Abstract
Over the past 20 years, there has been tremendous progress in endovascular aneurysm repair techniques and devices. The application of new third- and fourth-generation devices (from 2003 onward) has led to changes in the incidence and management of endoleaks. This comprehensive review aims to outline the most recent concepts with respect to pathophysiology/risk factors and management of Type 1 endoleaks.
Keywords: endovascular aneurysm repair, endoleak, Palmaz stent, EndoAnchors, aortic neck, iliac artery, interventional radiology
The goal of endovascular repair of an abdominal aortic aneurysm (AAA) is complete exclusion of the aneurysmal aortic segment reducing the risk of rupture, and ideally resulting in aortic remodeling. Patients who have had an endovascular stent graft repair of the aneurysm (EVAR) undergo lifelong surveillance to evaluate for the presence of aneurysm expansion and endoleaks. An endoleak is defined as persistent pressurization of the aneurysm sac following EVAR. In technically successful EVARs, endoleaks usually manifest as opacification of the aneurysmal sac on routine contrast-enhanced computed tomographic (CT) surveillance imaging. Endoleaks can, however, also present as persistent aneurysm growth in the absence of sac opacification. It is a triphasic CT angiography (CTA) that is the most commonly used imaging modality to evaluate postoperative EVAR success and is highly sensitive and specific at detecting endoleaks. 1 2 With modern generation devices, endoleaks are reported in 4 to 30% of patients after EVAR 3 4 and carry the risk of subsequent increase in aneurysm sac size and rupture. 3 4 5
Endoleaks can be classified into five types based on their etiology and location. This article will focus on Type 1 (T1) endoleaks which occur at the EVAR proximal and distal seal zones when incomplete apposition of the device with the native arterial wall allows continued flow into the aneurysmal aorta. T1 endoleaks can occur both at the time of device implantation or in the early post-operative period, suggesting failure of the index procedure, or in a delayed fashion due to aortic remodeling. The incidence of early and delayed (12 months) T1 endoleaks is 3.5 and 6.8%, respectively. 5 T1 endoleaks can be subclassified based on the location of the device failure occurring either in the proximal repair (T1a) or distal repair (T1b), or at the site of contralateral iliac occlusion in an aorto-uni-iliac (AUI) graft (T1c). T1c endoleaks are beyond the scope of this article and will not be discussed in further detail.
The importance of endoleaks, in particular T1, in relation to the risk of AAA rupture is related to the pressure within the aneurysm sac. Given that T1 endoleaks result in direct inline systemic blood flow to the aneurysm sac, management centers on aggressive intervention and repair. 6 7 Because as many as 10% of patients require reintervention due to T1 endoleaks seen on 30-day surveillance CTAs, it is essential to have optimal intraoperative imaging. Detection of a T1 endoleaks is essential, as 74% of post-EVAR ruptures are attributed to T1 endoleaks. 8
Risk Factors for Development of Type 1a Endoleak
The leading determinant of T1a endoleak risk is the quality of the proximal seal between the endograft and the native aortic wall. Prevention of a T1a endoleak is made difficult if there are anatomic features such as short neck, severe angulation or tortuosity, large neck diameter, thrombus, or calcification at the proximal landing zone 9 ( Table 1 ). Although many physicians (nearly 55%) often choose to utilize a liberal interpretation of anatomic characteristics deemed suitable for EVAR, 10 strict compliance with indications for use (IFU) anatomic guidelines for each stent graft reduces the incidence of endoleaks.
Table 1. Reported risk factors for Type 1a endoleaks after standard EVAR.
| Risk factors |
|---|
| Reversed taper anatomy 64 |
| Conical neck 64 |
| Stent graft deployment outside IFU 11 |
| Pronounced aortic curvature 65 |
| Aortic neck dilatation/remodeling (> 2 mm/y) 23 |
| Emergency EVAR (13–16) |
| Large neck (> 30 mm) |
| Short neck (< 15 mm) |
| Neck mural thrombus/calcifications (> 50%) |
| Steep neck angulation (> 60 degrees) 65 |
Abbreviations: EVAR, endovascular aneurysm repair; IFU, instructions for use.
Diameter of Proximal Neck
Depending on the aortic stent graft used, an infrarenal neck with a diameter of more than 30 mm is generally considered unsuitable, largely due to the need to oversize the graft by 20 to 30% with respect to the aortic wall. 11 Although newer generation stent grafts are capable of accommodating even larger infrarenal diameters, the aortic neck anatomy remains the primary determinant of an effective proximal seal, and thus of a subsequent T1 endoleak.
Length of Proximal Neck
To achieve an adequate seal between the stent graft and the aortic wall, snug apposition over a length of 10 to 15 mm is required. 11 Some limitations of the large diameter and short length of the infrarenal neck are overcome with the introduction of EndoAnchors as well as fenestrated and branched stent grafts, as will be discussed in the subsequent treatment section.
Angulation of the Proximal Neck
Aortic neck angulation is an important determinant of outcome after EVAR. 12 Angulation is more often seen with larger aneurysms (>60 mm). The current stent graft systems are designed primarily as straight neck sealing zone system. Although some specific stent grafts are more flexible, most are not engineered to seal in necks with greater than 60 degrees of angulation defined as the angle between the proximal aortic neck and longitudinal axis of the aneurysm ( Fig. 1a ).
Fig. 1.
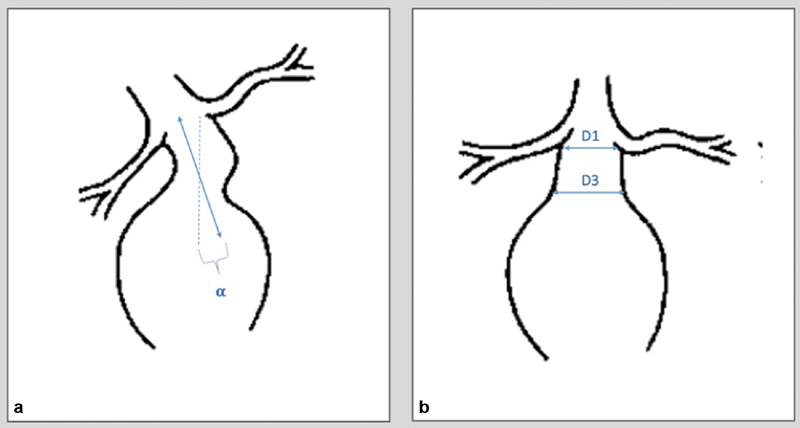
( a ) The aortic neck angulation is formed between points connecting the lowest renal artery and the origin of the aneurysm. The neck angle (α) should be < 60 degrees. ( b ) A conical/reversed taper neck is present when there is progressive increase of at least 3 mm from the lowest renal artery (D1) to the most proximal part of the aneurysm sac (D3).
During the index case, a several angulated neck increases the technical difficulty of accurate device deployment. Due to the challenge in positioning the device, there is an inherent inability of device to confirm to the aneurysm neck, resulting in inadequate seal zone and proper aneurysm exclusion. Over time, proximal angulation has negative long-term effects of the endovascular repair stability. Because of the geometry of the proximal seal zone, a high degree of angulation decreases the necessary down force required to dislodge the endograft, thus making distal migration of the stent graft more likely. 13 Although mild angulation (< 40 degrees) tends to not affect outcomes, moderate (40–59 degrees) and severe (> 60 degrees) angulation results in a higher risk of T1a endoleaks. 14
Conical Nature of the Proximal Neck
A cone-shaped or reversed tapered neck is regarded as a contraindication in most commercially available stent grafts. A conical neck is most commonly defined as a progressive increase of more than 3 mm between the lowest renal artery and the proximal sac ( Fig. 1b ). 15 A conical neck adds to the risk of postprocedural complications due to the difficulty of proximal stent fixation. 15 16 17 18
It is well known that oversizing the graft by 20 to 30% in the proximal seal zone significantly decreases the incidence of T1a endoleaks. 19 20 21 The ability to do this in the setting of conical neck presents a challenge. Due to the reverse taper nature of the neck as shown in Fig. 1b , when oversizing by the most distal and largest diameter of the neck, excessive oversizing at the proximal edge is likely to occur. Such excessive oversizing is thought to result in late aortic neck dilation, which increases the incidence of caudal device migration. 22 Furthermore, conical morphology in the aneurysm neck most likely represents an early stage in the process of aneurysmal dilatation and therefore can lead to neck dilation from disease progression. 19 20 Precise and thoughtful planning on morphologic evaluations of the proximal neck prior to EVAR delivery are essential to prevent T1a endoleaks.
Calcification and Mural Thrombus in Proximal Neck
Calcification and mural thrombus in the proximal neck are expressed in degrees of circumference. Mural thrombus in the neck of more than 90 degrees is considered a risk factor for endoleak due to poor apposition and the potential to remodel over time, whereas extensive calcification increases the probability of stent graft migration.
Aortic Remodeling
Aortic neck dilatation due to remodeling after EVAR deployment represents an important risk factor for T1a endoleak. To date, aortic remodeling and neck dilatation after EVAR represent a challenging reality related to late adverse events and ruptures. 23 Neck dilatation is defined as an increase in aortic neck size of >2 mm or 15%. 24 The pathophysiology of proximal aortic neck dilatation maybe linked to long-standing outward force of oversized self-expanding stents, although this is primarily speculative and the etiology of neck dilatation remains under study. 24
Overall, patients with any of the aforementioned anatomical characteristics, who receive an EVAR outside of the IFU, show a higher incidence of T1a endoleak before presenting with rupture (44% outside IFU vs. 6% within IFU; p < 0.001) and more frequently required an open repair (44 vs. 12%; p < 0.002) with higher perioperative mortality (10.3 vs. 0%; p < 0.01). 25
Risk Factors for Development of Type 1b Endoleak
Similar to T1a endoleaks, the leading determinant of T1b endoleak risk is the quality of the distal seal between the endograft and the native common iliac or external iliac arterial wall. Prevention of a T1b endoleak is made difficult if there are anatomic features such as aneurysmal iliac arteries, tortuosity of the iliac arteries, and short landing zones (<15 mm) ( Table 2 ). Much of the difficulty in reporting iliac complications is related to the ability to accurately assess and reproduce standardized measurements. As with proximal neck measurements, it is essential to ensure center-line measurements using anatomical landmarks to provide for standardization over time.
Table 2. Reported risks factors for Type 1b endoleaks after standard endovascular aneurysm repair.
Diameter and Length of the Distal Seal Zone
In standard EVAR placement, the distal stent graft seals in the common iliac artery. Similar to as in the proximal sealing zone, snug apposition of the graft and artery over a length of 10 to 15 mm is required to achieve an adequate seal. Depending on the device, it may not be possible to effectively seal in the common iliac artery. In these cases, the stent graft can be extended into the external iliac artery with either the use of branched iliac endografts or concomitant coil-embolize of the hypogastric artery.
Iliac Remodeling
Over 50% of T1b endoleaks occur within 6 months of the index operation for an EVAR. 9 Recent studies have outlined that both landing zones and iliac arteries are subjected to the outward radial force of the graft, potentially leading to T1b endoleaks. 9 26 27 The rate of iliac artery expansion/remodeling is greatest in the first 6 months at nearly a rate of 1 mm/month. 26 Similar to T1a endoleaks, the exact physiology for iliac artery remodeling is not well understood and primarily relies on in vitro studies. 28
Limb Retraction
Limb retraction occurs when the distance between the iliac bifurcation and the distal edge of the last seen has increased by ≥ 5 mm. As noted in Table 2 , iliac endografts with a diameter greater than 24 mm and of a “bell-bottom” design are associated with an increased risk of T1b endoleaks during midterm follow-up; this seems to be related to limb retraction at 5 years. 9 29 Only the length of initial iliac limb seal achieved during the initial operation is protective.
Extra attention should be given to close surveillance of patients with large aortic aneurysms and endograft limbs ≥ 24 mm, especially in the long term (3+ years from the index operation). A CTA is necessary to determine progressive loss of seal and/or retraction. As a result, these patients should have a surveillance schedule that includes CT scanning occasionally, as iliac limb complications are often a late phenomenon and thus CT scan cannot be waived completely overtime.
Diagnosis of Endoleak
T1 endoleaks are usually evident on the post stent graft deployment angiography. These can be seen as continued sac opacification resulting from insufficient proximal or distal seal. Reasonable attempts should be made to address these at the time of the index operation.
Even in the setting of technically successful EVAR, routine surveillance is essential. Surveillance modalities include radiography, ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). In general, CT angiography (CTA) is the accepted standard of care. 6 As a result, most patients undergoing EVAR have a CTA performed at 1, 6, and 12 months followed by yearly examinations thereafter, provided there are no complications or requirements for intervention based on prior imaging. On CTA/MRA, T1 endoleaks will be visualized as contrast adjacent to or in communication with the proximal or distal seal zone as well as in the aneurysm sac. They can frequently be seen on both the arterial phase and delayed phase images, in counter to other types of endoleaks that are more readily apparent on the delayed images. In general, T1 endoleaks seen on surveillance imaging should be treated promptly, with the aim of excluding the aneurysm from pressured circulation.
Type 1a Endoleak Treatment
Once an endoleak has been confirmed, management has generally consisted of aggressive endovascular repair. Given that with a T1a endoleak, there is a direct communication between the systemic blood flow and aneurysm sac, leading to pressurization and risk of rupture, it is essential to repair at the time of diagnosis. 30 31 32 For T1a endoleaks discovered at the index operation, the Society for Vascular Surgery recommends that every attempt should be made to resolve T1a endoleaks prior to leaving the endovascular suite. 33 There are several endovascular adjunct options that will be discussed in further detail later ( Table 3 ). 34 35 36 37 If an endovascular option is not available in reasonable timeframe and the patient is fit for open surgical repair, open conversion can be performed with acceptable results. 38
Table 3. Treatment modalities and their reported success rates for Type 1a endoleaks after standard EVAR.
| Treatment modalities | Technical success | Freedom from recurrent endoleak |
|---|---|---|
| Aortic balloon angioplasty +/− cuff/stent extension | 100% | 87% |
| Embolization (coils + glue) | 90–100% | 80% |
| EndoAnchors | 90–100% | 95–97% |
| Parallel stent grafts | 90–94.4% | 90–100% |
| Surgical conversion | > 90% | N/A |
Abbreviation: EVAR, endovascular aneurysm repair.
Balloon Dilation
If a T1a endoleak is noted on the completion angiography of the procedure, repeated intraoperative molding of the proximal seal zone with a compliant balloon to ensure apposition of the proximal stent graft to the aortic wall may result in resolution of the endoleak. The outcomes of this adjunct maneuver have shown 62 to 85% rate of resolution for the T1a endoleak. 39 This is most appropriate in situations in which the leading edge of the graft appears to be in good position immediately below the renal arteries.
Aortic Cuffs
If the initial endograft does not cover the entire aortic seal zone, or there is significant additional healthy aorta between the proximal edge of the graft and the renal arteries, the endoleak can be treated by placing a proximal extension cuff. This usually requires at least 10 mm additional sealable aorta to accommodate the cuff within the nonbifurcated portion of the main body. The overlap of the components should be generous (2 cm or more if possible) to maintain component stability.
Large-Caliber Balloon-Expandable Stents
If the aortic stent graft is believed to be placed in the optimal proximal location and a T1a endoleak persists after balloon dilation, stent graft apposition of the aortic wall might be achieved by placing a large, balloon-expandable stent proximally. The uncovered Palmaz stent ( Fig. 2 ; Palmaz P4014; Cordis, Miami Lakes, FL) 40 41 aims to improve graft alignment to the aortic wall. The overall success rate of the Palmaz stent is over 80% with only 5 to 10% requiring two Palmaz stents. 42
Fig. 2.
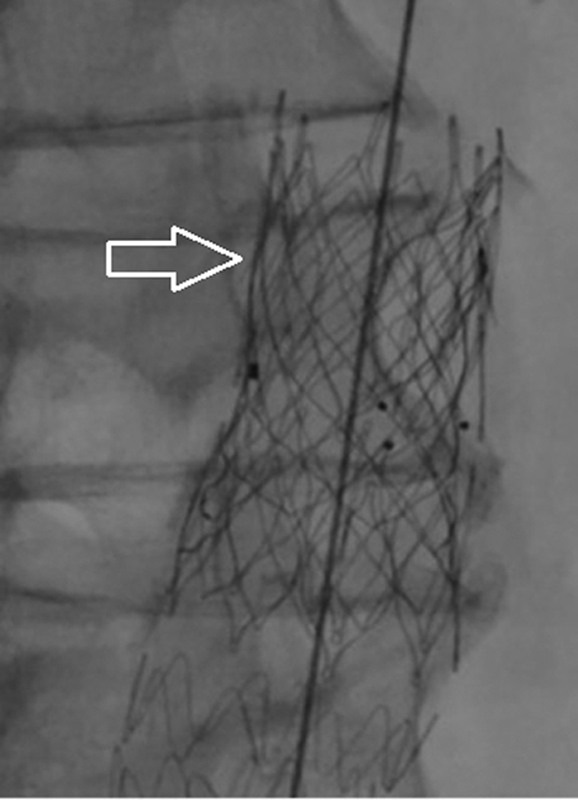
Deployment of a Palmaz stent (arrow) following infolding of the proximal part of the aortic stent graft, resulting in a T1a endoleak.
EndoAnchor
Heli-FX EndoAnchor systems (Medtronic Vascular, Santa Rosa, CA) are small metal, corkscrew-shaped screws that were originally developed to provide a better alignment of stent grafts in the setting of challenging aortic necks ( Fig. 3 ). They are deployed from a 16-Fr device at either the time of initial EVAR or in a delayed fashion. The theory behind their use is that directly fixing the graft to the aorta results in better apposition and decreased risk of graft migration. In a multicenter registry of 208 cases of primary prophylactic use of endostaples, technical failure (3/57, 5.3%) and T1a endoleak (2/45, 4.4%) were more prevalent in patients with aortic neck <10 mm as opposed to necks > 10 mm. 36 After a mean follow-up of 14 months in 130 patients, the prevalence of T1a endoleaks was 1.5% ( n = 2). Additionally in the prospective matched control study of Aneurysm Treatment Using the Heli-FX Aortic Securement System Global Registry (ANCHOR), the rate of sac regression was significantly higher in the patient cohort treated with EndoAnchors compared with the control (81.1 ± 9.5% vs. 48.7 ± 5.9%, p = 0.01). 43
Fig. 3.
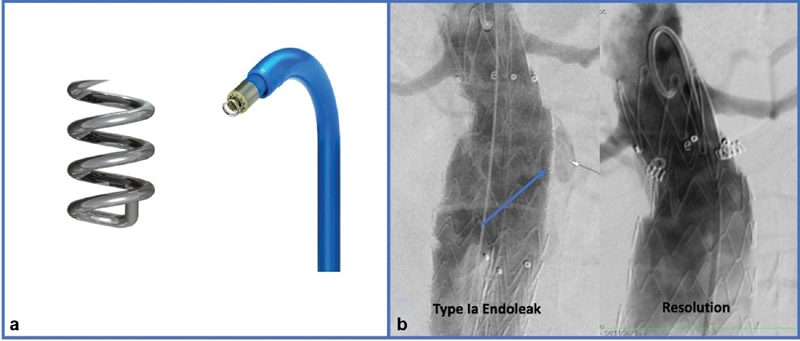
EndoAnchors. ( a ) EndoAnchor dimension of 4.5 mm in length and 3.5 mm in diameter. Current catheter used to directionally place the EndoAnchors in the proximal neck with a deflection tip. ( b ) Type 1a endoleak noted on completion angiography (blue arrow). Four EndoAnchors were placed in the proximal neck resulting in resolution of the T1a endoleak on the final angiography.
Aside from their use in primary prevention of T1 endoleaks, endostaples have been utilized to treat either immediate or delayed endoleaks, either by themselves or in combination with aortic extension cuffs. 44
Fenestrated Aortic Stent Grafts
Fenestrated aortic stent graft (FEVAR) offers a unique solution for endovascular treatment of juxtarenal aortic aneurysms. FEVAR was designed to allow for extension of the proximal seal zone into the visceral segment of the aorta through the use of devices that have fenestrations to allow for stent grafting of the renal and mesenteric arteries. In select cases where there is not adequate aortic length between the proximal endograft and the renal arteries to extend the construct with a traditional aortic extension cuff, it may be possible to use a proximal FEVAR device. For patients who present with a T1a endoleak on subsequent imaging or caudal device migration overtime resulting in a reduced landing zone, a fenestrated endograft provides a solution for proximal extension. 45
In the United States, the commercially available endograft that can be employed for this purpose is the Cook Zenith aortic stent graft which was approved by the Food and Drug Administration in 2012 for endovascular treatment of short-neck infrarenal and juxtarenal aneurysms. 29 Up to three fenestration or scallops are incorporated into the main body to allow continued flow into the visceral vessels while permitting extension of the proximal landing zone from the infrarenal aorta to the paravisceral region.
Fenestrated endografts are a growing option to treat challenging proximal aortic neck anatomy. However, FEVARs are expensive, time consuming to produce (often a minimum of 6 weeks), require renal artery manipulation during the procedure, and require advanced skills for successful implantation.
Chimney Grafts
The use of covered renal or visceral artery stents parallel to the aortic stent graft allows an extension of the aortic seal zone into the paravisceral segment; this approach to treating visceral aortic disease has been alternatively referred to as “snorkel,” “chimney,” or “periscope” grafting. 46 47 Chimney grafting was first described as the primary treatment for aortic aneurysms involving the renal and visceral segment but has been adapted to treat persistent T1a endoleaks. It has the advantage, as compared with FEVAR, of using commonly available, off-the-shelf devices. “Snorkeling” or “chimney” techniques (the so-called Ch-EVAR) refer to the placement of a covered stent into a branched vessel with the proximal part of the stent extending above the main aortic stent graft. The “periscope” or “reverse chimney” technique described placement of the covered stent below the distal edge of the main aortic stent graft.
Ch-EVAR is accomplished by obtaining access for both the main aortic device and the target renal or visceral arteries. It commonly involves bilateral femoral access for the main aortic device and left axillary access to assist with cannulation of the renal and/or visceral arteries. The branch vessel is cannulated from the arm access and a 6- to 8-Fr sheath is advanced to the targeted vessel. Through this sheath, a balloon-expandable or self-expanding stent is advanced. The main aortic stent graft is then delivered via the conventional femoral approach and deployed across the visceral sheaths. The chimney stent graft can then be adjusted as needed and then deployed. Molding balloons are simultaneously inflated in the main aortic stent graft and the chimney stent in a kissing fashion. It is essential to maintain guidewire access to the renal and/or visceral arteries until the completion angiography is performed.
Initial results on the technical feasibility and early results of the chimney technique were very promising. 48 49 50 However, recent reports have been more cautious, as there has been a 30-day procedure-related major complication rate of 25 to 40%. 51 52 Limitations of the Ch-EVAR technique include loss of wall apposition with resultant “gutter” leakage leading to persistent T1a endoleaks. Also this was not an insignificant rate of the stents becoming kinked, compressed, or occluded. 53
Embolization
When conventional techniques have failed or are unsuitable and there is a prohibitive surgical risk, transcatheter embolization offers an alternative management option. 54 The surgical approach commonly involves femoral access, although if an antegrade approach is more anatomically favorable, left brachial access can be used. A 2.4-Fr microcatheter (Terumo Interventional Systems, Somerset, NJ) is advanced inside a 6-Fr Cobra II-guiding catheter (Boston Scientific, Natick, MA). The glue or fibered microcoils can be released into the aortic wall neck and graft.
Intervention on T1a endoleaks with targeted embolization using Onyx glue is limited. The largest study to date from Ameli-Renani et al 10 reported 100% immediate technical success, with freedom from endoleak recurrence of 80% and freedom from sac growth of 85% at nearly 3.5 years; however, the numbers were small (27 embolizations in 25 unique patients). Embolization is usually a procedure of last resort when other bailout techniques have failed, are not possible, and the patient is not a candidate for open revision.
Type 1b Endoleak Treatment
Like T1a endoleaks, T1b endoleaks seen at the time of EVAR implantation should generally be addressed at the time of the index procedure if technically feasible ( Table 4 ). With rare exceptions, T1b endoleaks can be managed endovascularly and do not routinely require open surgical repair. In recent years, less aggressive oversizing of the endograft limbs has been suggested to reduce the risk of limb occlusions, retrograde displacement, as well as adverse iliac remodeling. 43 55
Table 4. Treatment modalities and their reported success rates for Type 1b endoleaks after standard EVAR.
| Treatment modalities | Technical success | Freedom from recurrent endoleak |
|---|---|---|
| Limb extension | 100% | 94% without embolization |
| Internal iliac artery embolization | 100% | 75% |
| Surgical/hybrid approach | > 90% | N/A |
Abbreviation: EVAR, endovascular aneurysm repair.
Limb Extension with Possible Internal Iliac Embolization
The most common and straightforward treatment of T1b endoleak is limb extension with or without embolization of the internal iliac artery depending on location of the new distal seal zone ( Fig. 4 ). Limb extension into the external iliac artery without embolization of the internal iliac is not advised, as the resultant T2 endoleak may lead to the expansion of the iliac artery and adverse remodeling. 9 Reappearance of the endoleak following extension and embolization may occur in 10% of cases. Unilateral internal iliac artery embolization is usually well tolerated; the greatest morbidity is buttock claudication for the patient which was reported in 6.7% of the cases. 9
Fig. 4.
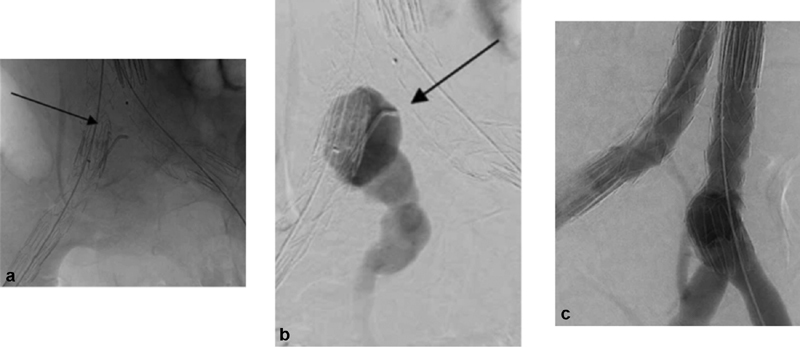
Endovascular aneurysm repair with embolization of the internal iliac system. ( a ) Aortic aneurysm involved the right common iliac system (arrow). Catheter is in the internal iliac artery. ( b ) Contrast is injected into the internal iliac artery origin (arrow). ( c ) Completion angiography with embolization of the internal iliac system and extension of the stent graft into the external iliac artery.
Iliac branched Device
The iliac branch devices allow extension of the distal seal zone into the external iliac artery while preserving perfusion to the hypogastric artery to facilitate a distal seal in short common iliac arteries or if there are concomitant iliac artery aneurysms ( Fig. 5 ). This technique can be performed bilaterally or after embolization of one of the hypogastric arteries. The goal is to reduce the risk of complications from bilateral hypogastric artery embolization such as buttock necrosis, bowel ischemia, ischemic colitis, and sexual dysfunction. 56 The benefit of the off shelf iliac branched devices is that it reduces the risk of hypogastric embolization and gutter leaks that can be associated with a snorkel. These devices were originally designed to be placed at the time of the index operation, but can be utilized as a secondary intervention for delayed T1b endoleaks. 57
Fig. 5.
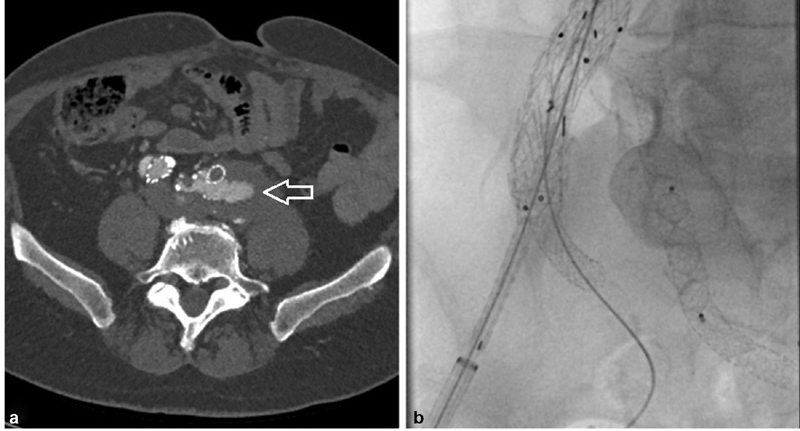
Iliac branch device. ( a ) One-month postoperative CTA that shows a T1b endoleak (arrow) from the distal landing zone in the left common iliac with corresponding increase in both the aortic and common iliac aneurysms. ( b ) Intraoperative angiography of the iliac branch endoprosthesis with wire access in both the external and internal iliac arteries.
Several companies have developed branched devices that allow stent graft deployment into both branches of the common iliac artery. Currently, the Gore Excluder Iliac Branch Endoprosthesis is approved for use in the United States. COOK Zenith Branch Endovascular Graft-Iliac Bifurcation is approved for use in Europe, but it is still under investigation in the United States. Both the U.S. GORE Pivotal trial 58 and the Global Registry for Endovascular Aortic Treatment (GREAT) 59 demonstrated nearly 95% patency, 100% freedom from T1 and T3 endoleaks, as well as less than 3% rate of intervention at 6 months.
Conversion to Aorto-Uni-Iliac Endograft
There are cases where endografting with a bifurcated endoprosthesis is contraindicated due to anatomical restrictions such as inadequate length to extend into the external iliac artery or possible thrombus or calcification. In these circumstances, AUI endograft and femorofemoral crossover bypass can overcome the limitations. An AUI is a particularly helpful option for high-risk patients with complex iliac artery anatomy with persistent T1b endoleak.
Watchful Waiting
There is a small, but coherent body of evidence that suggest that an T1a endoleaks detected at the intraoperative completion angiography may warrant a watchful waiting approach, 60 61 62 rather than immediate intervention. However, the decision to leave the operating room with a T1a endoleak is not to be taken lightly and dependent on multiple factors, including not only the rate of aneurysmal sac filling but also the possible interventions available at that time. A recent study 60 found that most T1a endoleaks resolve within a year without rupture or secondary intervention. This finding was further supported by Qazi et al 62 who noted that 92% of T1a endoleaks seen on final angiography resolve over the following 2.5 years without intervention. Further studies are necessary before advocating such an approach, and in lieu of further data, an aggressive stance toward management of T1 endoleaks should be maintained as is recognized in contemporary EVAR guidelines. 4 63
Conclusion
T1 endoleaks can frequently be treated by secondary endovascular interventions. Since the introduction of EVAR to the armamentarium for treatment of AAAs, the incidence of endoleaks and reinterventions has significantly decreased with the introduction of third- and fourth-generation devices. In addition, both treatment and prevention of T1 endoleaks has a high success rate, with low complication and endoleak recurrence.
References
- 1.Görich J, Rilinger N, Sokiranski R. Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology. 1999;213(03):767–772. doi: 10.1148/radiology.213.3.r99dc04767. [DOI] [PubMed] [Google Scholar]
- 2.Rozenblit A M, Patlas M, Rosenbaum A T. Detection of endoleaks after endovascular repair of abdominal aortic aneurysm: value of unenhanced and delayed helical CT acquisitions. Radiology. 2003;227(02):426–433. doi: 10.1148/radiol.2272020555. [DOI] [PubMed] [Google Scholar]
- 3.Rand T, Uberoi R, Cil B, Munneke G, Tsetis D. Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR) Cardiovasc Intervent Radiol. 2013;36(01):35–45. doi: 10.1007/s00270-012-0439-4. [DOI] [PubMed] [Google Scholar]
- 4.Chaikof E L, Dalman R L, Eskandari M K. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(01):2–7700. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Zaiem F, Almasri J, Tello M, Prokop L J, Chaikof E L, Murad M H. A systematic review of surveillance after endovascular aortic repair. J Vasc Surg. 2018;67(01):320–3.31E39. doi: 10.1016/j.jvs.2017.04.058. [DOI] [PubMed] [Google Scholar]
- 6.European Society for Vascular Surgery . Moll F L, Powell J T, Fraedrich G. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 01:S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Harris P L, Vallabhaneni S R, Desgranges P, Becquemin J P, van Marrewijk C, Laheij R J. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(04):739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 8.Mehta M, Paty P SK, Roddy S P. Treatment options for delayed AAA rupture following endovascular repair. J Vasc Surg. 2011;53(01):14–20. doi: 10.1016/j.jvs.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Bianchini Massoni C, Perini P, Tecchio T, Azzarone M, de Troia A, Freyrie A. A systematic review of treatment modalities and outcomes of type Ib endoleak after endovascular abdominal aneurysm repair. Vascular. 2018;26(01):90–98. doi: 10.1177/1708538117726468. [DOI] [PubMed] [Google Scholar]
- 10.Ameli-Renani S, Pavlidis V, Morgan R A. Early and midterm outcomes after transcatheter embolization of type I endoleaks in 25 patients. J Vasc Surg. 2017;65(02):346–355. doi: 10.1016/j.jvs.2016.06.101. [DOI] [PubMed] [Google Scholar]
- 11.Schuurmann R CL, van Noort K, Overeem S P. Aortic curvature is a predictor of late Type Ia endoleak and migration after endovascular aneurysm repair. J Endovasc Ther. 2017;24(03):411–417. doi: 10.1177/1526602817700378. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira N FG, Gonçalves F B, Hoeks S E. Long-term outcomes of standard endovascular aneurysm repair in patients with severe neck angulation. J Vasc Surg. 2018;68(06):1725–1735. doi: 10.1016/j.jvs.2018.03.427. [DOI] [PubMed] [Google Scholar]
- 13.Rahmani S, Grewal I S, Nabovati A, Doyle M G, Roche-Nagle G, Tse L W. Increasing angulation decreases measured aortic stent graft pullout forces. J Vasc Surg. 2016;63(02):493–499. doi: 10.1016/j.jvs.2014.06.115. [DOI] [PubMed] [Google Scholar]
- 14.Sternbergh W C, III, Carter G, York J W, Yoselevitz M, Money S R. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2002;35(03):482–486. doi: 10.1067/mva.2002.119506. [DOI] [PubMed] [Google Scholar]
- 15.Stanley B M, Semmens J B, Mai Q. Evaluation of patient selection guidelines for endoluminal AAA repair with the Zenith Stent-Graft: the Australasian experience. J Endovasc Ther. 2001;8(05):457–464. doi: 10.1177/152660280100800506. [DOI] [PubMed] [Google Scholar]
- 16.Chuter T AM. Stent-graft design: the good, the bad and the ugly. Cardiovasc Surg. 2002;10(01):7–13. doi: 10.1016/s0967-2109(01)00120-x. [DOI] [PubMed] [Google Scholar]
- 17.Lobato A C, Quick R C, Vaughn P L, Rodriguez-Lopez J, Douglas M, Diethrich E B. Transrenal fixation of aortic endografts: intermediate follow-up of a single-center experience. J Endovasc Ther. 2000;7(04):273–278. doi: 10.1177/152660280000700403. [DOI] [PubMed] [Google Scholar]
- 18.Dias N V, Resch T, Malina M, Lindblad B, Ivancev K. Intraoperative proximal endoleaks during AAA stent-graft repair: evaluation of risk factors and treatment with Palmaz stents. J Endovasc Ther. 2001;8(03):268–273. doi: 10.1177/152660280100800306. [DOI] [PubMed] [Google Scholar]
- 19.van Prehn J, Schlösser F JV, Muhs B E, Verhagen H JM, Moll F L, van Herwaarden J A. Oversizing of aortic stent grafts for abdominal aneurysm repair: a systematic review of the benefits and risks. Eur J Vasc Endovasc Surg. 2009;38(01):42–53. doi: 10.1016/j.ejvs.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 20.EUROSTAR Collaborators . Mohan I V, Laheij R JF, Harris P L. Risk factors for endoleak and the evidence for stent-graft oversizing in patients undergoing endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2001;21(04):344–349. doi: 10.1053/ejvs.2000.1341. [DOI] [PubMed] [Google Scholar]
- 21.Lin K K, Kratzberg J A, Raghavan M L. Role of aortic stent graft oversizing and barb characteristics on folding. J Vasc Surg. 2012;55(05):1401–1409. doi: 10.1016/j.jvs.2011.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conners M S, III, Sternbergh W C, III, Carter G, Tonnessen B H, Yoselevitz M, Money S R. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: a cautionary note. J Vasc Surg. 2002;36(03):476–484. doi: 10.1067/mva.2002.126561. [DOI] [PubMed] [Google Scholar]
- 23.Ribner A S, Tassiopoulos A K. Postoperative aortic neck dilation: myth or fact? Int J Angiol. 2018;27(02):110–113. doi: 10.1055/s-0038-1649516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouvelos G N, Oikonomou K, Antoniou G A, Verhoeven E LG, Katsargyris A. A systematic review of proximal neck dilatation after endovascular repair for abdominal aortic aneurysm. J Endovasc Ther. 2017;24(01):59–67. doi: 10.1177/1526602816673325. [DOI] [PubMed] [Google Scholar]
- 25.Zacharias N, Warner C J, Taggert J B. Anatomic characteristics of abdominal aortic aneurysms presenting with delayed rupture after endovascular aneurysm repair. J Vasc Surg. 2016;64:1629–1632. doi: 10.1016/j.jvs.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 26.Tsilimparis N, Dayama A, Ricotta J J., II Remodeling of aortic aneurysm and aortic neck on follow-up after endovascular repair with suprarenal fixation. J Vasc Surg. 2015;61(01):28–34. doi: 10.1016/j.jvs.2014.06.104. [DOI] [PubMed] [Google Scholar]
- 27.Coulston J, Baigent A, Selvachandran H, Jones S, Torella F, Fisher R. The impact of endovascular aneurysm repair on aortoiliac tortuosity and its use as a predictor of iliac limb complications. J Vasc Surg. 2014;60:585–589. doi: 10.1016/j.jvs.2014.03.279. [DOI] [PubMed] [Google Scholar]
- 28.Roos H, Tokarev M, Chernoray V. Displacement forces in stent grafts: influence of diameter variation and curvature asymmetry. Eur J Vasc Endovasc Surg. 2016;52(02):150–156. doi: 10.1016/j.ejvs.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Keisin Wang S, Drucker N A, Motaganahalli R L, Gupta A K, Fajardo A C. Fenestrated aortic endografts in the last 3 years: an update. Curr Surg Rep. 2016;4(11):1–8. [Google Scholar]
- 30.Harris P L, Vallabhaneni S R, Desgranges P, Becquemin J P, van Marrewijk C, Laheij R JF. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(04):739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 31.van Marrewijk C, Buth J, Harris P L, Norgren L, Nevelsteen A, Wyatt M G. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2002;35(03):461–473. doi: 10.1067/mva.2002.118823. [DOI] [PubMed] [Google Scholar]
- 32.Schurink G WH, Aarts N JM, Wilde J. Endoleakage after stent-graft treatment of abdominal aneurysm: implications on pressure and imaging--an in vitro study. J Vasc Surg. 1998;28(02):234–241. doi: 10.1016/s0741-5214(98)70159-4. [DOI] [PubMed] [Google Scholar]
- 33.Society for Vascular Surgery . Chaikof E L, Brewster D C, Dalman R L. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(04):S2–S49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Naughton P A, Garcia-Toca M, Rodriguez H E. Endovascular treatment of delayed type 1 and 3 endoleaks. Cardiovasc Intervent Radiol. 2011;34(04):751–757. doi: 10.1007/s00270-010-0020-y. [DOI] [PubMed] [Google Scholar]
- 35.Kim J K, Noll R E, Jr, Tonnessen B H, Sternbergh W C., III A technique for increased accuracy in the placement of the “giant” Palmaz stent for treatment of type IA endoleak after endovascular abdominal aneurysm repair. J Vasc Surg. 2008;48(03):755–757. doi: 10.1016/j.jvs.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Jordan W D, Jr, de Vries J-PPM, Ouriel K. Midterm outcome of EndoAnchors for the prevention of endoleak and stent-graft migration in patients with challenging proximal aortic neck anatomy. J Endovasc Ther. 2015;22(02):163–170. doi: 10.1177/1526602815574685. [DOI] [PubMed] [Google Scholar]
- 37.Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery . Chaikof E L, Blankensteijn J D, Harris P L. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35(05):1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 38.Scali S T, McNally M M, Feezor R J. Elective endovascular aortic repair conversion for type Ia endoleak is not associated with increased morbidity or mortality compared with primary juxtarenal aneurysm repair. J Vasc Surg. 2014;60(02):286–2940. doi: 10.1016/j.jvs.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell T FX, Corey M R, Deery S E. Select early type IA endoleaks after endovascular aneurysm repair will resolve without secondary intervention. J Vasc Surg. 2018;67(01):119–125. doi: 10.1016/j.jvs.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 40.Farley S M, Rigberg D, Jimenez J C, Moore W, Quinones-Baldrich W. A retrospective review of Palmaz stenting of the aortic neck for endovascular aneurysm repair. Ann Vasc Surg. 2011;25(06):735–739. doi: 10.1016/j.avsg.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Tzortzis E, Hinchliffe R J, Hopkinson B R. Adjunctive procedures for the treatment of proximal type I endoleak: the role of peri-aortic ligatures and Palmaz stenting. J Endovasc Ther. 2003;10(02):233–239. doi: 10.1177/152660280301000211. [DOI] [PubMed] [Google Scholar]
- 42.Abdulrasak M, Resch T, Sonesson B, Holst J, Kristmundsson T, Dias N V. The long-term durability of intra-operatively placed Palmaz stents for the treatment of Type Ia endoleaks after EVAR of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;53(01):69–76. doi: 10.1016/j.ejvs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Muhs B E, Jordan W, Ouriel K, Rajaee S, de Vries J P. Matched cohort comparison of endovascular abdominal aortic aneurysm repair with and without EndoAnchors. J Vasc Surg. 2018;67(06):1699–1707. doi: 10.1016/j.jvs.2017.10.059. [DOI] [PubMed] [Google Scholar]
- 44.Deaton D H. Improving proximal fixation and seal with the HeliFx aortic EndoAnchor. Semin Vasc Surg. 2012;25(04):187–192. doi: 10.1053/j.semvascsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Duvnjak S. Endovascular management of Type I endoleak with fenestrated aortic “cuff” and afterwards treatment of endoleak Type III. Int J Angiol. 2016;25(05):e111–e114. doi: 10.1055/s-0034-1544126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg R K, Clair D, Srivastava S. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38(05):990–996. doi: 10.1016/s0741-5214(03)00896-6. [DOI] [PubMed] [Google Scholar]
- 47.Criado F J. Chimney grafts and bare stents: aortic branch preservation revisited. J Endovasc Ther. 2007;14(06):823–824. doi: 10.1583/07-2247.1. [DOI] [PubMed] [Google Scholar]
- 48.Moulakakis K G, Mylonas S N, Avgerinos E. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012;55(05):1497–1503. doi: 10.1016/j.jvs.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Donas K P, Pecoraro F, Bisdas T. CT angiography at 24 months demonstrates durability of EVAR with the use of chimney grafts for pararenal aortic pathologies. J Endovasc Ther. 2013;20(01):1–6. doi: 10.1583/12-4029.1. [DOI] [PubMed] [Google Scholar]
- 50.Lee J T, Greenberg J I, Dalman R L.Early experience with the snorkel technique for juxtarenal aneurysms J Vasc Surg 20125504935–946., discussion 945–946 [DOI] [PubMed] [Google Scholar]
- 51.Banno H, Cochennec F, Marzelle J, Becquemin J P. Comparison of fenestrated endovascular aneurysm repair and chimney graft techniques for pararenal aortic aneurysm. J Vasc Surg. 2014;60(01):31–39. doi: 10.1016/j.jvs.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 52.Scali S T, Feezor R J, Chang C K.Critical analysis of results after chimney endovascular aortic aneurysm repair raises cause for concern J Vasc Surg 20146004865–873., discussion 873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kansagra K, Kang J, Taon M C. Advanced endografting techniques: snorkels, chimneys, periscopes, fenestrations, and branched endografts. Cardiovasc Diagn Ther. 2018;8 01:S175–S183. doi: 10.21037/cdt.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green N, Sidloff D A, Stather P W, Bown M J, Sayers R D, Choke E. Endoleak after endovascular aneurysm repair: current status. Rev Vasc Med. 2014;2(02):43–47. [Google Scholar]
- 55.Mantas G K, Antonopoulos C N, Sfyroeras G S. Factors predisposing to endograft limb occlusion after endovascular aortic repair. Eur J Vasc Endovasc Surg. 2015;49(01):39–44. doi: 10.1016/j.ejvs.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Wong S, Greenberg R K, Brown C R, Mastracci T M, Bena J, Eagleton M J. Endovascular repair of aortoiliac aneurysmal disease with the helical iliac bifurcation device and the bifurcated-bifurcated iliac bifurcation device. J Vasc Surg. 2013;58(04):861–869. doi: 10.1016/j.jvs.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 57.Bisdas T, Weiss K, Donas K P, Schwindt A, Torsello G, Austermann M. Use of iliac branch devices for endovascular repair of aneurysmal distal seal zones after EVAR. J Endovasc Ther. 2014;21(04):579–586. doi: 10.1583/14-4712R.1. [DOI] [PubMed] [Google Scholar]
- 58.Schneider D B, Matsumura J S, Lee J T, Peterson B G, Chaer R A, Oderich G S. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the Gore Iliac Branch Endoprosthesis. J Vasc Surg. 2017;66:775–785. doi: 10.1016/j.jvs.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 59.Ellozy S, Schneider D, Mastumura J. Outcomes at one year with the gore excluder iliac branch endoprosthesis. J Vasc Surg. 2016;64(03):832. [Google Scholar]
- 60.O'Donnell T FX, Corey M R, Deery S E. Select early type IA endoleaks after endovascular aneurysm repair will resolve without secondary intervention. J Vasc Surg. 2018;67(01):119–125. doi: 10.1016/j.jvs.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 61.Chisci E, Guidotti A, Pigozzi C. Long-term analysis of standard abdominal aortic endovascular repair using different grafts focusing on endoleak onset and its evolution. Int J Cardiol. 2019;276:53–60. doi: 10.1016/j.ijcard.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Qazi A A, Jaberi A, Mironov O. Conservative management of type 1A endoleaks at completion angiogram in endovascular repair of infra-renal abdominal aortic aneurysms with current generation stent grafts. Vascular. 2019;27(02):168–174. doi: 10.1177/1708538118811206. [DOI] [PubMed] [Google Scholar]
- 63.American College of Cardiology; American Heart Association . Fleisher L A, Fleischmann K E, Auerbach A D. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 64.Tadros R O, Faries P L, Ellozy S H. The impact of stent graft evolution on the results of endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2014;59(06):1518–1527. doi: 10.1016/j.jvs.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Pitoulias G A, Valdivia A R, Hahtapornsawan S. Conical neck is strongly associated with proximal failure in standard endovascular aneurysm repair. J Vasc Surg. 2017;66(06):1686–1695. doi: 10.1016/j.jvs.2017.03.440. [DOI] [PubMed] [Google Scholar]


