart (Hainsworth & Drinkhill 2007, Ivy & Scott 2015). The sympathoadrenal response is critical to surviving acute exposure to severe hypoxia (Seidler & Slotkin 1985, Slotkin & Seidler 1988), but it can become detrimental during chronic exposure to hypoxia at high altitude because it can result in increased blood pressure, increased cardiac workload, and impeded blood flow to some tissues (including the digestive and reproductive organs) (Bernardi et al. 1998, Calbet 2003, Hainsworth & Drinkhill 2007). Indeed, chronic hypoxia can lead to the long-term maintenance or enhancement of the hypoxic chemoreflex. Ventilatory acclimatization to hypoxia (VAH) occurs after days to weeks of hypoxia exposure, leading to further increases in breathing that arise from increased hypoxia sensitivity of the carotid bodies (Pardal et al. 2007, Powell et al. 1998) and from increased sensitivity of the brain to the afferent signals from the carotid bodies (Pamenter & Powell 2016, Pamenter et al. 2014). Sympathoadrenal activation also persists with chronic exposure to hypoxia (Bernardi et al. 1998, Calbet 2003, Hainsworth & Drinkhill 2007). Therefore, the effects of chronic hypoxia on the hypoxic chemoreflex elicit some physiological responses that are beneficial to pulmonary O2 uptake and tissue O2 supply (e.g., increases in ventilation) but other responses (e.g., persistent sympathoadrenal activation) that may compromise O2 supply to some tissues.
Figure 6.
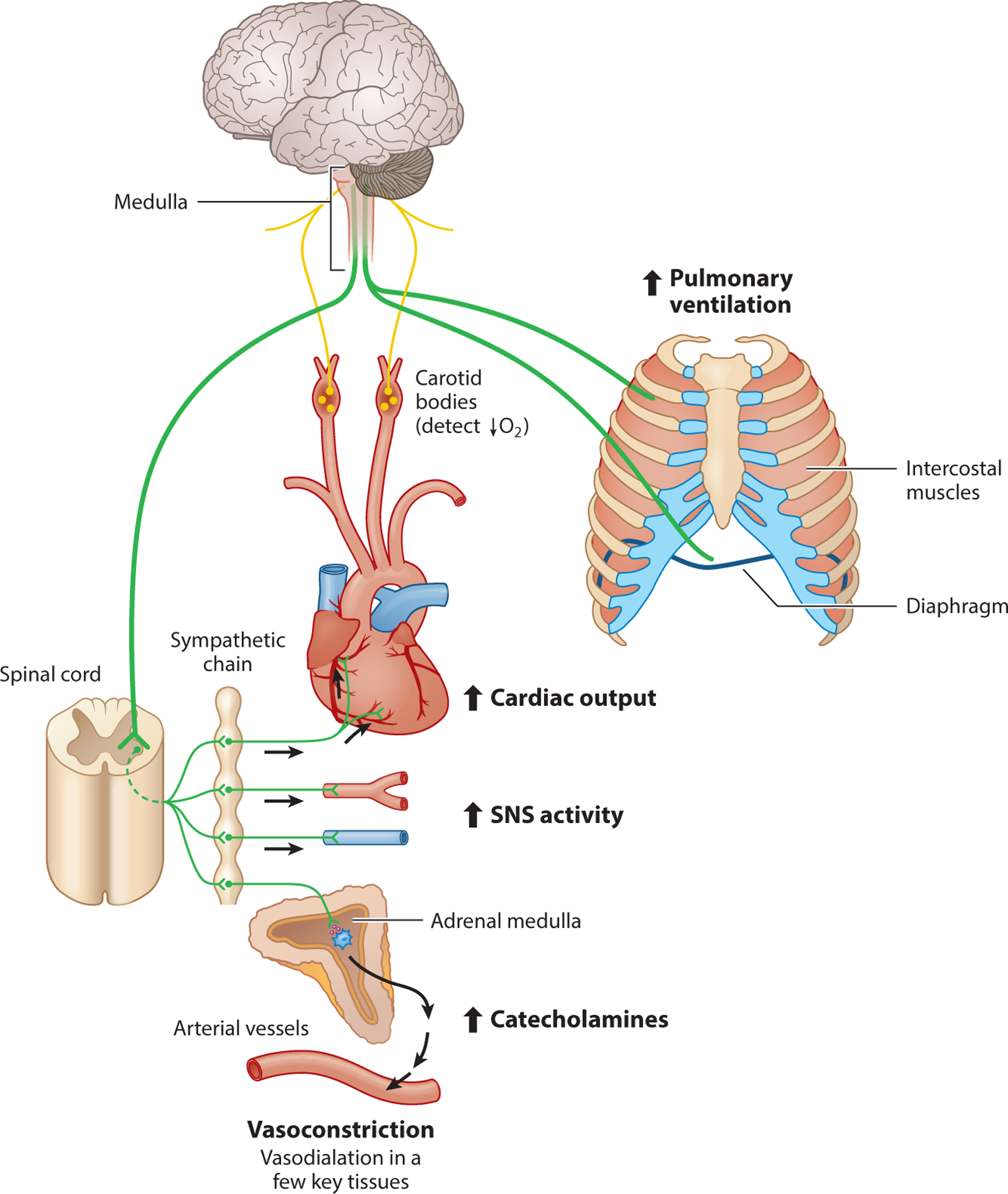
The hypoxic chemoreflex is a key control system regulating the ventilatory and circulatory responses to hypoxia. The carotid bodies detect a reduction in the partial pressure of O2 (PO2) of arterial blood, which increases the neural output of afferent neurons (yellow) sending information to the brain. This alters the output of efferent neurons (green) that innervate the respiratory muscles (diaphragm and intercostal muscles), which leads to an increase in pulmonary ventilation and also activates preganglionic neurons of the sympathetic nervous system (SNS). The latter increases the activity of postganglionic SNS neurons and leads to the release of catecholamines (epinephrine, norepinephrine, etc.) into the circulation from the adrenal medulla (the inner tissue of the adrenal gland). This sympathoadrenal response to hypoxia increases cardiac output, and it induces vasoconstriction in many tissues, with the exception of some key hypoxia-sensitive organs (i.e., brain and heart) that experience vasodilation.
How has the hypoxic chemoreflex and its plasticity evolved in high-altitude natives? There would likely be trade-offs associated with changes to the entire reflex: Maintaining or augmenting the reflex should benefit pulmonary O2 uptake, but blunting the effects of the reflex should attenuate the detrimental effects of chronic hypoxia on the cardiovascular system and on blood flow to some tissues. These trade-offs may explain why divergent changes in how the hypoxic chemoreflex controls breathing appear to have evolved in different high-altitude taxa. Pulmonary ventilation and the sensitivity of the HVR in some high-altitude mammals and birds are similar or enhanced compared with their low-altitude counterparts (Beall et al. 1997, Brutsaert 2007, Ivy et al. 2019, Moore 2000, Pichon et al. 2009, Scott & Milsom 2007) but are blunted in others (Brutsaert et al. 2005, Lague et al. 2017). Less is known about how the sympathoadrenal response to hypoxia has evolved, but some evidence suggests that the activation of the sympathoadrenal system in chronic hypoxia may be attenuated in high-altitude natives (Bernardi et al. 1998, Pichon et al. 2013, Scott et al. 2019). We have recently begun disentangling how the entire reflex has evolved in high-altitude deer mice. Our results suggest that a significant attenuation in the plasticity of the hypoxic chemoreflex in highlanders has evolved in conjunction with adjustments in breathing that help safeguard pulmonary O2 uptake.
Chronic hypoxia leads to significant changes in breathing in lowland deer mice but not in highland mice. Lowland mice exhibit VAH, in which exposure to chronic hypoxia increases pulmonary ventilation and makes the breathing pattern more effective (higher tidal volumes and lower breathing frequencies at a given total ventilation), in association with improvements in arterial O2 saturation under hypoxia (Ivy & Scott 2017a,b, 2018). VAH in lowland mice is associated with significant growth of the carotid bodies (Ivy & Scott 2017a), as occurs in other mammals (Pardal et al. 2007, Powell et al. 1998). In contrast, highland mice do not exhibit VAH, but they exhibit a fixed increase in breathing that is similar to hypoxia-acclimated lowlanders and maintain even higher arterial O2 saturations under hypoxia (Ivy & Scott 2017a, 2018). High-altitude mice have evolved a fixed increase in alveolar ventilation and pulmonary O2 uptake, consistent with genetic assimilation of the VAH response (a process whereby an environmentally induced phenotype becomes genetically encoded), but this has been achieved without a significant enlargement of the carotid bodies that is typical of VAH in lowlanders (Ivy & Scott 2017a). This latter finding is likely significant, because enlargement of the carotid bodies may increase the afferent signal to the brain that activates the sympathoadrenal system.
High-altitude deer mice have also evolved a significant reduction in catecholamine release from the adrenal medulla (Scott et al. 2019). The release of catecholamines in response to maximal stimulation (measured in vitro using carbon fiber amperometry on adrenal medulla slices) is much lower in highland deer mice compared with lowland conspecifics, which appeared to result from a reduction in cellular catecholamine synthesis. Furthermore, exposure to chronic hypoxia reduces the sensitivity of the adrenal medulla to stimulation in lowlanders, but chronic hypoxia has no effect on this trait in highlanders. Therefore, in addition to a potential reduction in the afferent signal to the brain from the carotid bodies, high-altitude deer mice have evolved a reduced capacity to release catecholamines into the circulation in response to this afferent signal (Scott et al. 2019). This reduction in catecholamine release represents a potential mechanism for attenuating the maladaptive effects of chronic sympathoadrenal activation and should lead to a counter-gradient pattern of altitudinal variation.
High-altitude deer mice appear to have evolved changes in the hypoxic chemoreflex that have improved their ability to maintain O2 homeostasis under chronic hypoxia. Within this complex system, we observe one regulated phenotype that enhances O2 transport (increases in breathing and pulmonary O2 uptake), which exhibits a pattern of genetic assimilation in highlanders, as well as the attenuation of another regulated phenotype (sympathoadrenal activation) that is believed to be maladaptive in chronic hypoxia. Chronic sympathetic activation represents a significant cost of the plasticity underlying VAH, and this cost is likely avoided by using a different mechanism to induce a fixed increase in breathing and pulmonary O2 uptake. The fixed increase in breathing and pulmonary O2 uptake should also mitigate potential costs of blunting sympathoadrenal activation by the hypoxic chemoreflex. Therefore, the fact that both beneficial and counterproductive changes can result from plasticity in a single physiological control system may influence how the plasticity in such systems evolve.
GLOBAL CLIMATE CHANGE, PHENOTYPIC PLASTICITY, AND ALTITUDINAL RANGE SHIFTS
With warming temperatures, lowland species are expected to shift their elevational ranges upwards, potentially putting alpine and subalpine specialists at risk of competitive displacement. Prospects for the upslope advance of lowland species will depend critically on modes of acclimatization and adaptation to hypoxia. For cases in which phenotypic plasticity is adaptive, it seems reasonable to expect that acclimatization capacity should be a strong predictor of a species’ colonization success. However, studies of hypoxia acclimatization in humans and other lowland mammals suggest ways in which plasticity may actually impede high-altitude adaptation. Research to date suggests the hypothesis that a critical component of high-altitude adaptation involves the evolution of mechanisms for short-circuiting maladaptive acclimatization responses that are elicited by chronic hypoxia.
ACKNOWLEDGMENTS
J.F.S. acknowledges grant support from the National Institutes of Health (HL087216) and the National Science Foundation (MCB-1517636 and OIA-1736249), and G.R.S. acknowledges support from the Canada Research Chairs program.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
References
- Bassett DR Jr., Howley ET. 2000. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc 32:70–84 [DOI] [PubMed] [Google Scholar]
- Beall CM, Reichsman AB. 1984. Hemoglobin levels in a Himalayan high altitude population. Am. J. Phys.Anthr 63:301–6 [DOI] [PubMed] [Google Scholar]
- Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Almasy LA, et al. 1997. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am. J. Phys. Anthr 104:427–47 [DOI] [PubMed] [Google Scholar]
- Bernardi L, Passino C, Spadacini G, Calciati A, Robergs R, et al. 1998. Cardiovascular autonomic modulation and activity of carotid baroreceptors at altitude. Clin. Sci 95:565–73 [DOI] [PubMed] [Google Scholar]
- Black CP, Tenney SM. 1980. Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir. Physiol 39:217–39 [DOI] [PubMed] [Google Scholar]
- Brutsaert T 2016. Why are high altitude natives so strong at high altitude? Nature versus nurture: genetic factors versus growth and development In Advances in Experimental Medicine and Biology, Vol. 903, ed. Roach R, Hackett P, Wagner P, pp. 101–12. Boston: Springer; [DOI] [PubMed] [Google Scholar]
- Brutsaert TD. 2007. Population genetic aspects and phenotypic plasticity of ventilatory responses in high altitude natives. Respir. Physiol. Neurobiol 158:151–60 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Araoz M, Soria R, Spielvogel H, Haas JD. 2000. Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared to European sojourners and Europeans born and raised at high altitude. Am. J. Phys. Anthr 113:169–81 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, León-Velarde F. 2005. Ancestry explains the blunted ventilatory response to sustained hypoxia and lower exercise ventilation of Quechua altitude natives. Am.J. Physiol. Reg. Integr. Comp. Physiol 289:R225–34 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Palacios, et al. 2003. Spanish genetic admixture is associated with larger decrement from sea level to 4,338 m in Peruvian Quechua. J. Appl. Physiol 95:519–28 [DOI] [PubMed] [Google Scholar]
- Burri PH, Weibel ER. 1971. Morphometric estimation of pulmonary diffusion capacity. II. Effect of Po2 on the growing lung adaption of the growing rat lung to hypoxia and hyperoxia. Respir. Physiol 11:247–64 [DOI] [PubMed] [Google Scholar]
- Calbet JA. 2003. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J. Physiol 551:379–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, et al. 1997. Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. J. Appl. Physiol 83:661–67 [DOI] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. 2012. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. PNAS 109:8635–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Storz JF. 2013. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J. Exp. Biol 216:1160–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Connaty AD, McClelland GB, Storz JF. 2014. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68:48–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover DO, Duffy TA, Hice LA. 2009. The covariance between genetic and environmental influences across ecological gradients: reassessing the evolutionary significance of countergradient and cogradient variation. Ann. N.Y. Acad. Sci 1168:100–29 [DOI] [PubMed] [Google Scholar]
- Conover DO, Schultz ET. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol 10:248–52 [DOI] [PubMed] [Google Scholar]
- Dane DM, Cao K, Lu H, Yilmaz C, Dolan J, et al. 2018. Acclimatization of low altitude-bred deer mice (Peromyscus maniculatus) to high altitude. J. Appl. Physiol 125:1411–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson NJ, Ivy CM, Alza L, Cheek R, York JM, et al. 2016. Mitochondrial physiology in the skeletal and cardiac muscles is altered in torrent ducks, Merganetta armata, from high altitudes in the Andes. J. Exp. Biol 219:3719–28 [DOI] [PubMed] [Google Scholar]
- Dawson NJ, Lyons SA, Henry DA, Scott GR. 2018. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol 223:e13030. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Morgan BJ. 2015. Humans in hypoxia: a conspiracy of maladaptation?! Physiology 30:304–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS. 1990. Selection in different environments: effects on environmental sensitivity (reaction norm) and on mean performance. Genet. Res 56:57–70 [Google Scholar]
- Falconer DS, Mackay TFC. 1996. Introduction to Quantitative Genetics Harlow, UK: Longman Sci. Technol; 4th ed. [Google Scholar]
- Galen SC, Natarajan C, Moriyama H, Weber RE, Fago A, et al. 2015. Contribution of a mutational hotspot to adaptive changes in hemoglobin function in high-altitude Andean house wrens. PNAS 112:13958–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R-L, Kubo K, Kobayashi T, Sekiguchi M, Honda T. 1998. Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am. J. Physiol. Heart Circ. Physiol 274:H1792–99 [DOI] [PubMed] [Google Scholar]
- Ge R-L, Lun G-WH, Chen Q-H, Li H-L, Gen D, et al. 1995. Comparisons of oxygen transport between Tibetan and Han residents at moderate altitude. Wilderness Environ. Med 6:391–400 [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol 21:394–407 [Google Scholar]
- Gilbert-Kawai ET, Milledge JS, Grocott MP, Martin DS. 2014. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology 29:388–402 [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Kirkton SD, Howlett RA, Britton SL, Koch LG, et al. 2006. Continued divergence in of rats artificially selected for running endurance is mediated by greater convective blood O2 delivery. J. Appl. Physiol 101:1288–96 [DOI] [PubMed] [Google Scholar]
- Grether GF. 2005. Environmental change, phenotypic plasticity, and genetic compensation. Am. Nat 166:E115–23 [DOI] [PubMed] [Google Scholar]
- Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, et al. 1993. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J. Appl. Physiol 74:312–18 [DOI] [PubMed] [Google Scholar]
- Günther H, Brunner R, Klußmann FW. 1983. Spectral analysis of tremorine and cold tremor electromyograms in animal species of different size. Pflügers Archiv. Eur. J. Physiol 399:180–85 [DOI] [PubMed] [Google Scholar]
- Hainsworth R, Drinkhill MJ. 2007. Cardiovascular adjustments for life at high altitude. Respir. Physiol. Neurobiol 158:204–11 [DOI] [PubMed] [Google Scholar]
- Hayes JP. 1989. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol. Zool 62:732–44 [Google Scholar]
- Hayes JP, O’Connor CS. 1999. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53:1280–87 [DOI] [PubMed] [Google Scholar]
- Henderson KK, Wagner H, Favret F, Britton SL, Koch LG, et al. 2002. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. J. Appl. Physiol 93:1265–74 [DOI] [PubMed] [Google Scholar]
- Horscroft JA, Kotwica AO, Laner V, West JA, Hennis PJ, et al. 2017. Metabolic basis to Sherpa altitude adaptation. PNAS 114:6382–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, et al. 2009. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J. Appl. Physiol 106:1819–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia CCW, Carbayo JJ, Yan X, Bellotto DJ. 2005. Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir. Physiol. Neurobiol 147:105–15 [DOI] [PubMed] [Google Scholar]
- Hsia CCW, Johnson RL, McDonough P, Dane DM, Hurst MD, et al. 2007. Residence at 3,800-m altitude for 5 mo in growing dogs enhances lung diffusing capacity for oxygen that persists at least 2.5 years. J. Appl. Physiol 102:1448–55 [DOI] [PubMed] [Google Scholar]
- Ivy CM, Lague SL, York JM, Chua BA, Alza L, et al. 2019. Control of breathing and respiratory gas exchange in ducks native to high altitude in the Andes. J. Exp. Biol 222:jeb198622. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR. 2015. Control of breathing and the circulation in high-altitude mammals and birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol 186:66–74 [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR. 2017a. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiol 221:266–82 [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR. 2017b. Ventilatory acclimatization to hypoxia in mice: methodological considerations. Respir. Physiol. Neurobiol 235:95–103 [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR. 2018. Evolved changes in breathing and CO2 sensitivity in deer mice native to high altitudes. Am. J. Physiol. Reg. Integr. Comp. Physiol 315:R1027–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Storz JF, Fago A. 2016. Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp. Biochem. Physiol. A Mol. Integr. Physiol 195:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Claassen H, Cerretelli P. 1991. Muscle structure and performance capacity of Himalayan Sherpas. J. Appl. Physiol 70:1938–42 [DOI] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. 1996. Muscle ultrastructure and biochemistry of lowland Tibetans. J. Appl. Physiol 81:419–25 [DOI] [PubMed] [Google Scholar]
- Kirkton SD, Howlett RA, Gonzalez NC, Giuliano PG, Britton SL, et al. 2009. Continued artificial selection for running endurance in rats is associated with improved lung function. J. Appl. Physiol 106:1810–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR. 2012. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr.Physiol 2:141–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lague SL, Chua B, Alza L, Scott GR, Frappell PB, et al. 2017. Divergent respiratory and cardiovascular responses to hypoxia in bar-headed geese and Andean birds. J. Exp. Biol 220:4186–94 [DOI] [PubMed] [Google Scholar]
- Lechner AJ. 1977. Metabolic performance during hypoxia in native and acclimated pocket gophers. J. Appl. Physiol 43:965–70 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Sanchez J, Bigard AX, Brunet A, Lesty C, Monge C. 1993. High altitude tissue adaptation in Andean coots: capillarity, fibre area, fiber type and enzymatic activities of skeletal muscle. J. Comp. Physiol. B 163:52–58 [DOI] [PubMed] [Google Scholar]
- Levins R 1969. Thermal acclimation and heat resistance in Drosophila species. Am. Nat 103:483–99 [Google Scholar]
- Lindsey BG, Nuding SC, Segers LS, Morris KF. 2018. Carotid bodies and the integrated cardiorespiratory response to hypoxia. Physiology 33:281–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Gonzalez-Rodriguez P, Gao L, Fernandez-Aguera MC, Pardal R, Ortega-Saenz P. 2016. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am. J. Physiol. Cell Physiol 310:C629–42 [DOI] [PubMed] [Google Scholar]
- Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, et al. 2015. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am. J. Physiol. Reg. Integr. Comp. Physiol 308:R779–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, McClelland GB, Scott GR. 2017. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J. Physiol 595:4785–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina JN, McCracken KG, Chua B, York JM, Milsom WK. 2017. Morphological and morphometric specializations of the lung of the Andean goose, Chloephaga melanoptera: a lifelong high-altitude resident. PLOS ONE 12:e0174395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Costello O, Agey PJ, Wu L, Szewczak JM, MacMillen RE. 1998. Increased fiber capillarization in flight muscle of finch at altitude. Respir. Physiol 111:189–99 [DOI] [PubMed] [Google Scholar]
- McClelland GB, Scott GR. 2019. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol 81:561–83 [DOI] [PubMed] [Google Scholar]
- Monge C, León-Velarde F. 1991. Physiological adaptation to high altitude: oxygen-transport in mammals and birds. Physiol. Rev 71:1135–72 [DOI] [PubMed] [Google Scholar]
- Monge CC, Arregui A, León-Velarde F. 1992. Pathophysiology and epidemiology of chronic mountain sickness. Int. J. Sports Med 13:S79–81 [DOI] [PubMed] [Google Scholar]
- Moore LG. 2000. Comparative human ventilatory adaptation to high altitude. Respir. Physiol 121:257–76 [DOI] [PubMed] [Google Scholar]
- Moore LG, Charles SM, Julian CG. 2011. Humans at high altitude: hypoxia and fetal growth. Respir. Physiol. Neurobiol 178:181–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Hoffman FG, Lanier HC, Wolf CJ, Cheviron ZA, et al. 2015a. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol. Biol. Evol 32:978–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann FG, Weber RE, Fago A, Witt CC, Storz JF. 2016. Predictable convergence in hemoglobin function has unpredictable molecular underpinnings. Science 354:336–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340:1324–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Jendroszek A, Kumar A, Weber RE, Tame JRH, et al. 2018. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLOS Genet 14:e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Munoz-Fuentes V, et al. 2015b. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLOS Genet 11:e1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikel KE, Shanishchara NK, Ivy CM, Dawson NJ, Scott GR. 2017. Effects of hypoxia at different life stages on locomotory muscle phenotype in deer mice native to high altitude. Comp. Biochem. Physiol. B Biochem. Mol. Biol 224:98–104 [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Carr JA, Go A, Fu Z, Reid SG, Powell FL. 2014. Glutamate receptors in the nucleus tractus solitarius contribute to ventilatory acclimatization to hypoxia in rat. J. Physiol 592:1839–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Powell FL. 2016. Time domains of the hypoxic ventilatory response and their molecular basis. Compr. Physiol 6:1345–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J. 2007. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131:364–77 [DOI] [PubMed] [Google Scholar]
- Pearson KG, Acharya H, Fouad K. 2005. A new electrode configuration for recording electromyographic activity in behaving mice. J. Neurosci. Methods 148:36–42 [DOI] [PubMed] [Google Scholar]
- Pearson OP, Pearson A. 1976. A stereological analysis of the ultrastructure of the lungs of wild mice living at low and high altitude. J. Morphol 150:359–68 [DOI] [PubMed] [Google Scholar]
- Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, et al. 2009. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am.J. Physiol. Reg. Integr. Comp. Physiol 297:R978–87 [DOI] [PubMed] [Google Scholar]
- Pichon A, Zhenzhong B, Marchant D, Jin G, Voituron N, et al. 2013. Cardiac adaptation to high altitude in the plateau pika (Ochotona curzoniae). Physiol. Rep 1:e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL. 2007. The influence of chronic hypoxia upon chemoreception. Respir. Physiol. Neurobiol 157:154–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. 1998. Time domains of the hypoxic ventilatory response. Respir. Physiol 112:123–34 [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ. 2017. Oxygen sensing by the carotid body: past and present. Adv. Exp. Med. Biol 977:3–8 [DOI] [PubMed] [Google Scholar]
- Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, et al. 2013. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. PNAS 110:20669–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J-M, Folkow LP, Blix AS. 2007. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol 69:113–43 [DOI] [PubMed] [Google Scholar]
- Rhodes J 2005. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J. Appl. Physiol 98:1092–100 [DOI] [PubMed] [Google Scholar]
- Schippers M-P, Ramirez O, Arana M, Pinedo-Bernal P, McClelland GB. 2012. Increase in carbohydrate utilization in high-altitude Andean mice. Curr. Biol 22:2350–54 [DOI] [PubMed] [Google Scholar]
- Scott AL, Pranckevicius NA, Nurse CA, Scott GR. 2019. Regulation of catecholamine release from the adrenal medulla is altered in deer mice (Peromyscus maniculatus) native to high altitudes. Am. J. Physiol. Reg. Integr. Comp. Physiol 317:R407–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR. 2011. Elevated performance: the unique physiology of birds that fly at high altitudes. J. Exp. Biol 214:2455–62 [DOI] [PubMed] [Google Scholar]
- Scott GR, Egginton S, Richards JG, Milsom WK. 2009a. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R. Soc. B 276:3645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. 2015a. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol. Biol. Evol 32:1962–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Hawkes LA, Frappell PB, Butler PJ, Bishop CM, Milsom WK. 2015b. How bar-headed geese fly over the Himalayas. Physiology 30:107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Milsom WK. 2006. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir. Physiol. Neurobiol 154:284–301 [DOI] [PubMed] [Google Scholar]
- Scott GR, Milsom WK. 2007. Control of breathing and adaptation to high altitude in the bar-headed goose. Am. J. Physiol. Reg. Integr. Comp. Physiol 293:R379–91 [DOI] [PubMed] [Google Scholar]
- Scott GR, Milsom WK. 2009. Control of breathing in birds: implications for high altitude flight In Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects, ed. Glass ML, Wood SC, pp. 429–48. Berlin: Springer-Verlag [Google Scholar]
- Scott GR, Richards JG, Milsom WK. 2009b. Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am. J. Physiol. Reg. Integr. Comp. Physiol 297:R1066–74 [DOI] [PubMed] [Google Scholar]
- Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK. 2011. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol 28:351–63 [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. 1985. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J. Physiol 358:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Laurie SS. 2014. HIF and pulmonary vascular responses to hypoxia. J. Appl. Physiol 116:867–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenmann C, Robach P, Lundby C. 2017. Regulation of blood volume in lowlanders exposed to high altitude. J. Appl. Physiol 123:957–66 [DOI] [PubMed] [Google Scholar]
- Signore AV, Yang Y-Z, Yang Q-Y, Qin G, Moriyama H, et al. 2019. Adaptive changes in hemoglobin function in high-altitude Tibetan canids were derived via gene conversion and introgression. Mol. Biol. Evol In press. 10.1093/molbev/msz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Huff CD, Witherspoon DJ, Prchal JT, Jorde LB. 2015a. Adaptive genetic changes related to haemoglobin concentration in native high-altitude Tibetans. Exp. Physiol 100:1263–68 [DOI] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Qin G, et al. 2015b. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J. Physiol 593:3207–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. 1988. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J. Dev. Physiol 10:1–16 [PubMed] [Google Scholar]
- Storz JF. 2010. Genes for high altitudes. Science 329:40–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2016. Hemoglobin-oxygen affinity in high-altitude vertebrates: Is there evidence for an adaptive trend? J. Exp. Biol 219:3190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2019. Hemoglobin: Insights into Protein Structure, Function, and Evolution Oxford, UK: Oxford Univ. Press [Google Scholar]
- Storz JF, Bridgham JT, Kelly SA, Garland T. 2015. Genetic approaches in comparative and evolutionary physiology. Am. J. Physiol. Reg. Integr. Comp. Physiol R197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Cheviron ZA, McClelland GB, Scott GR. 2019. Evolution of physiological performance capacities and environmental adaptation: insights from high-elevation deer mice (Peromyscus maniculatus). J. Mammal 100:910–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. 2010a. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol 213:2565–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, et al. 2009. Evolutionary and functional insights into the mechanism underlying high altitude adaptation of deer mouse hemoglobin. PNAS 106:14450–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA. 2010b. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol 213:4125–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson ER, Bärtsch P. 2014. High Altitude: Human Adaptation to Hypoxia New York: Springer [Google Scholar]
- Sylvester JT, Shimoda LA, Aaronson PI, Ward JPT. 2012. Hypoxic pulmonary vasoconstriction. Physiol. Rev 92:367–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate KB, Ivy CM, Velotta JP, Storz JF, McClelland GB, et al. 2017. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. J. Exp. Biol 220:3616–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts DM, Natarajan C, Revsbech IG, Projecto-Garcia J, Hoffman FG, et al. 2015. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol 32:287–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta JP, Ivy CM, Wolf CJ, Scott GR, Cheviron ZA. 2018. Maladaptive phenotypic plasticity in cardiac muscle growth is suppressed in high-altitude deer mice. Evolution 72:2712–27 [DOI] [PubMed] [Google Scholar]
- Wagner PD. 1996. A theoretical analysis of factors determining VO2max at sea level and altitude. Respir. Physiol 106:329–43 [DOI] [PubMed] [Google Scholar]
- Wagner PD, Araoz M, Boushel R, Calbet JAL, Jessen B, et al. 2002. Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J. Appl. Physiol 92:1393–400 [DOI] [PubMed] [Google Scholar]
- Wagner PD, Simonson TS, Wei G, Wagner HE, Wuren T, et al. 2015. Sea-level haemoglobin concentration is associated with greater exercise capacity in Tibetan males at 4200 m. Exp. Physiol 100:1256–62 [DOI] [PubMed] [Google Scholar]
- Weibel ER, Taylor CR, Hoppeler H. 1991. The concept of symmorphosis: a testable hypothesis of structure-function relationship. PNAS 88:10357–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JM, Scadeng M, McCracken KG, Milsom WK. 2018. Respiratory mechanics and morphology of Tibetan and Andean high-altitude geese with divergent life histories. J. Exp. Biol 221:jeb170738. [DOI] [PubMed] [Google Scholar]
- Zhu X, Guan Y, Signore AV, Natarajan C, DuBay SG, et al. 2018. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. PNAS 115:1865–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, et al. 1996. Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3658 m). Respir. Physiol 103:75–82 [DOI] [PubMed] [Google Scholar]


