Abstract
Background
Cryopreserved platelet products can be stored for years and are mainly used in military settings. Following thawing, cryopreserved platelets are activated, resulting in faster clot formation but reduced aggregation in vitro, rendering their efficacy in bleeding unknown. Also, concerns remain on the safety of these products. The aim was to investigate the efficacy and safety of cryopreserved platelets in a rat model of traumatic hemorrhage.
Study Design and Methods
After 1 hour of shock, rats (n = 13/group) were randomized to receive a balanced transfusion pack (1:1:1 red blood cell:plasma:platelet) made from syngeneic rat blood, containing either liquid stored platelets or cryopreserved platelets. Primary outcome was the transfusion volume required to obtain a mean arterial pressure (MAP) of 60 mmHg. Secondary outcomes were coagulation as assessed by thromboelastometry (ROTEM®) and organ failure as assessed by biochemistry and histopathology.
Results
The transfusion volume to obtain a MAP of 60 mmHg was lower in animals receiving cryopreserved platelets (5.4 [4.1‐7.1] mL/kg) compared to those receiving liquid stored platelets (7.5 [6.4‐8.5] mL/kg, p < 0.05). ROTEM® clotting times were shorter (45 [41‐48] vs. 49 [45‐53]sec, p < 0.05), while maximum clot firmness was slightly lower (68 [67‐68] vs. 69 [69‐71]mm, p < 0.01). Organ failure was similar in both groups.
Conclusions
Use of cryopreserved platelets required less transfusion volume to reach a targeted MAP compared to liquid stored platelets, while organ injury was similar. These results provide a rationale for clinical trials with cryopreserved platelets in (traumatic) bleeding.
1. INTRODUCTION
Trauma‐induced coagulopathy is characterized by platelet dysfunction. 1 , 2 Whereas platelet counts can be preserved during traumatic bleeding, platelet dysfunction occurs in approximately 45% of trauma patients. 1 , 2 , 3 In accordance, transfusion of high doses of platelets results in a reduction in early death following trauma compared to lower doses of platelets. 4 , 5 Liquid stored platelet products however, have a limited shelf‐life (5‐7 days at room temperature). Cryopreservation of platelets increases the lifespan of these products from days to years, rendering cryopreserved platelets suitable for use in austere and military environments. 6 Following thawing, cryopreserved stored platelets are activated and show impaired aggregation and adhesion in vitro. 7 Also, recovery in vivo following autologous transfusion of thawed cryopreserved platelets is reduced compared to fresh autologous platelet products in healthy volunteers. 8 However, despite these observations, initiation of clot formation occurs faster and fibrin formation is unaffected. 9 Cryopreserved platelet products have been used in military and civilian settings, and in terms of clinical outcome, appear to have a similar performance compared to other platelet products. 6 , 10 , 11 , 12 Despite these findings and the fact that cryopreservation could potentially resolve problems with availability or waste of platelet products, widespread use of cryopreserved platelets is hampered, which is probably due to a perceived loss of function of the platelets. 7 , 13 , 14 , 15 However, impaired aggregation in vitro and a reduced recovery in vivo do not correlate with the hemostatic potential of cryopreserved platelets. 11 In contrast, cryopreserved platelets induce a more rapid hemostatic control compared to standard stored products in vitro, presumably as a result of activation, a high platelet‐derived extracellular vesicle content and an increased thrombin generating capacity. 16 In addition to questions relating to the ability of cryopreserved platelet to control bleeding, questions concerning their safety remain. The advantage of the freeze‐thawing‐induced activation of the platelets for controlling bleeding may also have the disadvantage of an increased risk of thromboembolic events. 17 Furthermore, cryopreserved platelets have been shown to have immune‐modulating effects in vitro and to augment a pro‐inflammatory response with ensuing organ injury, as observed in mice. 18
The aim of this study is to compare the hemostatic capacity of cryopreserved with liquid stored platelets in a rat model of traumatic hemorrhagic shock and to investigate the impact on organ injury. We hypothesized that resuscitation with cryopreserved platelets requires less volume to restore hemostasis and shock when compared to liquid stored platelets.
2. MATERIALS AND METHODS
2.1. Ethics, species and intervention
This study was approved by the Institutional Animal Care and Use Committee of the Amsterdam University Medical Centers, location Academic Medical Center. The procedures were performed in accordance with the European Parliament directive (2010/63/EU) and the national law the Experiments on Animals Act (Wod, 2014). Animals were group housed (2 to 4 rats per cage) with access to water and food (2016 Teklad global 16% protein, Envigo, USA) ad libitum at least 7 days before the experiment. The light‐dark cycle was from 7 am until 7 pm. Experiments began at 8 am, traumatic shock was completed around 10 am and ended at 4 pm. A total 26 male Sprague Dawley rats weighing 350‐400 grams (Envigo, UK) were subjected to a model of multiple trauma with uncontrolled liver bleeding.
2.2. Experimental model
2.2.1. Blood component manufacturing methods
Forty syngeneic donor rats were used for the preparation of blood products as described before. 19 , 20 In short, whole blood was obtained through cardiac puncture and prepared and stored according to national blood bank standards. Whole blood was mixed in a 9‐to‐1 ratio with citrate‐phosphate‐dextrose solution, after which blood was immediately centrifuged (10 min, 1892G, 20°C) to separate the blood in three different components. Most plasma was removed and stored at −80°C for 1 day. Two buffy coats were removed and diluted with the rest of plasma until a hematocrit of 20% was obtained. Thereafter, to remove remaining red blood cells and leukocytes, the plasma diluted pooled buffy coat was centrifuged (10 min, 300G, 22°C) and platelet‐rich supernatant plasma was obtained. Platelet‐rich plasma was either kept on a roller bank in a temperature‐controlled stove at 22°C to be used in the next 2 days or was further processed to cryopreserved platelets. The remaining (buffy coat removed) red blood cells were diluted to a hematocrit of 60% with additive solution (saline adenosine glucose mannitol SAGM) and stored at 4°C to be used the next day. One day after blood component production and storage, the products were (thawed and) used for transfusion.
The process to manufacture small volumes cryopreserved platelets was developed by the Dutch military blood bank, mimicking the procedure for human cryopreserved platelets. 21 , 22 , 23 , 24 Dimethylsulfoxide (DMSO) 27% in saline (BloodStor® 27 NaCl Biopreservation Media; Biolife solutions Bothell, WA, USA) was added to fresh rat platelet‐rich plasma in four equal steps (30 sec between steps) while gently stirring to a final DMSO concentration of 6%. Next, this product was centrifuged (10 min, 1250G, 22°C, acceleration 9, brake 0, Eppendorf 4904R). Supernatant was removed (150 μL was left on the pellet) and the pelleted platelets of the product were suspended during 1 minute with slow pipetting movements. To lower the freezing rate, which makes the freezing process comparable to human products, the product‐tube was first wrapped in tissue paper in such a way that the wrapped tube fits in a 50 mL falcon tube. Thereafter, the package was placed upright in an −80°C freezer. The platelets were kept at −80°C before use. On the day of use, cryopreserved platelets were unwrapped and thawed for 5 minutes in a 37°C water bath, after which platelets were suspended with slow pipetting movements for 30 seconds while the tube was still in the water bath. Warm (35‐37°C) thawed plasma product was added rapidly to the suspended platelet pellet while the tube was mixed on a vortex in order to reconstitute the cryopreserved platelet product to the same volume of plasma as the liquid stored rat platelets. The thawed suspended rat platelets were used within 15 minutes after preparation.
When this process was piloted with small volume cryopreserved human platelets, it resulted in >80% in vitro recovery, which was comparable to recovery of standard volume cryopreserved human platelet products (data not shown). Also, the in vitro thromboelastography coagulation characteristics of small volume cryopreserved human platelets were comparable to that of normal volume cryopreserved human platelet products (data not shown).
2.2.2. Model of traumatic bleeding
Animals received induction anesthesia with ketamine (Alfasan, The Netherlands), dexmedetomidine (Orion Pharma, Finland) and atropine (Dechra, The Netherlands) and maintenance anesthesia with ketamine and dexmedetomidine intravenously. A tracheostomy was performed and rats were connected to a mechanical ventilator (Babylog 8000, Draeger, Germany). Pressure‐controlled ventilation was applied with respiratory rate set at 60/min, a positive end expiratory pressure of 3.5 kPa and inspiratory pressures of 10 kPa, yielding tidal volumes between 7‐8 mL/kg. Recruitment was performed every hour by increasing inspiratory pressures (Pinsp) to 25 kPa for 5 seconds. Respiratory rate was increased to 75/min during traumatic hemorrhagic shock. In case of a high PCO2 (>35 mmHg) with a low pH (<7.3), Pinsp was increased by 2 kPa. Monitoring of arterial blood pressure and blood sampling was done via an arterial catheter placed in the right carotid artery. The right jugular vein was cannulated and used for transfusion. Temperature was monitored with a rectal thermometer and kept between 36.5‐37.0°C using a heat table and heat lamp.
Traumatic injury was inflicted by a fracture of the right femur employing a guillotine. Following a median laparotomy, crush injury of the small intestine was inflicted by clamping the gut five times approximately 2 cm from the Treitz ligament with 0.2 cm in‐between the injuries. Isovolumic hemodilution was done with Ringerʼs Lactate (Baxter, USA) according to the formula (0.30(0.06 × weight + 0.770). 25 The liver was punctured at the left lateral lobe using a stance to penetrate a circumference of 0.5 cm of the liver, leading to uncontrolled bleeding. The episode of uncontrolled bleeding lasted for 15 minutes with an open abdomen. If blood pressure did not drop below a mean arterial pressure (MAP) of 40 mmHg, a maximum of 1.5 mL of blood (in addition to the isovolumic hemodilution) was drawn from the carotid artery line (controlled hemorrhage). If MAP dropped below 30 mmHg within the 15 minutes of uncontrolled bleeding period, the liver was packed with gauzes. After 15 minutes, the abdomen was closed to maintain adequate body temperatures. Total hemorrhage was the sum of controlled blood loss (baseline sample + maximum 1.5 mL during shock) and uncontrolled blood loss (estimation of blood loss in the abdomen). After a total shock duration of 1 hour (15 min uncontrolled bleeding +45 min of MAP<40 mmHg), rats were randomized to receive resuscitation with a 1:1:1 volume ratio of red blood cell:plasma:liquid stored platelets (liquid stored) or 1:1:1 volume ratio of red blood cell:plasma:cryopreserved platelets (cryopreserved). Transfusion was set at a speed of 8.0 mL/hour. After reaching a MAP of 60 mmHg for 5 minutes, transfusion was stopped. Thirty minutes before termination of the experiment (at 5.5 hours), a FITC‐labeled 70 kDa dextran was administered to assess endothelial barrier leakage. After euthanization by bloodletting, the circulation was flushed against gravitational force with 50 mL NaCl 0.9%. Both vascular supply of the left lung and left kidney were tied‐off during this process to assess wet/dry ratios of these organs. Wet weight was measured by measuring the weight of the left lung/kidney, drying the organ for 1 week at 60°C, and reevaluating the weight of the dried organ.
2.2.3. Measurements during the experiment
Blood samples were obtained just before trauma (T0), after 1 hour of shock and just before initiation of transfusion (T1), at 3 hours (T3), at 4 hours (T4) and after 6 hours at termination of the experiment and subsequent euthanization of the animals (T6). Blood was assessed for arterial blood gas and electrolytes at all time points and rotational thromboelastometry (ROTEM® Delta, Werfen, Spain) at four time points (T0, T1, T3, and T6). Thrombocyte count (XN‐9000, Sysmex, Japan), hemoglobin/ hematocrit (RAPIDPoint 500, Siemens Healtineers, Germany), prothrombin time and Clauss fibrinogen level (CS2500, Siemens Healthineers, Germany), aspartate transaminase, alanine transaminases, creatinine (Cobas c702, Roche, Switzerland) and urine protein (Cobas c502, Roche, Switzerland) were measured at two time points (T0, T6).
2.2.4. Rotational thromboelastometry
The EXTEM assay evaluates the extrinsic coagulation pathway, using tissue factor and phospholipids to initiate coagulation. Clotting time (CT) determines time to clot initiation, the alpha angle assesses the rate of clot formation, maximum clot firmness (MCF) represents the strength of the clot and the 30 minutes lysis index shows the percentage of amplitude decrease 30 minutes after maximum clot firmness, indicating clot lysis. The FIBTEM assay uses EXTEM initiators of coagulation plus a potent platelet inhibitor (cytocholasin D) to eliminate platelet contribution to evaluate the contribution of fibrinogen to clot formation. To assess the platelet component of clot formation, the FIBTEM MCF value is subtracted from the EXTEM MCF value.
2.2.5. Enzyme‐linked immunosorbent assays (ELISA)
Levels of circulating syndecan‐1, a component of the endothelial glycocalyx, and high mobility group protein B1 (HGMB‐1), 26 damage marker released from predominantly platelets, were measured with ELISAs according to manufacturerʼs guidelines (eLabscience, USA).
2.2.6. Organ assessments
The hematoxylin and eosin slides of the lung, liver, spleen, small intestine, and kidney were scored by a pathologist, who was blinded to treatment allocation. This scoring system has been used before. 19 , 20 The lung, for example, was scored based on lung edema, interstitial inflammatory cell infiltration, endothelialitis, and hemorrhage. The scale of each category consisted of a score of 0 (=absent) to 3 (=severe). For the full scoring list see Table S1, available as supporting information in the online version of this paper.
2.2.7. Immunostaining FITC‐dextran leakage
Deparaffinized blanco organ slides of the lung and kidney were colored using a rabbit ‐ anti‐FITC/anti‐rabbit horse radish peroxidase and NovaRed coloring method. The stopping times were kept constant for all slides. Five different images of each organ, blinded for the treatment allocation to the assessor, were obtained with microscope (10x zoom) and camera (BX51 with UC90, Olympus, Japan). Pictures were inverted using Image J. Five random inverted pictures were used to set a threshold indicating presence of FITC‐70 kDa dextran leakage. Median percentage of area intensity was used as measure for endothelial leakage.
2.3. Sample size calculation
Based on previous experiments, 19 given a treatment effect of 0.6 mL/kg and a standard deviation of 0.47 mL/kg, 11 rats per group have a power of 80% to detect a statistical significant difference in transfusion volume needed to reach a MAP of 60 mmHg. Because we previously experienced a mortality of 20% in our model, we added two additional rats per group, yielding 13 per experimental group.
2.4. Statistical analysis
Data were analyzed using SPSS (IBM, version 25.0), graphs were made using GraphPad Prism (version 8.0.2). All parameters were first assessed for distribution and checked for normality using a Kolmogorov‐Smirnov test and by visual inspection of histograms. Data were presented based on their distribution as median with interquartile range, mean with standard deviation or percentage. Parameters were tested for differences using the Mann‐Whitney U test, Student T test, or Chi‐Squared test. Paired (T0 vs. T6) nonparametric differences were tested within groups with a Friedman ANOVA. A p value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Trauma, shock, and transfusion needs
Traumatized animals were in severe shock in both groups, indicated by high base deficits, increased lactate levels and low blood pressures without significant differences between group (Table 1, Fig. 1). Controlled blood loss (baseline sample + additional bloodletting) plus estimated uncontrolled blood (estimated volume of blood loss in the abdomen) loss during the shock period were not different between cryopreserved and liquid stored platelet treated rats (total blood loss of 9.6 [8.8‐10.1] vs. 9.7 [9.0‐10.3] mL, p = 0.84). All animals survived the 6‐hour time point.
TABLE 1.
Parameters at baseline, after traumatic shock and after resuscitation strategy
| Liquid stored platelets | Cryopreserved platelets | |||||
|---|---|---|---|---|---|---|
| Parameter | Before injury (T0) | After injury and shock (T1) | After transfusion (T3) | Before injury (T0) | After injury and shock (T1) | After transfusion (T3) |
| Weight (gram) | 359 (344‐373) | ND | ND | 346 (339‐366) | ND | ND |
| pH | 7.39 (7.37‐7.45) | 7.41 (7.37‐7.43) | 7.41 (7.37‐7.42) | 7.43 (7.39‐7.47) | 7.39 (7.32‐7.45) | 7.40 (7.36‐7.42) |
| pCO2 (mmHg) | 34.9 (31.0‐41.4) | 24.9 (23.8‐27.4) | 28.8 (26.2‐30.1) | 36.5 (33.9‐38.9) | 23.8 (21.2‐32.1) | 28.3 (23.4‐32.3) |
| BE (mEq/L) | −0.7 (−2.3‐0.1) | −8.0 (−8.6‐−6.8) | −6.0 (−8.3‐−5.6) | 0.0 (−2.0‐1.6) | −8.0 (−11.0 ‐ −6.0) | −7.3 (−8.2 ‐ −6.1) |
| Lactate (mmol/L) | 0.7 (0.6‐0.8) | 3.7 (3.1‐4.9) | 2.3 (2.0‐2.9) | 0.7 (0.6‐0.7) | 3.8 (2.8‐7.4) | 2.1 (1.6‐3.4) |
| Na+ (mmol/L) | 139 (138‐140) | 130 (128‐134) | 137 (135‐140) | 139 (138‐141) | 131 (129‐132) | 138 (136‐139) |
| K+ (mmol/L) | 4.1 (3.9‐4.6) | 5.9 (5.7‐6.8) | 5.8 (5.4‐6.1) | 4.1 (3.9‐4.3) | 6.3 (6.0‐6.7) | 5.7 (5.3‐6.0) |
| Ca2+ (mmol/L) | 1.0 (1.0‐1.1) | 1.0 (0.9‐1.1) | 1.1 (1.0‐1.2) | 1.1 (1.1‐1.1) | 1.1 (1.0‐1.1) | 1.1 (1.0‐1.1) |
| Cl− (mmol/L) | 107 (105‐108) | 104 (100‐108) | 107 (106‐112) | 107 (105‐108) | 104 (101‐107) | 109 (108‐111) |
| Glucose (mmol/L) | 18.4 (17.1‐20.1) | 31.3 (29.9‐36.6) | 22.5 (19.8‐24.7) | 17.6 (17.3‐18.8) | 34.7 (30.4‐35.5) | 20.1 (17.9‐21.3) |
Note: Data are presented as median (IQR).
Abbreviations: BE, base excess; ND, not determined.
FIGURE 1.
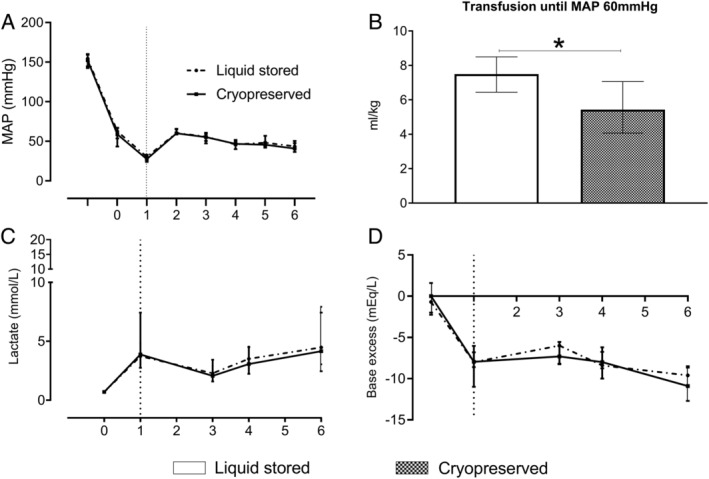
Shock and resuscitation needs. Data are median with interquartile ranges. (A) Mean arterial pressure. Between T0 and T1: 1 hour shock phase; between T1 and T2: transfusion phase. Three hours, 3 4 hours, 4 and 6 hours 6 after trauma additional blood samples were taken. (B) Transfusion until MAP 60 mmHg. (C) Lactate. (D) Base excess. Rats were treated with either a balanced 1:1:1 red blood cell:plasma:platelet with liquid‐stored platelets or a balanced transfusion ratio with cryopreserved platelets. Dotted vertical line represents when resuscitation was initiated. *p < 0.05 between groups
Quality assessments of the different blood products are described in the Table S2, available as supporting information in the online version of this paper. The rat cryopreserved platelet product yielded a median in vitro freeze‐thaw recovery of 87% [69‐100]. The volume of transfusion needed to restore circulation to the predetermined MAP of 60 mmHg was significantly lower in the cryopreserved platelet transfused rats compared to the liquid stored platelet transfused rats (5.4 [4.1‐7.1] mL/kg vs. 7.5 [6.4‐8.5] mL/kg, p = 0.02). The degree of shock as assessed by lactate levels and base deficits was similar in both groups. These variables remained similar between groups over the course of the experiment.
3.2. Coagulation
Our trauma and shock model resulted in decreased platelet counts, prolonged prothrombin time and decreased fibrinogen levels, without differences between groups. Thrombocyte counts were equal also at the end of the experiment (Table 2). Both groups remained coagulopathic after trauma and resuscitation, as shown by approximately 1.3 times prolonged prothrombin times compared to baseline, without differences between the cryopreserved (13.9 [12.2‐14.4] sec) and the liquid stored platelet treated group (14.2 [12.7‐15.8] sec, p = 0.36). Also, fibrinogen levels remained low in both groups 6 hours post injury, without differences in the cryopreserved platelet group (1.3 [1.1‐1.6] g/L) compared to the liquid stored platelet group (1.3 [1.0‐1.4] g/L, p = 0.34). The ROTEM® assays were measured at multiple time points (Fig. 2). Trauma, blood loss, and saline dilution reduced FIBTEM clot strength but did not significantly affect EXTEM clot strength or clotting times within 1 hour after trauma. Two hours following transfusion, EXTEM clotting time was significantly shorter in the cryopreserved platelet treated rats (45 [41‐48] sec) compared to the liquid stored platelet group (49 [45‐53] sec, p < 0.05), while maximum clot firmness was slightly but significantly decreased (68 [67‐68] mm vs. 69 [69‐71] mm, p < 0.01). The platelet contribution to clot formation was similar between the cryopreserved group compared to the liquid stored platelet group (57 [55‐57] mm vs. 59 mm [54‐60], p = 0.06). There was no lysis present during this experiment in both groups.
TABLE 2.
Coagulation, endothelial leakage, and biochemical assessment of organ injury
| Parameter | Liquid stored platelets | Cryopreserved platelets | ||
|---|---|---|---|---|
| Before injury (T0) | After 6 hours (T6) | Before injury (T0) | After 6 hours (T6) | |
| Hemoglobin (mmol/L) | 9.2 (8.9‐9.4) | 6.3 (5.7‐6.5) | 9.0 (8.6‐9.6) | 6.4 (5.8‐6.6) |
| Hematocrit (%) | 44 (42‐44) | 30 (27‐31) | 43 (41‐46) | 30 (27‐31) |
| Coagulation and HMGB‐1 | ||||
| Thrombocytes (*109/L) | 975 (917‐998) | 511 (399‐654)** | 904 (832‐1003) | 448 (376‐509)** |
| Fibrinogen (g/L) | 2.3 (2.1‐2.4) | 1.3 (1.0‐1.4)** | 2.2 (2.1‐2.4) | 1.3 (1.1‐1.6)** |
| PT (sec) | 10.8 (10.6‐10.9) | 14.2 (12.7‐15.8)** | 10.7 (10.5‐10.9) | 13.9 (12.2‐14.4)** |
| HMGB‐1 (ng/mL) | 1.4 (1.3‐1.8) | 25.5 (13.5‐33.1)** | 1.5 (1.3‐1.6) | 21.5 (10.2‐26.1)** |
| Endothelial leakage | ||||
| Syndecan‐1 (ng/mL) | 61.0 (42.7‐106.6) | 136.6 (108.7‐173.1)** | 54.9 (46.5‐64.4) | 105.1 (86.4‐150.8)** |
| Area FITC leakage‐lung (%) | NA | 13.2 (7.0‐20.0) | NA | 14.3 (9.5‐18.0) |
| Area FITC leakage‐kidney (%) | NA | 20.0 (13.4‐28.6) | NA | 25.6 (20.7‐29.8) |
| Biochemical assessment of organ injury | ||||
| ALT (U/L) | 49.5 (44.3‐53.5) | 1315.0 (872.0‐1822.0)** | 52.0 (48.5‐57.5) | 1093.0 (685.0‐1635.0)** |
| AST (U/L) | 58.0 (54.0‐65.0) | 2733.0 (1436.5‐3598.5)** | 61.0 (57.0‐63.0) | 1656 (1123.0‐3276.0)** |
| Creatinine (μmol/L) | 24.0 (24.0‐26.5) | 142.0 (104.5‐156.5)** | 24.0 (23.0‐25.5) | 145.0 (128.0‐155.0)** |
| Urine protein (g/L) | 0.6 (0.4‐1.0) | 1.7 (1.3‐1.9) * | 0.9 (0.7‐1.0) | 1.7 (1.6‐2.2)* |
Note: Data are presented as median (IQR). *p < 0.05 within group (T0‐T6), **p < 0.01 within group (T0‐T6). No significant between group differences were detected.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; FITC, fluorescein isothiocyanate; HMGB‐1, high mobility group protein B1; NA, not applicable; PT, prothrombin time.
FIGURE 2.
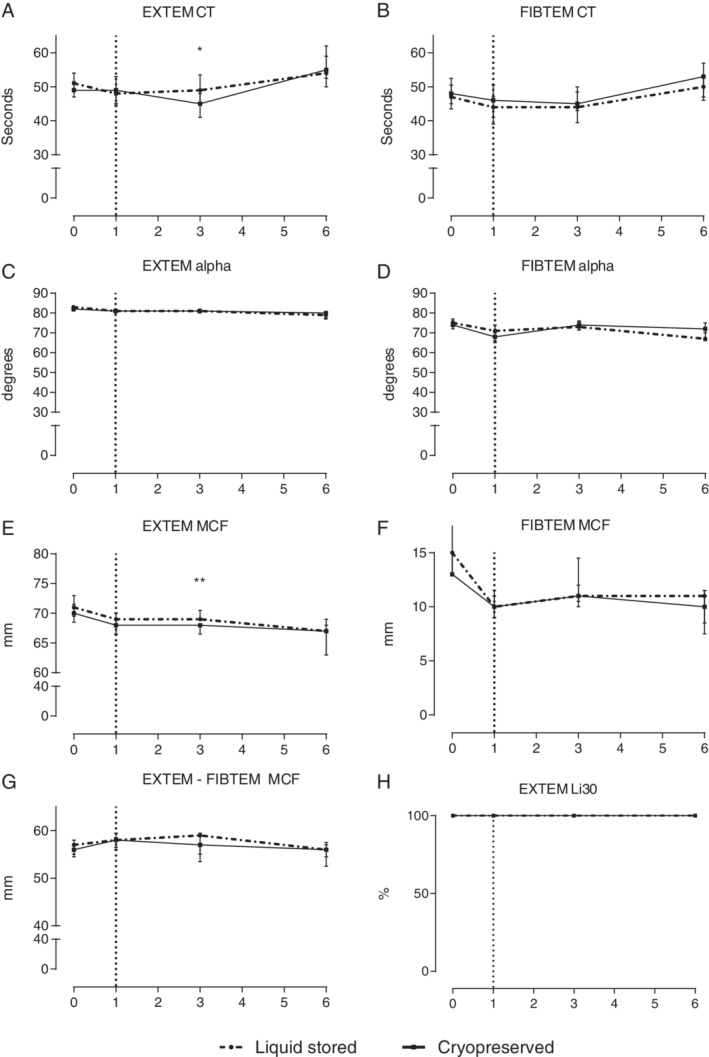
Rotational thromboelastometry. Data are represented as median with interquartile ranges. (A) EXTEM clotting time. (B) FIBTEM clotting time. (C) EXTEM alpha angle. (D) FIBTEM alpha angle. (E) EXTEM maximum clot firmness. (F) FIBTEM maximum clot firmness. (G) EXTEM MCF subtracted by FIBTEM MCF, depicting the platelet contribution to maximum clot firmness. (H) EXTEM lysis index at 30 minutes post maximum clot firmness. Dotted vertical line represents when resuscitation was initiated. *p < 0.05, **p < 0.01 between groups
3.3. Organ injury
Trauma resulted in acute lung, acute kidney, and acute liver injury (Table 2, Fig. 3). Lung injury did not reveal differences between cryopreserved and liquid stored platelet treated rats, depicted by similar lung wet/dry ratios (5.5 [5.2‐5.7] vs. 5.2 [4.7‐5.6], p = 0.22) and similar lung histology scores (Fig. 3). Kidney injury was not different, with similar creatinine levels and kidney wet/dry ratios in the cryopreserved platelet (4.3 [3.9‐4.7]) vs. liquid stored platelets (4.3 [3.7‐4.6], p = 0.88). In addition, groups did not differ in leakage of FITC‐dextran from the circulation into the lung and kidney (Table 2). Syndecan‐1, a marker for glycocalyx degradation, was elevated after trauma, but was not different between groups. Thereby, cryopreserved platelets compared to liquid stored platelets had no impact on endothelial permeability. Also, liver injury did not differ between groups, as determined by similar ALT (alanine transaminase) levels. Assessing organ histology scores revealed no differences between groups (Fig. 3). Thrombus formation was only observed on the site where injury was directly inflicted, without differences between groups. No thrombi were observed in organs without direct traumatic injury. Individual scores of the different organs are shown in Fig. S1, available as supporting information in the online version of this paper. HMGB‐1 is a damage molecule that is released from platelets. The level of HMGB‐1 was markedly elevated after traumatic shock, however there were no significant differences between the liquid stored platelet group and the cryopreserved platelet group.
FIGURE 3.
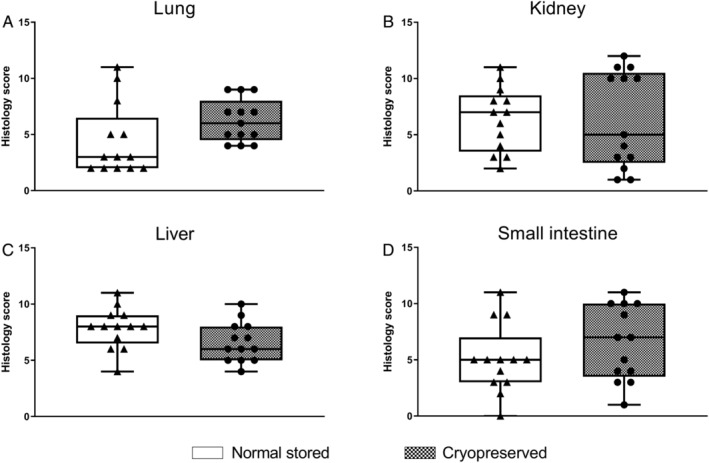
Organ histology scores. Data are presented as boxplot with total range. Cumulative scores of the (A) lung, (B) kidney, (C) liver, and (D) small intestine are shown. For individual scores and total spleen score: see Fig. S1, available as supporting information in the online version of this paper
4. DISCUSSION
In this model of uncontrolled traumatic bleeding, a balanced transfusion strategy containing cryopreserved platelets required 28% less blood product volume to reach a targeted MAP when compared to a strategy using liquid‐stored platelets. Reduced transfusion needs were associated with a faster initiation of clot formation as assessed by ROTEM®, but lower clot amplitude. Use of cryopreserved platelets was comparable to treatment with liquid stored platelets in terms of organ injury.
In our model, the lower volume of transfusion needed to restore MAP to a predefined target in the cryopreserved platelet treated rats, may be attributed to the faster clotting times that were observed with ROTEM®. These data are in line with previous in vitro data, that showed that cryopreserved platelets reduced clotting times compared to liquid stored platelets. 27 , 28 , 29 The reason why cryopreserved platelets compared to liquid stored platelets reduce ROTEM clotting times might be due to increased procoagulant microparticle content and increased thrombin generation. 16 , 30 We found that clot firmness was slightly reduced in the cryopreserved platelet group, although this effect was smaller than found in previous in vitro studies. 7 , 9 , 16 Reduction in clot firmness most likely was not due to lower fibrinogen activity, as fibrinogen levels as well as ROTEM® FIBTEM did not differ between groups. Thereby, the reduction in clot strength in the cryopreserved group may be due to reduced platelet function. In line with this, results of the MCF EXTEM minus FIBTEM were also lower in the cryopreserved platelet group when compared to the liquid platelet group. The differences in clot firmness were however very small (median difference of 1 mm) and it is not clear whether this reflects clinical relevance. Our data suggest that a balanced transfusion with cryopreserved platelets is possibly better for achieving hemostasis compared to liquid stored platelets, even when clot firmness is somewhat reduced. In line with this, cryopreserved platelets formed similar amounts of fibrin in in vitro flow models compared to liquid stored platelets, 21 which also suggests that decreased in vitro platelet functions of cryopreserved platelets such as clot strength, aggregation, and adhesion may not be clinically relevant to reduce bleeding in vivo.
Clinical data comparing the efficacy of cryopreserved platelets to liquid stored platelets in patients are sparse. In an older cardiac surgery trial, cryopreserved platelet transfusion resulted in less blood loss and less blood product use compared to liquid stored platelets. 11 This result was however not reproduced in a more recent pilot trial in cardiac surgery patients, in which no difference in blood loss was shown between groups. 12 Notably, patients treated with liquid stored platelet were twice more likely to have a postoperative bleeding compared to patients treated with cryopreserved platelets. 12 In observational studies in trauma patients, the efficacy of cryopreserved platelets was comparable to historical controls treated with liquid stored platelets. 10 Our data add to the rationale of designing a follow‐up trial investigating efficacy.
In terms of safety, we found no significant differences in endothelial leakage, edema, or organ injury between groups. Previously, in a mouse model of controlled bleeding, use of cryopreserved platelets was associated with high ALT levels, hepatic macrophage infiltration, and hepatic myeloperoxidase activity compared to liquid stored platelet treated mice. 31 An explanation for these different results may be the use of a different transfusion model. In the present study, cryopreserved platelets in plasma were administered in addition to red blood cells, whereas only platelets in plasma were transfused in the mouse model. 31 Furthermore, HMGB‐1 levels were not different between groups in this study. Damaged cells and platelets release HMGB‐1. HMGB‐1 acts as an alarmin and is positively correlated with neutrophil activation, thrombosis and organ failure in trauma. 17 , 32 , 33 , 34 We found no evidence that cryopreserved platelets resulted in HMGB‐1‐mediated inflammation.
The current model has some limitations. This study was designed employing a pressure‐fixed transfusion target as primary outcome. The group of cryopreserved platelets required less transfusion volume compared to liquid stored platelet group. As transfusion is associated with organ injury, we cannot exclude that transfusion of equal volumes would have resulted in higher organ injury scores in the cryopreserved group. However, in clinical practice, a pressure‐fixed target is commonly used as resuscitation goal to minimize transfusion. The resuscitation target of 60 mmHg was chosen based on a previous study in which a target of 60 mmHg compared to other targets did not result in increased mortality, did not increase interleukin levels, reduced blood loss and reduced amount of fluid therapy. 35 Furthermore, rats differ in their coagulation system compared to humans, including higher ranges of platelet counts, hampering translation to humans. 36 However, similar to humans, rats also encounter a platelet dysfunction due to traumatic shock. 37
In conclusion, transfusion of cryopreserved platelets in a rat trauma transfusion model results in improved clotting time and reduced blood transfusion requirements. In terms of organ injury, cryopreserved platelets appear to be as safe as liquid stored platelets. The results provide a rationale for clinical studies investigating the efficacy and safety of cryopreserved platelets in traumatic bleeding.
5. CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Fig. S1. Individual organ scores and total spleen score. Data are boxplots with total range. Individual scores from the A. Lung, B. Kidney, C. Liver, D. Small intestine, and E. Spleen are shown. F. Total histology score of the spleen.
Table S1. Organ failure assessment score
Table S2. Quality assessment of blood products
Kleinveld DJB, Sloos PH, Noorman F, et al. The use of cryopreserved platelets in a trauma‐induced hemorrhage model. Transfusion. 2020;60:2079–2089. 10.1111/trf.15937
Sources of support: Stichting Ziektekostenverzekering Krijgsmacht (SZVK) and the Dutch Ministry of Defense.
Funding information Dutch Ministry of Defense; Stichting Ziektekostenverzekering Krijgsmacht (SZVK)
REFERENCES
- 1. Vulliamy P, Gillespie S, Armstrong PC, et al. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A 2019;116:17444‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verni CC, Davila A Jr, Balian S, et al. Platelet dysfunction during trauma involves diverse signaling pathways and an inhibitory activity in patient‐derived plasma. J Trauma Acute Care Surg 2019;86:250‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg 2012;73:13‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardenas JC, Zhang X, Fox EE, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv 2018;2:1696‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noorman F, van Dongen TT, Plat MJ, et al. Transfusion: −80°C frozen blood products are safe and effective in military casualty care. PLoS One 2016;11:e0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson L, Reade MC, Hyland RA, et al. In vitro comparison of cryopreserved and liquid platelets: potential clinical implications. Transfusion 2015;55:838‐47. [DOI] [PubMed] [Google Scholar]
- 8. Dumont LJ, Cancelas JA, Dumont DF, et al. A randomized controlled trial evaluating recovery and survival of 6% dimethyl sulfoxide–frozen autologous platelets in healthy volunteers. Transfusion 2013;53:128‐37. [DOI] [PubMed] [Google Scholar]
- 9. Cid J, Escolar G, Galan A, et al. In vitro evaluation of the hemostatic effectiveness of cryopreserved platelets. Transfusion 2016;56:580‐6. [DOI] [PubMed] [Google Scholar]
- 10. Bohonek M, Kutac D, Landova L, et al. The use of cryopreserved platelets in the treatment of polytraumatic patients and patients with massive bleeding. Transfusion 2019;59(S2):1474‐8. [DOI] [PubMed] [Google Scholar]
- 11. Khuri SF, Healey N, MacGregor H, et al. Comparison of the effects of transfusions of cryopreserved and liquid‐preserved platelets on hemostasis and blood loss after cardiopulmonary bypass. J Thorac Cardiovasc Surg 1999;117:172‐83 discussion 83‐84. [DOI] [PubMed] [Google Scholar]
- 12. Reade MC, Marks DC, Bellomo R, et al. A randomized, controlled pilot clinical trial of cryopreserved platelets for perioperative surgical bleeding: the CLIP‐I trial. Transfusion 2019;59(9):2794‐2804. 10.1111/trf.15423. Epub 2019 Jul 10. [DOI] [PubMed] [Google Scholar]
- 13. Marks DC, Johnson L. Assays for phenotypic and functional characterization of cryopreserved platelets. Platelets 2019;30:48‐55. [DOI] [PubMed] [Google Scholar]
- 14. Spector JI, Yarmala JA, Marchionni LD, et al. Viability and function of platelets frozen at 2 to 3 C per minute with 4 or 5 per cent DMSO and stored at −80 C for 8 months. Transfusion 1977;17:8‐15. [DOI] [PubMed] [Google Scholar]
- 15. Valeri CR, MacGregor H, Giorgio A, et al. Circulation and hemostatic function of autologous fresh, liquid‐preserved, and cryopreserved baboon platelets transfused to correct an aspirin‐induced thrombocytopathy. Transfusion 2002;42:1206‐16. [DOI] [PubMed] [Google Scholar]
- 16. Johnson L, Coorey CP, Marks DC. The hemostatic activity of cryopreserved platelets is mediated by phosphatidylserine‐expressing platelets and platelet microparticles. Transfusion 2014;54:1917‐26. [DOI] [PubMed] [Google Scholar]
- 17. Vogel S, Bodenstein R, Chen Q, et al. Platelet‐derived HMGB1 is a critical mediator of thrombosis. J Clin Invest 2015;125:4638‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464:104‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleinveld DJB, Wirtz MR, van den Brink DP, et al. Use of a high platelet‐to‐RBC ratio of 2:1 is more effective in correcting trauma‐induced coagulopathy than a ratio of 1:1 in a rat multiple trauma transfusion model. Intensive Care Med Exp 2019;7(Suppl 1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wirtz MR, Jurgens J, Zuurbier CJ, et al. Washing or filtering of blood products does not improve outcome in a rat model of trauma and multiple transfusion. Transfusion 2019;59:134‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Six KR, Delabie W, Devreese KMJ, et al. Comparison between manufacturing sites shows differential adhesion, activation, and GPIbalpha expression of cryopreserved platelets. Transfusion 2018;58:2645‐56. [DOI] [PubMed] [Google Scholar]
- 22. Valeri CR, Feingold H, Marchionni LD. A simple method for freezing human platelets using 6 per cent dimethylsulfoxide and storage at −80 degrees C. Blood 1974;43:131‐6. [PubMed] [Google Scholar]
- 23. Valeri CR, Ragno G, Khuri S. Freezing human platelets with 6 percent dimethyl sulfoxide with removal of the supernatant solution before freezing and storage at −80 degrees C without postthaw processing. Transfusion 2005;45:1890‐8. [DOI] [PubMed] [Google Scholar]
- 24. Lelkens CC, Koning JG, de Kort B, et al. Experiences with frozen blood products in the Netherlands military. Transfus Apher Sci 2006;34:289‐98. [DOI] [PubMed] [Google Scholar]
- 25. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 1985;26:72‐6. [PubMed] [Google Scholar]
- 26. Mardente S, Mari E, Massimi I, et al. From human megakaryocytes to platelets: effects of aspirin on high‐mobility group box 1/receptor for advanced glycation end products axis. Front Immunol 2018;8:1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slichter SJ, Dumont LJ, Cancelas JA, et al. Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion 2018;58:2129‐38. [DOI] [PubMed] [Google Scholar]
- 28. Napolitano M, Mancuso S, Lo Coco L, et al. Buffy coat‐derived platelets cryopreserved using a new method: results from in vitro studies. Transfus Apher Sci 2018;57:578‐81. [DOI] [PubMed] [Google Scholar]
- 29. Johnson L, Tan S, Jenkins E, et al. Characterization of biologic response modifiers in the supernatant of conventional, refrigerated, and cryopreserved platelets. Transfusion 2018;58:927‐37. [DOI] [PubMed] [Google Scholar]
- 30. Raynel S, Padula MP, Marks DC, et al. Cryopreservation alters the membrane and cytoskeletal protein profile of platelet microparticles. Transfusion 2015;55:2422‐32. [DOI] [PubMed] [Google Scholar]
- 31. Zhao J, Sun Z, You G, et al. Transfusion of cryopreserved platelets exacerbates inflammatory liver and lung injury in a mice model of hemorrhage. J Trauma Acute Care Surg 2018;85:327‐33. [DOI] [PubMed] [Google Scholar]
- 32. Orlova VV, Choi EY, Xie C, et al. A novel pathway of HMGB1‐mediated inflammatory cell recruitment that requires Mac‐1‐integrin. EMBO J 2007;26:1129‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan J, Li Y, Levy RM, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1‐TLR4 signaling. J Immunol 2007;178:6573‐80. [DOI] [PubMed] [Google Scholar]
- 34. Giannoudis PV, Mallina R, Harwood P, et al. Pattern of release and relationship between HMGB‐1 and IL‐6 following blunt trauma. Injury 2010;41:1323‐7. [DOI] [PubMed] [Google Scholar]
- 35. Lin GS, Chou TH, Wu CY, et al. Target blood pressure for hypotensive resuscitation. Injury 2013;44:1811‐5. [DOI] [PubMed] [Google Scholar]
- 36. Letson HL, Dobson GP. Differential contributions of platelets and fibrinogen to early coagulopathy in a rat model of hemorrhagic shock. Thromb Res 2016;141:58‐65. [DOI] [PubMed] [Google Scholar]
- 37. Darlington DN, Wu X, Keesee JD, et al. Severe trauma and hemorrhage leads to platelet dysfunction and changes in cyclic nucleotides in the rat. Shock 2019;53(4):468‐75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Individual organ scores and total spleen score. Data are boxplots with total range. Individual scores from the A. Lung, B. Kidney, C. Liver, D. Small intestine, and E. Spleen are shown. F. Total histology score of the spleen.
Table S1. Organ failure assessment score
Table S2. Quality assessment of blood products


