Abstract
We are experiencing seminal times in computing that seem to define a fourth industrial revolution. This may fundamentally change the way we live, work, and relate to one another. Embracing data and digital information is a top priority for most industries these days, and Life Sciences is no exception. The pharmaceutical industry in particular is fundamentally a data‐driven business. Inspired by a desire to “Go Big on Data,” we developed a strategic roadmap defining a digital transformation to reimagine the way we work in Novartis Global Drug Development, leveraging data science to generate and inject actionable insights into our best practices. We launched a program called Nerve Live, and built a state‐of‐the‐art data and analytics platform to harness past and present operational data, providing access to decades of drug development “experience” buried across multiple sources. The platform enabled the systematic application of machine learning and predictive analytics to generate “intelligence”: new insights across multiple functional areas. To action the insights and create “value,” we crafted skillfully designed end‐user applications for domain experts to plan, track, predict, compare and monitor domain activities, optimize costs, and maximize quality. Today, the Nerve Live program enables insights‐driven decision making at scale, unlocking productivity, and providing transparency across the Novartis Global Drug Development organization and beyond. We identified three main drivers making the Nerve Live program successful and enabling the associated digital transformation to flourish. We discuss the challenges, highlight the benefits, and see the importance of leading the way to become future proof.
The Potential for Data and Digital in Drug Development
After the PC reached the mainstream and revolutionized the way we work, 1 after the web fundamentally changed the way people interact and connect with each other, after smartphones brought computing to almost half the world population 2 profoundly changing people's lives, we are in the midst of the next data and digital revolution. Four elements are powering it: (1) the steady acceleration of the rate at which new data is generated; (2) cloud computing that offers universally available data storage and exponentially growing computing power; (3) the explosion of machine learning and artificial intelligence (AI); and largely important, (4) the will of people to embrace the technology revolution to transform every aspect of our life. Whether it is the aerospace or automotive industry, electricity suppliers, or the banking sector: big data tools and digital technologies are changing every aspect of how companies operate. We are living in unprecedented times for data and digital disruption—and Life Sciences is no exception.
How can data and digital further help the pharmaceutical industry to bring better medicines to patients faster and more efficiently? A few years ago, we tackled this question for global drug development in our company. Simply stated, the role of drug development is to take molecules identified in research laboratories as biologically active on targets linked to disease, and transform them into effective medicines that can be prescribed safely to millions of patients worldwide. To prove safety and efficacy, program teams design clinical studies and execute them through medical centers enrolling patients around the world. The scale of such operations is impressive, as our company currently runs over 500 trials across > 70 countries, with over 80,000 patients participating. In parallel, technical development teams design and develop the drug formulations, products, and devices that maximize the patients’ experience with our medicines.
The pharmaceutical industry keeps improving practices and processes used in drug development to develop new medicines. However, historically, the sector has not been at the frontline of the data and digital revolution, taking advantage of the opportunities that it brings. 3 The drug development process involves tedious, time‐consuming tasks that can often take days or even weeks to complete manually. Consider the example of clinical trials management. Summarizing the status of just one study implied a strenuous review of many spreadsheets to obtain first the country, then the regional, and finally the global overview. All data coming from different systems through manual, one‐time extractions, at different time points, and often following different assumptions and rules.
When we then assessed the situation in our company, we realized that the transformative potential to boost drug development with data and digital is enormous—from significantly faster trials to lower cost, and higher quality of operations. However, we had to take a radical new approach in the way we manage our data and support our processes if we wanted to benefit from the possibilities offered by the technology revolution.
A Goldmine of Operational Data
Data science starts with data and Life Sciences is a sector notoriously rich in data. Just think about the exponential explosion of ‐omics data over the last 20 years, or the vast amount of datasets built from having conducted thousands of clinical studies across countless diseases, and developed all along hundreds of drug products. Data sources separate between two main domains: “patient data,” our wealth of clean, curated, longitudinal and interventional data going back decades, as well as “operational data,” like project management, finance, quality, trial management, drug manufacturing data… i.e., all the data that has been and continue being created daily to support the transactions, processes, and workflows that move the drug development machine forward.
This paper focuses on our work with “operational data,” and the steps we took to bring the digital revolution into Novartis Global Drug Development and reimagine the way we work powered by insights‐driven decision making. Most of what we uncovered during this journey may apply well and at a comparable scale to “patient data”: examples include clinical end points, electrophysiological and other biomarkers, vital signs, laboratory and other data types from clinical trials, medical imaging data, genomics, proteomics and other data from biological molecules, as well as real‐world data collected from patients, providers, pharmacies, payers, and others, once medicines are made available to patients. However “operational data,” being fundamentally of non‐human origin, is generally not subject to the requirements mandated by data protection and privacy regulations (e.g., General Data Protection Regulation (GDPR) 4 ), patient informed consent for storage and use of patient data, and several data integrity and compliance regulations issued by health authorities (e.g., 21 Code of Federal Regulations (CFR) Part 11 5 ). Therefore, we identified “operational data” as ideal domain for starting our digital transformation in Global Drug Development.
Why is old data so valuable? Historical “operational data” carries a quantitative and precise representation of our “experience” with many of the tasks supporting drug development. A few examples: for each past clinical study, historical trial management data indicate exactly how successful each medical center was at recruiting patients; for each investigational drug, past data shows how well clinical drug supply followed the initial plans, and finance data can tell the entire spend story behind a development program. For an organization like Global Drug Development, the wealth of operational data collected over the last 20 years is a true goldmine that can be explored with the tools offered by data science to build predictive models for how future activities will go. One of the most valuable assets we have at our fingertips.
Building an Advanced Analytics Platform
The pharmaceutical industry is fundamentally a data‐driven business. 6 All along the value chain from research to market, we wrap our molecules around with data: to prove their mode of action, their safety, and efficacy, and eventually to inform healthcare practitioners on how to best use them when treating patients to improve or extend their lives. When it came to operational data, however, we had to break new ground in many areas and fundamentally rethink how we integrate, analyze, and use that data to support decisions. Operational data seems as locked into silos. It typically resides in multiple internal dedicated systems (e.g., project management data in the project management system). Data extraction from those systems is difficult, and requires time‐consuming manual efforts that only information technology (IT) personnel with special access rights can execute. In general, operational data landscapes emerge as very fragmented.
Overcoming these challenges requires ensuring continuous access to the right data, logical data integration and proper data curation, availability of the latest data engineering, and data science tools and methods to structure and interrogate the data, and finally the ability to turn all of this into actionable insights. We soon realized that we had to start our digital transformation by building a state‐of‐the‐art advanced analytics platform, that we called Nerve Live (Figure 1 ). We chose a new hybrid architecture, with a local data ingestion server and private cloud‐based storage and computation. In doing this 4 years ago, we believe we have been leading the way in the pharmaceutical industry, 7 leveraging unprecedented computing power and advances in data management systems to store, organize, and optimize data for analysis and insight at scale. As such, Nerve Live enables three main things:
to ingest data automatically from different sources, clean it, link it, and make it ready for analytics;
to build sophisticated machine learning algorithms to analyze the data and generate actionable insights;
to create dedicated web‐based front‐end user modules (also called “solutions” or “applications”) for employees to use in their daily workflows to make better, insights‐driven decisions.
Figure 1.
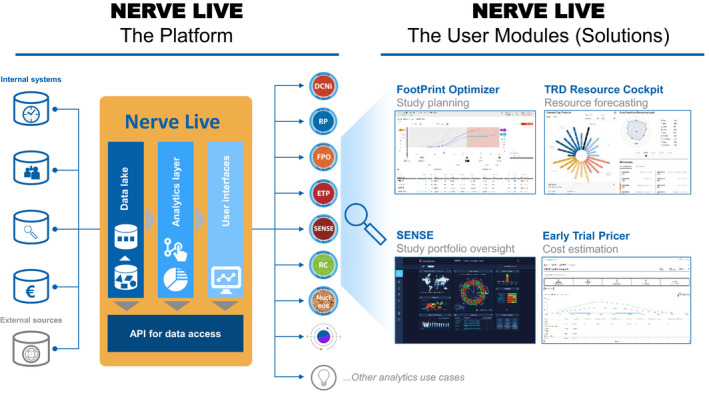
Left panel: High‐level schematic representing sample data sources and the Nerve Live platform through its main logical components: data lake, analytics layer, data access application programming interface (API) and end‐user interfaces (modules or solutions). The current eight Nerve Live modules are indicated by colored round icons with an acronym (see Table 1 for a complete description). Right panel: Illustration of the Nerve Live program through example screenshot from four Nerve Live modules (solutions).
This foundational step was far from easy. First, the new platform architecture was not a company standard and required many discussion rounds before approval. Then, besides being locked in silos, data were owned by different functions, displayed inconsistencies, and was difficult to access. Operational data are not generated with the intention of doing analytics. It typically just supports temporary tasks and transactions. One of the monumental efforts behind Nerve Live has been “connecting” and “curating” that data so that it could be seamlessly utilized across the operational landscape. This involved re‐processing decades of data from a variety of systems, each with their own formats and identifiers—getting the data ready for analytics was at the foundation for our digital transformation. In general, the amount of work required to bring all data from different sources together to an analyzable format is largely underestimated in the public excitement about AI and machine learning. 8
Modules Bring Actionable Insights into Decision Making
With the Nerve Live program (Figure 1 ), we developed a strategic roadmap defining a digital transformation to reimagine the way we work in Novartis Global Drug Development. To date, we have focused on areas of priority, including clinical trial operations, technical research and development, and financial and resource planning. Our goals were to simplify planning, budgeting, and execution activities, enable predictive, faster decision making, streamline operational processes, and ease access to information.
In essence, Nerve Live enables our development organization to access “experience,” create “intelligence,” and unlock “value” across the value chain. For each use case, the approach has been to combine decades of operational data from multiple internal systems (a digital representation of our collective drug development “experience”), apply machine learning and advanced analytics to generate new insights (“intelligence”), and create application modules for our associates that can action insights (unlocking “value”) to plan, track, predict, compare, and monitor domain activities, optimize costs, and maximize quality. This approach enables us to maximize efficiency (“faster, better, cheaper”) and make smarter, data‐driven‐decisions all across drug development. Machine learning has been essential to build representations (models) of process elements (e.g., patient enrollment) based on historical data from multiple domains, identifying, rather than prescribing, the putative drivers behind such events with minimal assumptions.
To date, the Nerve Live program delivered eight different solutions (similar to apps on a smartphone) that are serving > 4,000 associates. Solutions deliver intuitive user experiences, elegantly combining advanced analytics with well thought application design and the right data context—this smart combination is what makes Nerve Live products special. We started with one module, called DCN Insights, to predict and monitor trial enrollment, trial cost, and trial quality, and enable historical comparison across different studies or countries. Notably, and for the first time, DCN Insights delivered machine learning predictions for timing of “end of enrollment” across > 300 studies simultaneously and through intuitive visualizations, allowed for root cause analysis down to single site activities, or provided machine learning‐based risk for quality events across thousands of clinical sites globally. Importantly, it acted as a “technology demonstrator” to show that we could deliver on our vision and truly augment decision making in critical areas of drug development at scale. The success of DCN Insights allowed us to gain the necessary organizational trust that empowered the program to continue along the Nerve Live roadmap and develop new use cases. The scope for the current eight Nerve Live modules is summarized in Table 1 .
Table 1.
The current eight Nerve Live modules
| Module | Full name | Primary user | Short description |
|---|---|---|---|
| DCNi | DCN insights | GDD | Provides insights on patient enrollment, quality and cost, and enables comparison across different studies or countries |
| FPO | Footprint optimizer | GDO | Enables study enrollment scenario planning and identification of optimal study locations (countries and sites) based on historical data |
| RP | Resource planner | GDO | Forecasts resource requirements, such as staffing and time commitment needed, for each clinical study |
| ETP | Early trial pricer | GDO | Enables early prediction of clinical trial costs for various scenarios, allowing teams to choose the most suitable one |
| SENSE | SENSE | GDD | The “control tower” for Novartis clinical studies, helping to monitor the portfolio of studies and identify any potential risks to timelines or costs |
| RC | Resource cockpit | TRD | Visualizes project portfolio across technical units, and enables prediction and optimization of resource allocation (internal and external) |
| Nucleus | Nucleus | TRD | Enables design of optimal study drug supply plans and minimizes supply risk |
| DYNAMO | Dynamic allocation with machine optimization | Finance | Forecasts total and phased costs for every new (planning) and ongoing (tracking) clinical trial |
GDD, Global Drug Development; GDO, Global Development Operations; TRD, Technical Research and Development.
Most are identified by an acronym. The Primary User is the GDD Line Function that acts as business owner for the solution and typically brings the largest number of users.
One of the modules, SENSE, is particularly interesting because it is the first with a room built around it: a physical “Insights Center” (Figure 2 ). The purpose of SENSE is to provide a predictive oversight to our portfolio of > 500 global clinical studies, identify potential risks along the clinical trial process, and drive action to prevent problems before they may happen. All of this in real‐time and fully automated. SENSE provides the big picture—a clear understanding of the status of all our clinical studies, enabling better oversight, preventive course correction, and greater efficiencies. In addition, SENSE provides the opportunity to determine if our trial process elements are fit for purpose, indicating areas needing process improvement. Inspired by the aviation world, SENSE has been referred to as “The Control Tower for clinical studies.” 9
Figure 2.

The SENSE Insights Center on the Novartis Campus in Basel, Switzerland, inaugurated on December 10, 2018.
Enablers
Reflecting on the key drivers making the Nerve Live program successful and enabling the associated digital transformation to flourish, three main factors emerge: (i) senior management sponsorship, (ii) the Nerve Live core team setup, and (iii) our approach and method.
As it is the case for political will behind the leading success of the digitalization of Estonia, 10 a fundamental constant driver behind the Nerve Live transformation has been the widespread cross‐functional support for its transformative agenda. It was indeed critical to have principal sponsorship from the Head of Global Drug Development and broad endorsement for the program across his leadership team. A digital transformation breaks a lot of new ground and questions the “status quo” in many areas. “Change immunity” of an expert organization like Global Drug Development may tend to excessively question the new approaches and ways of working as they force people to leave established comfort zones (e.g., we had to ensure regular communications from the top as part of, e.g., town hall meetings, leadership blogs, and internal social media), explaining the potential to be unlocked by the Nerve Live program and asking everyone across the organization to support its development.
Building the right team was the second critical success factor. A strong team of talented product owners capable to deliver solutions representing the vision developed closely with the future users of each Nerve Live module. The primary responsibility of product owners is to lead product development, including: ideation, team building, deep scoping, data mapping, data science discovery, directing development, and ensuring delivery. Our product owners’ mixed set of core competencies are not easy to find in a single individual: data science expertise (as ex‐practitioners), business problem “translation” to computationally tractable questions, a high learning agility, because products integrate information across multiple domains of expertise, focused attention to users’ needs, and strong command of leading approaches, including professional project management, Design Thinking for structured ideation, and Agile software development for adaptive implementation (Agile comprises various approaches under which requirements and solutions evolve over fast implementation cycles and through the collaborative effort of self‐organizing and cross‐functional teams and their end‐users. For a review, see ref. 11). We were fortunate to identify a few qualified individuals by meticulously screening dozens of profiles and conducting technical interviews, taking some calculated risks on a few competences that may have been initially weak but showed potential for rapid upgrade. Product owners also need to know well how to navigate our IT process governance, which covers solution architecture approval, quality, security, compliance classification, and software applications deployment. It is important to mention that we established a long‐term partnership with our IT organization, aligning goals, values, and finding a way to work together toward a common goal.
The third major strength behind the Nerve Live program was a relentless entrepreneurial approach with product mind‐set: think big, start small. The program started small by building the platform and just one module (DCN Insights) with a small team. Thanks to this lean model, the program was able to deliver tangible results in a short time, build credibility and trust, and deliver value early on. The early success fueled a next wave of investment and the build of more modules. A yearly funding cycle (“waves”) has repeated successfully over the last 4 years, and we are working on the next one. Our product teams were kept small, 12 involved users early on, and were empowered to take all decisions needed to design, develop, and deploy data‐driven, predictive solutions that help the business reimagine the way they work.
Final Remarks
Our goal is embedding insights‐driven decision making in the fabric of drug development, putting data science center stage. Leading such a digital transformation requires skills with innovative technologies, but not only. Most technology challenges can be overcome relatively easily. The difficult challenges are those involving people, behaviors, and the change required for the transformation to succeed. Building trust in both data and data science methods is vital. Although trust requires data science excellence, education, and high data quality standards, trust also needs a shared understanding that things may not look perfect at the beginning, and people should keep adopting new solutions and ways of working with imperfect data and insights. As we look at versions 2.x and 3.x that are being developed for the various Nerve Live modules, thanks to the Agile methodology, these acquire new functionalities, improve existing functionalities, and overall make the user experience better and better. However, those advanced versions would have never been developed if users would have not adopted initial versions 1.0.
Although we are just starting to assess systematically the benefit from the program, initial projections indicate that productivity gains in the order of 10% are already achievable across the portfolio of activities. To illustrate initial successes, across clinical sites, we have measured less inactive sites and faster enrollment periods through insights‐driven planning and preventive operations management (Footprint Optimizer and SENSE), lower study budgets through optimized early cost planning (Early Trial Pricer), and more efficient resource allocation (Resource Planner). Another benefit from the digital revolution, beyond efficiency is transparency. Having integrated separate data systems through a common data model and built data‐driven insights modules designed to be generally accessible means that everyone, from the senior director to the assistant laboratory scientist, sees the same data picture. So, instead of spending energy telling each other stories about the pieces of data one does have access too, everyone can combine forces to address the strategic questions that emerge from a full, comprehensive, and shared perspective. This is a powerful change, and likely to be a disruptive change. It challenges not only work methods and processes, but also existing hierarchies, power‐systems, and mindsets.
Originally developed for use in Global Drug Development (GDD), the Nerve Live footprint has now extended beyond GDD, ingesting data from the Novartis Institutes for BioMedical Research (NIBR), and supporting NIBR and Global Medical Affairs studies through various modules, as well as helping on operational study design for Sandoz, our generic pharmaceuticals and biosimilars division. The simplicity of the “experience → intelligence → value” concept, the successful results, and the learnings collected with Nerve Live in GDD gave us the opportunity to scale the concept of enterprise data and analytics platform further across the organization. We started creating other “Insights Centers” like SENSE (Figure 2 ) in other business units (e.g., Novartis Technical Operations, our drug manufacturing organization), to provide transparency and support larger parts of the company with augmented intelligence.
We believe the biggest benefit of all is that through this transformation we are setting ourselves up to be future proof, ready to seize the next advances in data science and AI that promises to generate new knowledge. 13
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to sincerely thank the members of the core Nerve Live team, as well as the representatives from the business line functions and IT colleagues who have passionately sponsored, strongly contributed, or wisely advised on the development of the various solutions and the data and analytics platform. Without them Nerve Live would not exist today.
References
- 1. Schwab, K. The fourth industrial revolution – what it means and how to respond. Foreign Affairs <https://www.foreignaffairs.com/articles/2015‐12‐12/fourth‐industrial‐revolution> (2015). Accessed September 30, 2019.
- 2. Holst, A. Global smartphone penetration rate as share of population from 2016 to 2020. Statista <https://www.statista.com/statistics/203734/global‐smartphone‐penetration‐per‐capita‐since‐2005/> (2019). Accessed September 30, 2019.
- 3. Jain, S.H. The health care innovation bubble. Healthcare 5, 231–232 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) <http://data.europa.eu/eli/reg/2016/679/oj> (2019).
- 5. US Food and Drug Administration . 21 CFR Part 11 <https://gov.ecfr.io/cgi‐bin/text‐idx?SID=a9a7ea74f55b223a7ebc058e54c6c58b&mc=true&tpl=/ecfrbrowse/Title21/21cfr11_main_02.tpl>.
- 6. Mijuk, G. Drug development gets big data analytics boost. Novartis <https://www.novartis.com/stories/discovery/drug‐development‐gets‐big‐data‐analytics‐boost> (2018). Accessed September 30, 2019.
- 7. Ghangurde, A. McKinsey Execs on Expanding Pharma's Digital Push, Lessons From Amazon. Scrip <https://scrip.pharmaintelligence.informa.com/SC141824/McKinsey‐Execs‐On‐Expanding‐Pharmas‐Digital‐Push‐Lessons‐From‐Amazon> (2020).
- 8. Shaywitz, D. Novartis CEO who wanted to bring tech into pharma now explains why it's so hard. Forbes <https://www.forbes.com/sites/davidshaywitz/2019/01/16/novartis‐ceo‐who‐wanted‐to‐bring‐tech‐into‐pharma‐now‐explains‐why‐its‐so‐hard/> (2019). Accessed September 30, 2019.
- 9. A touch of “mission control” for Novartis clinical trials. Daily Dispatch, BioPharmaDispatch, Australia, September 4, 2019 <https://pharmadispatch.com/news/a‐touch‐of‐mission‐control‐for‐novartis‐clinical‐trials>. Accessed September 30, 2019.
- 10. Kattel, R. & Mergel, I. Estonia's digital transformation: mission mystique and the hiding hand. UCL Institute for Innovation and Public Purpose Working Paper Series (IIPP WP 2018–09) <https://www.ucl.ac.uk/bartlett/public‐purpose/wp2018‐09> (2018). Accessed September 30, 2019.
- 11. Dybå, T. & Dingsøyr, T. Empirical studies of agile software development: a systematic review. Inf. Softw. Technol. 50, 833–859 (2008). [Google Scholar]
- 12. Hackman, J.R. Rethinking team leadership or team leaders are not music directors In: New directions in the psychology of leadership (eds. Messick D.M. & Kramer R.M.) 115–142 (Lawrence Erlbaum Associates, Mahwah, NJ, 2005). [Google Scholar]
- 13. LeCun, Y. , Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015). [DOI] [PubMed] [Google Scholar]


