Abstract
Achondroplasia is a genetic disorder that results in disproportionate short stature. The true prevalence of achondroplasia is unknown as estimates vary widely. This systematic literature review and meta‐analysis was conducted to better estimate worldwide achondroplasia birth prevalence. PubMed, Embase, Scielo, and Google Scholar were searched, complemented by manual searching, for peer‐reviewed articles published between 1950 and 2019. Eligible articles were identified by two independent researchers using predefined selection criteria. Birth prevalence estimates were extracted for analysis, and the quality of evidence was assessed. A meta‐analysis using a quality effects approach based on the inverse variance fixed effect model was conducted. The search identified 955 unique articles, of which 52 were eligible and included. Based on the meta‐analysis, the worldwide birth prevalence of achondroplasia was estimated to be 4.6 per 100,000. Substantial regional variation was observed with a considerably higher birth prevalence reported in North Africa and the Middle East compared to other regions, particularly Europe and the Americas. Higher birth prevalence was also reported in specialized care settings. Significant heterogeneity (Higgins I 2 of 84.3) was present and some indication of publication bias was detected, based on visual asymmetry of the Doi plot with a Furuya‐Kanamori index of 2.73. Analysis of pooled data from the current literature yields a worldwide achondroplasia birth prevalence of approximately 4.6 per 100,000, with considerable regional variation. Careful interpretation of these findings is advised as included studies are of broadly varying methodological quality.
Keywords: achondroplasia, birth prevalence, epidemiology, meta‐analysis, systematic review
Abbreviations
- CIs
confidence intervals
- FGFR3 gene
fibroblast growth factor receptor 3 gene
- LFK index
Luis Furuya‐Kanamori index
1. INTRODUCTION
Achondroplasia (OMIM 100800), the most common form of disproportionate short stature, is caused by a variant in the fibroblast growth factor receptor 3 (FGFR3) gene, which leads to inhibition of endochondral bone development (Horton, Hall, & Hecht, 2007; Rousseau et al., 1994; Shiang et al., 1994). Achondroplasia is inherited as an autosomal dominant condition, although it is estimated that approximately 80% of cases occur due to a de novo germ cell mutations in unaffected parents (Horton et al., 2007). In some studies, this is related to advanced paternal age (Orioli, Castilla, Scarano, & Mastroiacovo, 1995; Waller et al., 2008; Wilkin et al., 1998).
Achondroplasia can result in medical complications that significantly increase morbidity and mortality across the lifespan. Common medical complications include delayed motor and speech development in children (Hunter, Bankier, Rogers, Sillence, & Scott Jr., 1998; Ireland et al., 2010; Ireland et al., 2011; Ireland et al., 2012), otolaryngeal problems such as otitis media associated with hearing loss (Afsharpaiman, Sillence, Sheikhvatan, Ault, & Waters, 2011; Hunter et al., 1998; Tunkel et al., 2012), respiratory dysfunction including obstructive sleep apnea (Afsharpaiman et al., 2011; Hunter et al., 1998) spinal stenosis and compression (Hunter et al., 1998; Shirley & Ain, 2009), and dental malocclusions (Hunter et al., 1998). Furthermore, these medical complications can cause significant pain and diminish physical function and quality of life (Alade et al., 2013; Dhiman et al., 2017; Gollust, Thompson, Gooding, & Biesecker, 2003; Mahomed, Spellmann, & Goldberg, 1998; Matsushita et al., 2019). Mortality rates are elevated in individuals with achondroplasia at all ages due to an increased risk of sudden death in young children attributed to brainstem compression resulting from foramen magnum stenosis and greater mortality risk in adulthood related to cardiovascular disease, neurological complications, and accidents (Hashmi et al., 2018; Hecht, Francomano, Horton, & Annegers, 1987; Simmons, Hashmi, Scheuerle, Canfield, & Hecht, 2014; Wynn, King, Gambello, Waller, & Hecht, 2007). While there is currently no approved effective pharmacological treatment for skeletal dysplasias caused by variants of FGFR3 gene, efforts to develop disease‐specific therapies for achondroplasia are underway (Breinholt et al., 2019; Garcia et al., 2013; Komla‐Ebri et al., 2016; Savarirayan et al., 2019). Treatment with growth hormone does not have substantial effect (Harada et al., 2017) and while limb lengthening can be successful, it is a major undertaking associated with significant complications (Donaldson, Aftab, & Bradish, 2015; Kim, Balce, Agashe, Song, & Song, 2012; Ko, Shim, Chung, & Kim, 2019; Leiva‐Gea et al., 2020; Venkatesh et al., 2009).
Achondroplasia is a rare disease. In the United States, a rare disease is defined as a condition that affects fewer than 200,000 people (Orphan Drug Act of 1983), and in the European Union, a disease is defined as rare when it affects fewer than 1 in 2,000 people (GARD, 2019; Orphanet, 2019). Deriving accurate prevalence estimates in a rare disease is especially challenging due to small population sizes, incomplete disease characterization, and rapidly evolving diagnostic methods. Furthermore, prevalence data can vary by population studied, geography, year of birth and the method of diagnosis, and these elements are not always robustly reported and accounted for in the epidemiologic literature. Reported estimates of achondroplasia birth prevalence vary widely, ranging from 1 in 10,000 to 40,000 newborns worldwide (GARD, 2019; Horton et al., 2007; Ornitz & Legeai‐Mallet, 2017; Orphanet, 2019; Pauli, 2019; Unger, Bonafé, & Gouze, 2017) and reports are often based on a few selected references (Horton et al., 2007; Pauli, 2019; Unger et al., 2017).
Accurate prevalence rates are critical for health economics, public health, and research purposes. This systematic literature review with meta‐analysis aims to provide a pooled estimate of achondroplasia birth prevalence in the general population. A secondary objective is to gain insight into the distribution of the birth prevalence of achondroplasia across regions of the world.
2. METHODS
The Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines were used as a guidance for the reporting of this systematic review. The study protocol was registered to Prospero, (van den Bosch et al., PROSPERO 2020 CRD42020148316).
2.1. Identification of eligible publications
PubMed (MEDLINE) and Embase were searched for articles reporting on the birth prevalence of achondroplasia between January 1950 up to and including July 29, 2019. PubMed was searched using the following search strategy: “Achondroplasia”[MeSH Terms] OR “Achondroplasia”[All Fields]) OR Achondroplastic[All Fields] OR “Skeletal dysplasia”[all fields] AND “Prevalence”[Mesh] OR Prevalen*[tiab] OR “Epidemiology”[Mesh] OR “Epidemiology”[subheading] OR Epidemiol*[tiab] OR Burden[tiab] OR “Incidence”[Mesh] OR Inciden*[tiab]. A comparable search strategy was formulated for Embase. The complete search queries can be found in Appendix I. Additional relevant articles were identified in Scielo and Google Scholar using the terms “Achondroplasia” AND “Prevalence” OR “Incidence.” The reference lists of narrative and systematic reviews with focus on achondroplasia prevalence and the reference lists of eligible articles were checked for additional eligible articles.
Articles were considered eligible if they were peer‐reviewed and had an abstract available in the English language. Only articles that reported the birth prevalence of achondroplasia in an unselected population (i.e., individuals captured in a study setting that is likely representative for the general population) were included. The following exclusion criteria were applied: Did not report primary data, presented overlapping results from identical datasets (in which case only one report was included), review article, letter, comment or conference abstract, animal study, case report or case series, or clinical trial. Case series and clinical trials were excluded since they risked representing selected populations rather than the population at large.
2.2. Study screening
Selection of peer‐reviewed articles was based on title and abstract screening, followed by screening of the full‐text in potentially eligible articles. The title and abstract selection and the full‐text screening was done in duplicate by two independent reviewers (FK and JB). After the screening process there was less than 5% discrepancy between the two researchers. The results were compared and discussed, and any disagreements were adjudicated by a third researcher (PKF) until consensus was reached.
2.3. Data extraction and quality assessment
Data from eligible studies were extracted into Microsoft Excel by two researchers (FK, PKF). Data extraction tables were then reviewed by a second researcher (JB). Information identified from the studies included geographical region, country, birth period, study design, setting, (i.e., specialized care, defined as a referral hospital or tertiary care center, versus other settings, such as community hospitals), study population characteristics, and study outcomes (i.e., sample size, birth prevalence). When studies included multiple study estimates (e.g., birth prevalence estimates stratified by birth year or country), data‐extraction was performed separately for each study estimate.
As studies varied dramatically with respect to study methodology and the completeness of reporting, a quality assessment tool was devised to assist in evaluating the quality of the evidence presented in each study. The quality of evidence was assessed across five domains: Data source (i.e., context of case ascertainment), diagnostic method, appropriateness of the numerator and of the dominator used in determining birth prevalence, and the statistical adequacy of population size surveyed (95% confidence) (Naing, Winn, & Rusli, 2006). The quality assessment tool is detailed in Table 1. No studies were excluded based on study quality.
TABLE 1.
Quality of evidence scoring tool
| Score | ||||
|---|---|---|---|---|
| Scoring domain | Strong | Moderate | Weak | |
| Data source | Was the data source complete and representative of the population as a whole? |
|
|
|
| Diagnostic method | Was the method(s) used for the case definition definitive? a |
|
|
|
| Numerator | Was reporting of the numerator sufficient (describes any combination of live births, still born, spontaneous abortions/pregnancy terminations)? |
|
|
|
| Denominator | Was reporting of the denominator sufficient and appropriate? |
|
|
|
| Population size | Was the population size adequate to estimate birth prevalence with 95% confidence? (Naing et al., 2006) |
|
|
|
Abbreviation: NR, not reported.
When methods varied among sites or across time, the study was assigned the value of the lowest scoring method.
2.4. Statistical analysis
Birth prevalence was defined as the total number of achondroplasia cases among births in a predefined population, divided by the sample size of the predefined population, multiplied by 100,000. A meta‐analysis was performed to assess the overall birth prevalence of achondroplasia, as well as birth prevalence stratified by region (North America, South America, Europe, North Africa/Middle East, Sub‐Saharan Africa and South Asia, South‐East Asia/Oceania) and by study setting. For study setting a distinction was made between specialized care (i.e., women who gave birth in a tertiary hospital or referral center) and other settings. A meta‐analysis was performed only when at least three estimates were available per stratification category.
Meta‐analyses were performed using raw data reported in the articles. To prevent bias resulting from small values where variance approaches zero, prevalence estimates were transformed using the double arcsine method (Barendregt, Doi, Lee, Norman, & Vos, 2013). Using this method, confidence intervals (CIs) are forced within the 0% and 100% range. The final pooled result and 95% CIs were back transformed for ease of interpretation (Barendregt et al., 2013; Schwarzer, Chemaitelly, Abu‐Raddad, & Rucker, 2019).
A quality effects approach based on the inverse variance fixed effect model was used for the main analysis. In this model, the redistribution of inverse variance weights is done using a quality parameter between zero (lowest quality) and one (highest quality) (Al Khalaf, Thalib, & Doi, 2011; Deeks, Altman, & Bradburn, 2001; Doi & Thalib, 2008; Doi & Thalib, 2009). The rating of the study quality for the quality effects model was performed as follows: For each question of the quality assessment tool two points were allocated when the study scored “strong,” one point when the study scored “moderate” and zero points when the study scored “weak.” The sum of the individual scores was determined and normalized to a value between 0 and 1 by dividing by the maximum possible score (8). Question Q5 (regarding population size) was omitted from the quality scoring for the meta‐analysis, as the study population size is included in the weights using the inverse variance method. The quality index, which is computed for each analysis (Table 3), expresses the extent (%) to which the weights are redistributed by the application of the quality effect weights.
The more commonly used random effects inverse variance model (DerSimonian & Laird, 1986) was also conducted.
The level of study heterogeneity was assessed by computing the Higgins I 2 statistic, along with a visual assessment using forest plots (Higgins, Thompson, Deeks, & Altman, 2003). A p‐value for the chi‐square test of less than .05 was considered statistically significant. I 2 values of less than 25%, 25–50%, 50–75%, and more than 75% were considered as very low, low, medium, and high heterogeneity, respectively (Huedo‐Medina, Sanchez‐Meca, Marin‐Martinez, & Botella, 2006). Heterogeneity was assessed for I 2 values of 75% or higher using sensitivity analysis.
Publication bias was investigated by assessment of the Doi plot along with the interpretation of the Luis Furuya‐Kanamori (LFK) index (Furuya‐Kanamori, Barendregt, & Doi, 2018). When a symmetrical Doi plot is presented, no publication bias is expected. The LFK‐index quantifies the differences between the two sides of the plot. An index within ±1 was associated with no asymmetry, an index between ±1 and ± 2 indicated minor asymmetry, and an index above ±2 was interpreted as the presence of major asymmetry (Barendregt & Doi, 2011–2016; Furuya‐Kanamori et al., 2018). All analyses were conducted using the MetaXL version 5.3 (www.epigear.com) add‐in for Microsoft Excel.
3. RESULTS
The combined PubMed and Embase search yielded 866 unique hits, of which 68 were selected for full text evaluation (Figure 1). In addition, 65 articles from Google Scholar and Scielo, and 24 articles identified by hand searching the reference lists of eligible articles or systematic reviews were identified for the full text evaluation. From these 156 articles, 52 articles were eligible for inclusion (Figure 1). The 52 included studies reported 101 achondroplasia birth prevalence estimates. Eleven study estimates were excluded, because of double inclusion of data, or lack of reporting of numerator and denominator (further details can be found in Table 2).
FIGURE 1.
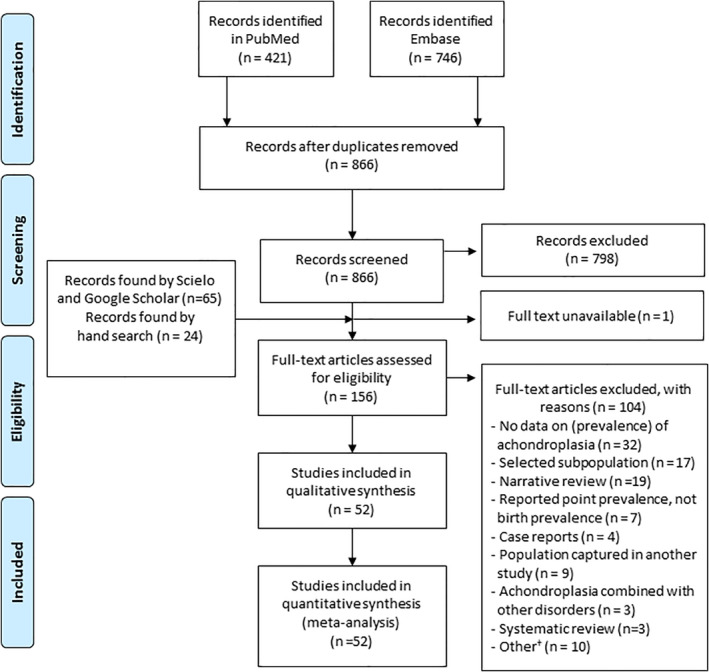
Flow chart of selection process (Moher, Liberati, Tetzlaff, & Altman, 2009). † For example, Modeling study, no original data, no English abstract [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Summary statistics of reported achondroplasia birth prevalence
| Region | Birth prevalence per 100,000 median (IQR) a | Number of studies; number of estimates (% of total estimates) | Population size (% of overall population surveyed in the included studies) |
|---|---|---|---|
| Worldwide | 4.73 (3.10–10.83) | 52 b ; 90 (100%) | 48,453,349 (100%) |
| North America | 4.00 (3.57–4.95) | 9; 15 (16.7%) | 16,748,130 (34.57%) |
| South America | 3.20 (1.95–4.66) | 5; 6 (6.7%) | 8,463,833 (17.47%) |
| Europe c | 3.62 (2.71–5.54) | 13; 40 (44.4%) | 19,945,267 (41.16%) |
| North Africa/Middle East | 34.31 (16.53–52.25) | 13; 13 (14.4%) | 218,831 (0.45%) |
| Sub‐Saharan Africa | 12.60 (7.47–16.53) | 5; 5 (5.6%) | 224,680 (0.46%) |
| South and South‐East Asia/Oceania | 10.58 (4.39–12.82) | 11; 11 (12.2%) | 2,852.608 (5.89%) |
| Populations investigated in specialized care d | |||
| Yes | 13.43 (7.61–2,921) | 14; 14 (15.6%) | 524,538 (1.08%) |
| No | 4.08 (2.94–6.43) | 38; 76 (84.4%) | 47,928,811 (98.92%) |
Abbreviation: IQR, interquartile range.
Birth prevalence rates based on the numerator and denominators reported in the articles (i.e., number of cases/population size × 100,000).
Two studies reported results stratified for multiple regions (Kallen et al., 1993; Orioli et al., 1995). Eleven study estimates were excluded, all extracted from the study of Coi et al. (2019): four because numerator and denominator were not reported, and seven to prevent double inclusion of data (i.e., the overall data from all regions were excluded because that data were also included separately per region).
For the study of Coi et al., 2019 the data from the separate European countries are included in the analysis, instead of data of the countries combined.
Referral center/tertiary hospital.
3.1. Characteristics of studies
Table 2 summarizes the birth prevalence estimates and the main characteristics of the included studies. The included studies spanned six geographical regions and 90 estimates comprising births between 1951 and 2015. In total, outcomes from 48,453,349 births were reported, with 1896 reported cases of achondroplasia (Table 2). The median birth prevalence worldwide was 4.7 cases per 100,000 births. Substantial regional variation was observed with a considerably higher birth prevalence reported in North Africa and the Middle East than in other regions, particularly Europe and the Americas. The reported birth prevalence was also notably higher in reports deriving data from specialized care settings (referral centers/tertiary hospitals), compared with other settings. Individual data for each birth prevalence estimate are shown in Table 3. More than half of the birth prevalence estimates represented births from 1990 to 2015 (Table 3). Almost half of the total population surveyed were in Europe, followed by North America and South America. Sub‐Saharan Africa, North Africa/Middle East. Asia/Oceania combined represented less than 7% of the total population surveyed. Approximately 16% of the birth prevalence estimates were retrieved from studies conducted in specialized care settings (1.1% of the total included study population). For 68 of 90 estimates (75.5%) the study population comprised of a combination of live born and still born infants. More than half of these estimates (n = 35) included pregnancy terminations (Coi et al., 2019; Jaruratanasirikul et al., 2016; Langlois & Scheuerle, 2015; Rasmussen et al., 1996; Stevenson, 1957; Waller et al., 2008). For the remaining estimates it was unclear whether pregnancy terminations were considered. Fifteen estimates (16.7%) were based on livebirths and for seven estimates (7.8%) it was unclear whether livebirths, stillbirths and/or terminations were taken into account.
TABLE 3.
Individual birth prevalence reports and study characteristics by region and by country
| Study quality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (s), year | Country | Sub‐National | Birth prevalence | Birth period | Study design/data source | Study population | Specialized care a | Sample size | Data source | Diagnostic method | Numerator | Denominator | Population size |
| North America | |||||||||||||
| (Alonso Lotti et al., 1998) | Cuba | 13 of the 15 regions | 4.42 | 1985/03–1996/12 | RECUMAC | Live births, stillbirths | No | 520,578 | Strong | Moderate | Strong | Strong | Strong |
| (Guzmán‐Huerta et al., 2012) | Mexico | UNIMEF | 10.99 b | 1995/01–2009/12 | Review of hospital charts of patients seen at the National Institute of Perinatal Medicine | Live births, stillbirths | Yes | 81,892 | Moderate | Strong | Strong | Strong | Weak |
| (Kallen et al., 1993) | Mexico | NR | 2.51 b | 1978–1988 | Programa Mexicano de Registro y vigilancia epidemiogica de malformaciones congentias externas | Live births, stillbirths | No | 359,000 | Strong | Weak | Strong | Strong | Strong |
| (Curran, Sigmon, & Opitz, 1974) | USA | New Jersey | 4.00 b | NR (“past 10 years,” <1973) | Records from the Margaret Hague Maternity Hospital | Live births | No | 75,000 | Moderate | Strong | Strong | Strong | Weak |
| (Langlois & Scheuerle, 2015) | USA | Texas | 2.66 b | 1999–2009 | Records in the Texas Birth Defects Registry | Live births, stillbirths, elective terminations | No | 4,207,898 | Strong | Weak | Strong | Weak | Strong |
| (Rasmussen et al., 1996) | USA | Boston, Massachusetts | 2.37 | 1972/02–1975/02, 1979/01–1990/12 | Brigham and Women's Hospital active malformation surveillance system | Live births, stillbirths >20 w, elective terminations | Yes | 126,316 | Moderate | Strong | Strong | Strong | Moderate |
| (Stevenson, Carey, Byrne, Srisukhumbowornchai, & Feldkamp, 2012) | USA | Utah | 3.53 | 1999–2008 | UBDN | Live births, stillbirths, elective terminations | No | 509,286 | Strong | Strong | Strong | Strong | Strong |
| (Waller et al., 2008) | USA | Arkansas | 5.20 | 1993–1999 | Arkansas Reproductive Health Monitoring System | Live births, stillbirths >20 w, elective terminations >20 w | No | 250,000 | Strong | Moderate | Strong | Weak | Strong |
| Atlanta | 3.89 | 1968–2001 | Atlanta Congenital Defects Program | 1,129,972 | Strong | Moderate | Strong | Weak | Strong | ||||
| California | 4.70 | 1983–1997 | California Birth Defects Monitoring System | 3,572,233 | Strong | Moderate | Strong | Weak | Strong | ||||
| Iowa | 4.09 | 1983–2001 | Iowa Register for Congenital and Inherited Disorders | 733,196 | Strong | Moderate | Strong | Weak | Strong | ||||
| New York | 3.60 | 1992–2001 | New York State Congenital Malformations Registry | 2,664,131 | Strong | Moderate | Strong | Strong | Strong | ||||
| Oklahoma | 5.99 | 1994–2003 | Oklahoma Birth Defects Registry | 484,013 | Strong | Moderate | Strong | Weak | Strong | ||||
| Texas | 3.87 | 1996–2002 | Texas Birth Defects Epidemiology and Surveillance Branch | 2,042,554 | Strong | Moderate | Strong | Weak | Strong | ||||
| (Woolf & Turner, 1969) | USA | Salt Lake City, Utah | 13.43 b | 1951–1961 | Retrospective review of nursery records in the Latter‐day Saints Hospital | Live births | No | 59,561 | Moderate | Weak | Strong | Strong | Weak |
| South America | |||||||||||||
| (Barbosa‐Buck et al., 2012) | Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Paraguay, Uruguay, and Venezuela | NR | 4.40 | 2000/01–2007/12 | ECLAMC | Live births, stillbirths >500 g | No | 1,544,496 | Strong | Moderate | Strong | Strong | Strong |
| (Duarte et al., 2018) | Argentina | 24 jurisdictions | 4.75 | 2009/11–2016/12 | RENAC | Live births, stillbirths >500 g | No | 1,663,610 | Strong | Moderate | Strong | Strong | Strong |
| (Kallen et al., 1993) | All South American Countries | NR | 1.93 b | 1967–1989 | ECLAMC | Live births, stillbirths | No | 2,278,000 | Strong | Weak | Strong | Strong | Strong |
| (Orioli et al., 1995) | South America | NR | 1.64 | 1967–1981 | ECLAMC | Live births | No | 852,893 | Strong | Strong | Strong | Strong | Strong |
| South America | NR | 2.00 | 1982–1992 | No | 2,054,682 | Strong | Strong | Strong | Strong | Strong | |||
| (Sánchez, Brito‐Arreaza, Alvarez‐Arratia, & Ramírez, 1991) | Venezuela | Ciudad Bolívar | 14.25 | 1978/04–1990/08 | Congenital malformations surveillance program at Ruiz y Paez Hopital | Live births until 1979–12, live births, stillbirths thereafter | No | 70,152 | Moderate | Strong | Strong | Strong | Weak |
| Europe | |||||||||||||
| (Coi et al., 2019) | Austria | Styria | 1.62 | 1991–2012 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 247,210 | Strong | Moderate | Strong | Strong | Strong |
| Belgium | Antwerp | 5.49 | 1991–2014 | No | 400,634 | Strong | Moderate | Strong | Strong | Strong | |||
| Croatia | Zagreb | 3.73 | 1991–2015 | No | 160,988 | Strong | Moderate | Strong | Strong | Moderate | |||
| (Andersen Jr & Hauge, 1989) | Denmark | Fyn | 1.28 | 1970/01/01–1983/12/31 | County hospital records | Live births, stillbirths | No | 77,977 | Moderate | Moderate | Strong | Strong | Weak |
| (Coi et al., 2019) | Denmark | Odense | 5.22 | 2000–2014 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 76,625 | Strong | Moderate | Strong | Strong | Weak |
| (Kallen et al., 1993) | Denmark | NR | 0.61 b | 1983–1988 | Danish National Board of Health: Registry of Congenital Malformations | Live births, stillbirths | No | 328,000 | Strong | Weak | Strong | Strong | Strong |
| (Coi et al., 2019) | France | Auvergne | 3.89 | 1991–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 334,612 | Strong | Moderate | Strong | Strong | Strong |
| France | Isle de Reunion | 5.94 | 2001–2015 | No | 218,796 | Strong | Moderate | Strong | Strong | Strong | |||
| France | Paris | 6.11 | 1991–2015 | No | 768,885 | Strong | Moderate | Strong | Strong | Strong | |||
| (Stoll, Dott, Roth, & Alembik, 1989) | France | City of Strasbourg (urban area) and “Département du Bas‐Rhin” (rural area) | 6.64 | 19 79/01–1986/12 | Registry of all newborn children in Strasbourg and Department du Bas‐Rhin | Live births, stillbirths | No | 105,374 | Strong | Strong | Strong | Strong | Moderate |
| (Coi et al., 2019) | Germany | Saxony Anhalt | 4.76 | 1991–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 357,516 | Strong | Moderate | Strong | Strong | Strong |
| (Kallen et al., 1993) | Italy | NR | 3.42 b | 1978–1988 | Italian birth defects monitoring system (IPIMC) | Live births, stillbirths | No | 1,256,000 | Strong | Weak | Strong | Strong | Strong |
| (Camera, 1980) | Italy | Genoa | 1.86 b | 1960–1980/02 | Records of osteochondroplasias encountered in the maternity ward of a single hospital | NR | No | 53,700 | Moderate | Weak | Weak | Weak | Weak |
| (Camera & Mastroiacovo, 1988) | Italy | NR | 3.70 | 1978–1985 | IMMSBD | Live births, stillbirths | No | 838,717 | Strong | Strong | Strong | Strong | Strong |
| (Coi et al., 2019) | Italy | Emilia Romagna | 5.70 | 1991–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 806,485 | Strong | Moderate | Strong | Strong | Strong |
| (Coi et al., 2019) | Tuscany | 5.06 | 1991–2015 | No | 672,268 | Strong | Moderate | Strong | Strong | Strong | |||
| (Orioli et al., 1995) | Italy | NR | 3.61 | 1978–1991 | IPIMC | Live births, stillbirths | No | 1,494,756 | Strong | Weak | Strong | Strong | Strong |
| (Coi et al., 2019) | Ireland | Cork&Kerry | 3.34 | 1996–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 179,563 | Strong | Moderate | Strong | Strong | Strong |
| Malta | NR | 6.35 | 1991–2015 | No | 110,174 | Strong | Moderate | Strong | Strong | Moderate | |||
| Netherlands | Northern region | 3.01 | 1991–2015 | No | 465,261 | Strong | Moderate | Strong | Strong | Strong | |||
| Norway | NR | 2.39 | 1999–2012 | No | 836,535 | Strong | Moderate | Strong | Strong | Strong | |||
| Poland | Wielkopolska | 4.47 | 1999–2015 | No | 626,876 | Strong | Moderate | Strong | Strong | Strong | |||
| Spain | Basque County | 2.72 | 1991–2015 | No | 441,896 | Strong | Moderate | Strong | Strong | Strong | |||
| Spain | Valencia region | 2.69 | 2007–2015 | No | 446,903 | Strong | Moderate | Strong | Strong | Strong | |||
| (Martínez‐Frías et al., 1991) | Spain | 16 of the 17 Spanish Regions (Comunidades Autonomas) | 2.53 | 1976/04–1988/12 | ECEMC | Live births | No | 710,815 | Strong | Moderate | Strong | Strong | Strong |
| (Gustavson & Jorulf, 1975) | Sweden | Uppsala | 6.75 b | 1970/02–1974/08 | Prospective collection of neonatal disorders and anomalies of the skeleton at the University Hospital in Uppsala | Live births, stillbirths | No | 14,816 | Moderate | Strong | Strong | Strong | Weak |
| (Kallen et al., 1993) | Sweden | NR | 1.64 b | 1965–1989 | Swedish register of congenital malformations | Live births, stillbirths | No | 2,375,000 | Strong | Weak | Strong | Strong | Strong |
| (Coi et al., 2019) | Switzerland | Vaud | 3.63 | 1991–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 192,684 | Strong | Moderate | Strong | Strong | Strong |
| UK | Wessex | 4.07 | 1994–2015 | No | 615,000 | Strong | Moderate | Strong | Strong | Strong | |||
| UK | Wales | 3.48 | 1998–2015 | No | 602,776 | Strong | Moderate | Strong | Strong | Strong | |||
| UK | South West England | 3.12 | 2005–2015 | No | 545,302 | Strong | Moderate | Strong | Strong | Strong | |||
| UK | Northern England | 3.03 | 1991–2015 | No | 824,745 | Strong | Moderate | Strong | Strong | Strong | |||
| UK | Thames Valley | 1.94 | 1991–2015 | No | 411,928 | Strong | Moderate | Strong | Strong | Strong | |||
| (Gardner, 1977) | UK | Edinburgh | 1.93 b | 1964/04–1968/10 | Edinburgh Register of the Newborn | Live births, stillbirths | No | 51,836 | Strong | Strong | Strong | Strong | Weak |
| 2.73 b | 1968/11–1973/12, 1968/11–1972/11 | Birth records at the Simpson Memorial Maternity Pavilion of the Royal Infirmary and at the Eastern General Hospital | Live births, stillbirths | No | 36,569 | Moderate | Strong | Strong | Strong | Weak | |||
| (Harris & Patton, 1971) | UK | Manchester | 6.26 b | 1951–1969 | Reassessment of cases of achondroplasia from birth records at St. Mary's Hospital, Manchester | Live births, stillbirths | No | 63,934 | Moderate | Moderate | Strong | Strong | Weak |
| (Sokal, Tata, & Fleming, 2014) | UK | Whole country | 7.56 b | 1990–2009 | Prospectively collected primary care data from THIN | Live births | No | 794,169 | Moderate | Weak | Strong | Strong | Strong |
| (Stevenson, 1957) | UK | Belfast | 28.34 | 1938/01–1956/06 | Records of the Royal Maternity Hospital | Live births, stillbirths | No | 31,753 | Moderate | Moderate | Strong | Strong | Weak |
| (Coi et al., 2019) | Ukraine | OMNI‐net | 6.00 | 2005–2015 | EUROCAT | Live births, stillbirths ≥20 w, elective terminations | No | 333,189 | Strong | Moderate | Strong | Strong | Strong |
| Northern Africa/Middle East | |||||||||||||
| (Golalipour, Ahmadpour‐Kacho, & Vakili, 2005) | Iran | Gorgan | 40.02 | 1998/01–1999/08 | Prospective collection of congenital malformation frequency at a referral hospital in Gorgan | Live births, stillbirths | Yes | 9,996 | Moderate | Moderate | Strong | Strong | Weak |
| (Golalipour, Kaviany, Golalipour, Mirfazeli, & Behnampour, 2018) | Iran | Gorgan, Golestan Provincein | 33.44 | 2007/03–2011 | Prospective collection of frequencies of congenital limb defects in 3 hospitals in Gorgan | Live births | Yes | 32,895 | Strong | Moderate | Strong | Strong | Weak |
| (Alaani, Al‐Fallouji, Busby, & Hamdan, 2012) | Iraq | Fallujah | 16.53 b | 2009/11–2010/09 | Records from a single pediatric clinic | Live births | Yes | 6,049 | Moderate | Weak | Strong | Strong | Weak |
| (Al‐Ani et al., 2012) | Iraq | Al‐Anbar governorate | 52.25 | 2010/10–2011/10 | WICCARS | Live births | Yes | 5,742 | Strong | Moderate | Strong | Strong | Weak |
| (Al‐Janabi, 2007) | Iraq | Al‐Anbar governorate | 241.60 | 2000/07–2002/06 | Prospective collection of congenital malformation frequency at the Maternal and Children Hospital in Al‐Anbar governate | Live births, stillbirths | No | 12,831 | Moderate | Moderate | Strong | Strong | Weak |
| (Al‐Obaidi, Mahmood, & Al‐Dalla Ali, 2013) | Iraq | Ramadi | 66.93 | 2009/02–2009/10 | Prospective collection of congenital malformation frequency at the Maternity and Children Teaching Hospital in Ramadi | Live births, stillbirths | No | 1,494 | Moderate | Moderate | Strong | Strong | Weak |
| (Al‐Rubaii, Al‐Tufaily, & Fakhri, 2009) | Iraq | Babylon | 62.82 b | 2007/01–2008/01 | Records from Babylon Maternity and Pediatrics Teaching Hospital | Live births | No | 9,551 | Moderate | Moderate | Strong | Weak | Weak |
| (Taboo, 2012) | Iraq | Mosul | 34.21 b | 2009/01–2010/12 | Prospective study of congenital abnormalities at Lahore General Hospital | Live births, stillbirths | No | 46,775 | Moderate | Moderate | Strong | Strong | Weak |
| (Madi, Al Naggar, Al Awadi, & Bastaki, 2005) | Kuwait | Al‐Jahra Region | 12.92 b | 2000/01–2001/12 | Data from the newborn register at AL‐Jahra Hospital | Live births, stillbirths | No | 7,739 | Moderate | Strong | Strong | Strong | Weak |
| (Bittar, 1998) | Libanon | Southern sector of Beirut, Baalbak, Hermel and South Lebanon | 25.87 | 1991/02–1993/07 | Prospective collection of congenital malformation frequency at a large hospital in south Beirut | Live births, stillbirths | No | 3,865 | Moderate | Moderate | Strong | Strong | Weak |
| (Al‐Jama, 2001) | Saudi Arabia | Al‐Khobar | 6.77 | 1992/01–1997/12 | Retrospective examination of delivery room records | Singleton live births | Yes | 14,762 | Moderate | Strong | Strong | Strong | Weak |
| (Sallout et al., 2015) | Saudi Arabia | Riyadh | 48.14 | 2007/01–2012/12 | Prospective collection of data on congenital anomalies in the obstetrics and gynecology ultrasound unit King Fahad Medical City | Live births | Yes | 29,084 | Moderate | Moderate | Strong | Strong | Weak |
| (Al‐Gazali et al., 2003) | UAE | Al Ain Medical District | 10.51 | 1996/01–2000/12 | Active malformation surveillance system in Al Ain Medical District | Live births, stillbirths | No | 38,048 | Strong | Strong | Strong | Strong | Weak |
| Sub‐Saharan Africa | |||||||||||||
| (Charlotte, Aurore, Charlotte, Esther, & Eugene, 2015) | Camaroon | NR | 16.53 b | 2008/01–2012/06 | Prospective collection of congenital malformation frequency at Doala General Hospital | Live births, stillbirths | Yes | 6,048 | Moderate | Moderate | Strong | Strong | Weak |
| (Ekanem et al., 2008) | Nigeria | Cross River and Akwa Ibom states | 3.13 | 1980–2003 | Records from University of Calabar Teaching Hospital, St Luke's Hospital Anua, Uyo, and St Mary's Hospital Uruakpan | NR | Yes | 127,929 | Moderate | Weak | Weak | Weak | Moderate |
| (Ekanem, Bassey, Mesembe, Eluwa, & Ekong, 2011) | Nigeria | Port Harcourt, Rivers state | 12.60 b | 1990–2003 | Records from 2 major hospitals in Port Harcourt | NR | Yes | 39,693 | Moderate | Weak | Weak | Weak | Weak |
| (Sunday‐Adeoye, Okonta, & Egwuatu, 2007) | Nigeria | Afiko, Ebonyi State | 38.62 b | 1980/01–1999/12 | Records births at the Mater Misericordiae Hospital | NR | No | 33,659 | Strong | Moderate | Strong | Strong | Weak |
| (Delport, Christianson, Van den Berg, Wolmarans, & Gericke, 1995) | South‐Africa | Pretoriaz | 5.76 | 1986/05–1989/04 | Prospective collection of congenital malformation frequency at the Kalafong Hospital | Live births | Yes | 17,351 | Moderate | Moderate | Strong | Strong | Weak |
| South‐East Asia/Oceania | |||||||||||||
| (Oberklaid, Danks, & Jensen, 1979) | Australia | Victoria | 3.85 | 1969–1975 | Royal Children's Hospital records and surveys of all pediatricians, radiologists, orthopedic surgeons in Victoria (1968–1970). Newspaper and television publicity, Little Peoples' Association of Australasia, and personal visits to rural areas to ascertain additional cases. | NR | No | 492,889 | Weak | Weak | Weak | Weak | Strong |
| (Kallen et al., 1993) | Australia | NR | 4.93 b | 1981–1989 | Data from Australian National data systems for (1) congenital malformations and (2) for pregnancies resulting from in vitro fertilization. | Live births, stillbirths | No | 1,946,000 | Strong | Weak | Strong | Strong | Strong |
| (Jaikrishan et al., 2013) | India | NR | 11.38 | 1995/08–2011/06 | Prospective collection of congenital malformation frequency in 7 government hospitals serving people from high and normal national radiation areas | Live births, stillbirths >28 w | No | 140,558 | Moderate | Moderate | Strong | Strong | Moderate |
| (Kusumalatha et al., 2017) | India | Kakinada | 14.40 b | 2016/01–2016/12 | Hospital‐based cross‐sectional study | Live births, stillbirths | No | 13,893 | Moderate | Moderate | Strong | Strong | Weak |
| (Rasheed & Haseeb, 2016) | India | Maharashtra | 14.26 | 1994/03–1995/04 | Prospective collection of frequencies of congenital anomalies at Marden Medical Complex | Live births, stillbirths | Yes | 7,012 | Moderate | Moderate | Strong | Strong | Weak |
| (Higurashi et al., 1990) | Japan | Tokyo | 10.92 | 1972/07–1985/12 | Records from consecutive births in a single large maternity hospital in Tokyo | Live births | No | 27,472 | Moderate | Moderate | Strong | Strong | Weak |
| (Peng, 1988) | Malaysia | State of Kedah | 10.12 | 1984/04–1987/03 | Records of live births occurring in Alor Setar General Hospital | Live births | Yes | 19,769 | Moderate | Moderate | Strong | Strong | Weak |
| (Qadir, Amir, & Bano, 2017) | Pakistan | Mardan | 10.58 b | 2016/05–2017/04 | Prospective collection of frequencies of congenital anomalies at Government Medical College and Hospital | NR | No | 9,453 | Moderate | Moderate | Weak | Weak | Weak |
| (Tariq, 2010) | Pakistan | Lahore | 34.82 b | 2007/01–2007/12 | Prospective study of congenital abnormalities at Al‐Batool Teaching Hospital of Obstetrics and Gynecology | NR | No | 2,872 | Moderate | Moderate | Weak | Weak | Weak |
| (Nasreen, Naib, & Ibrar, 2016) | Pakistan | Peshawar | 0.00 b | 2007/06–2009/06 | Prospective collection of data on congenital anomalies at Khyber Teaching Hospital | NR | No | 6,297 | Moderate | Moderate | Weak | Weak | Weak |
| (Jaruratanasirikul et al., 2016) | Thailand | Songkhla, Trang and Phatthalung | 2.68 b | 2009/01–2013/12 | Records from Bureau of Policy and Strategy, Ministry of Public Health | Live births, stillbirths, elective terminations | No | 186,393 | Strong | Moderate | Strong | Strong | Strong |
Abbreviations: ECEMC, Spanish Collaborative Study of Congenital Malformations; ECLAMC, Latin American Collaborative Study of Congenital Malformations; EUROCAT, European network of population‐based registries for the epidemiological surveillance of congenital anomalies; g, grams; IMMSBD, Italian birth defects monitoring system; NR, not reported; OMNI‐NET, Ukraine Birth Defects Program; RECUMAC, Registry of Congenital Malformations; RENAC, Records from National Network of Congenital Anomalies of Argentina; THIN, the Health Improvement Network; UBDN, Utah Birth Defect Network; UK, United Kingdom; UNIMEF, Department of Maternal Fetal Medicine; USA, United States of America; W, weeks; WICCARS, Western Iraq Center for Congenital Anomalies Registry and Surveillance.
Yes: Women who gave birth at a referral center or tertiary hospital. No: Women who gave birth in other settings.
Calculated per 100,000 births based on raw data provided in the article.
Only three studies (Camera & Mastroiacovo, 1988; Orioli et al., 1995; Stevenson et al., 2012) scored strong across all four domains of the quality assessment tool (Table 3). As shown in Figure 2, the most common domain (40% of estimates) on which an estimate may have received a weak score was population size (i.e., the investigated population was too small to estimate birth prevalence with 95% confidence). The description of the numerator was weak (it was unclear if the numerator included still births and/or elective terminations) in 15.5% of all estimates and the denominator was not congruent to the numerator (e.g., numerator included still births and/or elective terminations and the denominator included only livebirths) in 16.7%. Case definition method was weak in 17.8% of all estimates. Only one study scored weak on data source (1.1%).
FIGURE 2.
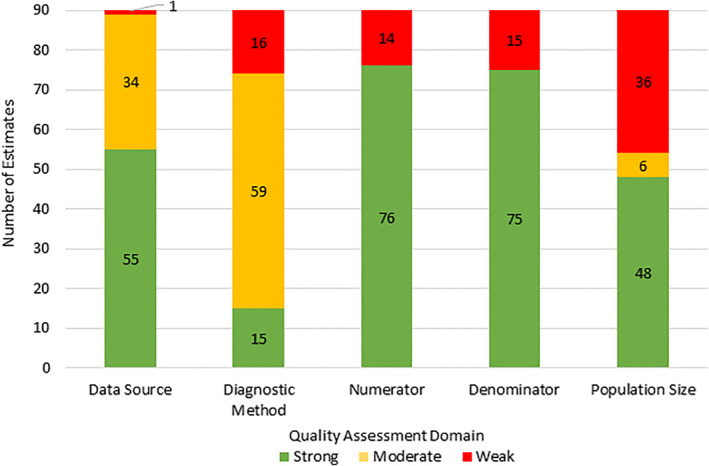
Quality assessment of included estimates (N = 90). For numerator and denominator a moderate score was not an option (Table 1) [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Meta‐analyses
Pooled analysis based on the quality effects model showed a worldwide achondroplasia birth prevalence of 4.6 cases per 100,000 births (Table 4). Figure 3 shows an overview of the global pooled prevalence of achondroplasia and the pooled estimate per region using the quality effects model.
TABLE 4.
Meta‐analysis of reported achondroplasia birth prevalence stratified by study setting and by region
| Pooled birth prevalence per 100,000 | Higgins I 2 test (95% CI) | ||||
|---|---|---|---|---|---|
| Quality effects model (95% CI) | Random effects model (95% CI) | p value b | N studies; N estimates | Quality index a | |
| Worldwide | 4.6 (3.9–5.4) | 4.5 (4.1–5.0) |
84.3 (81.3–86.9) <.001 |
52; 90 | 23.0 |
| Specialized care c | 13.3 (5.3–24.6) | 16.4 (8.8–26.3) |
78.4 (64.3–87.0) <.001 |
14; 14 | 30.2 |
| Other settings d | 4.2 (3.5–4.9) | 4.2 (3.7–4.6) |
84.2 (80.8–87.0) <.001 |
38; 76 | 18.8 |
| North America | 4.2 (3.5–5.0) | 4.2 (3.5–4.9) |
71.18 (51.3–82.9) <.001 |
9; 15 | 52.5 |
| South America | 3.5 (2.1–5.3) | 3.9 (2.5–5.7) |
91.4 (84.1–95.4) <.001 |
5; 6 | 11.0 |
| Europe | 3.5 (3.0–4.2) | 3.6 (3.2–4.0) |
76.2 (67.9–82.4) <.001 |
13; 40 | 14.5 |
| North Africa and Middle east | 35.1 (14.9–63.0) | 43.1 (23.0–69.3) |
82.6 (71.5–89.4) <.001 |
13; 13 | 18.6 |
| Sub‐Saharan Africa | 17.9 (3.0–42.8) | 12.8 (2.2–30.6) |
82.7 (60.5–92.5) <.001 |
5; 5 | 65.4 |
| South and Southeast Asia/Oceania | 5.9 (2.9–10.0) | 6.3 (3.8–9.4) |
61.9 (26.7–80.3) <.001 |
11;11 | 33.1 |
Abbreviation: CI, confidence intervals.
The quality index represents the extent to which (percent) the weights of the inverse variance fixed effect model are redistributed by the application of the quality effect weights.
Chi2 p‐value.
Women who gave birth in a specialized care setting (i.e., referral center or tertiary hospital).
Women who gave birth in other settings (not a referral center or tertiary hospital).
FIGURE 3.
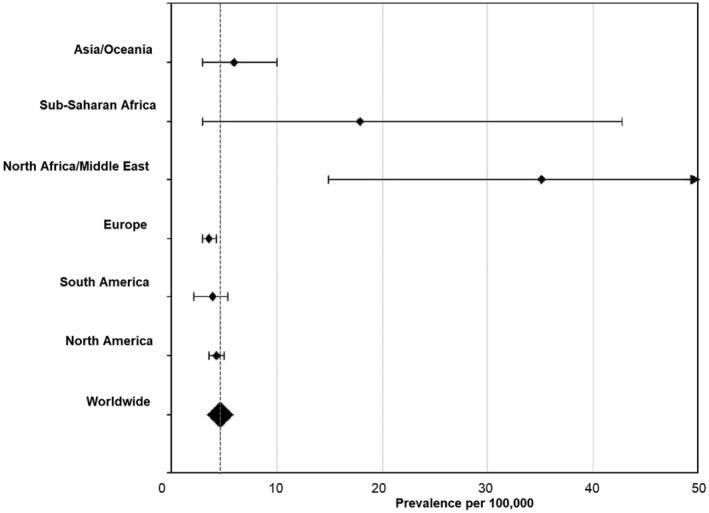
Forest plot of achondroplasia pooled birth prevalence estimates. Prevalence was estimated using the quality effects model
The pooled birth prevalence estimate was substantially higher in North Africa/Middle East (35.1 per 100,000, based on 13 estimates) and Sub‐Saharan Africa (17.9 per 100,000, based on five estimates), compared with other regions. All of the studies conducted in these regions were relatively small, resulting in very large confidence intervals (Figure 3). One study conducted in North Africa/Middle East (N = 12,831) (Al‐Janabi, 2007) reported a birth prevalence of 240.6 cases per 100,000 births. When this study was omitted from the regional analysis, the estimated I 2 changed to 54.5% (p = .052) and the pooled birth prevalence changed to 24.4 cases (95%CI 9.1–46.5) per 100,000 births, which is still higher than observed in other regions. The lowest pooled prevalence estimates were found in South America (3.5 per 100,000, based on six estimates) and Europe (3.5 per 100,000, based on 40 estimates). Results were generally similar to those obtained in the random effects model.
The pooled birth prevalence differed by the setting in which subjects gave birth with a ~3‐fold higher birth prevalence (13.3 cases per 100,000 births) in specialized care settings compared with other settings (4.2 cases per 100,000 births). Results obtained using the fixed effects model were generally similar to those obtained in the quality effects model (Table 4).
3.3. Heterogeneity and bias
A high level of heterogeneity was observed among the studies (Table 4). Heterogeneity persisted even after stratification by region and study date (e.g., omitting papers conducted on or before 1975, data not shown).
The visual asymmetry of the Doi plot suggested publication bias, where smaller studies reported higher birth prevalence estimates (Figure 4). The LFK index of 3.78 suggested positive asymmetry of the plot. However, when stratifying the results by region, major asymmetry suggesting publication bias was only present for reports of birth prevalence in North America, Sub‐Saharan Africa and the Asia/Oceania region, with LFK indexes of 2.73, 2.56 and 7.23, respectively. Minor asymmetry was detected in South America, Europe, North Afica/Middle East, with LFK indexes of 1.02, 1.18, and 1.37, respectively.
FIGURE 4.
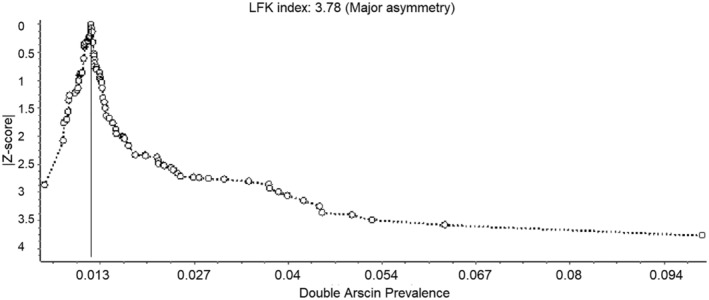
Doi plot to evaluate publication bias
4. DISCUSSION
Meta‐analysis of the studies included in this systematic literature review estimated the pooled birth prevalence of achondroplasia in the general population worldwide to be 4.6 cases (95%CI 3.9–5.4) per 100,000 births, based on 52 studies and 90 study estimates.
A high degree of heterogeneity was observed among estimates of birth prevalence. Several factors may contribute to this heterogeneity, including reporting of birth prevalence in all pregnancies vs. restricted to live births (18%). In the article by Coi et al., 18.9% of diagnosed cases resulted in terminations of pregnancy for fetal anomaly, a factor that may apply in other studies as well (Coi et al., 2019). The inclusion of stillbirths and elective terminations in some of the studies may have led to some degree of overestimation of achondroplasia birth prevalence. In addition, reported birth prevalence tended to be higher in smaller studies and in those reporting data deriving from specialized care settings. This latter observation may reflect the fact that mothers in whom fetal anomalies are suspected may be more likely to give birth in these centers, especially in regions (or in past eras) where home‐births are more common. Other factors that could account, in part, for discrepancies among the reports include completeness of ascertainment and diagnostic accuracy; however, these could not be readily assessed.
The birth prevalence appeared substantially higher in North Africa and the Middle East, and in Sub‐Saharan Africa than in other regions. These large regional variances were not in accordance with our expectations, as the preponderance of achondroplasia cases arise from spontaneous dominant mutations (Horton et al., 2007), which would not necessarily be expected to give rise to isolated “hotspots.” The studies that reported unusually high birth prevalence tended to be smaller studies (scoring weak on the population size domain of the quality assessment tool, Table 3) and did not provide evidence‐based explanations for the high number of congenital malformation cases observed. Because of the small population sizes surveyed, the precision of the estimate for these regions is notably lower (i.e., the confidence intervals are larger, see Figure 3) than for other regions. In addition, the proportion of estimates for which data were derived from specialized care settings was substantially higher in North Africa and the Middle East, and in Sub‐Saharan Africa compared with other regions (80% for Sub‐Saharan Africa and46% for North Africa and Middle East, as compared to 18% for South and Southeast Asia/Oceania, 13% for North America, and 0% for South America and for Europe), which may also contribute to higher apparent prevalence. While the estimates for North Africa and the Middle East, and for Sub‐Saharan Africa may reflect the genuine birth prevalence, they should be interpreted in the context of these limitations. However, achondroplasia birth prevalence has been linked to race, ethnicity, and social factors (Orioli et al., 1995; Waller et al., 2008; Wilkin et al., 1998), such as advanced paternal age (Duarte et al., 2018). Also, there is growing (though inconclusive) evidence that higher incidences of congenital abnormalities can be due to prenatal exposure to environmental pollution (e.g., air‐ and water‐pollution as a result of urbanization and industrialization; Dolk & Vrijheid, 2003; Vrijheid et al., 2011). Such factors may have the potential to have contributed to a truly elevated birth prevalence in these regions.
The visual assessment of the Doi plot and the positive LFK index indicated major asymmetry, suggesting that the pooled birth prevalence resulting from our analysis may be slightly overestimated (Furuya‐Kanamori et al., 2018). However, the asymmetry could arise from sources other than publication bias (e.g., data irregularities and/or heterogeneity; Egger, Davey Smith, Schneider, & Minder, 1997; Sterne, Egger, & Smith, 2001; Sterne & Harbord, 2004). When publication bias was assessed by region, major asymmetry was only detected in North America, Sub‐Saharan Africa and Asia/Oceania.
In the course of developing this systematic literature review, it became apparent that there were deficiencies both in the way studies were conducted as well as in the way in which results were reported, which undoubtedly contributed to the heterogeneity discussed previously. The fact that only three of the reports scored strongly across all domains in our quality rating scale reflects a need for better study design and reporting in the epidemiological literature.
In an attempt to compensate for some of the quality differences, a quality effects model was used as the primary model for meta‐analysis in this study. This model was designed to take differences in study quality into account, by giving lower weights to studies of lower quality (Doi & Thalib, 2008). However, one limitation of this approach is that the mean quality over all domains was used in the calculations implying that each domain was of equal importance. In addition, the model did not necessarily distinguish deficiencies in the quality in the reporting from the quality of the study design and execution. As the random effects model is also a well‐known and widely used approach, results are also presented using this model (Table 4). For most analyses, no large differences in pooled birth prevalence were observed between the models and confidence intervals were overlapping. Thus, while we feel the quality evaluation was worthwhile and informative, it clearly does not account for all for the heterogeneity among the studies.
5. CONCLUSION
Based on 52 studies and 90 prevalence estimates, this systematic review and meta‐analysis estimated the achondroplasia birth prevalence worldwide, as well as for different regions of the world. The worldwide pooled birth prevalence using a quality effects model was 4.6 cases (95%CI 3.9–5.4) per 100,000 births or 1 in 22,000 births (95% CI 18,500 to 26,000). To our knowledge this is the most comprehensive estimate of achondroplasia birth prevalence available. Careful interpretation of these results is advised, as most reports lacked key study design and/or reporting elements and moderate to high heterogeneity was present.
CONFLICT OF INTEREST
The review team members P. K. F., S. L., and R. S. are employed by BioMarin Pharmaceutical, Inc.
AUTHOR CONTRIBUTIONS
Pamela K. Foreman, Renée Shediac, Sarah Landis, Judith van den Bosch, and Femke van Kesse involved in conception and design of the work. Pamela K. Foreman, Judith van den Bosch, Femke van Kesse, and Rosa van Hoorn involved in acquisition, analysis of the data. Pamela K. Foreman, Judith van den Bosch, Femke van Kesse, Rosa van Hoorn, Renée Shediac, and Sarah Landis involved in interpretation of the data. Pamela K. Foreman, Renée Shediac, Sarah Landis, Judith van den Bosch, and Femke van Kesse involved in development of the quality of evidence tool. Pamela K. Foreman, Renée Shediac, Sarah Landis, Judith van den Bosch, Femke van Kesse, and Rosa van Hoorn involved in drafting and revising the article.
ACKNOWLEDGMENTS
This systematic review is funded by BioMarin Pharmaceutical, Inc. We thank the statisticians Gianni Amato from BioMarin Pharmaceutical, Inc. and Edwin Martens (self‐employed) for their advice on the methodology of the meta‐analysis.
Appendix I.
SEARCH STRINGS
PubMed (MEDLINE)
#1 Achondroplasia
(“achondroplasia”[MeSH Terms] OR “achondroplasia”[All Fields]) OR achondroplastic[All Fields] OR “skeletal dysplasia”[all fields]
#2 Birth prevalence
“Prevalence”[Mesh] OR prevalen*[tiab] OR “epidemiology”[Mesh] OR “epidemiology”[subheading] OR epidemiol*[tiab] OR burden[tiab] OR “Incidence”[Mesh] OR inciden*[tiab]
Limits:
• Publication date: from first release date to 29‐07‐2019.
• Language: all languages with abstract in English
#1 and #2 yielded 421 hits.
I.1.
Embase
#1 Achondroplasia
‘achondroplasia’/exp OR ‘achondroplasia’ OR achondroplastic or ‘skeletal dysplasia’
#2 Birth prevalence
‘prevalence’/exp OR ‘prevalen*’:ab, ti OR ‘epidemiology’/exp OR epidemiology:lnk OR epidemiol*:ab, ti OR burden:ab, ti OR ‘incidence’/exp OR inciden*:ab, ti
Limits:
• Publication date: from first release date to 29‐07‐2019.
• Language: all languages with abstract in English
#1 and #2 yields 746 hits.
Foreman PK, van Kessel F, van Hoorn R, van den Bosch J, Shediac R, Landis S. Birth prevalence of achondroplasia: A systematic literature review and meta‐analysis. Am J Med Genet Part A. 2020;182A:2297–2316. 10.1002/ajmg.a.61787
Pamela K. Foreman and Femke van Kessel contributed equally to this work.
Funding information BioMarin Pharmaceutical, Inc
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- Afsharpaiman, S. , Sillence, D. O. , Sheikhvatan, M. , Ault, J. E. , & Waters, K. (2011). Respiratory events and obstructive sleep apnea in children with achondroplasia: Investigation and treatment outcomes. Sleep & Breathing, 15(4), 755–761. 10.1007/s11325-010-0432-6 [DOI] [PubMed] [Google Scholar]
- Al Khalaf, M. M. , Thalib, L. , & Doi, S. A. (2011). Combining heterogenous studies using the random‐effects model is a mistake and leads to inconclusive meta‐analyses. Journal of Clinical Epidemiology, 64(2), 119–123. 10.1016/j.jclinepi.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Alaani, S. , Al‐Fallouji, M. A. , Busby, C. , & Hamdan, M. (2012). Pilot study of congenital anomaly rates at birth in Fallujah, Iraq, 2010. The Journal of IMA, 44(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alade, Y. , Tunkel, D. , Schulze, K. , McGready, J. , Jallo, G. , Ain, M. , … Hoover‐Fong, J. (2013). Cross‐sectional assessment of pain and physical function in skeletal dysplasia patients. Clinical Genetics, 84(3), 237–243. 10.1111/cge.12045 [DOI] [PubMed] [Google Scholar]
- Al‐Ani, Z. R. , Al‐Haj, S. A. , Al‐Ani, M. M. , Al‐Dulaimy, K. M. , Al‐Maraie, A. K. , & Al‐Ubaidi, B. K. (2012). Incidence, types, geographical distribution, and risk factors of congenital anomalies in Al‐Ramadi Maternity and Children's Teaching Hospital, Western Iraq. Saudi Medical Journal, 33(9), 979–989. [PubMed] [Google Scholar]
- Al‐Gazali, L. I. , Bakir, M. , Hamid, Z. , Varady, E. , Varghes, M. , Haas, D. , … Dawadu, A. (2003). Birth prevalence and pattern of osteochondrodysplasias in an inbred high risk population. Birth Defects Research. Part A, Clinical and Molecular Teratology, 67(2), 125–132. 10.1002/bdra.10009 [DOI] [PubMed] [Google Scholar]
- Al‐Jama, F. (2001). Congenital malformations in newborns in a teaching hospital in eastern Saudi Arabia. Journal of Obstetrics and Gynaecology, 21(6), 595–598. [DOI] [PubMed] [Google Scholar]
- Al‐Janabi, M. K. (2007). Congenital malformations in the west of Iraq. Journal of the Faculty of Medicine Baghdad, 49(3), 295–299. [Google Scholar]
- Al‐Obaidi, B. K. , Mahmood, N. S. , & Al‐Dalla Ali, F. J. (2013). Incidence of birth defects at birth among babies delivered at maternity and children teaching hospital in Ramadi. Al‐Anbar Medical Journal, 11(1), 1–10. [Google Scholar]
- Alonso Lotti, F. , Cendán Muñiz, I. , Ferrero Oteiza, M. E. , Roca Ortiz, J. , Castillo González, P. , Petizco Hernández, A. , & Ferreiro, A. (1998). Caracterización patogénica de los recién nacidos con malformaciones múltiples. Revista Cubana de Pediatría, 70(2), 73–78. [Google Scholar]
- Al‐Rubaii, B. J. , Al‐Tufaily, Y. A. , & Fakhri, M. (2009). Congenital anomalies admitted to Intensive Neonatal Care Unit in Babylon Maternity and Pediatric Teaching Hospital. Kerbala Jorunal of Medicine, 2(5), 594–598. [Google Scholar]
- Andersen, P. E., Jr. , & Hauge, M. (1989). Congenital generalised bone dysplasias: A clinical, radiological, and epidemiological survey. Journal of Medical Genetics, 26(1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa‐Buck, C. O. , Orioli, I. M. , da Graça Dutra, M. , Lopez‐Camelo, J. , Castilla, E. E. , & Cavalcanti, D. P. (2012). Clinical epidemiology of skeletal dysplasias in South America. American Journal of Medical Genetics, Part A, 158A(5), 1038–1045. 10.1002/ajmg.a.35246 [DOI] [PubMed] [Google Scholar]
- Barendregt, J. J. , & Doi, S. A. (2011. –2016). MetaXL User Guide, version 5.3. Sunrise Beach, Queensland, Australia: EpiGear International Pty Ltd; Retrieved from www.epigear.com [Google Scholar]
- Barendregt, J. J. , Doi, S. A. , Lee, Y. Y. , Norman, R. E. , & Vos, T. (2013). Meta‐analysis of prevalence. Journal of Epidemiology and Community Health, 67(11), 974–978. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- Bittar, Z. (1998). Major congenital malformations presenting in the first 24 hours of life in 3865 consecutive births in south of Beirut. Incidence and pattern. Le Journal Médical Libanais—The Lebanese Medical Journal, 46(5), 256–260. [PubMed] [Google Scholar]
- Breinholt, V. M. , Rasmussen, C. E. , Mygind, P. H. , Kjelgaard‐Hansen, M. , Faltinger, F. , Bernhard, A. , … Hersel, U. (2019). TransCon CNP, a sustained‐release C‐type natriuretic peptide prodrug, a potentially safe and efficacious new therapeutic modality for the treatment of comorbidities associated with fibroblast growth factor receptor 3‐related skeletal dysplasias. The Journal of Pharmacology and Experimental Therapeutics, 370(3), 459–471. 10.1124/jpet.119.258251 [DOI] [PubMed] [Google Scholar]
- Camera, G. (1980). On the frequency of achondroplasia. Acta Medica Auxologica, 12(2), 127–128. [Google Scholar]
- Camera, G. , & Mastroiacovo, P. (1988). Birth prevalence and mutation rate of achondroplasia in the Italian multicentre monitoring system for birth defects. Basic Life Sciences, 48, 11–15. [DOI] [PubMed] [Google Scholar]
- Charlotte, T. N. , Aurore, N. D. , Charlotte, B. , Esther, B. , & Eugene, B. P. (2015). Prenatal diagnosis of congenital malformations in Douala General Hospital. Open Journal of Obstetrics and Gynecology, 5(15), 839–848. [Google Scholar]
- Coi, A. , Santoro, M. , Garne, E. , Pierini, A. , Addor, M. C. , Alessandri, J. L. , … Barišić, I. (2019). Epidemiology of achondroplasia: A population‐based study in Europe. American Journal of Medical Genetics, Part A, 179(9), 1971–1978. 10.1002/ajmg.a.61289 [DOI] [PubMed] [Google Scholar]
- Curran, J. P. , Sigmon, B. A. , & Opitz, J. M. (1974). Lethal forms of chondrodysplastic dwarfism. Pediatrics, 53(1), 76–85. [PubMed] [Google Scholar]
- Deeks J. J., Altman D. G., & Bradburn M. J. (Eds.). (2001). Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic reviews in health care: Meta‐analysis in context. London: BMJ Publishing Group. [Google Scholar]
- Delport, S. , Christianson, A. , Van den Berg, H. , Wolmarans, L. , & Gericke, G. (1995). Congenital anomalies in black South African liveborn neonates at an urban academic hospital. South African Medical Journal, 85(1), 11–15. [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dhiman, N. , Albaghdadi, A. , Zogg, C. K. , Sharma, M. , Hoover‐Fong, J. E. , Ain, M. C. , & Haider, A. H. (2017). Factors associated with health‐related quality of life (HRQOL) in adults with short stature skeletal dysplasias. Quality of Life Research, 26(5), 1337–1348. 10.1007/s11136-016-1455-7 [DOI] [PubMed] [Google Scholar]
- Doi, S. A. , & Thalib, L. (2009). An alternative quality adjustor for the quality effects model for meta‐analysis. Epidemiology, 20(2), 314 10.1097/EDE.0b013e318196a8d0 [DOI] [PubMed] [Google Scholar]
- Doi, S. A. R. , & Thalib, L. (2008). A quality‐effects model for meta‐analysis. Epidemiology, 19(1), 94–100. [DOI] [PubMed] [Google Scholar]
- Dolk, H. , & Vrijheid, M. (2003). The impact of environmental pollution on congenital anomalies. British Medical Bulletin, 68(1), 25–45. 10.1093/bmb/ldg024 [DOI] [PubMed] [Google Scholar]
- Donaldson, J., Aftab, S., & Bradish, C. (2015). Achondroplasia and limb lengthening: Results in a UK cohort and review of the literature. Journal of Orthopaedics, 12(1), 31–34. 10.1016/j.jor.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, S. P. , Rocha, M. E. , Bidondo, M. P. , Liascovich, R. , Barbero, P. , & Groisman, B. (2018). Bone dysplasias in 1.6 million births in Argentina. European Journal of Medical Genetics, 62, 103603 10.1016/j.ejmg.2018.12.008 [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal (Clinical Research ed.), 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanem, T. , Bassey, I. , Mesembe, O. , Eluwa, M. , & Ekong, M. (2011). Incidence of congenital malformation in 2 major hospitals in Rivers state of Nigeria from 1990 to 2003. Eastern Mediterranean Health Journal, 17(9), 701–705. [PubMed] [Google Scholar]
- Ekanem, T. B. , Okon, D. E. , Akpantah, A. O. , Mesembe, O. E. , Eluwa, M. A. , & Ekong, M. B. (2008). Prevalence of congenital malformations in Cross River and Akwa Ibom states of Nigeria from 1980–2003. Congenital Anomalies, 48(4), 167–170. [DOI] [PubMed] [Google Scholar]
- Furuya‐Kanamori, L. , Barendregt, J. J. , & Doi, S. A. R. (2018). A new improved graphical and quantitative method for detecting bias in meta‐analysis. International Journal of Evidence‐Based Healthcare, 16(4), 195–203. 10.1097/xeb.0000000000000141 [DOI] [PubMed] [Google Scholar]
- Garcia, S. , Dirat, B. , Tognacci, T. , Rochet, N. , Xavier, M. , Bonnafous, S. , … Gouze, E. (2013). Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Science Translational Medicine, 5, 203ra124 10.1126/scitranslmed.3006247 [DOI] [PubMed] [Google Scholar]
- GARD . (2019). The genetic and rare diseases information center Retrieved from https://rarediseases.info.nih.gov/.
- Gardner, R. (1977). A new estimate of the achondroplasia mutation rate. Clinical Genetics, 11(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Golalipour, M. , Ahmadpour‐Kacho, M. , & Vakili, M. (2005). Congenital malformations at a referral hospital in Gorgan, Islamic Republic of Iran. Eastern Mediterranean Health Journal, 11(4), 707–715. [PubMed] [Google Scholar]
- Golalipour, M. J. , Kaviany, N. , Golalipour, E. , Mirfazeli, A. , & Behnampour, N. (2018). Prevalence and patterns of congenital limb defects in the north of Iran (2007–2011). Iranian Journal of Neonatology, 9(1), 60–64. 10.22038/ijn.2018.23880.1301 [DOI] [Google Scholar]
- Gollust, S. E. , Thompson, R. E. , Gooding, H. C. , & Biesecker, B. B. (2003). Living with achondroplasia in an average‐sized world: An assessment of quality of life. American Journal of Medical Genetics. Part A, 120a(4), 447–458. 10.1002/ajmg.a.20127 [DOI] [PubMed] [Google Scholar]
- Gustavson, K. H. , & Jorulf, H. (1975). Different types of osteochondrodysplasia in a consecutive series of newborns. Helvetica Paediatrica Acta, 30(3), 307–314. [PubMed] [Google Scholar]
- Guzmán‐Huerta, M. E. , Morales, A. S. , Benavides‐Serralde, A. , Camargo‐Marín, L. , Velázquez‐Torres, B. , Gallardo‐Gaona, J. M. , … Ramírez‐Calvo, J. A. (2012). Prenatal prevalence of skeletal dysplasias and a proposal ultrasonographic diagnosis approach. Revista de Investigación Clínica, 64(5), 429–436. [PubMed] [Google Scholar]
- Harada, D. , Namba, N. , Hanioka, Y. , Ueyama, K. , Sakamoto, N. , Nakano, Y. , … Seino, Y. (2017). Final adult height in long‐term growth hormone‐treated achondroplasia patients. European Journal of Pediatrics, 176(7), 873–879. 10.1007/s00431-017-2923-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, R. , & Patton, J. T. (1971). Achondroplasia and thanatophoric dwarfism in the newborn. Clinical Genetics, 2(2), 61–72. [DOI] [PubMed] [Google Scholar]
- Hashmi, S. S. , Gamble, C. , Hoover‐Fong, J. , Alade, A. Y. , Pauli, R. M. , Modaff, P. , … Hecht, J. T. (2018). Multicenter study of mortality in achondroplasia. American Journal of Medical Genetics. Part A, 176(11), 2359–2364. 10.1002/ajmg.a.40528 [DOI] [PubMed] [Google Scholar]
- Hecht, J. T. , Francomano, C. A. , Horton, W. , & Annegers, J. (1987). Mortality in achondroplasia. American Journal of Human Genetics, 41(3), 454–464. [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. British Medical Journal (Clinical Research ed.), 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi, M. , Oda, M. , Iijima, K. , Iijima, S. , Takeshita, T. , Watanabe, N. , & Yoneyama, K. (1990). Livebirth prevalence and follow‐up of malformation syndromes in 27,472 newborns. Brain and Development, 12(6), 770–773. 10.1016/S0387-7604(12)80004-0 [DOI] [PubMed] [Google Scholar]
- Horton, W. A. , Hall, J. G. , & Hecht, J. T. (2007). Achondroplasia. Lancet, 370(9582), 162–172. 10.1016/s0140-6736(07)61090-3 [DOI] [PubMed] [Google Scholar]
- Huedo‐Medina, T. B. , Sanchez‐Meca, J. , Marin‐Martinez, F. , & Botella, J. (2006). Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. 10.1037/1082-989x.11.2.193 [DOI] [PubMed] [Google Scholar]
- Hunter, A. G. , Bankier, A. , Rogers, J. G. , Sillence, D. , & Scott, C. I., Jr. (1998). Medical complications of achondroplasia: A multicentre patient review. Journal of Medical Genetics, 35(9), 705–712. 10.1136/jmg.35.9.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland, P. J. , Donaghey, S. , McGill, J. , Zankl, A. , Ware, R. S. , Pacey, V. , … Johnston, L. M. (2012). Development in children with achondroplasia: A prospective clinical cohort study. Developmental Medicine and Child Neurology, 54(6), 532–537. 10.1111/j.1469-8749.2012.04234.x [DOI] [PubMed] [Google Scholar]
- Ireland, P. J. , Johnson, S. , Donaghey, S. , Johnston, L. , McGill, J. , Zankl, A. , … Townshend, S. (2010). Developmental milestones in infants and young Australasian children with achondroplasia. Journal of Developmental and Behavioral Pediatrics, 31(1), 41–47. 10.1097/DBP.0b013e3181c72052 [DOI] [PubMed] [Google Scholar]
- Ireland, P. J. , McGill, J. , Zankl, A. , Ware, R. S. , Pacey, V. , Ault, J. , … Johnston, L. M. (2011). Functional performance in young Australian children with achondroplasia. Developmental Medicine and Child Neurology, 53(10), 944–950. 10.1111/j.1469-8749.2011.04050.x [DOI] [PubMed] [Google Scholar]
- Jaikrishan, G. , Sudheer, K. , Andrews, V. , Koya, P. , Madhusoodhanan, M. , Jagadeesan, C. , & Seshadri, M. (2013). Study of stillbirth and major congenital anomaly among newborns in the high‐level natural radiation areas of Kerala, India. Journal of Community Genetics, 4(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruratanasirikul, S. , Tangtrakulwanich, B. , Rachatawiriyakul, P. , Sriplung, H. , Limpitikul, W. , Dissaneevate, P. , … Tantichantakarun, P. (2016). Prevalence of congenital limb defects: Data from birth defects registries in three provinces in Southern Thailand. Congenital Anomalies, 56(5), 203–208. [DOI] [PubMed] [Google Scholar]
- Kallen, B. , Knudsen, L. B. , Mutchinick, O. , Mastroiacovo, P. , Lancaster, P. , Castilla, E. , & Robert, E. (1993). Monitoring dominant germ cell mutations using skeletal dysplasias registered in malformation registries: An international feasibility study. International Journal of Epidemiology, 22(1), 107–115. [DOI] [PubMed] [Google Scholar]
- Kim, S.‐J. , Balce, G. C., Agashe, M. V., Song, S.‐H., & Song, H.‐R. (2012). Is bilateral lower limb lengthening appropriate for achondroplasia?: Midterm analysis of the complications and quality of life. Clinical Orthopaedics and Related Research, 470(2), 616–621. 10.1007/s11999-011-1983-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, K. R. , Shim, J. S., Chung, C. H., & Kim, J. H. (2019). Surgical Results of Limb Lengthening at the Femur, Tibia, and Humerus in Patients with Achondroplasia. Clinics in Orthopedic Surgery, 11(2), 226 10.4055/cios.2019.11.2.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komla‐Ebri, D. , Dambroise, E. , Kramer, I. , Benoist‐Lasselin, C. , Kaci, N. , Le Gall, C. , … Legeai‐Mallet, L. (2016). Tyrosine kinase inhibitor NVP‐BGJ398 functionally improves FGFR3‐related dwarfism in mouse model. The Journal of Clinical Investigation, 126(5), 1871–1884. 10.1172/jci83926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumalatha, P. , Rani, K. R. , Anuradha, B. , Rani, B. S. , Rao, K. S. S. , & Suneetha, K. S. (2017). Study of congenital malformations in a Tertiary Care Centre, Government General Hospital, Kakinada. Journal of Evolution of Medical and Dental Sciences, 6(81), 5712–5717. [Google Scholar]
- Langlois, P. H. , & Scheuerle, A. E. (2015). Descriptive epidemiology of birth defects thought to arise by new mutation. Birth Defects Research Part A – Clinical and Molecular Teratology, 103(11), 913–927. 10.1002/bdra.23412 [DOI] [PubMed] [Google Scholar]
- Leiva‐Gea, A. , Delgado‐Rufino, F. B., Queipo‐de‐Llano, A., Mariscal‐Lara, J. , Lombardo‐Torre, M., & Luna‐González, F. (2020). Staged upper and lower limb lengthening performing bilateral simultaneous surgery of the femur and tibia in achondroplastic patients. Archives of Orthopaedic and Trauma Surgery. 10.1007/s00402-020-03360-3 [DOI] [PubMed] [Google Scholar]
- Madi, S. , Al Naggar, R. , Al Awadi, S. , & Bastaki, L. (2005). Profile of major congenital malformations in neonates in Al‐Jahra region of Kuwait. Eastern Mediterranean Health Journal, 11(4), 700–706. [PubMed] [Google Scholar]
- Mahomed, N. N. , Spellmann, M. , & Goldberg, M. J. (1998). Functional health status of adults with achondroplasia. American Journal of Medical Genetics, 78(1), 30–35. [DOI] [PubMed] [Google Scholar]
- Martínez‐Frías, M. , Cereijo, A. , Bermejo, E. , López, M. , Sánchez, M. , & Gonzalo, C. (1991). Epidemiological aspects of Mendelian syndromes in a Spanish population sample: I. Autosomal dominant malformation syndromes. American Journal of Medical Genetics, 38(4), 622–625. [DOI] [PubMed] [Google Scholar]
- Matsushita, M. , Kitoh, H. , Mishima, K. , Yamashita, S. , Haga, N. , Fujiwara, S. , … Ishiguro, N. (2019). Physical, mental, and social problems of adolescent and adult patients with achondroplasia. Calcified Tissue International, 104(4), 364–372. 10.1007/s00223-019-00518-z [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing, L. , Winn, T. , & Rusli, B. (2006). Practical issues in calculating the sample size for prevalence studies. Archives of Orofacial Sciences, 1, 9–14. [Google Scholar]
- Nasreen, A. , Naib, J. M. , & Ibrar, M. (2016). Frequency of birth defects and associated risk factors. Pakistan Journal of Medical and Health Sciences, 10(2), 541–543. [Google Scholar]
- Oberklaid, F. , Danks, D. M. , & Jensen, F. (1979). Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. Journal of Medical Genetics, 16(2), 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli, I. M. , Castilla, E. E. , Scarano, G. , & Mastroiacovo, P. (1995). Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. American Journal of Medical Genetics, 59(2), 209–217. 10.1002/ajmg.1320590218 [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M. , & Legeai‐Mallet, L. (2017). Achondroplasia: Development, pathogenesis, and therapy. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 246(4), 291–309. 10.1002/dvdy.24479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanet . (2019). Retrieved from https://www.orpha.net.
- Pauli, R. M. (2019). Achondroplasia: A comprehensive clinical review. Orphanet Journal of Rare Diseases, 14(1), 1–1. 10.1186/s13023-018-0972-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng . (1988). Major congenital anomalies in livebirths in Alor Setar General Hospital during a three‐year period. The Medical Journal of Malaysia.43(2), 138–149. [PubMed] [Google Scholar]
- Qadir, M. , Amir, S. , & Bano, S. (2017). Prevalence and associated risk factors of congenital anomalies at a tertiary care hospital. Pakistan Journal of Medical and Health Sciences, 11(3), 942–945. [Google Scholar]
- Rasheed, S. , & Haseeb, M. (2016). Incidence and distribution of congenital malformations in newborns: A hospital based study. International Journal of Contemporary Pediatrics, 3(3), 806. [Google Scholar]
- Rasmussen, S. A. , Bieber, F. R. , Benacerraf, B. R. , Lachman, R. S. , Rimoin, D. L. , & Holmes, L. B. (1996). Epidemiology of osteochondrodysplasias: Changing trends due to advances in prenatal diagnosis. American Journal of Medical Genetics, 61(1), 49–58. [DOI] [PubMed] [Google Scholar]
- Rousseau, F. , Bonaventure, J. , Legeai‐Mallet, L. , Pelet, A. , Rozet, J. M. , Maroteaux, P. , … Munnich, A. (1994). Mutations in the gene encoding fibroblast growth factor receptor‐3 in achondroplasia. Nature, 371(6494), 252–254. 10.1038/371252a0 [DOI] [PubMed] [Google Scholar]
- Sallout, B. , Obedat, N. , Shakeel, F. , Mansoor, A. , Walker, M. , & Al‐Badr, A. (2015). Prevalence of major congenital anomalies at King Fahad Medical City in Saudi Arabia: A tertiary care centre‐based study. Annals of Saudi Medicine, 35(5), 343–351. 10.5144/0256-4947.2015.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, O. , Brito‐Arreaza, A. , Alvarez‐Arratia, M. C. , & Ramírez, N. (1991). Prevalence of bone dysplasias in newborns at the Ruíz y Páez Hospital in Bolívar City. Venezuela. 1978‐1990. Investigación Clínica, 32(2), 67–76. [PubMed] [Google Scholar]
- Savarirayan, R. , Irving, M. , Bacino, C. A. , Bostwick, B. , Charrow, J. , Cormier‐Daire, V. , … Hoover‐Fong, J. (2019). C‐type natriuretic peptide analogue therapy in children with achondroplasia. New England Journal of Medicine, 381(1), 25–35. 10.1056/NEJMoa1813446 [DOI] [PubMed] [Google Scholar]
- Schwarzer, G. , Chemaitelly, H. , Abu‐Raddad, L. J. , & Rucker, G. (2019). Seriously misleading results using inverse of Freeman‐Tukey double arcsine transformation in meta‐analysis of single proportions. Research Synthesis Methods, 10(3), 476–483. 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang, R. , Thompson, L. M. , Zhu, Y. Z. , Church, D. M. , Fielder, T. J. , Bocian, M. , … Wasmuth, J. J. (1994). Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell, 78(2), 335–342. 10.1016/0092-8674(94)90302-6 [DOI] [PubMed] [Google Scholar]
- Shirley, E. D. , & Ain, M. C. (2009). Achondroplasia: Manifestations and treatment. The Journal of the American Academy of Orthopaedic Surgeons, 17(4), 231–241. 10.5435/00124635-200904000-00004 [DOI] [PubMed] [Google Scholar]
- Simmons, K. , Hashmi, S. S. , Scheuerle, A. , Canfield, M. , & Hecht, J. T. (2014). Mortality in babies with achondroplasia: Revisited. Birth Defects Research Part A: Clinical and Molecular Teratology, 100(4), 247–249. [DOI] [PubMed] [Google Scholar]
- Sokal, R. , Tata, L. J. , & Fleming, K. M. (2014). Sex prevalence of major congenital anomalies in the United Kingdom: A national population‐based study and international comparison meta‐analysis. Birth Defects Research Part A: Clinical and Molecular Teratology, 100(2), 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. , Egger, M. , & Smith, G. D. (2001). Systematic reviews in health care: Investigating and dealing with publication and other biases in meta‐analysis. British Medical Journal (Clinical Research ed.), 323(7304), 101–105. 10.1136/bmj.323.7304.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. C. , & Harbord, R. M. (2004). Funnel plots in meta‐analysis. The Stata Journal, 4(2), 127–141. 10.1177/1536867X0400400204 [DOI] [Google Scholar]
- Stevenson, A. (1957). Achondroplasia: An account of the condition in Northern Ireland. American Journal of Human Genetics, 9(1), 81–91. [PMC free article] [PubMed] [Google Scholar]
- Stevenson, D. A. , Carey, J. C. , Byrne, J. L. B. , Srisukhumbowornchai, S. , & Feldkamp, M. L. (2012). Analysis of skeletal dysplasias in the Utah population. American Journal of Medical Genetics, Part A, 158 A(5), 1046–1054. 10.1002/ajmg.a.35327 [DOI] [PubMed] [Google Scholar]
- Stoll, C. , Dott, B. , Roth, M. P. , & Alembik, Y. (1989). Birth prevalence of skeletal dysplasias. Clinical Genetics, 35(2), 88–92. [DOI] [PubMed] [Google Scholar]
- Sunday‐Adeoye, I. , Okonta, P. , & Egwuatu, V. (2007). Congenital malformations in singleton and twin births in rural Nigeria. The Nigerian Postgraduate Medical Journal, 14, 277–280. [PubMed] [Google Scholar]
- Taboo, Z. A.‐A. (2012). Prevalence and risk factors for congenital anomalies in Mosul city. Iraqi Academic Scientific Journal, 11(4), 458–470. [Google Scholar]
- Tariq, S. (2010). Congenital anomalies in newborn. The Professional Medical Journal, 17(01), 135–139. [Google Scholar]
- Tunkel, D. , Alade, Y. , Kerbavaz, R. , Smith, B. , Rose‐Hardison, D. , & Hoover‐Fong, J. (2012). Hearing loss in skeletal dysplasia patients. American Journal of Medical Genetics. Part A, 158a(7), 1551–1555. 10.1002/ajmg.a.35373 [DOI] [PubMed] [Google Scholar]
- Unger, S. , Bonafé, L. , & Gouze, E. (2017). Current care and investigational therapies in achondroplasia. Current Osteoporosis Reports, 15(2), 53–60. 10.1007/s11914-017-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch, J. , van Kessel‐de Bruijn, F. , Landis, S. , Shediac, R. , Foreman, F. , & van Hoorn, R. (PROSPERO 2020. CRD42020148316). Systematic review on the birth prevalence of achondroplasia Retrieved from https://www.crd.york.ac.uk/PROSPERO/. [DOI] [PMC free article] [PubMed]
- Venkatesh, K. P. , Modi, H. N., Devmurari, K., Yoon, J. Y., Anupama, B. R., & Song, H. R. (2009). Femoral lengthening in achondroplasia. The Journal of Bone and Joint Surgery. British Volume, 91‐B(12), 1612–1617. 10.1302/0301-620x.91b12.22418 [DOI] [PubMed] [Google Scholar]
- Vrijheid, M. , Martinez, D. , Manzanares, S. , Dadvand, P. , Schembari, A. , Rankin, J. , & Nieuwenhuijsen, M. (2011). Ambient air pollution and risk of congenital anomalies: A systematic review and meta‐analysis. Environmental Health Perspectives, 119(5), 598–606. 10.1289/ehp.1002946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller, D. K. , Correa, A. , Vo, T. M. , Wang, Y. , Hobbs, C. , Langlois, P. H. , … Hecht, J. T. (2008). The population‐based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. American Journal of Medical Genetics. Part A, 146a(18), 2385–2389. 10.1002/ajmg.a.32485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin, D. J. , Szabo, J. K. , Cameron, R. , Henderson, S. , Bellus, G. A. , Mack, M. L. , … Sykes, B. (1998). Mutations in fibroblast growth‐factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. The American Journal of Human Genetics, 63(3), 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf, C. M. , & Turner, J. A. (1969). Incidence of congenital malformations among live births in Salt Lake City, Utah, 1951–1961. Social Biology, 16(4), 270–279. [DOI] [PubMed] [Google Scholar]
- Wynn, J. , King, T. M. , Gambello, M. J. , Waller, D. K. , & Hecht, J. T. (2007). Mortality in achondroplasia study: A 42‐year follow‐up. American Journal of Medical Genetics. Part A, 143a(21), 2502–2511. 10.1002/ajmg.a.31919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


