Abstract
Context:
Medullary thyroid carcinoma (MTC), being an aggressive disease, requires meticulous follow-up and multidisciplinary management. The clinical presentation, management, outcome of MTC varies among different populations.
Aims:
An audit was conducted to evaluate the demography, clinical presentation, management, and outcome of MTC in a tertiary care center in South India.
Settings and Design:
A retrospective analysis was conducted of data from hospital records of patients with MTC treated at our center from 2004 to 2019.
Statistical Analysis:
All analyses were performed with SPSS software (version 16).
Results:
Among the 82 patients (M 42, F 40), mean age was 42.07 years (SD 14.5), 46 were operated at our center and 36, outside. Follow-up data were not available for all patients. Median duration of the disease was 36 months and median follow-up was 28 months. Lymphnode dissection was more common (37/46) in patients operated at our center than outside operated patients (17/36) (P < 0.01). At presentation, more than half of the patients had stage IV disease and 8 had distant metastases. Bone, lung, and liver were the common sites of metastases. Persistent hypercalcitoninemia >50 pg/mL was seen in 49.9%. Salvage surgeries of the neck were necessary in 29 patients (38.2%). Mean survival was 66 months and 10-year survival was 35%. Male gender (P = 0.008) and Stage IV disease at presentation (P = 0.038) were associated with poorer survival.
Conclusion:
MTC, in our population, presented at an advanced stage. Male gender and stage IV at presentation had poor survival. Early diagnosis, aggressive initial neck clearance, close follow-up with tumor markers, appropriate imaging, along with prompt surgical intervention will help to improve outcome.
Keywords: Differentiated thyroid carcinoma, medullary thyroid carcinoma, multiple endocrine neoplasia, survival
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a rare thyroid malignancy originating from parafollicular C-cells[1] and accounts for 5% of all thyroid carcinomas with disease specific mortality upto 13%.[2] Western literature states that the presentation is most common in the 4th or 5th decade of life with a female preponderance.[3] There are only few studies from India on MTC especially from south India.[4,5]
MTC has clear guidelines for management from various expert sources. Calcitonin (Ctn) and Carcino Embryonic Antigen (CEA) are the most common tumor markers used for diagnosis and monitoring as they correlate well with the tumor burden and stage of the disease. On follow-up, these markers are measured every 3 to 6 months. Based on the blood levels of these markers, periodic imaging using CT scan of the neck and chest, MRI, and/or FDG PET/CT were performed for assessing residual or recurrent disease to help decision making regarding therapy. MTC can present sporadically or in a familial form which necessitates genetic counseling and testing for RET germline mutations in these patients. The distribution of sporadic and familial MTC was 82% and 18%, respectively, in a previously published Indian Study.[6]
Aims and Objectives
The present study was undertaken to evaluate the clinicopathological features, management and outcome of MTC patients seen during a period of 15 years at Amrita Institute of Medical Sciences (AIMS), Kochi, Kerala, South India, a tertiary care institution.
METHODS
Retrospective analysis was performed from electronic hospital records of patients with MTC who were seen at AIMS from January 2004 to January 2019, after obtaining Institutional permission.
Hospital protocol
The thyroid cancer clinic used the approach for management recommended by the ATA Guidelines.[7] Initial surgery carried out at AIMS was total thyroidectomy with Lymph Node Dissection (LND) as needed. In patients with preoperative diagnosis of MTC, Ctn, CEA levels were assessed and appropriate imaging was advised to evaluate for Lymph node (LN) or distant metastasis (DM). For incidentally detected MTC, these markers were drawn 1 month after surgery.[8] On follow-up, Ctn and CEA were checked 3 and 6 months postoperatively. For Ctn <150 pg/mL, follow-up was with tumor markers and neck Ultrasonography (USG) at 6 months intervals for the first two years. If Ctn was high or increasing, further imaging appropriate for the clinical status was carried out. Salvage surgeries of the neck were performed whenever required with curative or palliative intent. Ctn and CEA were repeated 3 months after the procedure to assess response. In those with persistent biochemical disease, Ctn doubling time determined the frequency and type of testing, imaging modality, (CT scan, MRI, FDG PET CT or a DOTA NOC PET/CT) and therapy. TSH was kept in the normal range with thyroxine supplementation. External Beam Radiation Therapy (EBRT) was given to those who had local unresectable disease and to metastatic lesions when indicated. Tyrosine Kinase Inhibitors (TKI) were prescribed after consultation with Oncology for a few with advanced disease.
Good Outcome was defined as either no evidence for active disease (disease free) and or those where disease progression was not noted by available structural imaging and change in Ctn and/or CEA rise was not more than 50% of baseline (stable disease).
Poor Outcome was defined as patients whose disease progressed structurally by imaging or biochemically (>50% consistent rise in biomarkers), had appearance of new lesions and when they expired.
Laboratory assays
Calcitonin and CEA levels were measured by a variety of assays during the period of data collection. As it was not possible to obtain the methodology and other details used in the earlier years, any value above the upper limit of the manufacturer's reference range was used as the cut off for an abnormal value in Ctn and CEA. CEA testing became available in our laboratory from 2011 using an Electrochemiluminescence immunoassay (ECLIA). From 2017 onwards, the Roche ECLIA was used. The limit of detection was 0.2 ng/mL and the normal range, 0.0-5.0 ng/mL.
Institutional Ethics Committee had approved this study.
Statistical analysis
Data entered was analyzed using SPSS version 16. All categorical variables were compared using Chi-square tests and survival was plotted with Kaplan Meier survival analysis and compared using Log Rank Test.
RESULTS
The cohort consisted of 82 patients with MTC seen during the period 2004 -2019. Gender distribution was equal in our patients [Table 1]. Mean age of onset was similar in both genders (M 41.7 years vs F 42.3 years). Preoperative Ctn levels were available in 51 patients and showed correlation with extent of metastases, DM was seen only in patients with Ctn level >500 pg/mL.
Table 1.
Distribution of common variables in the study sample (n=82)
| Variables | Number (%) |
|---|---|
| Gender | M 42 F 40 |
| Age at diagnosis | |
| Mean age | 42.07 (SD14.7 years) |
| Median duration of disease | 36 months |
| Metastasis at diagnosis n (52) | |
| Yes | 49 (80.9%) |
| Lymph nodal metastasis alone | 41 |
| Distant metastasis | 8 (Bone 3 Liver 3 Lung 2) |
| Associated papillary thyroid carcinoma | 4 |
| Genetic analysis done | 33 |
| Positive | 8 |
| Multiple endocrine neoplasia | |
| MEN2A | 7 |
| MEN2B | 1 |
| Preoperative calcitonin levels (n=51) | |
| Calcitonin median range | 862 pg/mL |
| Calcitonin Level categories and Metastasis | |
| 7 (13.7) ( 3 had nodal metastases) | |
| 5-100 pg/ml | 16 (31.4) (7 had nodal metastases) |
| 100-500 pg/ml | 28 (54.9)(20 had nodal metastases |
| >500 pg/ml | 4 had nodal along with Distant metastases while 4 had Distant metastasis only |
Surgical management
Of the 82 patients, 46 were operated at AIMS and 36 were operated at outside centers. Among these, 31/46 (75.6%) had a preoperative diagnosis of MTC by fine-needle aspiration cytology (FNAC). These details were, however, not available for patients operated outside. Initial LND along with thyroidectomy was more frequent in patients operated at our center (37/46) than outside operated patients (17/36), this difference being statistically significant (P < 0.01). Among the 36 patients who had initial surgery outside, 16 needed re-exploration of neck at AIMS for residual disease.
Staging and histopathological details
AJCC Staging (8th Edition)[9] was available only in 69 patients and 39 had Stage IV disease at presentation. Among the 68 patients whose initial staging was available, males had more stage IVc disease (11/34) than females (6/34) but this difference was not statistically significant (P = 0.07).
Histopathological data showed that 37/55 tumors were over 2 cm in size, 21 had extrathyroidal extension and perinodal extension was seen in 25/45 patients with metastatic nodes.
Details of treatment and follow-up
Among the 55 patients whose postoperative Ctn levels available, <5 pg/mL was seen in 16 patients and >500 pg/mL was seen in 10 patients.
Median follow-up was 28 months. Totally 29 patients required salvage surgeries for recurrent disease, some more than once. The need for salvage surgeries was significantly lower in AIMS operated patients (11/46) compared to patients operated outside 18/36 (P < 0.01). EBRT was given to 19 patients and 11 received TKIs.
Outcome and survival
Among the 62 patients with adequate follow-up data, good outcome was seen in 25 patients (40.3%) and poor outcome in 37 patients (59.7%). Almost all disease-free patients belonged to stage I and II. As stage at presentation advanced, so did the severity of outcome, maximum deaths being seen in stage IV [Figure 1].
Figure 1.
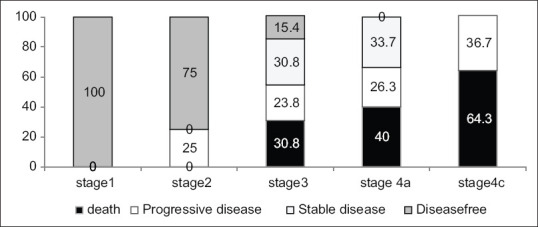
Outcome of patients according to the stage at presentation as % (n = 62) (P < 0.01)
Four patients died during follow-up and three more deaths were reported later on telephone enquiry. Another 10 patients were having terminal disease and were taken home for palliative care and presumed to have died soon after discharge. At the time of the last visit, 20 patients had DM. Though there was no gender preponderance in the cohort as a whole, DMs were strikingly more in men (65%). Lung and bone were the commonest sites of DMs in this cohort followed by liver. All patients with liver metastases died. Four patients were suspected to have tumor dedifferentiation based on histopathology reports of the repeat lymph node resection. These patients also had high levels of CEA (939-2878 ng/mL) and all expired.
A univariate analysis showed that male gender, advanced stage at presentation, perinodal extension, high pre and postoperative Ctn level, residual disease, and need for salvage surgeries to have a significant association with poor outcome. Despite increased morbidity from completion surgery and salvage surgeries, 10/33 (30.3%) patients operated outside had good outcome, compared to 22/45 (48.9%) operated at AIMS, but this difference was not statistically significant (P = 0.078) possibly due to our aggressive and meticulous follow-up. Lack of sufficient data precluded a multivariate analysis. Hence Kaplan-Meier survival estimates were used to study the association of above variables with survival.
Survival estimates using Kaplan–Meier method showed that median survival of these subjects was 5½ years (66 months) and only 35% of these patients were alive after 10 years [Figure 2].
Figure 2.
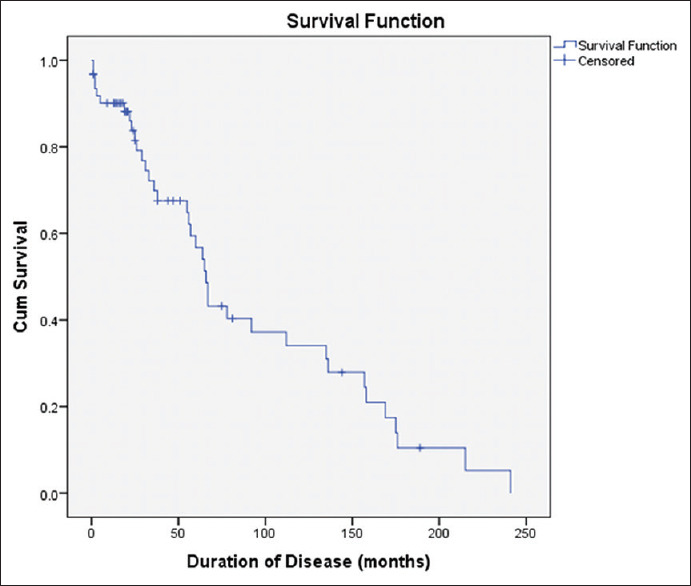
Kaplan–Meier survival plot with log rank (Mantel–Cox) test
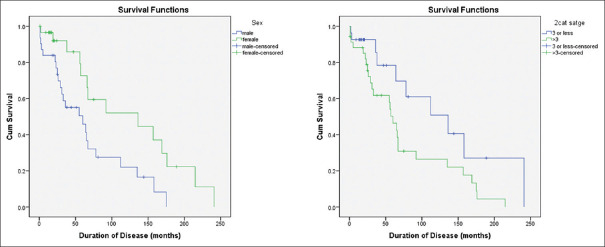
When factors associated with survival were compared using Log Rank test [Figure 3], male gender was associated with poor survival (median survival 60 months) compared to females (136 months) and this difference was statistically significant (P < 0.01) The AJCC stage at disease presentation also had negative association with survival. While stage III and below had median survival of 136 months, higher stage patients had significantly lower survival (60 months) (P 0.036).
Figure 3.
Comparison of survival estimates of gender and stage of presentation
Only 33 patients underwent genetic testing for multiple endocrine neoplasia type 2 of which, 8 tests were positive.
DISCUSSION
This retrospective data collection of MTC for 15 years, planned to evaluate the clinicopathologic features and outcomes, served as an audit of our management.
Table 2 shows a comparison of this study with 2 other Indian studies and one Japanese study by Ito et al.[10] regarding salient parameters that might have influenced the survival.
Table 2.
Comparison between four studies
| Parameters | This study | South Indian study[4] | North Indian Study[4] | Yasuhiro Ito et al.[4] |
|---|---|---|---|---|
| Study period | 2004-2019 15 years | 2008-2016 8 years | 1990-2009 19 years | 1975-2014 39 years |
| No. of patients | 82 | 90 | 71 | 233 |
| M F | M42 F 40 | M 47 F43 | M 45 F 26 | M 60 F 173 |
| Mean age | 42.07 (SD | 40 years (range 14-70 years) | 39.9±14.1 years | |
| 14.7 years) | ||||
| LND | 78.3% | 93.3% | 67% | 90% |
| Therapeutic | ?? | 48 patients | ||
| Prophylactic | ?? | 164 patients | ||
| AJCC 8 stage IV at diagnosis (%) | 56.4% | NA | 63.6% | 46/233 (19.7%) |
| Distant metastases | 20 | 16 | 14 | 19 |
| Died | 13/20 | 8/16 | 3/14 | 12/19 |
| Kaplan-Meier survival estimates | 5 year OS 54% | 5-year OS 86.3% | 5 year OS 74.6% 10-year OS 58% | 10 year CSS |
| 10 year OS 35% | 10 year OS 81.2% | 97% |
NA=Not available, OS=Overall survival, CSS=Cause-specific survival
Ten-year overall survival was much lower in the present study and worse than the other 2 Indian studies. Ito et al., however, had very high survival which, the authors attributed to, the aggressive LND (90%) at the outset. This was likely as all their patients except one had the preoperative diagnosis of MTC with FNAC and/or Ctn. Knowing the diagnosis beforehand would have helped the surgeons to be aggressive with the initial surgery. Stage IV presentation, an established adverse prognostic factor[11,12] was seen in almost half of this cohort which was lower than the 63.5% in the North Indian study and much higher compared to the Japanese study (19%), perhaps pointing to the benefit of early diagnosis. Although metastatic disease was low in Ito et al. as compared with the other studies, once it developed, prognosis in that series also was uniformly poor.
Lung and bone were the commonest metastatic sites as was seen in other series.[10] Liver metastases conferred high mortality. Male gender was associated with poor outcome compared to females despite both groups being comparable in age and stage at presentation. DM was more common in males and possibly contributed to poor survival. Genetic testing was positive in 8 out of 33 patients. One was a MEN2 B mutation, another a 634 mutation from 1 member of a family of 3, one V804L mutation and another, an S891A mutation in a family of five. Two Indian studies found that the 634 mutation is the commonest in Indian subjects.[6,13] Despite counseling, number of patients tested was low due to socioeconomic factors or inherent fear and anxiety denying the chance of early detection in family members. The 5 member family, which included a healthcare worker was, however, receptive and was able to obtain “cure” because of early detection. As excellent results were seen in hereditary MTC because of prompt early intervention, patients with MTC should be encouraged to undergo genetic testing.
This study points out the need for improving awareness among public of the advantages of early diagnosis and aggressive evaluation periodically on follow-up for residual/recurrent disease especially for development of DM so that early and appropriate measures can be instituted to contain the disease and reduce mortality. In patients where MTC is a “histological surprise", strong consideration should be given for re-surgery for a complete clearance including LND, especially if Ctn is detectable postoperatively.
The very strength of this study is its exhaustive nature.
Limitations of the study: Incompleteness of data, especially the histopathological details in patients initially operated outside, insufficient investigations for socioeconomical reasons and loss of patients to follow-up. Another drawback is that not all patients had pheochromocytoma screening as it was done only in patients who had preoperative diagnosis, syndromic MTC, and those who had hypertension or suggestive symptoms.
CONCLUSION
Results of this retrospective study showed that MTC patients at AIMS mostly presented with advanced disease and showed poor outcome with only about one third having 10-year survival. Male gender and stage IV of disease at presentation were associated with poor prognosis. Initial surgical management appeared to be suboptimal for many patients necessitating further salvage surgeries. Follow-up remained inadequate probably due to socioeconomic reasons and many patients were lost to follow-up. This study highlights the need for combined and coordinated efforts to promote early diagnosis, optimal neck dissection at the outset, close follow-up with imaging and tumor markers for progression of disease and early detection of metastases in order to improve outcome in MTC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114–8. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randle RW, Balentine CJ, Leverson GE, Havlena JA, Sippel RS, Schneider DF, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161:137–46. doi: 10.1016/j.surg.2016.04.053. https://doiorg/101016/jsurg201604053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raue F. German medullary thyroid carcinoma/multiple endocrine neoplasia registry German MTC/MEN Study Group Medullary thyroid carcinoma/multiple endocrine neoplasia type 2. Langenbecks Arch Surg. 1998;383:334–6. doi: 10.1007/s004230050143. [DOI] [PubMed] [Google Scholar]
- 4.Cherian AJ, Ramakant P, Pai R, Manipadam MT, Elanthenral S, Chandramohan A, et al. Outcome of treatment for medullary thyroid carcinoma-Asingle centre experience. Indian J Surg Oncol. 2018;9:52–8. doi: 10.1007/s13193-017-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrotra PK, Mishra A, Mishra SK, Agarwal G, Agarwal A, Verma AK. Medullary thyroid cancer: Clinico-pathological profile and outcome in a tertiary care center in North India. World J Surg. 2011;35:1273–80. doi: 10.1007/s00268-011-1086-7. [DOI] [PubMed] [Google Scholar]
- 6.Yadav M, Agrawal V, Pani KC, Verma R, Jaiswal S, Mishra A, et al. C-cell hyperplasia in sporadic and familial medullary thyroid carcinoma. Indian J Pathol Microbiol. 2018;61:485–8. doi: 10.4103/IJPM.IJPM_478_17. [DOI] [PubMed] [Google Scholar]
- 7.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed SR, Ball DW. Clinical review: Incidentally discovered medullary thyroid cancer: Diagnostic strategies and treatment. J Clin Endocrinol Metab. 2011;96:1237–45. doi: 10.1210/jc.2010-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. [Google Scholar]
- 10.Ito Y, Miyauchi A, Kihara M, Higashiiyama T, Fukushima M, Miya A. Static prognostic factors and appropriate surgical designs for patients with medullary thyroid carcinoma: The second report from a single-institution study in Japan. World J Surg. 2018;42:3954–66. doi: 10.1007/s00268-018-4738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–42. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 12.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–48. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Mahesh DM, Nehru AG, Seshadri MS, Thomas N, Nair A, Pai R, et al. RET mutations in a large Indian family with medullary thyroid carcinoma. Indian J Endocrinol Metab. 2014;18:516–20. doi: 10.4103/2230-8210.137508. [DOI] [PMC free article] [PubMed] [Google Scholar]