Abstract
Objectives This study aims to assess the blood flow and the vascularity pattern across the newly bridged vascularized bone grafts for scaphoid nonunions using three-dimensional (3D) high frequency power Doppler ultrasonography and its role in the union.
Materials and Methods A total of 26 patients with scaphoid nonunions were operated with 1,2-intercompartmental supraretinacular artery (ICSRA) graft. CT scan and 3D high frequency power Doppler ultrasonography were performed in all patients between 12 and 18 weeks, and its results were analyzed.
Results Doppler ultrasonography confirmed the pulsatile flow and vascularity across the pedicle and vascularized bone graft incorporation into the scaphoid nonunion site.
Conclusions 3D high frequency power Doppler ultrasonography is a simple, noninvasive, nonradiation, reproducible, and well-reliable diagnostic modality in assessing the blood flow and vascularity of the bone grafts used for scaphoid nonunions.
Level of Evidence This is a Level IV study.
Keywords: scaphoid, nonunion, 2-ICSRA graft, 3D high frequency power Doppler, union
Vascularized bone grafting from distal radius with headless cancellous screw fixation is a treatment option for scaphoid nonunion with or without avascular necrosis (AVN) of the proximal pole. 1 There have been various studies and reviews so far on 1,2-intercompartmental supraretinacular artery (ICSRA) based vascularized graft for scaphoid nonunions 2 3 and the use of radial styloidectomy for better exposure and tension reduction in these vascularized grafts. 4 The functional outcomes, results, and scaphoid union rates are quite variable. Progressive radioscaphoid arthritis is seen in 21% patients. 2 The union is assessed by computed tomography (CT) scan performed between 12 and 16 weeks. 5
Vascularized bone grafts in scaphoid nonunions import osteogenic progenitors to enhance the healing potential of the fracture and, together with its inherent blood supply, improves graft survival rather than depending on the unreliable vasculature of the scaphoid. In addition, the vascularity accelerates graft incorporation, thus providing durability and better viability. 6
Three-dimensional high frequency power Doppler ultrasonography (3D-HFP-DU) is a noninvasive integrated Doppler power spectrum highly sensitive to slow and fast flow and detects neovascularization in fracture healing. In revascularization, local blood perfusion and assessment of microcirculation can also be better understood. 7 In addition to the functional healing of fracture, which is defined by the mechanical competence of the callus, there is a growing potential for noninvasive, diagnostic methods to reveal the progress of biological process, which occur during fracture healing. 8
The aim of this study was to analyze the role of 3D-HF-PDU in all postoperative patients to assess the microvasculature of the vascularized graft, neoangiogenesis, revascularization across the fracture site, and subsequent progress to union, whereby possible implications can be interpreted.
Materials and Methods
A total of 26 patients with scaphoid nonunion admitted and operated between April 2012 and January 2015 were analyzed. All were men, and the most common mode of injury was a bike fall. Of the 26 patients, 16 had right side involvement and 10 had left side involvement. The mean age of the patient was 23.8 (range: 20–29 years). The average delay from the injury to the surgery was 203 days (range: 182–256 days). The common reasons for the delay were conservative treatment with immobilization and no treatment. All of them presented with pain, scaphoid tenderness, stiffness, loss of extension, restriction of radial and ulnar deviations, and marked restriction of daily activities. Radiographs of the wrist (posteroanterior, lateral, and oblique wrist views, and a posteroanterior ulnar deviation view to extend the scaphoid) were taken and nonunion scaphoid was documented. Three-dimensional CT scans of the wrist were performed to study the pattern and displacement of nonunions. Magnetic resonance imaging (MRI) was also performed to assess the bone marrow vascularity around the nonunion and to pick up proximal pole AVN. All these fractures were at waist level and classified as Lichtman type 1 (simple nonunion) and type 2 (unstable nonunion). Ten patients had unstable nonunion, with joint effusion, synovial thickening, and minimal erosions. The patient with Lichtman types 3 to 5 were excluded from the study as they needed different treatment modalities.
All patients were operated with curvilinear dorsoradial incision under supraclavicular brachial block anesthesia and tourniquet. The 1,2-ICSRA was visualized on the surface of the extensor retinaculum. Perforating vessels from the 1,2-ICSRA to the dorsal distal radius were found. Dorsal radial bone grafts with maximum perforators were elevated precisely to reach the scaphoid nonunion site. Scaphoid nonunion site was freshened, curetted, and compressed with Herbert's cannulated screw. The vascularized bone graft was transposed to reach the nonunion site where it was gently overlaid into the nonunion site as a dorsal onlay and stabilized with a short-cut smooth Kirschner (K) wire. These grafts were harvested in appropriate size so that they snug fit over the scaphoid nonunion site to prevent complications such as migration of grafts and conflicts with radio- and midcarpal joints. In four patients who had humpback deformity, scaphocapitate K-wire was used in addition to vascularized bone grafts ( Table 1 ) using the same approach. Serial postoperative radiographs were taken and documented. Three-dimensional CT scan of the wrist was performed in all cases to confirm the union and trabeculae bridging over the nonunion between 12 and 18 weeks postoperative. At the same time point, all patients received 3D-HFPDU assessment over the dorsal wrist and scaphoid area using high-frequency (center frequency at 55 MHz) In Vivo Micro-Imaging System (Mindray, Shenzhen, China). Doppler spectral analysis was performed to assess the flow of the 1,2-ICSRA based bone graft across the union site. In addition, the blood flow signals in the scaphoid and peripheral soft tissues were also observed for interpretations.
Table 1. Patient's details and the results.
| S.no | Age | Side | Associated deformity | Additional treatment | Delay (days) time interval injury- surgery | Time interval surgery – bony union (days) (radiographs, CT, 3D USG) | Mean flexion | Mean extension | Grip strength (% compared with normal side) | Mean radial deviation | Mean ulnar deviation | Wrist score (modified Mayo scoring) | VAS score | DASH score | Follow-up (months) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | Right | Nil | 240 | 180 | 75 | 40 | 96 | 15 | 30 | 80 | 1 | 3.3 | 26 | Nil | |
| 2 | 22 | Left | Humpback | Scaphocapitate with K-wire | 226 | 185 | 75 | 45 | 98 | 20 | 20 | 80 | 0 | 8.3 | 28 | Delayed union. Screw prominence Minimal radiocarpal arthritis |
| 3 | 26 | Left | Nil | 200 | 184 | 75 | 45 | 95 | 20 | 20 | 85 | 0 | 9.2 | 36 | Nil | |
| 4 | 21 | Right | Nil | 182 | 189 | 70 | 40 | 94 | 20 | 25 | 85 | 0 | 3.3 | 32 | Nil | |
| 5 | 20 | Right | Nil | 196 | 189 | 70 | 35 | 92 | 20 | 35 | 85 | 0 | 6.7 | 36 | Minimal radiocarpal arthritis | |
| 6 | 22 | Right | Nil | 194 | 176 | 70 | 35 | 98 | 25 | 35 | 85 | 0 | 8.3 | 28 | Nil | |
| 7 | 24 | Right | Nil | 199 | 168 | 75 | 30 | 96 | 15 | 20 | 80 | 2 | 17.5 | 27 | Nil | |
| 8 | 25 | Right | Nil | 220 | 194 | 70 | 35 | 98 | 20 | 20 | 80 | 0 | 5.8 | 32 | Nil | |
| 9 | 26 | Left | Humpback | Scaphocapitate with K-wire | 206 | 185 | 75 | 30 | 98 | 20 | 20 | 80 | 0 | 3.3 | 34 | Nil |
| 10 | 27 | Right | Nil | 209 | 189 | 70 | 40 | 98 | 20 | 30 | 85 | 0 | 8.3 | 33 | Nil | |
| 11 | 22 | Right | Nil | 214 | 188 | 70 | 40 | 96 | 20 | 30 | 80 | 0 | 3.3 | 32 | Nil | |
| 12 | 21 | Left | Nil | 256 | 180 | 70 | 45 | 96 | 20 | 30 | 80 | 0 | 6.7 | 28 | Nil | |
| 13 | 22 | Left | Nil | 201 | 174 | 75 | 40 | 98 | 20 | 30 | 85 | 0 | 3.3 | 29 | Nil | |
| 14 | 24 | Right | Nil | 205 | 186 | 70 | 35 | 92 | 15 | 25 | 80 | 1 | 13.3 | 30 | Minimal radiocarpal arthritis | |
| 15 | 25 | Left | Humpback | Scaphocapitate with K-wire | 195 | 190 | 70 | 35 | 90 | 15 | 25 | 80 | 1 | 11.7 | 32 | Nil |
| 16 | 21 | Right | Nil | 189 | 198 | 70 | 35 | 94 | 20 | 20 | 80 | 0 | 9.2 | 27 | Nil | |
| 17 | 22 | Left | Nil | 202 | 201 | 75 | 40 | 90 | 15 | 20 | 80 | 1 | 10.8 | 30 | Nil | |
| 18 | 23 | Right | Nil | 209 | 196 | 75 | 45 | 94 | 20 | 30 | 80 | 0 | 9.2 | 29 | Nil | |
| 19 | 24 | Right | Nil | 217 | 206 | 75 | 45 | 94 | 20 | 30 | 85 | 0 | 9.2 | 26 | Minimal radiocarpal arthritis | |
| 20 | 25 | Right | Nil | 218 | 200 | 75 | 45 | 95 | 20 | 30 | 85 | 0 | 12.5 | 33 | Nil | |
| 21 | 22 | Right | Humpback | Scaphocapitate with K-wire | 195 | 190 | 75 | 40 | 95 | 20 | 30 | 85 | 0 | 3.3 | 20 | |
| 22 | 26 | Left | Nil | 197 | 199 | 70 | 40 | 95 | 20 | 30 | 85 | 1 | 6.7 | 22 | ||
| 23 | 27 | Right | Nil | 184 | 190 | 70 | 30 | 95 | 20 | 30 | 85 | 0 | 5.8 | 22 | ||
| 24 | 25 | Left | Nil | 180 | 195 | 70 | 30 | 95 | 20 | 30 | 85 | 0 | 3.3 | 21 | ||
| 25 | 29 | Right | Nil | 170 | 185 | 70 | 30 | 95 | 20 | 30 | 85 | 0 | 3.3 | 20 | Minimal radiocarpal arthritis | |
| 26 | 26 | Left | Nil | 180 | 180 | 70 | 30 | 95 | 15 | 25 | 85 | 1 | 5.8 | 21 |
Abbreviations: CT, computed tomography; DASH, Disabilities of the Arm, Shoulder, and Hand; K-wire, Kirschner wire; VAS, visual analog scale; 3D USG, three-dimensional ultrasonography.
Note: interpretation of 1,2-intercompartment supraretinacular artery based vascularized bone graft for scaphoid nonunion with postoperative 3D high frequency-power Doppler ultrasonography.
Figure 1 shows the clinical picture of the dorsum of the operated wrist with markings of the radial artery (red line) proximally in the volar aspect of distal forearm, its continuation over the anatomical snuffbox dorsum, origin of 1,2-ICSRA, and vascularized graft (green box) harvested from the dorsum of the radius and rotated over the scaphoid nonunion site (blue marking). The longitudinal position of the probe over the anatomical snuffbox clearly showed large size caliber (0.12-cm) radial artery and its direction of flow to form a dorsal radiocarpal arch. The origin of its dorsal branch of 0.19-cm vessel diameter at this juncture was also appreciated. The oblique position of the probe far away from the anatomical snuff box over the scaphoid showed the vascularized bone graft 1,2-ICSRA pedicle of small caliber (0.05 cm) containing small perforators and the pulsatile wave form in Doppler spectral analysis.
Fig. 1.

( A ) The clinical of the dorsum of the operated wrist with schematic markings of the radial artery course (red line solid and dotted), 1,2-ICSRA, dorsal bone graft, and its rotation over scaphoid nonunion site. ( B ) The direction and position of the Doppler probe over anatomical snuffbox and ( C ) the corresponding Doppler images showing 0.12-cm large sized radial artery and its origin of dorsal branch, which is measured as 0.19 cm. The radial artery continues to form a dorsal radiocarpal arch. ( D ) The different direction and position of the Doppler probe far from anatomical snuffbox and over the scaphoid shows the vascularized bone graft with 0.05-cm small vessel 1,2-ICSRA. The reverse pattern of 1,2-ICSRA vessel confirms its rotation from the dorsal radius. ICSRA, intercompartmental supraretinacular artery.
Once the union was confirmed with CT scan, progressive strengthening and wrist range of movements were started at 14 weeks (range: 12–18 weeks). K-wire was not removed in all of the cases since it was cut short and buried. In 9 of 26 patients who had skin prominence with irritations, discomfort, and pain, the K-wire was removed. Full weight-lifting and sports activities were started at 9 months. All patients' functional outcomes were evaluated using the modified Mayo Wrist Scoring system ( Table 1 ) and the Disabilities of the Arm, Shoulder, and Hand (DASH) score. Range of motion, grip strength, and pain were also measured by goniometer and hydraulic dynamometer.
Results
The average follow-up in our study was 22 months (range: 20–36 months). Radiographs and CT scan showed bridging trabeculae and good union in 25 patients ( Fig. 2 ) and delayed union in one patient ( Fig. 3 ). The time taken to confirm the union differed. Radiographic follow-up at 12 weeks had shown to be quite unreliable to determine fracture healing in vascularized bone grafts, whereas CT scan confirmed it between 12 and 18 weeks. The summation of average time taken from the surgery to bony union assessed by X-ray, CT, or 3D Doppler study was 189.5 days (range: 168–206 days). One patient had prominence of scaphoid screw, and four patients had progressive radio-scaphoid arthritis (15%). The average visual analog score of all postoperative patients was 0.3 (range: 0–2). The functional results, according to the modified Mayo Wrist Scoring system ( Table 1 ), were rated as good in all of the patients, with eight patients having 85 points. The mean DASH score was 5.2 (range: 3.3–17.5). The mean wrist flexion, extension arc, radial deviation, and ulnar deviation were 72, 37, 19, and 28 degrees, respectively. All the patients returned to their previous work with 95% average grip strength.
Fig. 2.

( A, D ) A 21-year-old male patient with radiographs showing scaphoid nonunion treated with 1,2-intercompartmental supraretinacular artery based vascularized graft, Herbert cancellous screw fixation, and Kirschner wire fixation of the graft. ( C, F ) CT (computed tomography) scan at 16 weeks shows union. ( B, E ) Postoperative radiographs at the final follow-up.
Fig. 3.
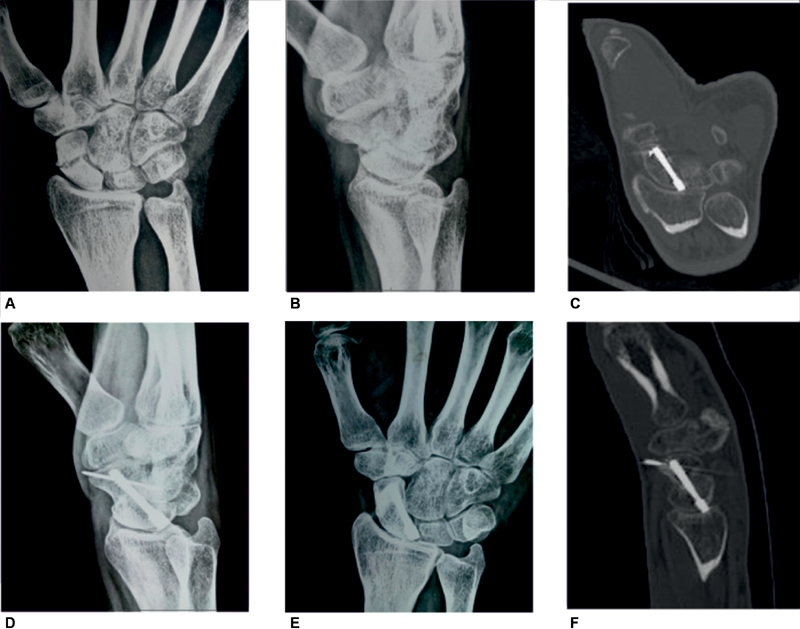
( A, B, D, E ) A 22-year-old male patient with scaphoid nonunion treated with 1, 2-intercompartmental supraretinacular artery, Herbert cancellous screw fixation, and Kirschner wire fixation of the graft. ( C, F ) CT (computed tomography) scan at 14 weeks confirms union with trabecular bridging across the nonunion site. ( B, E ) The radiographs union at follow-up.
In all patients, 3D-HFP-DU showed blood flow signals in the graft, scaphoid, and peripheral soft tissues. It provided accurate assessments of microvasculature from the vascularized graft into the scaphoid nonunion site. The reverse pattern of pulsatile blood flow in the 1,2-ICSRA was appreciated in all cases ( Figs. 4 and 5 ). There was no kinking of the long pedicle of 1,2-ICSRA or pedicle thrombosis. Assumption of pedicle injury or blockade resulting in conversion of a vascularized graft to a nonvascularized bone graft was ruled out. All grafts remained vascular preserving its flow and integrity. Persistent, consistent, and pulsatile flow pattern across the graft incorporated into the scaphoid nonunion site was the most important and key point we analyzed in our study. The position of Doppler probe, identification of vascularized grafts, scaphoid nonunion site, and small vessel of ICSRA differentiate it from large vessels.
Fig. 4 (A).

Three-dimensional high frequency power Doppler ultrasonography pictures shows the distal radius, (RAD). ( C ) 1,2-intercompartmental supraretinacular artery (ICSRA) is identified (V) and its small perforators below the BG is seen. ( B,D ) 1,2-ICSRA vessel (V) and pulsatile wave flow patterns over the scaphoid nonunion (SCA). BG, bone graft; VBG, vascularized bone graft and scaphoid.
Fig. 5.

Three-dimensional high frequency power Doppler ultrasonography images showing the BG ( A ) and confirming the pedicle of the 1,2-ICSRA vascularized BG ( B ). ( C ) The position of bone graft over the scaphoid and the reverse flow of small-sized (0.05 cm) 1,2-ICSRA pedicle whose orientation, location, and directions are perceptibly different from radial artery and its branches. ( D ) Pulsatile flow and wave patterns. ICSRA, intercompartmental supraretinacular artery. BG, bone graft.
Discussion
Zaidemberg et al first described the use of the 1,2-ICSRA graft for scaphoid nonunion in 1991 and reported 100% union in 11 patients. 9 Subsequently, many authors have popularized this technique and published their results with satisfactory-to-good functional outcomes. The overall benefit and greatest advantages in this technique are the anatomical reliability of the dorsal vascular network, long pedicle, relative simplicity of the procedure, and consistent favorable outcomes. 1 2 Conventional radiographs and 3D CT are useful and quantitative methods of assessing the fracture healing postoperative. 10 The measurement of callus volume and density predicts the extent of fracture consolidation.
Conventional two-dimensional Doppler instruments are sophisticated equipment that combine the real-time B-mode ultrasound imager for imaging the anatomical structures, providing information about vascular flow and movement patterns. These instruments allow evaluation of the assumed direction of blood flow with respect to the ultrasound beam. 11 The only drawback is picking Doppler signal instantaneously from scatterers and reflectors anywhere within the beam of the transducer since the transducer is excited continuously.
However, a 3D-HF-PDU allows discrimination of Doppler signals from different depths and detects moving interfaces and scatterers from within a well-defined sample volume, which can then be positioned anywhere along the axis of the ultrasound beam.
Furthermore, 1,2-ICSRA originates from the radial artery approximately 5 cm proximal to the radiocarpal joint, runs beneath the brachioradialis muscle, and lies on the dorsal surface of the extensor retinaculum; 1,2-ICSRA can be exceedingly difficult to visualize near its origin, and entire course with its branching segment from the radial artery is not always well seen intraoperatively. Because the vessel is small and fragile, the vascularized graft is always harvested with a pedicle of surrounding retinacular soft tissue for protection of the vessel. 3 4 Therefore, postoperative visualization, orientation of the vessels, differentiating between the radial artery and 1,2-ICSRA, and its new location after being harvested with the graft are the main challenges.
3D-HF-PDU is a valuable tool in addressing those challenges. The 1,2-ICSRA pedicle to the bone grafts over the scaphoid nonunion site, radial artery, and its anatomical variations of branches can be easily appreciated. The 1,2-ICSRA vessel diameter was measured in all of our cases, which averaged 0.05 cm. This small vessel caliber and its location away from the anatomical snuffbox differentiated 1,2-ICSRA from radial artery, which averaged 0.12 cm in diameter in our study. Identification of 1,2-ICSRA pedicle, bone graft over the scaphoid nonunion site, and Doppler spectral analysis with pulsatile wave forms are the hallmark features of 3D-HFP-DU.
The vascular volume assessment and the relative number of erythrocytes by 3D-HFP-DU have a declining trend from 2 to 8 weeks, which is similar to other studies on neovascularization in bone repair. 7 Raines et al observed a decrease in neovascular volume in the marrow cavity from the peak value at 14 days after bone drilling in tibial marrow ablation rats. 12 Zhang et al found a high expression of vascular endothelial growth factor lasting from 14 to 28 days postfemoral fracture in rats; then it decreased gradually until 6 weeks. 13 There is decreased blood perfusion during callus mineralization and remodeling. Considering these postfracture declining trend in the neovascularization, 3D-HFP-DU was performed with CT scan between 12 and 18 weeks for all of our patients. In addition, 3D geometric evaluation can improve the accuracy, objectivity, and integrity of the microcirculation information. 7
Studies of the biology of fracture healing have demonstrated definitively and consistently that for secondary fracture healing, angiogenesis is a critical process in the time course of healing, and that for any instance of successful bone healing, the injury site must be vascularized. Thus, methods for noninvasive assessments of the presence of vessels and the vascularity of the callus tissues can be very valuable for gauging the status of the healing process. 8 However, despite the known importance of angiogenesis in fracture healing, it is not common clinical practice to assess vasculature for fracture healing. This became the aim of our study, and the biology of fracture healing was demonstrated in precarious blood supplied scaphoid waist fractures treated with 1,2-ICSRA based vascularized bone grafts.
In vivo assessment of microvasculature during femoral fracture healing in rats and evaluation of vascularization and blood flow at the fracture site by 3D-HFP-DU was comparable to micro-CT based microangiography. 7 3D-HFP-DU is non radiation and highly sensitive in distinguishing between normal and impaired angiogenic response, thereby differentiating delayed and nonunion during fracture healing. It is noninvasive and real-time, and can be used for longitudinal follow-up for both vascularization and blood flow quantifications.
3D-HFP-DU can be used in various aspects of surgery with vascularized grafts at different sites amenable. We are sure that the scope of 3D-HFP-DU in future will be beyond vascularized grafts as its microvasculature assessment, volume, accuracy, differentiating or quantifying delayed union or nonunion, incorporation of vascularized grafts into the fracture site, neovascularization, neoangiogenesis, and other parameters may replace or adjunct CT angiography. Many more studies should garner such as ours to harbinger how 3D-HFP-DU is going to suite amidst the X-rays and CT scans in various fields.
The limitations of this study are the small sample size and the retrospective nature of study without analyzing the difference before and after harvesting the graft using the 3D-HFP-DU. The application of 3D-HFP-DU has limitations. The detection of flow signals is limited by the penetration depth. With the use of higher Doppler frequency, penetration depth would be decreased; therefore, researchers must compromise between accuracy and detection depth. Having said that, invasive CT angiography and noninvasive MR (magnetic resonance) angiogram can still be a futuristic option to assess the blood flow and vasculature in the pedicle of these vascularized bone grafts used for scaphoid nonunions.
1,2-ICSRA vascularized bone grafting has completely changed the treatment modalities in scaphoid nonunion by achieving good bony union, symptom relief, and excellent functional outcomes. The use of 3D-HFP-DU in postoperative scaphoid nonunion with vascularized grafts is a possible modality to demonstrate vascular supply to scaphoid. CT scan confirms the union. Considering the vascularized grafts over the scaphoid nonunion site and K-wire producing artifacts in CT scans and time delay in radiographs confirming the union, 3D-HFP-DU is suitably advantageous and definitely a different modality replacing both of them in this given scenario.
3D-HFP-DU is a noninvasive, nonradiation imaging modality that does not interfere with artifacts and offers high-resolution anatomical visualization of neovascular networks. The feasibility and reproducibility in detecting the small-sized 1,2-ICSRA and its visualization amidst larger vessels are the hallmark features of 3D-HFP-DU. It provides a robust approach in demonstrating the biology of fracture healing by angiogenesis and revascularization across the fracture site, which can be a valuable method in assessing the status of scaphoid union.
Acknowledgments
The authors thank the patients, who have been the backbone of this study, and the radiology team, who supported with images and results. IRB approved this study and remained always a constant support in this endeavor.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Chang M A, Bishop A T, Moran S L, Shin A Y. The outcomes and complications of 1,2-intercompartmental supraretinacular artery pedicled vascularized bone grafting of scaphoid nonunions. J Hand Surg Am. 2006;31(03):387–396. doi: 10.1016/j.jhsa.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann S P, Bishop A T, Berger R A. Use of the 1,2 intercompartmental supraretinacular artery as a vascularized pedicle bone graft for difficult scaphoid nonunion. J Hand Surg Am. 2002;27(03):391–401. doi: 10.1053/jhsu.2002.32077. [DOI] [PubMed] [Google Scholar]
- 3.Hirche C, Heffinger C, Xiong L. The 1,2-intercompartmental supraretinacular artery vascularized bone graft for scaphoid nonunion: management and clinical outcome. J Hand Surg Am. 2014;39(03):423–429. doi: 10.1016/j.jhsa.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Waitayawinyu T, Pfaeffle H J, McCallister W V, Nemechek N M, Trumble T E. Management of scaphoid nonunions. Hand Clin. 2010;26(01):105–117. doi: 10.1016/j.hcl.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Gray R RL, Shin A Y. Vascularized bone grafting of scaphoid nonunions. Oper Tech Sports Med. 2010;18:155–162. [Google Scholar]
- 6.Pao V S, Chang J.Scaphoid nonunion: diagnosis and treatment Plast Reconstr Surg 2003112061666–1676., quiz 1677, discussion 1678–1679 [DOI] [PubMed] [Google Scholar]
- 7.Sun M H, Leung K S, Zheng Y P. Three-dimensional high frequency power Doppler ultrasonography for the assessment of microvasculature during fracture healing in a rat model. J Orthop Res. 2012;30(01):137–143. doi: 10.1002/jor.21490. [DOI] [PubMed] [Google Scholar]
- 8.Augat P, Morgan E F, Lujan T J, MacGillivray T J, Cheung W H. Imaging techniques for the assessment of fracture repair. Injury. 2014;45 02:S16–S22. doi: 10.1016/j.injury.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Zaidemberg C, Siebert J W, Angrigiani C. A new vascularized bone graft for scaphoid nonunion. J Hand Surg Am. 1991;16(03):474–478. doi: 10.1016/0363-5023(91)90017-6. [DOI] [PubMed] [Google Scholar]
- 10.den Boer F C, Bramer J A, Patka P.Quantification of fracture healing with three-dimensional computed tomography Arch Orthop Trauma Surg 1998117(6-7):345–350. [DOI] [PubMed] [Google Scholar]
- 11.Caruso G, Lagalla R, Derchi L, Iovane A, Sanfilippo A. Monitoring of fracture calluses with color Doppler sonography. J Clin Ultrasound. 2000;28(01):20–27. doi: 10.1002/(sici)1097-0096(200001)28:1<20::aid-jcu3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Raines A L, Sunwoo M H, Thacker K.
- 13.Zhang G, Qin L, Hung W Y. Flavonoids derived from herbal Epimedium Brevicornum Maxim prevent OVX-induced osteoporosis in rats independent of its enhancement in intestinal calcium absorption . Bone. 2006;38(06):818–825. doi: 10.1016/j.bone.2005.11.019. [DOI] [PubMed] [Google Scholar]


