Abstract
Endoleak remains a significant challenge to endovascular aneurysm repair, particularly as evolving techniques and devices have allowed treatment of increasingly complex aneurysm anatomy with increasing number of device components. Intervention is recommended for both type I and III endoleaks due to their risk of rupture, and endovascular techniques are the favored modality with placement of a bridging endograft over the endoleak defect. Conversion to open surgical repair remains the definitive option in cases where less invasive methods have failed or are precluded. In this article, the authors review evidence on the etiology, incidence, diagnosis, and current techniques for type III endoleak management.
Keywords: endoleak, aortic aneurysm, surgery, stent graft, interventional radiology, endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) has gained wide acceptance as the most common and usually preferred method for the treatment of aortic aneurysms. As graft technology has advanced, EVAR is now associated with lower 30-day mortality and morbidity rates as well as earlier discharge compared with traditional open aneurysm repair. 1 2 3 However, the most significant drawback of EVAR is its association with increased rate of secondary intervention compared with open repair, with endoleaks being the most common indication. 4 5
Endoleak is defined as a persistent blood flow to the aneurysm sac after endovascular stent placement. 6 7 Type III endoleaks describe the situation in which this persistent blood flow is attributed to either defects between components in modular grafts (type IIIa) or from a defect in the endograft itself, such as a fabric tear or stent fracture (type IIIb). 8 9 Type IIIb endoleaks can further be subdivided into a major or minor on the basis of whether the endograft defect is ≥ 2 mm in size. 8 9
Etiology
In infrarenal EVAR, type IIIa endoleaks are seen with separation of the main device body from the contralateral iliac limb, or separation of the main body from an ipsilateral distal extension cuff or proximal extension cuff. 8 10 Type III endoleaks associated with thoracic endovascular aortic repair (TEVAR) procedures are rare with newer third-generation devices, but have been described when proximal and distal components separate. 11 Historically, endografts placed before 1998 are considered to be first- and second-generation devices, and those placed after 1998 are considered third-generation devices. 12
Similar to type I endoleaks, type III endoleaks can be classified as either early or late. Early type IIIa endoleaks are diagnosed on completion angiography and are attributed to either insufficient overlap between graft components or inadequate balloon expansion at component junctions. 8 Early type IIIb endoleaks are rare with modern devices, and entail a preexisting endograft fabric tear or injury to the endograft during placement or manipulation. 12 Late type III endoleaks develop months to years later, with a median time interval of 5.6 years. 12 Late type IIIa endoleaks are generally attributed to conformational change in the aneurysm sac leading to component separation, endograft migration, or dilation of aortic and/or iliac attachment sites ( Fig. 1 ). Large aneurysm size, especially over 6.5 cm, has consistently been associated with late development of endoleaks in both TEVAR and EVAR, including type III endoleaks. 13
Fig. 1.
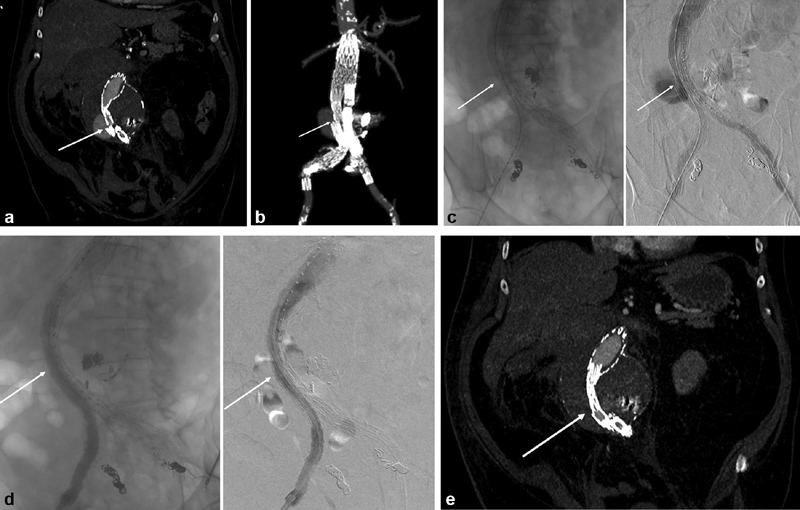
Late type III endoleak due to iliac component separation in a Cook Zenith placed 10 years prior to presentation. The patient had been lost to follow-up for several years and presented with a contained rupture. ( a ) Reformatted CT angiography at presentation, demonstrating separation between the main body of the graft and the right iliac limb. Arrow denotes the separation between the main body and the right iliac limb. ( b ) Three-dimensional reconstruction of presenting CT angiography. Arrow again denotes the separation between the main body and the right iliac limb. ( c ) Intraoperative angiography demonstrating extravasation. Arrow denotes the separation between the main body and the right iliac limb. ( d ) intraoperative angiography after placement of a bridging iliac limb with appropriate covering of the prior graft component separation (arrow). ( e ) CT angiography performed prior to discharge demonstrating successful repair, with arrow denoting position of the bridging iliac limb.
Incidence
According to the Veterans Affairs Open Surgery Versus Endovascular Repair (OVER) trial in 2015, approximately 30% of those undergoing EVAR develop an endoleak, with 3% of those being classified as type III. 14 Other studies, using the EUROSTAR registry in 2006, also conclude that due to material fatigue and limb disconnection, earlier generation devices had higher rates of type III endoleaks of 3 to 4.5%, and as high as 8 to 12% in specific devices. 8 12 Newer third-generation EVAR devices have a decreased rate of type III endoleak, around 1%, attributed to improvement in material properties and increased component overlap, but this may be falsely low, given these newer devices have shorter follow-up times. 15 Given device improvement, it is likely that type IIIa endoleaks are more common compared with type IIIb. In first- and second-generation grafts, 53% of type III endoleaks were type IIIa; in third-generation devices, 83% of type III endoleaks were type IIIa. 12 16 Of note, the Endologix AFX Endovascular Graft System was recalled by the Food and Drug Administration (FDA) in 2018 due to its high rate of type III endoleaks, and this device has since been replaced by a redesigned AFX device.
Complex fenestrated EVAR procedures have an increased number of endograft junctions, which has led to an associated increase in type IIIa endoleaks. Reports vary, but early type III endoleaks have been seen up to 25% on completion angiography. 17 The rate of type III endoleaks is low after TEVAR, with initial studies having an estimated rate of 6% in 2006, but there remains limited literature on the subject with many studies performed on stents in place for dissection. 18 The estimated rate in modern TEVAR devices in 2017 has been shown to be 2%, 19 and device studies of the Zenith TX2 showed no type III endoleaks and only one seen in the Gore TAG. 11 20 21 22
Diagnosis
After EVAR placement, the updated 2017 guidelines from the SVS recommend surveillance imaging with CT scan at 1 month, 6 months, 12 months, and yearly thereafter. Type III endoleaks are often asymptomatic, with most diagnosed on surveillance imaging, and rarely due to high clinical suspicion. Those with rapid aneurysm sac growth or aneurysm rupture may present with abdominal pain and hemodynamic instability. Some small series have suggested that 10% of those with a type III endoleak present with aneurysm rupture. 12 Rarely bowel ischemia, retroperitoneal bleeding, or device component separation causing acute lower extremity ischemia can be presenting symptoms. 12 16
Early type IIIa endoleaks should be diagnosed on completion angiography, but the sensitivity and specificity of angiography is highly variable depending on the study (60–90%), and many early type IIIa endoleaks may be first seen on follow-up imaging. 22 Both computed tomographic angiography (CTA) and ultrasonography are commonly used for post-EVAR surveillance, with the diagnosis of a late endoleak being made when these images demonstrate contrast or blood flow within the sac but outside of the endograft. 23 After endoleak identification, if not already obtained, a three-phase CTA should be performed to accurately define endoleak type as well as delineate aneurysm sac size. CTA has a sensitivity and specificity of more than 90% for type III endoleak detection, and is currently the standard means of diagnosis due to its speed, reproducibility, and excellent spatial and contrast resolution. 18 24 25 The three-phase CTA includes a (1) precontrast, (2) contrast-enhanced, and (3) delayed phase which delineate (1) the structural integrity of a modular stent graft, including the determination of any graft crimping, stent fracture, migration, or component disconnection; (2) the presence of any high-flow endoleaks (indicating high aneurysm sac pressure); or (3) the presence of more subtle, delayed, low-flow endoleaks. False-positive findings that mimic endoleaks include calcifications within both the aortic wall or within mural thrombus, which can be identified using the precontrast images, or graft billowing between stented portions of the stent graft which may appear as isolated outpouching resembling a type III endoleak. 18
Magnetic resonance angiography (MRA) has also been shown to be an acceptable alternative to CTA if necessary, with some reports of increased sensitivity in the ability of MRA to detect endoleaks over CTA. 26 27 Cine MRA, with its ability to assess for aneurysm sac motion, may be able to differentiate type I and III endoleaks which lead to a pulsatile aneurysm sac, from type II, IV, or V endoleaks. 28 A historical advantage of MRA over CTA was the avoidance of iodinated contrast in those with renal insufficiency, but this advantage is no longer apparent after identification of gadolinium-associated nephrogenic systemic sclerosis. 29
Unfortunately, many endograft systems are made with ferromagnetic components which create significant scattering artifact immediately adjacent to the endograft and limit the utility of MRA. Endograft devices made with nonferromagnetic materials like nitinol do not suffer from this drawback. Another limitation of MRA is that it cannot be performed in patients with indwelling ferromagnetic hardware such as pacemakers or intracranial aneurysm clips. Perhaps the biggest advantages of CTA over MRA are patient convenience due to short acquisition time and ease of interpretation on behalf of the surgeon.
Contrast-enhanced ultrasound is another imaging modality that has been shown in select studies to have comparable accuracy to CTA in identifying endoleak location, but its use is somewhat limited, given that it is operator dependent and loses accuracy in patients with a large body habitus or large amounts of bowel gas. 30
A combination of CTA, plain abdominal X-rays, and contrast-enhanced ultrasonography will be sufficient to diagnose more than 90% of type III endoleaks. 18 24 25 The diagnosis of type IIIb endoleaks can be very challenging on noninvasive imaging, and many type IIIb endoleaks may be misdiagnosed as type I endoleaks. 12 31 Small series have suggested that the majority of type IIIb endoleaks are identified on digital subtraction angiography or during surgical intervention. Detection of small type III endoleaks may require angiography techniques such as balloon occlusion distal to the region of interest with selective angiography under pressure. 31 32 For even smaller “microleaks,” both proximal and distal balloon occlusion may be necessary during angiography. 32
Management
The Society of Vascular Surgery (SVS) reporting standards for EVAR (2002) and TEVAR (2010) classifies the presence of either a type I or type III endoleak at the completion of the index procedure (early endoleak) to be a technical failure. 6 33 The secondary reintervention rate for both EVAR and TEVAR has been shown to be between 7 and 12% in large datasets, mostly commonly for endoleaks. 18 34 35 36 It is obvious that prevention of endoleaks, with careful preoperative planning, endograft size selection, assessment of proximal and distal landing zones, and appropriate endograft overlap, is the best strategy to avoid type III endoleaks. Prior analysis in 2000 of the EUROSTAR registry found that with respect to EVAR, those with a late type III endoleak have a ninefold increased risk of aneurysm rupture compared with other registry patients. 37 Based on these outcomes, current guidelines indicate that type III endoleaks are as serious as type I endoleaks, with both leading to pressurization of the aneurysm sac, and should be treated when they are identified to prevent future aneurysm rupture. 6 In previous data published by Eng et al, endovascular intervention for type III endoleaks was implemented in 68% of patients, open surgical repair in 10%, and hybrid procedures in 18%. 38 Recent literature has supported that selective type III endoleaks can spontaneously resolve after a fenestrated EVAR, 17 but observation alone of type III endoleaks is currently only recommended in those patients who are either unfit or refuse intervention.
Early
Early type III endoleaks detected on completion angiography should be treated at the time of diagnosis. 6 This often can be achieved by repeat ballooning at areas of component overlap or additional endograft placement to achieve better component overlap. 39
Late
Main Body and Iliac Limb Separation
Initial endovascular treatment of late type III endoleaks is most commonly attempted, with deployment of additional endograft components to bridge region of disconnected components or cover the fabric defect. The simplest presentation of a type IIIa endoleak is separation of the main body and iliac limb. The primary challenge in repair in this situation is cannulation of the main body through the separated limb, which may have been significantly displaced. 8 12 If retrograde cannulation via femoral access fails, an antegrade brachial or axillary approach can be attempted with snaring of the guidewire from femoral access. Once cannulation has been achieved, a new iliac limb graft can be deployed to serve as a bridge to the separated component. 40 It is imperative to avoid having the wire pass between the struts of the migrated endografts, which may make placement of a bridging device impossible. In cases where a bridging stent graft cannot be placed, multiple sites of type IIIa endoleaks, or the proximal landing zone has had significant migration, placement of a new bifurcated stent graft within the previous device may be necessary. 41 If isolated endovascular intervention is not feasible, placement of an aorto-uni-iliac (AUI) device with contralateral common iliac plug/coil placement and surgical femoral–femoral bypass is an additional option. 12 42 It is optimal to place the AUI device on the contralateral side of the endoleak, but other considerations such as internal iliac patency and size should also be considered. 42 The coil/plugs should be placed in the native common iliac artery and proximal to the origin of the internal iliac artery to preserve flow into the pelvis from the femoral–femoral bypass. 42
Main Body and Proximal Aortic Extension Separation
Treating type III endoleaks that are the result of component separation of the main body from a proximal aortic extension cuff is often more complex. In situations where the aortic neck has not significantly increased in diameter, a new aortic extension cuff can be deployed to bridge the defect. Given the short length of aortic cuffs, it may not be possible to achieve sufficient overlap; rates of recurrent type IIIa endoleaks were as high as 25% in first- and second-generation devices with cuff relining. 11 43 Another option that has been described in this scenario is deployment of a new bifurcated endograft inside the prior separated device. 43 With the placement of a new bifurcated device, the integrity of aneurysm exclusion no longer relies on the migrated endograft and additional short proximal aortic extension cuffs, which are prone to future endoleak development, are avoided. 11 One significant limitation is that to place a new bifurcated device, there must be sufficient length between the proximal landing zone and the flow divider of the migrated endograft to allow for deployment of the contralateral limb of the new device within the migrated device. 11 41 43 Similar to type IIIa endoleaks due to iliac limb separation, if endovascular intervention alone is not feasible, a AUI device with contralateral common iliac occlusion and surgical femoral–femoral bypass can be performed. 42
In cases where the proximal aortic extension has separated from the main body, and either there is a significant distance that must be bridged or the aneurysm neck has increased in diameter, larger endograft devices are likely to be necessary. For this scenario, thoracic endovascular devices, which have a large diameter and increased length, are valid options that can be used to bridge the defect. 41 Lastly, if no endovascular options are available or if they have failed, conversion to open surgical repair may be considered.
Intervention Outcomes and Follow-up
There are no surveillance imaging guidelines specific to patients who had an endoleak intervention. Most practitioners use the surveillance guidelines recommend after initial EVAR placement, with CT scan at 1 month, 6 months, 12 months, and yearly thereafter. Long-term follow-up data after type III endoleak intervention is limited to small series: in these, 22 to 25% of patients who underwent intervention for a type III endoleak needed an additional intervention for recurrent endoleak within 10 years. 12 44 In those studies, 80% of recurrent type III endoleaks were caused by fabric tears in first- or second-generation devices, and the majority were managed with surgical revision. 12 It is well demonstrated that over 50% of patients have incomplete surveillance after EVAR, and given the high rate of recurrence after type III endoleak intervention and its association with aneurysm rupture, diligent radiographic follow-up is strongly advocated. 45
Conclusion
Endoleaks are the most frequent complication after EVAR and the most common indication for secondary intervention. Type III endoleaks are an uncommon subgroup that are less common in newer generation devices. Most literature in the area is based on first- and second-generation devices which had a higher rate of type IIIb endoleak compared with third-generation devices. Like type I endoleaks, type III endoleaks lead to a pressurized aneurysm sac, and up to 10% of those with a type III endoleak present with aneurysm rupture. Society guidelines recommend that all diagnosed type III endoleaks should be repaired. In the modern device era, type III endoleaks are most often associated with endograft component separation (type IIIa), and the majority are treated by endovascular techniques to stent over the junctional endograft defect. However, these endovascular reinterventions are not free of adverse events and endoleak recurrence can occur, highlighting the need for continued radiologic surveillance in these patients.
Footnotes
Conflict of Interest None declared.
References
- 1.Parodi J C, Palmaz J C, Barone H D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(06):491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 2.Dua A, Kuy S, Lee C J, Upchurch G R, Jr, Desai S S. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59(06):1512–1517. doi: 10.1016/j.jvs.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group . Blankensteijn J D, de Jong S E, Prinssen M. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352(23):2398–2405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 4.EVAR Trial Investigators Patel R, Sweeting M J, Powell J T, Greenhalgh R M.Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial Lancet 2016388(10058):2366–2374. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn M L, Buck D B, O'Malley A J. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med. 2015;373(04):328–338. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery . Chaikof E L, Blankensteijn J D, Harris P L. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35(05):1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 7.Görich J, Rilinger N, Sokiranski R. Treatment of leaks after endovascular repair of aortic aneurysms. Radiology. 2000;215(02):414–420. doi: 10.1148/radiology.215.2.r00ma22414. [DOI] [PubMed] [Google Scholar]
- 8.Bucci F, Fiengo L, Valerio N, Ferdani M.Late type IIIb endoleak after endovascular aneurysm repair: case report and review of the literature G Chir 201132(6-7):329–333. [PubMed] [Google Scholar]
- 9.Cronenwett J. Philadelphia, PA: Saunders Elsevier; 2010. Rutherford’s Vascular Surgery, 7th Edition. Cronenwett and Johnson, Eds. [Google Scholar]
- 10.Ueda T, Takaoka H, Petrovitch I, Rubin G D. Detection of broken sutures and metal-ring fractures in AneuRx stent-grafts by using three-dimensional CT angiography after endovascular abdominal aortic aneurysm repair: association with late endoleak development and device migration. Radiology. 2014;272(01):275–283. doi: 10.1148/radiol.14130920. [DOI] [PubMed] [Google Scholar]
- 11.Leszczyński J, Macioch W, Chudziński W, Gałązka Z. Late type III endoleak after thoracic endovascular aneurysm repair and previous infrarenal stent graft implantation - a case report and review of the literature. Wideochir Inne Tech Malo Inwazyjne. 2017;12(03):320–324. doi: 10.5114/wiitm.2017.69239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maleux G, Poorteman L, Laenen A. Incidence, etiology, and management of type III endoleak after endovascular aortic repair. J Vasc Surg. 2017;66(04):1056–1064. doi: 10.1016/j.jvs.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 13.Parmer S S, Carpenter J P, Stavropoulos S W. Endoleaks after endovascular repair of thoracic aortic aneurysms. J Vasc Surg. 2006;44(03):447–452. doi: 10.1016/j.jvs.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 14.OVER Veterans Affairs Cooperative Study Group . Lal B K, Zhou W, Li Z. Predictors and outcomes of endoleaks in the Veterans Affairs Open Versus Endovascular Repair (OVER) Trial of Abdominal Aortic Aneurysms. J Vasc Surg. 2015;62(06):1394–1404. doi: 10.1016/j.jvs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Tadros R O, Faries P L, Ellozy S H. The impact of stent graft evolution on the results of endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2014;59(06):1518–1527. doi: 10.1016/j.jvs.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Theodoridis P G, Staramos D N, Ptochis N. Combined Type III and Type II endoleaks after endovascular aneurysm repair: presentation of 2 cases and a literature review. Ann Vasc Surg. 2019;55:3.08E7–3.08E12. doi: 10.1016/j.avsg.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow N J, McCallum J C, Liang P. Select type I and type III endoleaks at the completion of fenestrated endovascular aneurysm repair resolve spontaneously. J Vasc Surg. 2019;70(02):381–390. doi: 10.1016/j.jvs.2018.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleason T G. Endoleaks after endovascular aortic stent-grafting: impact, diagnosis, and management. Semin Thorac Cardiovasc Surg. 2009;21(04):363–372. doi: 10.1053/j.semtcvs.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Appoo J J, Moser W G, Fairman R M. Thoracic aortic stent grafting: improving results with newer generation investigational devices. J Thorac Cardiovasc Surg. 2006;131(05):1087–1094. doi: 10.1016/j.jtcvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 20.Scali S T, Goodney P P, Walsh D B. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53(06):1499–1505. doi: 10.1016/j.jvs.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TX2 Clinical Trial Investigators Matsumura J S, Cambria R P, Dake M D, Moore R D, Svensson L G, Snyder S.International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results J Vasc Surg 20084702247–257., discussion 257 [DOI] [PubMed] [Google Scholar]
- 22.Ellozy S H, Carroccio A, Minor M. Challenges of endovascular tube graft repair of thoracic aortic aneurysm: midterm follow-up and lessons learned. J Vasc Surg. 2003;38(04):676–683. doi: 10.1016/s0741-5214(03)00934-0. [DOI] [PubMed] [Google Scholar]
- 23.Armerding M D, Rubin G D, Beaulieu C F. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology. 2000;215(01):138–146. doi: 10.1148/radiology.215.1.r00ap28138. [DOI] [PubMed] [Google Scholar]
- 24.Görich J, Rilinger N, Sokiranski R. Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology. 1999;213(03):767–772. doi: 10.1148/radiology.213.3.r99dc04767. [DOI] [PubMed] [Google Scholar]
- 25.Baum R A, Carpenter J P, Tuite C M. Diagnosis and treatment of inferior mesenteric arterial endoleaks after endovascular repair of abdominal aortic aneurysms. Radiology. 2000;215(02):409–413. doi: 10.1148/radiology.215.2.r00ma17409. [DOI] [PubMed] [Google Scholar]
- 26.Cohen E I, Weinreb D B, Siegelbaum R H. Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair. J Magn Reson Imaging. 2008;27(03):500–503. doi: 10.1002/jmri.21257. [DOI] [PubMed] [Google Scholar]
- 27.Pitton M B, Schweitzer H, Herber S. MRI versus helical CT for endoleak detection after endovascular aneurysm repair. AJR Am J Roentgenol. 2005;185(05):1275–1281. doi: 10.2214/AJR.04.0729. [DOI] [PubMed] [Google Scholar]
- 28.Faries P L, Agarwal G, Lookstein R. Use of cine magnetic resonance angiography in quantifying aneurysm pulsatility associated with endoleak. J Vasc Surg. 2003;38(04):652–656. doi: 10.1016/s0741-5214(03)00944-3. [DOI] [PubMed] [Google Scholar]
- 29.Hasebroock K M, Serkova N J. Toxicity of MRI and CT contrast agents. Expert Opin Drug Metab Toxicol. 2009;5(04):403–416. doi: 10.1517/17425250902873796. [DOI] [PubMed] [Google Scholar]
- 30.Kapetanios D, Kontopodis N, Mavridis D, McWilliams R G, Giannoukas A D, Antoniou G A. Meta-analysis of the accuracy of contrast-enhanced ultrasound for the detection of endoleak after endovascular aneurysm repair. J Vasc Surg. 2019;69(01):280–2.94E8. doi: 10.1016/j.jvs.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura J S, Ryu R K, Ouriel K.Identification and implications of transgraft microleaks after endovascular repair of aortic aneurysms J Vasc Surg 20013402190–197., discussion 369–370 [DOI] [PubMed] [Google Scholar]
- 32.Rubin B G, Marine L, Parodi J C. An algorithm for diagnosis and treatment of type II endoleaks and endotension after endovascular aneurysm repair. Perspect Vasc Surg Endovasc Ther. 2005;17(02):167–172. doi: 10.1177/153100350501700222. [DOI] [PubMed] [Google Scholar]
- 33.Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards Fillinger M F, Greenberg R K, McKinsey J F, Chaikof E L.Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg 201052041022–1033., 1033.e15 [DOI] [PubMed] [Google Scholar]
- 34.Conrad M F, Adams A B, Guest J M. Secondary intervention after endovascular abdominal aortic aneurysm repair. Ann Surg. 2009;250(03):383–389. doi: 10.1097/SLA.0b013e3181b365bd. [DOI] [PubMed] [Google Scholar]
- 35.Lange C, Aasland J K, Ødegård A, Myhre H O. The durability of EVAR–what are the evidence and implications on follow-up? Scand J Surg. 2008;97(02):205–212. doi: 10.1177/145749690809700227. [DOI] [PubMed] [Google Scholar]
- 36.VALOR Investigators . Fairman R M, Criado F, Farber M. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48(03):546–554. doi: 10.1016/j.jvs.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Harris P L, Vallabhaneni S R, Desgranges P, Becquemin J P, van Marrewijk C, Laheij R J. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(04):739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 38.Eng M L, Brewer M B, Rowe V L, Weaver F A. Treatment options for late type III endoleaks after endovascular aneurysm repair. Ann Vasc Surg. 2015;29(03):5.94E7–5.94E11. doi: 10.1016/j.avsg.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 39.White S B, Stavropoulos S W. Management of endoleaks following endovascular aneurysm repair. Semin Intervent Radiol. 2009;26(01):33–38. doi: 10.1055/s-0029-1208381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao P, De Rango P, Verzini F, Parlani G. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg (Torino) 2010;51(01):53–69. [PubMed] [Google Scholar]
- 41.Teutelink A, van der Laan M J, Milner R, Blankensteijn J D. Fabric tears as a new cause of type III endoleak with Ancure endograft. J Vasc Surg. 2003;38(04):843–846. doi: 10.1016/s0741-5214(03)00416-6. [DOI] [PubMed] [Google Scholar]
- 42.Schuurman J P, Fioole B, van den Heuvel D A, de Vries J P. Endovascular therapy for recurrent type III endoleak. Vasc Endovascular Surg. 2010;44(02):123–125. doi: 10.1177/1538574409345025. [DOI] [PubMed] [Google Scholar]
- 43.Jim J, Rubin B G, Sanchez L A. Use of a bifurcated endovascular graft for treatment of endograft migration with major endoleak. Vascular. 2012;20(01):49–53. doi: 10.1258/vasc.2011.cr0285. [DOI] [PubMed] [Google Scholar]
- 44.van Lammeren G W, Fioole B, Waasdorp E J, Moll F L, van Herwaarden J A, de Vries J P. Long-term follow-up of secondary interventions after endovascular aneurysm repair with the AneuRx endoprosthesis: a single-center experience. J Endovasc Ther. 2010;17(03):408–415. doi: 10.1583/10-3086.1. [DOI] [PubMed] [Google Scholar]
- 45.Garg T, Baker L C, Mell M W. Postoperative surveillance and long-term outcomes after endovascular aneurysm repair among Medicare beneficiaries. JAMA Surg. 2015;150(10):957–963. doi: 10.1001/jamasurg.2015.1320. [DOI] [PubMed] [Google Scholar]


