Abstract
Anxiety and depression are strikingly more prevalent in women compared with men. Dysregulation of corticotropin-releasing factor (CRF) binding to its cognate receptor (CRFR1) is thought to play a critical role in the etiology of these disorders. In the present study, we investigated whether there were sex differences in the effects of chronic variable stress (CVS) on CRFR1 cells using CRFR1-GFP reporter mice experiencing a 9-day CVS paradigm. Brains were collected from CVS and stress naïve female and male mice following exposure to the open field test. This CVS paradigm effectively increased anxiety-like behavior in female and male mice. In addition, we assessed changes in activation of CRFR1 cells (co-localization with c-Fos and phosphorylated CREB (pCREB)) in stress associated brain structures, including two sexually dimorphic CRFR1 cell groups in the anteroventral periventricular nucleus (AVPV/PeN; F>M) and paraventricular hypothalamus (PVN; M>F). CVS increased CRFR1-GFP cell number as well as the number of CRFR1/pCREB co-expressing cells in the female but not male AVPV/PeN. In the PVN, the number of CRFR1/pCREB co-expressing cells was overall greater in males regardless of treatment and CVS resulted in a male-specific reduction of CRFR1/c-Fos cells. In addition, CVS induced a female-specific reduction in CRFR1/c-Fos cells within the anteroventral bed nucleus of the stria terminalis and both sexes exhibited a reduction in CRFR1/c-Fos co-expressing cells following CVS within the ventral basolateral amygdala. Overall, these sex-specific effects of CVS on CRFR1 populations may have implications for sex differences in stress-induction of mood disorders.
Keywords: corticotropin releasing factor, sex difference, chronic stress, anxiety
1. Introduction
Within the United States, women are at approximately double the risk of men for being diagnosed with a stress-related mood disorder, such as anxiety or depression (Kornstein et al., 2000; Kessler et al., 2005). Together, these disorders that create a significant economic burden estimated at $210 billion annually (Greenberg et al., 2015). At present, there is limited information about sex differences in the central nervous system that contribute to the observed lifetime prevalence of stress-related mood disorders. Considering the striking sex differences in anxiety and depression, it is important to investigate the underlying neural circuitry. The interplay between corticotropin-releasing factor (CRF) and its receptor 1 subtype (CRFR1) may contribute to the observed sex differences.
CRF signaling at metabotropic CRFR1 regulates the hypothalamic-pituitary adrenal (HPA) axis and behavioral responses to stress (Perrin et al., 1993; Heinrichs et al., 1995; Smith et al., 1998; Subbannayya et al., 2013). Dysregulation of CRF/CRFR1 signaling has been shown to influence the onset of stress-related mood disorders such as anxiety and depression (Chrousos 2009; Garcia-Carmona et al., 2015; da Silva et al., 2016; Holly et al., 2016; Schussler et al., 2016). Rodent models show that chronic stress, which is associated with sustained release in CRF, leads to a dysregulation of HPA axis function and increases in anxiety- and depressive-like behaviors (Aguilera, 1994; Hodes et al., 2015; Algamal et al., 2018; Berry et al., 2012). One potential mechanism through which these alterations might occur is through chronic stress-induced alterations in the CRF/CRFR1 system. Previous studies have demonstrated that chronic stress can alter expression of CRF and CRFR1 in several key brain regions known to regulate the HPA axis and behavioral stress responses (Anisman et al., 2007; Choi et al., 2008). Chronic stress can also alter activation patterns of CRF and CRFR1 cells to modulate changes in the HPA axis and stress-related behaviors. For example, chronic social defeat increases activation of CRFR1 neurons in the paraventricular hypothalamus (PVN) in male mice; demonstrated by an elevated presence of c-Fos in CRFR1 neurons (Ramot et al., 2017).
Female and male rodents exhibit differences in neural and behavioral adaptations to chronic stress (Carvalho-Netto et al., 2011; Mallo et al., 2009; Renard et al., 2005). A study investigating rats after chronic stress demonstrated sex-dependent alterations in cAMP response element-binding protein (CREB) and phosphorylated CREB (pCREB), with males showing reductions in multiple limbic regions after chronic stress and females showing no effects of chronic stress (Lin et al., 2008). Furthermore, a chronic variable mild stress paradigm has been shown to induce sex- and region-specific alterations in CRF gene methylation as well as c-Fos within key stress-regulating brain regions including the PVN and extended amygdala (Sterrenburg et al., 2011). Sex differences in behavioral and HPA axis adaptations have also been reported in some variable stress paradigms (Hodes et al., 2015; Williams et al., 2019; Mallo et al., 2009; Renard et al., 2005). For example, short-term variable stress exposure results in elevated corticosterone (CORT) levels and increased indices of anxiety- and depression-like behavior in female, but not male, mice (Hodes et al., 2015; Williams et al., 2019). Sex differences in dysregulation of the CRF/CRFR1 system is a potential key contributor to the observed sex differences in neural and behavioral stress adaptation.
Using a validated CRFR1 reporter mouse line (bacterial artificial chromosome identified by green fluorescent protein (BAC CRFR1-GFP; Justice et al., 2008)) we previously identified two sexually dimorphic clusters of CRFR1 cells; one has higher levels within the female anteroventral periventricular nucleus (AVPV/PeN; Rosinger et al., 2017; 2019a), and the second shows higher levels of CRFR1 in the male PVN (Rosinger et al., 2019b). Both CRFR1 cell groups also exhibit sex differences in activation following an acute restraint stress with females showing an increase in CRFR1 cells that co-localize the transcription marker pCREB in the AVPV/PeN and males showing increased CRFR1/pCREB co-localization in the PVN. Both the AVPV/PeN and PVN CRFR1 cell groups also show a high level of co-expression with glucocorticoid receptor (Rosinger et al., 2019a, Ramot et al., 2017), which suggests that high levels of glucocorticoids, resulting from chronic stress, may alter these cell clusters. The present investigation therefore aimed to explore chronic stress-induced changes in CRFR1 levels and neural activation of CRFR1 cells in the AVPV/PeN and PVN, as well as other brain regions that are known to regulate behavioral and hormonal stress responses, including the extended amygdala. To do this, we exposed female and male BAC CRFR1-GFP reporter mice to chronic variable stress (CVS). Following 9 days of CVS, we show increased anxiety-like behavior independent of sex, and report region- and sex-specific alterations in CRFR1 levels and stress-induced activation of CRFR1 cells.
2. Methods
2.1. Animals
Subjects were 70–90 day old bacterial artificial chromosome (BAC GFP-CRFR1) mice on a C57BL6/J background (Justice et al., 2008) (N=24, 12 female/12 male). Mice were maintained on a 12/12 L/D cycle (lights on at 0700h), with food and water available ad libitum, aside from portions of the CVS paradigm that included overnight food deprivation. All procedures in this study were approved by the University at Albany Institutional Animal Care and Use Committee and are in accord with National Institutes of Health guidelines.
2.2. Chronic Variable Stress (CVS)
Adult male and freely cycling female CRFR1-GFP mice were randomly divided into chronic stress or home cage control conditions (n= 6/sex per treatment). CVS animals were housed in an adjacent vivarium. Control animals were group housed and not handled until the final open field assessment. Stressed animals were singly housed and subjected to a 9-day variable stress paradigm (described in Figure 1). Briefly, animals were exposed to one or two variable stressors, daily. On days in which two stressors were present, there was a minimum of 2 hours between stressors, including the mornings following overnight food deprivation (e.g., food reinstated at 0800h ad libitum, next stressor commenced at 1000h). The current protocol is a modified version of our previously validated paradigm, which was shown to affect HPA axis tone, and increase behavioral indices of anxiety (Johnson et al., 2015). Animals were exposed to the following battery of variable stressors: Wet cage (3hr), food deprivation overnight (12 hr), 45° cage tilt (3hr), tail suspension (10min), cold water swim (5min, or 10min), naphthalene exposure (1hr), and restraint stress (30min).
Figure 1. Chronic variable stress (CVS) timeline.
The CVS timeline illustrates conditions to which CVS-exposed mice were subjected, and the final open field exposure that all mice, CVS and NS, were exposed to on day 10. On day 10, animals were euthanized at 90 minutes following the onset of the open field exposure.
2.2.1. Wet Cage
Animals from the CVS treatment group were exposed to wet bedding in their home cage. Animals had 300mL of water poured into their home cage bedding and were left undisturbed for three hours. The water was poured over the entire base of the home cage to make it entirely wet. After three hours, the bedding was replaced with fresh bedding, and the animal was returned and left undisturbed until the next stressor was administered.
2.2.2. Tail Suspension
CVS mice had their tails taped and were suspended by their tail, one foot above the ground, for 10 minutes. After the 10 minutes were complete, the animals were returned to their home cage and left undisturbed until the next stressor was administered.
2.2.3. Cold Water Swim
Animals from the CVS treatment group were placed into the center of a beaker (12cm diameter X 24cm height) that was filled to 12cm with cold water (between 16–18°C). The mice were given 5 minutes for the cold swim before they were removed, dried off with a paper towel, and placed back into their home cage. Animals were monitored to ensure they were responsive and not drowning; however, no animals needed to be removed early. Following each animal exposure, the apparatus was emptied and cleaned with 70% ethanol.
2.2.4. Naphthalene Exposure
Crystalline naphthalene (~1.5g; Fisher Chemical) was wrapped into a sterile Kimwipe (Kimtech) and suspended on the left side of the home cage. Following one hour of exposure, the naphthalene was removed, and the animal was left undisturbed until the next stressor was administered.
2.2.5. Restraint Stress
Mice from the CVS treatment group were placed into a restraint tube (L: 6–4/5”, W: 3–9/10”, H: 2–3/5”) and left in their home cage for 30 minutes. The restraint tube was cleaned with 70% ethanol between each test.
2.3. Open Field
All animals from both treatment groups were exposed to a final 10-minute video-recorded open field assessment between 0800–1000h. Lighting in the open field testing area was 275–300 lux. This test was utilized both to induce a neural stress response and to determine effects of CVS on anxiety-like behaviors. Behaviors were analyzed during the first 5 minutes of open field exposure since this time period has been validated for assessing anxiety-like behaviors in mice (Bailey and Crawley, 2009; Gould et al., 2009; Zuloaga et al., 2008). The 10-minute duration of open field exposure was utilized in order to ensure a robust stress response that would induce c-Fos and pCREB. The apparatus consisted of an opaque Plexiglas cube (16” x 16” x 16”) with an open top. Mice were individually placed into one corner and allowed to roam freely for 10 minutes. After 10 minutes, animals were removed and singly housed in a new cage until sacrifice 80 minutes later. The apparatus was cleaned with 70% ethanol between each test. The open field was illuminated from above, and a camera was mounted on the ceiling for behavior recording. AnyMaze software (Stoelting Co.) was used to superimpose inner (8″ × 8″) and outer areas over the apparatus, from which the total locomotion, latency to inner area entry, number of inner area entries, and total time spent in the inner area were all calculated as previously performed (Jacobskind et al., 2018). We validated the accuracy of this assessment by hand scoring behavior in a subset of mice. We found a high level of concordance between automated and hand scoring for time spent in inner and outer areas, as well as latency to enter center area. Additionally, defecations were counted following the removal of each animal from the open field apparatus.
2.4. Tissue Collection for immunohistochemistry
All animals underwent cervical dislocation followed by rapid decapitation 90 minutes after the onset of open field exposure. Trunk blood was collected and brains were removed and placed in 4% paraformaldehyde, then stored overnight at 4° C. The next day, brains were transferred into a 30% sucrose cryoprotectant solution, where they remained at 4° C until sectioned. Brains were coronally sectioned at 40μm, into 3 series using a cryostat (Microm HM505E, MICROM international GmbH, Walldorf, Germany). Tissues were placed into cryopreservative and stored at 4° C until immunohistochemistry was performed.
2.5. Immunohistochemistry (IHC)
Dual-label fluorescent IHC was performed to determine co-expression of CRFR1-GFP+ cells with the neural activation marker c-Fos, the transcription marker pCREB, or an indicator of dopamine synthesis, tyrosine hydroxlase. Although both c-Fos and pCREB are commonly used as markers of neural activation, they differ with regard to effects of chronic stress. For example, chronic stress has been shown to induce robust attenuation of stress-induced c-Fos but not pCREB responses in brain regions including the PVN and arcuate nucleus (Kwon et al., 2006). Furthermore, pCREB is upstream of c-Fos in the signaling cascade and thus has the potential to modify transcription of a broader range of genes. Therefore, assessing c-Fos versus pCREB might reveal unique alterations in CRFR1 neural activation profiles following chronic stress. Tissue was covered with aluminum foil to avoid degradation of the endogenous GFP signal, rinsed in phosphate-buffered saline (PBS; pH 7.6), and incubated in 4% normal donkey serum (4% NDS) with 0.3% Triton-X in PBS (PBS-TX) for 60 minutes. Tissue was then placed directly into either primary antisera for c-Fos (Santa Cruz; rabbit; RRID: AB2106783; lot no. 12514; 1:250), pCREB (Cell Signaling; rabbit; RRID: AB2561044; lot no. 14; 1:500), or tyrosine hydroxylase (Millipore; anti-rabbit; AB390204; lot no. 2745367; 1:500) and incubated overnight at room temperature. The next morning, tissue was rinsed in PBS, then placed into secondary antisera (Jackson Labs; donkey anti-rabbit 594; cat no. 711585152; 1:500) mixed in 4% NDS and PBS-TX for 2.5 hours. After, tissue was transferred to the second primary antisera GFP (Abcam; chicken; RRID: AB300798; lot no. GR3190550–18; 1:2000) in 4% NDS and PBS-TX at room temperature overnight. On the third day, tissue was rinsed in PBS, and transferred to the second secondary antisera (Jackson Labs; donkey anti-chicken 488; cat no. 703545155; 1:1500) for 2.5 hours. After a final rinse in PBS, tissue was mounted and coverslipped with Santa Cruz hard set mounting media, containing DAPI, when dry. Specificity of labeling was assessed by performing immunohistochemistry using no primary and no secondary antibody controls. No tissue labeling was found in these validation tests. All primary antibodies (c-Fos, pCREB, GFP) used have also been previously been validated within our lab as well as others (Rosinger et al., 2019a; 2019b; Zuloaga et al., 2014). Furthermore, for inducible proteins (c-Fos, pCREB) we demonstrated very little labeling in amygdala and hypothalamic regions of mice not exposed to a stressor prior to sacrifice (Rosinger et al., 2019b; Zuloaga et al., 2014).
2.6. Microscopy
Assessment of CRFR1-GFP, c-Fos, pCREB, and tyrosine hydroxylase labeling was performed on a Nikon 80i microscope using a digital camera, at 20X magnification. We used the Allen Institute mouse brain coronal reference atlas (https://mouse.brain-map.org/static/atlas) to identify the brain regions of interest. Specifically, quantitative assessment of CRFR1-GFP, c-Fos, pCREB, TH, and co-labeled cells was performed after fixing proper regions of interest (ROIs) on images of the AVPV/PeN (rectangle; plate 53–54), arcuate nucleus (triangle; plate 72–73), PVN (triangle; plate 62–63), bed nucleus of the stria terminalis dorsolateral (oval; plate 52–53) and anteroventral (oval; plate 52–53), and central (oval; plate 70–71) and ventral basolateral amygdalae (oval; plate 70–71). Cells were quantified using ImageJ software to estimate various cell densities within the selected regions using methods previously described (Jacobskind et al., 2017; 2018; Rosinger et al., 2019a; 2019b). Co-labeling of c-Fos and pCREB with CRFR1-GFP cells was determined by the presence of red nuclear label (c-Fos or pCREB) within green neurons (CRFR1-GFP). Quantifications were performed within two sections bilaterally for each brain region. Approximate anatomical locations and ROIs within which CRFR1-GFP was quantified are shown in Figure 2.
Figure 2. Anatomical locations within which CRFR1-GFP and co-labeled cells were quantified.
Plate numbers correspond to locations determined by the Allen Mouse Brain Reference Atlas (https://mouse.brain-map.org/static/atlas). Approximate ROI shapes for the AVPV/PeN (A), BSTdl (B, top), BSTav (B, bottom), PVN (C), CeA (D, top), BLAv (D, bottom), and ARC (E). AC; anterior commissure, OT; optic tract.
2.7. Corticosterone Radioimmunoassay
Trunk blood was collected into EDTA-coated tubes 90 minutes after the onset of open field exposure. Following centrifugation for 10 minutes at 5500 RCF, supernatant was collected and frozen at −80 °C until radioimmunoassay was performed. Corticosterone was assessed using an I-125 corticosterone radioimmunoassay kit according to the manufacturer’s instructions (MP Biochemicals, LLC, Orangeburg, NY, USA). The intra-assay coefficient of variation was 3.9%.
2.8. Statistical Analyses
Two-way ANOVA was used for statistical analyses with sex and stress as factors for brain and behavioral analyses. Significant interactions and pre-planned group comparisons were further analyzed using T-tests where appropriate. Pre-planned comparisons were performed to assess within treatment effects of sex since our overarching hypothesis was that stress would sex specifically alter the CRFR1 system. Pearson’s correlations were also performed to assess the association between CRFR1-GFP, CRFR1/Fos, and CRFR1/pCREB labeled cells and the primary anxiety-like measure of the open field (center area time). These correlational analyses were performed separately, within males and females, in order to identify key CRFR1 cell groups that are associated with anxiety-like responses in males, females, and in both sexes. Correlations were not further performed within stress condition (No stress/CVS) of males and females due to insufficient power to perform these analyses. Significance level was set at p≤0.05 with data reported as means ± standard error of the mean (SEM).
3. Results
3.1. Anxiety-Like Behavior (Open Field)
A 2-way ANOVA revealed a significant effect of CVS treatment on the latency to first center entry in the open field (F(1,20) = 9.89; p< 0.01, where CVS-treated animals independent of sex took significantly longer to enter the center area (Figure 3a). A significant effect of treatment was also found for the number of center entries in the open field (F(1,20) = 7.66; p< 0.05), and the amount of time in the center of the open field (F(1,20) = 10.50; p< 0.01), with CVS mice showing fewer entries into the center and spending significantly less time in the center compared with non-stressed mice (Figure 3b–c). There was no significant difference in total distance traveled regardless of sex or treatment, which indicates no effect of CVS on general locomotor activity (Figure 3d). No significant effects of sex or interaction between chronic stress and sex were found for any behavioral measures, nor were there any significant differences in defecations in the open field (Means ± SEM, Female no stress= 0.67 ± 0.49, Male no stress= 0.83 ± 0.54, Female CVS= 1.00 ± 0.62, Male CVS= 2.00 ± 0.67).
Figure 3. Effects of CVS on anxiety-like behavior in the open field.
On day 10 following CVS onset, male and female CVS and non-stressed mice were exposed to a final open field exposure within which anxiety-like behavior was assessed. (A) The latency to first entry, was significantly longer for CVS animals than non-stressed animals. (B) Total number of center entries, showing that CVS mice made significantly fewer center entries than non-stressed counterparts. (C) The total amount of time spent in the center was significantly reduced in CVS-exposed compared to non-stressed mice. (D) No significant group differences were found for total distance traveled. * Main effect of treatment, p < 0.05.
3.2. Corticosterone Radioimmunoassay
No significant effects of chronic stress were found. However, there was a main effect of sex for plasma corticosterone collected at 90 minutes after open field onset, showing that all males had significantly less serum corticosterone than females, regardless of stress condition ((F(1,19) = 4.685) p=0.0434, (Means ± SEM, Female no stress= 84.96 ng/mL ± 24.75, Female CVS= 60.54 ng/mL ± 8.36; Male no stress= 45.67 ng/mL ± 10.11, Male CVS= 44.39 ng/mL ± 3.64)).
3.3. CVS effects on CRFR1-GFP, c-Fos, pCREB, tyrosine hydroxylase, and co-localized cells
3.3.1. AVPV/PeN
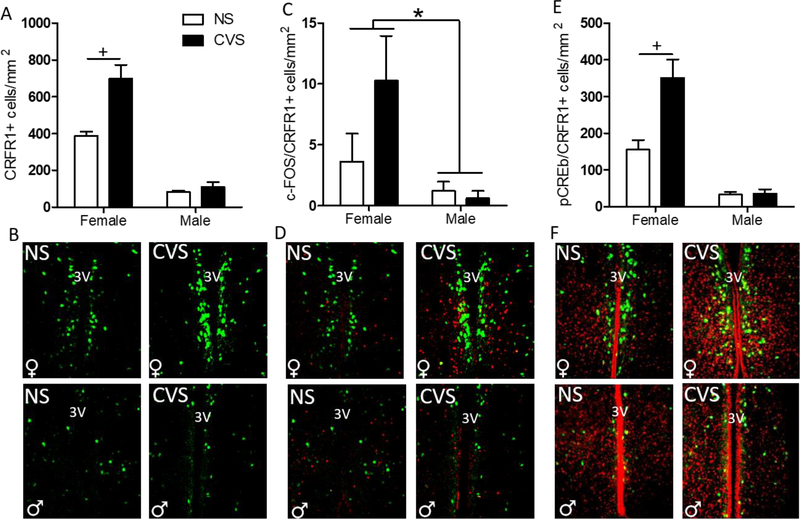
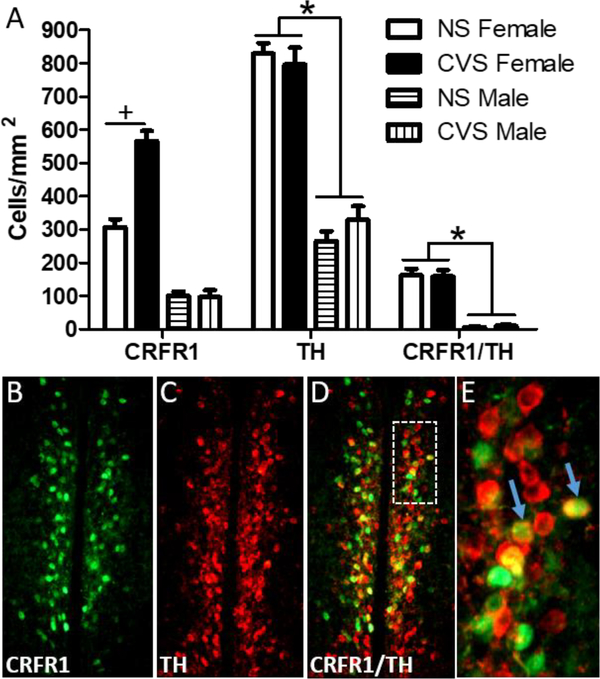
Analysis of CRFR1-GFP-ir within the AVPV/PeN revealed significant main effects of sex (Females>Males) (F(1,20) = 121.37; p≤0.001), and an interaction of sex and treatment (F(1,20) = 12.19; p≤0.01). Further post-hoc analysis showed an increase in CRFR1-GFP cell number in CVS females compared to control females (p≤0.01; Figure 4a–b) with no differences in males. Another 2-way ANOVA comparing c-Fos levels revealed a main effect of sex (F(1,20) = 12.52; p≤0.01; Table 1), where females had greater c-Fos-immunoreactivity (-ir) than males. There was also a trend toward increased c-Fos in the CVS compared to the no stress group (F(1,20) = 3.10; p=0.09). Furthermore, the number of c-Fos/CRFR1-GFP+ cells was elevated in females compared to males (F(1,20) = 12.52; p≤0.01; Figure 4c–d). Additionally, analysis of pCREB/CRFR1-GFP co-labeled cells revealed main effects of sex (F(1,20) = 54.99; p≤0.001), stress (F(1,20) = 11.32; p≤0.01), and an interaction between sex and stress (F(1,20) = 10.65; p≤0.01; Figure 4e–f). Post hoc comparisons indicated that CVS increased the number of pCREB/CRFR1 cells in females (p≤0.001) but not in males. Total pCREB-ir did not differ by sex or treatment (Table 1). Since CRFR1-GFP cell number showed a large increase in females following CVS we sought to investigate whether this increase occurred within a sexually dimorphic population of tyrosine hydroxylase neurons, which are abundant in this region (Simerly, 1989; Simerly et al., 1997; Semaan et al., 2010; Brock, et al. 2015; Scott et al., 2015). However, although our separate 2-Way ANOVAs replicate the increase in CRFR1-GFP following CVS in females (p≤0.001), and again report a sex difference in TH (females>males) (F(1,20) = 172.56; p≤0.001), no effects of CVS on the number of CRFR1-GFP/TH co-localized cells were found in either sex (Figure 5).
Figure 4. Effects of CVS on the number of AVPV/PeN CRFR1-GFP, c-Fos/CRFR1, and pCREB/CRFR1 cells.
(A) CVS females showed a significant increase in CRFR1-ir compared to all other treatment groups. (B) Representative images of CVS and NS female and male AVPV/PeN CRFR1-GFP. (C) c-Fos/CRFR1-GFP co-expression was significantly higher in all female AVPV/PeN than male, regardless of treatment condition. (D) Representative images of CVS and NS female and male c-Fos/CRFR1-GFP showing a female-specific reduction in c-Fos/CRFR1-GFP cells. (E) Females, regardless of treatment, had increased pCREB/CRFR1-GFP in the AVPV/PeN compared to males, and CVS females specifically had higher pCREB/CRFR1-GFP than any other groups. (F) Representative images of NS and CVS female and male pCREB/CRFR1-GFP in the AVPV/PeN showing female specific increased pCREB. 3V, third ventricle; NS, non-stressed; CVS, chronic variable stress. * Indicates a main effect of sex, (F>M; p < 0.05). + indicates a significant increase in CVS compared to NS females (p < 0.01).
Table 1.
Total c-FOS and pCREB immunoreactivity in various brain regions.
| Region | Marker | NS | CVS | ||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| AVPV/PeN | c-FOS | 82.7 ± 15.4* | 50.1 ± 12.9 | 138.3 ± 25.5* | 53.1 ± 7.3 |
| pCREB | 3249.4 ± 441.4 | 2976.4 ± 523.3 | 3164.9 ± 338.8 | 2768.1 ± 401.1 | |
| PVN | c-FOS | 596.5 ± 60.4^ | 574.1 ± 99.2^ | 207.6 ± 20.3 | 244.5 ± 43.4 |
| pCREB | 2466.9 ± 218.9 | 2676.2 ± 285.0 | 2471.2 ± 234.4 | 2633.6 ± 217.9 | |
| ARC | c-FOS | 159.1 ± 20.9^ | 172.7 ± 33.8^ | 103.8 ± 26.4 | 51.8 ± 7.9 |
| pCREB | 1812.2 ± 261.1 | 2152.5 ± 242.0 | 2268.7 ± 246.2 | 2004.9 ± 183.2 | |
| BSTav | c-FOS | 330.6 ± 32.2^ | 318.5 ± 25.9^ | 239.4 ± 13.8 | 243.4 ± 24.3 |
| pCREB | 1166.3 ± 118.4 | 1158.0 ± 111.5 | 1130.2 ± 106.8 | 1054.8 ± 126.9 | |
| BSTdl | c-FOS | 269.3 ± 18.2^* | 206.3 ± 11.8^ | 181.8 ± 23.9* | 173.7 ± 8.1 |
| pCREB | 573.3 ± 167.8 | 651.2 ± 138.7 | 562.3 ± 65.6 | 483.6 ± 90.4 | |
| BLAv | c-FOS | 111.3 ± 19.9^ | 129.5 ± 14.0^ | 62.7 ± 13.2 | 68.2 ± 7.45 |
| pCREB | 529.0 ± 94.0 | 890.2 ± 221.8 | 543.9 ± 119.1 | 737.7 ± 187.2 | |
| CeA | c-FOS | 709.7 ± 34.8 | 798.1 ± 54.5 | 825.7 ± 43.2 | 738.0 ± 48.0 |
| pCREB | 659.8 ± 62.4 | 1046.9 ± 93.1# | 927.9 ± 123.5 | 664.1 ± 131.7 | |
indicates main effect of sex (Female > Male).
indicates main effect of chronic stress (NS > CVS).
indicates interaction of chronic stress and sex, with further post-hoc analysis showing CVS males had significantly reduced pCREB compared to NS males.
NS, non-stressed; CVS, chronic variable stress; AVPV/PeN, rostral anteroventral periventricular nucleus; PVN, paraventricular nucleus of the hypothalamus; ARC, arcuate nucleus; BSTav, anteroventral portion of the bed nucleus of the stria terminalis; BSTdl, dorsolateral portion of the bed nucleus of the stria terminalis; BLAv, ventral basolateral amygdala; CeA, central amygdala. Data reported as mean cells/mm2 ± SEM, with the significance threshold set at p≤0.05.
Figure 5. Co-localization of AVPV CRFR1-GFP with TH following CVS.
(A) The number of CRFR1/TH co-localized cells was unaffected in male and female mice following CVS. This indicates that the increase in CRFR1-GFP labeled cells in CVS females occurs in an independent set of neurons. (B-D) Representative images of CRFR1-GFP, TH, and a merged image from a CVS female mouse, respectively. White inset box (D) indicates the area further magnified in (E). Arrows show examples of co-labeled neurons. * indicates a greater number of CRFR1-GFP, TH, and co-labeled cells in females compared to males, p < .001. + indicates p < .001 (NS compared to CVS females).
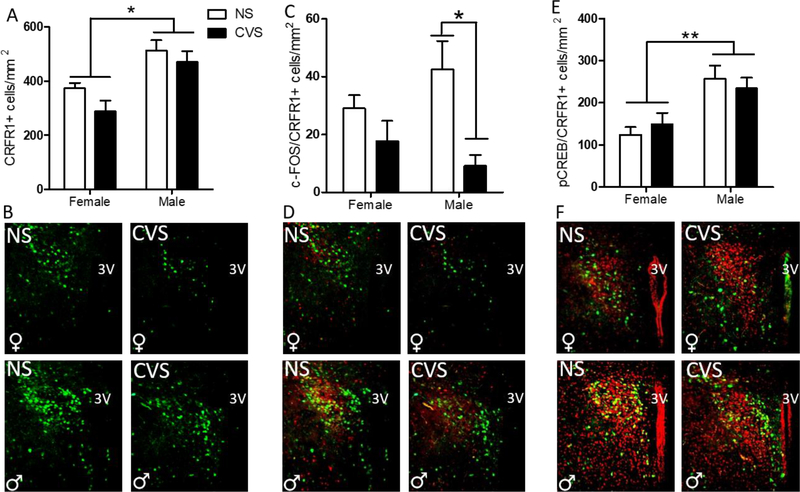
3.3.2. PVN
A 2-way ANOVA of PVN CRFR1 cells revealed a main effect of sex for CRFR1-GFP cell number (F(1,20) = 21.12; p≤0.001), where males of both conditions had significantly more CRFR1-GFP-ir cells than females (Figure 6a–b). A within sex planned comparison Student’s t-test demonstrated that CVS males had significantly fewer c-Fos/CRFR1-GFP cells than non-stressed males (t(10)=3.54, p≤0.01; Figure 6c–d). Analysis of total c-Fos-ir within the PVN revealed a significant main effect of treatment, where CVS animals had significantly reduced c-Fos-ir within the PVN compared with non-stressed animals (F(1,20) = 32.70; p≤0.001; Table 1). Finally, there was a main effect of sex on the total number of pCREB/CRFR1-GFP co-expressing cells, where males regardless of stress treatment had significantly increased co-expression compared to females (F(1,20) = 17.40; p≤0.001; Figure 6e–f). No group differences were found for total amount of pCREB in the PVN (Table 1).
Figure 6. Effects of CVS on the number of PVN CRFR1-GFP, c-Fos/CRFR1, and pCREB/CRFR1 cells.
(A) The female PVN had significantly less CRFR1-GFP than the male, regardless of treatment, with no effect of CVS on CRFR1-GFP in either sex. (B) Representative PVN CRFR1-GFP images for NS and CVS female and male mice. (C) CVS males had significantly fewer c-Fos/CRFR1 co-localized cells than non-stressed male counterparts, with no differences between female groups. (D) Representative c-Fos/CRFR1-GFP labeling in female and male mice from NS and CVS treatments. (E) Males had an overall greater amount of pCREB/CRFR1 co-labeled cells than females, regardless of treatment condition, with representative PVN pCREB/CRFR1 images from NS and CVS female and male mice shown in (F). NS, non-stressed; CVS, chronic variable stress. Data are presented as mean ± SEM, and significance threshold set to p<0.05. * Indicates statistical significance p<0.05. ** indicates p<.01.
3.3.3. BSTav
A 2-way ANOVA of total c-Fos within the BSTav revealed a significant main effect of treatment (F(1,20) = 11.04; p≤0.01), where CVS animals showed significantly less total c-Fos than non-stressed counterparts (Table 1). Further, the number of c-Fos/CRFR1 co-expressing cells was decreased in CVS-exposed animals (F(1,20) = 7.39; p≤0.05; Figure 7b). An additional within sex planned comparison t-test revealed that CVS reduced c-Fos/CRFR1-GFP expressing cells in females (t(10)=3.35, p≤0.01), but not in males (Figure 7b). No significant differences were found for total CRFR1-GFP-ir or pCREB expressing cells (Figure 7a,c; Table 1).
Figure 7. Effects of CVS on CRFR1-GFP cell number and co-localization with c-Fos and pCREB in various brain regions.
CRFR1-GFP, CRFR1/c-Fos, and CRFR1/pCREB co-labeled cells were quantified within the anteroventral portion of the bed nucleus of the stria terminalis (BSTav; A-C), dorsolateral portion of the bed nucleus of the stria terminalis (BSTdl; D-F), central amygdala (CeA; G-I), ventral basolateral amygdala (BLAv; J-L), and arcuate nucleus (ARC; M-O). CVS selectively reduced c-Fos/CRFR1-GFP co-labeled cells in female but not male BSTav (B). In the BLAv, the number of CRFR1/c-Fos co-labeled cells was significantly reduced in both sexes (K). There was also a trend toward a reduction in c-Fos/CRFR1 co-labeled cells in CVS mice within the arcuate nucleus (p=0.056; N), with CVS animals regardless of sex, trending toward reduced co-expression. No significant differences were found within the BSTdl or CeA. Data are reported as mean cells/mm2 ± SEM. + Indicates main effect of stress on outcome, p≤0.05. * Indicates an effect of sex on outcome, p≤0.05. & indicates a trend toward main effect of treatment, p = 0.056. NS, non-stressed; CVS, chronic variable stress.
3.3.4. BNSTdl
Analysis of total c-Fos revealed a significant main effect of sex (F(1,20) = 4.56; p≤0.05) and treatment (F(1,20) = 13.03; p≤0.01) with females showing overall greater levels of c-Fos than males and c-Fos was reduced by CVS. No significant differences were found for c-Fos/CRFR1-GFP or pCREB/CRFR1-GFP co-expression or total pCREB (Figure 7d–f; Table 1).
3.3.5. CeA
There were no significant effects of sex or stress condition on total CRFR1-GFP-ir, total c-Fos, c-Fos/CRFR1-GFP co-expression, or pCREB/CRFR1-GFP co-expression (Figure 7g–i; Table 1). However, there was a significant interaction of sex and stress for total pCREB (F(1,20) = 9.38; p≤0.05), which indicated a reduction in CeA pCREB in CVS males compared to control males (p≤0.05) (Table 1).
3.3.6. BLAv
A 2-way ANOVA revealed a significant reduction in c-Fos following CVS (F(1,20) = 14.66; p≤0.01; Table 1). There was also a significant main effect of treatment in the number of c-Fos/CRFR1-GFP cells (F(1,20) = 15.66; p≤0.001; Figure 7k) with CVS mice having decreased c-Fos/CRFR1-GFP co-localized cells compared to non-stressed mice. No group differences were found for total pCREB or pCREB/CRFR1-GFP co-expressing cells (Table 1; Figure 7j,l).
3.3.7. Arcuate Nucleus of the Hypothalamus (ARC)
Analysis of total c-Fos-ir within the ARC revealed a significant main effect of treatment (F(1,20) = 13.27; p≤0.01), where CVS mice had significantly less c-Fos-ir than non-stressed, regardless of sex (Table 1). There was also a trend toward a decrease in the number of c-Fos/CRFR1-GFP co-expressing cells in CVS mice (p=0.056; Figure 7n). No effect of sex or treatment was found for the number CRFR1-GFP-ir cells, pCREB, or pCREB/CRFR1-GFP co-labeled cells within the arcuate (Figure 7m,o).
3.4. Correlations between CRFR1, CRFR1/Fos, and CRFR1/pCREB labeled cells and center time in the open field
Pearson’s correlational analyses performed separately within males and females revealed a significant positive correlation between center time and CRFR1-GFP/c-Fos co-labeled cells within the BSTav and BLAv of both sexes. In females, a significant negative correlation was found between AVPV CRFR1-GFP and CRFR1-GFP/pCREB cells and center time, while a positive correlation was identified between CeA CRFR1-GFP cells and center time. In males, a significant positive correlation was found between PVN CRFR1-GFP/c-Fos co-labeled cells and time in the center area. No other correlations reached statistical significance in females or males; results are shown in Figure 8.
Figure 8. Correlations between time spent in the center area of the open field and CRFR1-GFP, CRFR1/c-Fos, and CRFR1/pCREB labeled cells within various brain regions in female (top) and male (bottom) mice.
Dark gray cells indicate a significant correlation (p<.05). A positive number indicates a positive association between time spent in the center area and the number of CRFR1-GFP, CRFR1/c-Fos, and CRFR1/pCREB labeled cells. This analysis reveals correlations with behavior that occur in both sexes (CRFR1/c-Fos in the BSTav and BLAv), only in females (CRFR1 and CRFR1/pCREB in AVPV/PeN, CRFR1 in CeA), and only in males (CRFR1/c-Fos in PVN). R1; CRFR1.
4. Discussion
The current study investigated the effects of chronic variable stress on CRFR1 cells within select rodent forebrain structures, including two sexually dimorphic clusters previously reported within the AVPV/PeN and PVN (Rosinger et al., 2017; Rosinger et al., 2019b). We found that our 9-day CVS paradigm produces an anxiety-like behavioral phenotype, independent of sex. In addition, we report that this CVS paradigm alters levels of CRFR1, and patterns of CRFR1 cell activation, in a region- and sex- dependent manner.
4.1. CVS induction of Anxiety-like Behavior
Following CVS exposure, both female and male mice exhibited increased markers of anxiety-like behavior in the final open field challenge, compared with stress-naïve mice. Specifically, CVS-exposed female and male mice had a greater latency to center entry, fewer center entries, and less time in the center compared with non-stressed mice (Figure 3a–c), independent of general locomotor activity (Figure 3d). The increased anxiety-like behavior in both female and male mice after CVS reflects findings of other studies using CVS paradigms (Mineur et al., 2006; Cotella et al., 2019). However, studies that have utilized a sub-chronic variable stress model have demonstrated that females may be more vulnerable to stress-related behavioral alterations than males (Hodes et al., 2015; Williams et al., 2019). This shorter duration stress paradigm induced depressive-like behavior only in females, which accompanied sex-specific patterns of transcription regulation in response to stress (Hodes et al., 2015). Therefore, while we report no sex differences in anxiety-like behavior in the open field using a 9-day CVS paradigm, it is possible that an attenuated CVS model may produce sexually dimorphic behavioral responses. Furthermore, while both sexes displayed stress-induced anxiety-like behavior in the open field, our findings support the idea that there are likely different mechanisms and neural networks involved in female versus male adaptations to chronic stress (Hodes et al., 2015; Lin et al., 2009; Bourke et al., 2012). It is important to note that sex differences in behavioral motivation have been reported in specific anxiety-related behavior tests, such as the elevated plus maze. In this task, male behaviors are shown to be primarily driven by anxiety while female behavior is primarily driven by activity (File, 2001; Fernandes et al., 1999). While we are unaware if similar sex differences in behavioral motivation exist in the open field test, the possibility of such differences should be considered when interpreting sex differences in stress-related behaviors. It also remains possible that assessment in a different anxiety-related behavioral test (e.g. novelty suppressed feeding) might have revealed a sex-specific effect of CVS (Hodes et al., 2015) or alternatively provided further validation of the present results which suggest elevated anxiety-like responses in both sexes.
CVS has been shown to cause hypersecretion of corticosterone following subsequent stress exposure (Herman, Adams, and Prewitt, 1995; Cotella et al., 2019), and specific CVS paradigms have elicited sex-specific effects on corticosterone levels (McCormick et al., 2005; Hodes et al., 2015). Our data show no effects of chronic stress within either female or male mice on serum corticosterone. However, the blood in our study was collected at 90 minutes after the open field (at the time of brain collection) and levels likely reflect recovery, not peak, serum corticosterone. Therefore, it is quite possible that peak or even basal corticosterone levels may have been altered by the current CVS paradigm. Regardless, our data show elevated recovery corticosterone levels in females compared to males as previously reported (Goel and Bale, 2010).
4.2. AVPV/PeN
A major finding in this study is that a 9-day CVS exposure nearly doubles CRFR1-GFP in the AVPV/PeN of female mice, with no effect in males. Following the final open field stress, CVS females also show an increase in CRFR1 cells that co-express the transcription marker pCREB compared to non-stressed females. Furthermore, CVS females showed a trend toward increased CRFR1/c-Fos co-labeled cells, although the effect did not reach statistical significance. These findings build on our previous report which demonstrated that an acute restraint stress increased pCREB co-localization within AVPV CRFR1-GFP neurons in females only. Our correlational analysis further demonstrates that high levels of AVPV CRFR1-GFP and CRFR1/pCREB co-labeled cells were associated with increased anxiety-like behavior in the open field test within females while no association was found in males. Together, these reports indicate that both acute and chronic stress selectively affect this population of CRFR1 neurons only in females and further suggest a link of AVPV CRFR1 with anxiety-like responses only within females. However, whether this increase in AVPV CRFR1 has a causal effect on behavioral responses remains unclear. Our present findings also support a previous study which indicates that CRFR1-expressing neurons are modified by chronic stress. Ramot et al. (2017) reported a recruitment of cellular activity within CRFR1 neurons of the male PVN following chronic social defeat stress (Ramot et al., 2017). Together, these findings suggest that the function of multiple subpopulations of CRFR1 cells can be modulated by chronic stress exposure.
The mechanisms through which these alterations in AVPV/PeN CRFR1 might occur is currently unknown, although sustained release of glucocorticoids (resulting from persistent stress) and binding to glucocorticoid receptors likely contribute to effects on AVPV/PeN CRFR1 neurons. The majority of CRFR1 neurons in the AVPV/PeN co-express glucocorticoid receptors (Rosinger et al., 2019) and glucocorticoid actions through GR have been shown to increase CRFR1 levels in distinct brain regions (Ramot et al., 2017). Alternatively, stress-induced increases in CRF signaling through CRFR1 within the AVPV/PeN might directly regulate elevations in CRFR1 and subsequent stress-induced activation of CRFR1 cells. CRF binding to CRFR1 induces phosphorylation of CREB and this persistent increase in phosphorylation of CREB can potentially induce alterations in CRFR1 itself, as well as c-Fos and many other genes involved in cell function and survival (Ahn et al., 1998; Mayr and Montminy, 2001; Lonze et al., 2002; Ortega-Martinez, 2015; Uribe-Marino et al., 2016). Future studies using local injections of antagonists for CRFR1 and GR could be used to assess the mechanisms that regulate stress-induced changes in AVPV CRFR1 neurons.
In order to identify a specific phenotype of neurons within which CRFR1 increased, we performed dual-label immunohistochemistry to determine co-localization of CRFR1 with a sexually dimorphic population of dopaminergic (tyrosine hydroxylase-expressing) neurons in the AVPV. Our results definitively show that CRFR1 is not upregulated in this population of neurons. Another possibility is that CRFR1 might increase within kisspeptin neurons in the AVPV, which are also more abundant in females (Poling and Kauffman, 2013). However, approximately 90% of kisspeptin neurons co-express TH, so the absence of CVS effects on CRFR1/TH co-labeling suggests that CRFR1 is likely not increased in the kisspeptin population either (Stephens et al., 2017). The AVPV is known to exert control over puberty onset, the estrus cycle, maternal behaviors, and other reproductive functions (Herbison, 2008; Scott et al., 2015). Therefore, an elevation in CRFR1 due to chronic stress could potentially affect these functions. Neurons in the AVPV also project to several brain regions that control behavioral and hormonal stress responses, including the PVN, BST, lateral septum, arcuate nucleus, and periaqueductal gray (Gu & Simerly, 1997). These projection patterns raise the possibility that CRFR1 neurons in the AVPV might be a sex-specific cell cluster for mediating stress responses, although the afferent projection sites of AVPV CRFR1 neurons are yet to be determined.
4.3. PVN
In a previous study from our lab we reported that adult males, compared to females, had a greater number of CRFR1-GFP neurons, as well as an increased number of CRFR1-GFP neurons that co-express pCREB following an acute restraint stress (Rosinger et al., 2019b). In the current investigation, we replicate these findings and also demonstrate that the sex difference in stress-induced pCREB within PVN CRFR1 neurons is sustained in mice following CVS. On the contrary, PVN CRFR1/c-Fos co-localized cells decreased after CVS exclusively in males. Previous studies in mice have shown that psychological stress induces high levels of both pCREB and c-Fos protein in the PVN at 1–2 hours following psychological stress (Kwon et al., 2006; Veening et al., 2004). However, prior chronic stress attenuates the c-Fos response to a final stressor, while pCREB levels are unaffected by chronic stress (Kwon et al., 2006). Our findings of CVS attenuation of PVN c-Fos, but an unaltered pCREB response, are consistent with this study. Phosphorylation of CREB is an important regulator of several immediate early genes, including c-Fos (Yamamoto et al., 1988; Ginty et al., 1993; Konradi et al., 1994) and can mediate plasticity of these genes (Moore et al., 1996). Therefore, repeated variable stress-induced elevations in pCREB may serve as an upstream regulator of c-Fos expression in the PVN as a whole, including specific attenuation within CRFR1 neurons.
CRFR1 neurons in the PVN have been shown to regulate neuroendocrine and behavioral stress responses (Ramot et al., 2017; Jiang et al., 2018). PVN CRFR1 neurons synapse on CRF neurons in the same region and are proposed to contribute to a local microcircuit that modulates CRF cell activity, CRF release, and ultimately release of corticosterone (Jiang et al., 2018; Jiang et al., 2019). CRFR1 neurons have also been shown to regulate behavioral adaptations to repeated social defeat in male mice (Ramot et al., 2017). The decreased c-Fos response, which occurs selectively in males following CVS, may be a male-specific mechanism that mediates alterations in behavioral and/or neuroendocrine stress responses. For example, activation of PVN CRFR1 neurons serve to suppress CRF secretion, therefore attenuated function of CRFR1 neurons could be a part of a mechanism that contributes to chronic stress-induced hyper-activation of the HPA axis in males. Interestingly, the correlation analysis indicated a significant positive association between CRFR1-GFP/c-Fos co-labeled cells and decreased anxiety-like behavior in males only. This further suggests that CRFR1 neurons in the PVN may have a sex-specific role in stress-associated behaviors.
4.4. Other Extended Amygdala and Hypothalamic Structures
Aside from the AVPV/PeN, no other regions examined showed changes in levels of CRFR1 following CVS. In addition, none of the regions we examined exhibited effects of CVS or sex differences in pCREB/CRFR1 co-expression, other than the AVPV/PeN and the PVN, described above. However, CVS significantly decreased c-Fos/CRFR1 co-expression in two regions (BSTav and BLAv) and another region (arcuate nucleus) showed a trend toward a decrease in c-Fos/CRFR1 co-expression following CVS.
The BST is a critical component of the stress response that has been repeatedly implicated in regulating behavioral responses to stress, as well as adaptations to chronic stress (Conrad et al., 2011; Roman et al., 2014). Numerous studies also demonstrate that CRF acts via CRFR1 within various divisions of the BST to control these functions (Sahuque et al., 2006; Pomrenze et al., 2019). The BST contains several divisions that are sexually dimorphic in cell volume and cell phenotype (Hines et al., 1985; van Leeuwen et al., 1985; Micevych et al., 1988; Forger et al., 2004) and has thus been proposed as a site through which gonadal hormones act to produce sexually dimorphic behavioral and neuroendocrine stress responses (Chen et al., 2016; Viau et al., 2001; Viau, 2002). Interestingly, the dorsolateral division of the BST has been shown to express higher levels of CRF in females than in males (Uchida et al., 2019; Funabashi et al., 2004). Here we report a novel sex difference, specifically that there is a significant reduction in c-Fos/CRFR1-ir cells in the anteroventral BST (BSTav) following CVS exclusively in female mice. The BSTav contains high levels of androgen and estrogen receptors that might play a role in mediating sex-specific changes in the function of these neurons following chronic stress. Our unpublished findings indicate that BSTav CRFR1 neurons also express high levels of androgen and estrogen receptors (Zuloaga, unpublished data). It is therefore possible that gonadal steroid hormone binding to these receptors contributes to the sex-specific effects of chronic stress on the BSTav. Given the known role of the BST in regulating anxiety- and depressive-like behaviors, this alteration in BSTav cell activation may be a mechanism that contributes to behavioral adaptations to chronic stress in females. Unlike the BSTav, no effects of CVS were found on CRFR1 cells in the BSTdl; however, there was overall greater c-Fos in females and a reduction in both sexes following chronic stress. The phenotype of the neurons that are sex-specifically activated and attenuated in the BSTdl by chronic stress is thus unknown, although the sexually dimorphic group of CRF-expressing cells in this region is an intriguing possibility (Uchida et al., 2019). CRF neurons in the BSTdl have been implicated as a key part of the circuit that regulates stress-induced anxiety responses (Pomrenze et al., 2019). Correlational analyses further revealed a positive correlation between CRFR1/c-Fos in the BSTav and center area time of both sexes, while no significant correlations were found for the BSTdl. This suggests a greater relationship between anxiety-like behavior and CRFR1 neurons in the BSTav as compared to the BSTdl, although causality remains to be determined.
The BLA has been consistently implicated in fear responses as well as anxiety- and depressive-like behaviors (Walker, Toufexis, and Davis, 2003; Carvalho-Netto et al., 2011; Wang et al., 2019; Choi et al., 2015). Previous work has shown that BLA function is modified by chronic stress exposure. Repeated restraint stress increases synaptic density in the BLA, which has been positively correlated with increased anxiety-like behavior in the elevated plus maze (Vyas et al., 2006). Moreover, others have demonstrated a critical role for CRFR1 activity within the BLA in anxiety- and depressive-like behaviors (Sotnikov et al., 2014; Chen et al., 2018), fear memories (Hollis et al., 2016), and stress-induced anorexia (Jochman et al., 2005). In the present study, we investigated effects of CVS on a population of CRFR1 neurons within the ventral division of the BLA in which CRFR1 is highly expressed, in contrast to lower levels in the dorsal BLA (Rosinger et al., 2017; Figure 2). We report a significant reduction in both c-Fos and c-Fos/CRFR1+ cells of the BLAv following CVS in both sexes. The reduction in c-Fos is in line with other studies in rats which demonstrate a reduction in total c-Fos expression following CVS (Ostrander et al., 2009), and we further show that this reduction also occurs within CRFR1 neurons of the BLAv. This alteration in cell activity likely contributes to changes in behaviors associated with CRFR1 signaling in the BLA, particularly the increase in anxiety-like behavior that occurs in both sexes following CVS. In contrast to the BLA, no effects of CVS were found within CRFR1 cells, or total c-Fos in the central amygdala (CeA). Long term stress paradigms (e.g. 21 days) have been shown to enhance CeA c-Fos responses to a final stressor (Hoffman et al., 2014), therefore it is possible that a prolonged stress paradigm is necessary to induce effects on c-Fos and/or CRFR1 neurons in this region. Although no effects of chronic stress were found for the number of CRFR1-GFP neurons, there was a positive correlation between CRFR1-GFP cell number and time in the center area within females. This suggests a link between CeA CRFR1 and anxiety-like behaviors, although the exact relationship between the two warrants further exploration.
Within the arcuate nucleus of the hypothalamus, there was a significant reduction in total c-Fos and a trend toward a decrease in c-Fos/CRFR1+ cells in CVS mice. Others have shown that chronic stress leads to reduced c-Fos, but not pCREB, in the arcuate nucleus (Kwon et al., 2006). Our data suggest that this reduction also occurs within CRFR1-expressing cells in the arcuate. The arcuate nucleus mediates several functions including glucose intake, cardiovascular regulation, and hormone release involving neuroendocrine signaling to the pituitary (Palkovits, 2008; Sapru, 2014; Hussain et al., 2015). Further, CRF actions within the arcuate have been proposed to modulate gonadotropin releasing hormone pulses and stress-induced suppression of appetite (Li et al., 2010; Stengel et al., 2009; Wang et al., 2017). It is thus possible that the reduced expression of c-Fos in arcuate CRFR1 cells could contribute to stress-induced effects on these functions.
It is important to note that the presence of c-Fos or pCREB within CRFR1 neurons are not necessarily the result of CRF signaling to these neurons. Stress signaling also involves other neuropeptides and neurotransmitters that might also be co-expressed in these CRFR1-expressing cells. Future studies using antagonists of CRFR1 are needed to determine whether changes in c-Fos or pCREB within CRFR1 expressing neurons are indeed induced by CRF. Assuming these changes are induced by CRF, it will be important for future studies to determine the neuroanatomical source of CRF inputs to these CRFR1 cell groups that mediate functional changes in CRFR1 neurons. Clusters of CRF expressing neurons are located in close proximity to CRFR1 cell groups that are altered by chronic stress in this study. These include dense CRF populations in the BST oval nucleus, medial preoptic area (near AVPV/PeN), and PVN (Walker et al., 2019). This indicates the possibility for local CRF signaling to modulate changes in neural activation of CRFR1 neurons. However, it also remains likely that CRF inputs from distal locations might also synapse on and regulate function of the CRFR1 populations assessed in this study. For example, PVN CRF neurons project to a number of limbic brain regions including CRFR1 containing regions assessed in the present study (BLA, CeA, ventral and dorsal BST, arcuate nucleus, as well as dense projections throughout the medial preoptic area, although it is unclear as to whether this specifically indicates the periventricular portion defined in this study as the AVPV/PeN (Zhang et al., 2017)).
Another limitation involves the use of CRFR1-GFP to identify CRFR1 containing cells. Although the CRFR1-GFP line has been validated for faithful reporting of Crfr1 expression and functional responses to CRF in many brain regions (Justice et al., 2008, Ramot et al., 2017, Jiang et al., 2018, Hunt et al., 2018), it should be noted that GFP expression does not formally demonstrate that GFP+ cells express functional CRFR1. In the context of CVS where we show an increase in the number of GFP+ cells in the AVPV/PeN, we have not yet characterized whether cells that initiate CRFR1 expression reported by GFP respond to CRF, or how CRF modulates activity of these cells. It is also important to note that estrous phase was not assessed in the present study and may have been altered by the CVS paradigm. Anxiety-like behaviors, as well as stress-induced c-Fos, have been reported to vary by estrous phase in rats (Frye et al., 2000; Figueiredo et al., 2002), therefore it is possible that c-Fos within CRFR1 neurons might also vary depending on estrous cycle. The AVPV, in particular, is highly sensitive to ovarian hormones and c-Fos expression in this region is modified by estrogen (Peterfi et al., 2004). Therefore, variability in estrous phase and potential stress-induced alterations in cyclicity should be considered in the interpretation of these results.
5. Conclusions
Chronic stress precipitates the onset of stress-related mood disorders, which exhibit a sex bias in lifetime prevalence rates (females>males), and dysregulation/alteration in CRFR1 function has been implicated in the onset of these conditions (Chrousos 2009; Garcia-Carmona et al., 2015; da Silva et al., 2016; Holly et al., 2016; Schussler et al., 2016). In the present study, we report that CVS exposure selectively impacts discrete CRFR1-containing cell groups in the female and male mouse, and these alterations occurred in tangent with CVS-induced anxiety-like behavior in both sexes. Sex differences in alterations in CRFR1 neurons and CRFR1 neuron activation reported in this study within the AVPV/PeN, PVN, and BSTav may represent sexually dimorphic circuits involved in behavioral and neuroendocrine adaptations to chronic stress.
Supplementary Material
Highlights.
Chronic variable stress (CVS) increases anxiety-like behavior in male and female mice.
CVS increased CRFR1-GFP cells and CRFR1/pCREB co-expressing cells in the female AVPV.
Sex-specific changes in CRFR1 cells were also found in the PVN and ventral BST.
Sex-specific effects of CVS on CRFR1 may contribute to sex differences in mood disorders.
Acknowledgments
The authors acknowledge Kristen Zuloaga and Lisa Robison for their technical assistance in behavioral analysis. Support was provided by University at Albany Research Initiation Funds (DZ), NIH-MH118692 (DZ), NIH-HD09641101, NIH-HD097331, NIH-DC017149 (PF), and NIH-MH112768 (NJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilera G (1994), Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendo 15:321–350. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Olive M, Aggarwal S, Krylove D, Ginty DD, Vinson C (1998), A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Bio 18(2):967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algamal M, Ojo JO, Lungmus CP, Muza P, Cammarata C, Owens MJ, Mouzon BC, Diamond DM, Mullan M, Crawford F (2018), Chronic Hippocampal Abnormalities and Blunted HPA Axis in an Animal Model of Repeated Unpredictable Stress. Front Behav Neurosci 12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisman H, Prakash P, Merali Z, Poulter MO (2007), Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res 181(2):180–90. [DOI] [PubMed] [Google Scholar]
- 5.Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice (2009). In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; Chapter 5. [Google Scholar]
- 6.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F (2012), Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 37(6):762–72. [DOI] [PubMed] [Google Scholar]
- 7.Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012. August;62(3):210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock O, De Mees C, Bakker J (2015), Hypothalamic expression of oestrogen receptor α and androgen receptor is se-, age-, and region-dependent in mice. J Neuroendocrinol 27(4):264–276. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho-Netto EF, Myers B, Jones K, Solomon MB, Herman JP (2011), Sex differences in synaptic plasticity in stress-responsive brain regions following chronic variable stress. Phys and Behav 104(2):242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CV, Brummet JL, Jordan CL, Breedlove SM (2016), Down, But Not Out: Partial Elimination of Androgen Receptors in the Male Mouse Brain Does Not Affect Androgenic Regulation of Anxiety or HPA Activity. Endocrinology 157(2):764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Li S, Cai J, Wei TJ, Liu LY, Zhao HY, Liu BH, Jing HB, Jin ZR, Liu M, Wan Y, Xing GG (2018), Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav Brain Res 338:134–142. [DOI] [PubMed] [Google Scholar]
- 12.Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP (2008), The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology 149(2):818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, Kim JE, Kim TK, Park JY, Lee JE, Kim H, Lee EH, Han PL (2015), TRH and TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology 97:346–56. [DOI] [PubMed] [Google Scholar]
- 14.Chrousos GP (2009), Stress and disorders of the stress system. Nat Rev Endocrinol 5(7):374–381. [DOI] [PubMed] [Google Scholar]
- 15.Conrad KL, Louderback KM, Gessner CP, Winder DG (2011), Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav 104(2):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotella EM, Gomez AS, Lemen P, Chen C, Fernandez G, Hansen C, Herman JP, Paglini MG (2019), Long-term impact of chronic variable stress in adolescence versus adulthood. Prog Neuropsychopharm & Biol Psychiatr 88:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Da Silva BS, Rovaris DL, Schuch JB, Mota NR, Cupertino RB, Aroche AP, Bertuzzi GP, Karm RG, Vitola ES, Tovo-Rodrigues L, Grevet EH, Bau CHD (2016), Effects of corticotropin-releasing hormone receptor one SNPs on major depressive disorder are influenced by sex and smoking status. J Affect Disord 205:282–288. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo HF, Dolgas CM, Herman JP (2002) Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology 143(7):2534–40. [DOI] [PubMed] [Google Scholar]
- 19.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ (2004), Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101(37):13666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frye CA, Petralia SM, Rhodes ME. (2000), Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 67(3):587–96. [DOI] [PubMed] [Google Scholar]
- 21.Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F (2004), Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology 29(4):475–85. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carmona JA, Baroja-Mazo A, Milanes MV, Laorden ML (2015), Sex differences between CRF1 receptor deficient mice following Naloxone-precipitated morphine withdrawal in a conditioned place aversion paradigm: implication of HPA axis. PLoS ONE 10(4):e0121125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME (1993), Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260(5105):238–41. [DOI] [PubMed] [Google Scholar]
- 24.Goel N, Bale TL (2010), Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology 151(4):1784–1794. doi: 10.1210/en.2009-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015), The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76(2):155–162. [DOI] [PubMed] [Google Scholar]
- 26.Gu GB, Simerly RB (1997), Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 384:142–164. [PubMed] [Google Scholar]
- 27.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008. March;57(2):277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman AN, Lorson NG, Sanabria F, Foster Olive M, Conrad CD. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem. 2014. July;112:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF (1995), The role of CRF in behavioral aspects of stress. Ann NY Acad Sci 771:92–104. [DOI] [PubMed] [Google Scholar]
- 30.Herman JP, Adams D, Prewitt C (1995), Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61(2):180–90. [DOI] [PubMed] [Google Scholar]
- 31.Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA (1985), Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci 5(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Tureki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ (2015), Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35(50):16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollis F, Sevelinges Y, Grosse J, Zanoletti O, Sandi C (2016), Involvement of CRFR1 in the basolateral amygdala in the immediate fear extinction deficit. Cog and Behav 3(5):e0084–16.2016 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA (2016), Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci 36(14):4093–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt AJ Jr, Dasgupta R, Rajamanickam S, Jiang Z, Beierlein M, Chan CS, Justice NJ (2018), Paraventricular hypothalamic and amygdalar CRF neurons synapse in the external globus pallidus. Brain Struct Funct 223(6):2685–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussain S, Richardson E, Ma Y, Holton C, De Backer I, Buckley N, Dhillo W, Berwick G, Zhang S, Carling D, Bloom S, Gardiner J (2015), Glucokinase activity in the arcuate nucleus regulates glucose intake. J Clin Invest 125(1):337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobskind JS, Rosinger ZJ, Zuloaga DG (2017), Hypothalamic-pituitary-adrenal axis responsiveness to methamphetamine is modulated by gonadectomy in males. Brain Res 1677:74–85. [DOI] [PubMed] [Google Scholar]
- 38.Jacobskind JS, Rosinger ZJ, Gonzalez T, Zuloaga KL, Zuloaga DG (2018), Chronic Methamphetamine Exposure Attenuates Neural Activation in Hypothalamic-Pituitary-Adrenal Axis-Associated Brain Regions in a Sex-specific Manner. Neurosci 380:132–145. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Z, Rajamanickam S, Justice NJ (2018), Local cortocitropin-releasing-factor signaling in the hypothalamic paraventricular nucleus. J Neurosci Epub ahead of print. pii: 1492–1517. doi: 10.1523/JNEUROSCI.1492-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Z, Rajamanickam S, Justice NJ. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress. 2019. August 10;11:100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jochman KA, Newman SM, Kalin NH, Bakshi VP (2005), Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci 119(6):1448–1458. [DOI] [PubMed] [Google Scholar]
- 42.Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, Raber J (2015), ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations. Neuropsychopharm 40(10):2443–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008), Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511(4):479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005), Lifetime prevalence and age-of-onset distributions for DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiat 62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 45.Konradi C, Cole RL, Heckers S, Hyman SE (1994), Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci 14(9):5623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Ryan CE, Hess AL, Harrison W, Davis SM, Keller MB (2000), Gender differences in chronic major and double depression. J Affect Disord 60(1):1–11. [DOI] [PubMed] [Google Scholar]
- 47.Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW (2006), The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus, and locus coeruleus. Neurosci 142:1281–1292. [DOI] [PubMed] [Google Scholar]
- 48.Li XF, Knox AMI, O’Byrne KT (2010), Corticotrophin-releasing factor and stress-induced inhibition of gonadotrophin-releasing hormone pulse generator in the female. Brain Res 1364:153–163. [DOI] [PubMed] [Google Scholar]
- 49.Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C (2009), Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex 19:1978–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lonze BE, Riccio A, Cohen S, Ginty DD (2002), Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacing CREB. Neuron 34(3):371–385. [DOI] [PubMed] [Google Scholar]
- 51.Mällo T, Matrov D, Kõiv K, Harro J. Effect of chronic stress on behavior and cerebral oxidative metabolism in rats with high or low positive affect. Neuroscience. 2009. December 15;164(3):963–74. [DOI] [PubMed] [Google Scholar]
- 52.Mayr B, Montminy M (2001), Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Bio 2:599–609. [DOI] [PubMed] [Google Scholar]
- 53.McCormick CM, Robarts D, Kopeikina K, Kelsey JE (2005), Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 48(1):64–74 [DOI] [PubMed] [Google Scholar]
- 54.Micevych P, Akesson T, Elde R (1988), Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol 269(3):381–91. [DOI] [PubMed] [Google Scholar]
- 55.Mineur YS, Belzung C, Crusio WE (2006), Effects of unpredictable mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175(1):43–50. [DOI] [PubMed] [Google Scholar]
- 56.Moore AN, Waxham MN, Dash PK (1996), Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem 271(24):14214–20. [DOI] [PubMed] [Google Scholar]
- 57.Ortega-Martinez S (2015), A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8:46. doi: 10.3389/fnmol.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, Herman JP (2009), Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression. Stress 12(6):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palkovits M (2008), Stress-induced activation of neurons in the ventromedial arcuate nucleus: a blood-brain-CSF interface of the hypothalamus. Ann NY Acad Sci 1148:57–63. [DOI] [PubMed] [Google Scholar]
- 60.Paxinos G, Franklin KBJ (2004), The mouse brain in stereotaxic coordinates, compact second edition. Elsevier Science (USA), ISBN: 0–12-388721–6. [Google Scholar]
- 61.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993. December; 133(6):3058–61. [DOI] [PubMed] [Google Scholar]
- 62.Peterfi Z, Churchill L, Hajdu I, Obal F Jr, Krueger JM, Parducz A (2004), Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 124(3):695–707. [DOI] [PubMed] [Google Scholar]
- 63.Poling MC, Kauffman AS (2013), Organizational and activational effects of sex steroids on kisspeptin neuron development. Front Neuroendocrinol 34(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, Morikawa H, Hopf FW, Messing RO. A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J Neurosci. 2019. February 6;39(6):1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A (2017), Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci 20(3):385–388. [DOI] [PubMed] [Google Scholar]
- 66.Renard GM, Suárez MM, Levin GM, Rivarola MA. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiol Behav. 2005. June 30;85(3):363–9. [DOI] [PubMed] [Google Scholar]
- 67.Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V (2014), PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47:151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosinger ZJ, Jacobskind JS, De Guzman RM, Zuloaga DG (2019b), A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience 409:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosinger ZJ, Jacobskind JS, Bulanchuk N, Malone M, Fico D, Justice NJ, Zuloaga DG (2019a), Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group. J Comp Neurol 527(6):1056–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, Zuloaga DG (2017), Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: a novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience 361:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF (2006), Psychopharm 186(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sapru HN (2014), Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci 175(0):38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schussler P, Kluge M, Gamringer W, Wetter TC, Yassouridis A, Uhr M, Rupprecht R, Steiger A (2016), Corticotropin-releasing hormone induces depression-like changes of sleep electroencephalogram in healthy women. Psychoneuroendocrino 74:302–307. [DOI] [PubMed] [Google Scholar]
- 74.Scott N, Prigge M, Yizhar O, Kimchi T (2015), A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525(7570):519–522. [DOI] [PubMed] [Google Scholar]
- 75.Semaan SJ, Murray EK, Poling MC, Dhamja S, Forger NG, Kauffman AS (2010), BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Neuroendo 151(12):5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simerly RB (1989), Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res 6(4):297–310. [DOI] [PubMed] [Google Scholar]
- 77.Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS (1997), Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA 94:14077–14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF (1998), Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20(6):1093–1102. [DOI] [PubMed] [Google Scholar]
- 79.Sotnikov SV, Markt PO, Malik V, Chekmareva NY, Naik RR, Sah A, Singewald N, Holsboer F, Czibere L, Landgraf R (2014), Bidirectional rescue of extreme genetic predispositions to anxiety: impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Transl Psychiatry February 11;4:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, Taché Y, Wang L (2009), Corticotropin-releasing factor-overexpressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology 150(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephens SBZ, Rouse ML, Tolson KP, Liaw RB, Parra RA, Chahal N, Kauffman AS (2017), Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T (2011), Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One 6(11):e28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subbannayya T, Balakrishnan L, Sudarshan G, Advani J, Kumar S, Mahmood R, Keshava Prasad TS (2013), An integrated map of corticotropin-releasing hormone signaling pathway. J Cell Commun Signal com 7:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uchida K, Otsuka H, Morishita M, Tsukahara S, Sato T, Sakimura K, Itoi K (2019), Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ 10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uribe-Marino A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, Dournes C, Fries GR, Masana M, Labermeier C, Wang XD, Hafner K, Schmid B, Rein T, Chen A, Deussing JM, Schmidt MV (2016), Prefrontal cortex corticotropin-releasing factor receptor 1 conveys acute stress-induced executive dysfunction. Biol Psychiat 80:743–753. [DOI] [PubMed] [Google Scholar]
- 86.van Leeuwen FW, Caffe AR, De Vries GJ (1985), Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res 325(1–2):391–4. [DOI] [PubMed] [Google Scholar]
- 87.Veening JG, Bouwknecht JA, Joosten HJ, Dederen PJ, Zethof TJ, Groenink L, van der Gugten J, Olivier B (2004), Stress-induced hyperthermia in the mouse: c-fos expression, corticosterone and temperature changes. Prog Neuropsychopharmacol Biol Psychiatry 28(4):699–707. [DOI] [PubMed] [Google Scholar]
- 88.Viau V (2002), Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol 14(6):506–13. [DOI] [PubMed] [Google Scholar]
- 89.Viau V, Soriano L, Dallman MF (2001), Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 13(5):442–52. [DOI] [PubMed] [Google Scholar]
- 90.Vyas A, Jadhav S, Chattanrji S (2006), Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neurosci 143(2):387–393. [DOI] [PubMed] [Google Scholar]
- 91.Walker LC, Cornish LC, Lawrence AJ, Campbell EJ (2019), The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: A validation study. Neuropharmacology 154:96–106. [DOI] [PubMed] [Google Scholar]
- 92.Walker DL, Toufexis DJ, Davis M (2003), Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463(1–3):199–216. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Goebel-Stengel M, Yuan PQ, Stengel A, Taché Y (2017), Corticotropin-releasing factor overexpression in mice abrogates sex differences in body weight, visceral fat, and food intake response to a fast and alters levels of feeding regulatory hormones. Biol Sex Differ 13;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Liu Y, Xiong J, Di T, Yuan Z, Wu J, Chen L (2019), Reduced serotonin impairs long-term depression in basolateral amygdala complex and causes anxiety-like behaviors in a mouse model of perimenopause. Exp Neurol 321:113030. [DOI] [PubMed] [Google Scholar]
- 95.Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, Moeser A, Jordan C, Leinninger G, Robison AJ (2019), Androgen-Dependent Excitability of Mouse Ventral Hippocampal Afferents to Nucleus Accumbens Underlies Sex-Specific Susceptibility to Stress. Biol Psychiatry pii: S0006–3223(19)31618-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR (1988), Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334(6182):494–8. [DOI] [PubMed] [Google Scholar]
- 97.Zhang R, Asai M, Mahoney CE, Joachim M, Shen Y, Gunner G, Majzoub JA (2017) Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol Psychiatry 22(5):733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuloaga DG, Johnson LA, Agam M, & Raber J (2014), Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J Neurochem 129(3):495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM (2008), Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav 54(5):758–766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.