Abstract
The increasing demand for global food security in the face of a warming climate is leading researchers to investigate the physiological and molecular responses of cereals to rising ambient temperatures. Wheat and barley are temperate cereals whose yields are adversely affected by high ambient temperatures, with each 1 °C increase above optimum temperatures reducing productivity by 5–6%. Reproductive development is vulnerable to high-temperature stress, which reduces yields by decreasing grain number and/or size and weight. In recent years, analysis of early inflorescence development and genetic pathways that control the vegetative to floral transition have elucidated molecular processes that respond to rising temperatures, including those involved in the vernalization- and photoperiod-dependent control of flowering. In comparison, our understanding of genes that underpin thermal responses during later developmental stages remains poor, thus highlighting a key area for future research. This review outlines the responses of developmental genes to warmer conditions and summarizes our knowledge of the reproductive traits of wheat and barley influenced by high temperatures. We explore ways in which recent advances in wheat and barley research capabilities could help identify genes that underpin responses to rising temperatures, and how improved knowledge of the genetic regulation of reproduction and plant architecture could be used to develop thermally resilient cultivars.
Keywords: Barley, cereals, high temperature, reproductive development, thermal resilience, wheat
We outline recent advancements in our understanding of the reproductive developmental traits and genetic pathways underpinning responses of wheat and barley to high ambient temperatures, and propose new strategies for developing thermotolerant cultivars.
Introduction
Global agriculture is challenged by increasing populations and climate change. In particular, cereals including bread wheat (Triticum aestivum), durum wheat (Triticum turgidum), and barley (Hordeum vulgare) are major sources of human nutrition for which grain yield and quality are significantly affected by high temperature (Wardlaw et al., 1989; Wallwork et al., 1998; Battisti and Naylor, 2009; Lobell et al., 2011). For example, a temperature increase of 2 °C reduces global wheat yields by 11%; based on 2017 production, this equates to a yield loss of 84.8 Mt, which is higher than the total amount of wheat produced annually in Northern Europe (Zaveri and Lobell, 2019). Climate models predict that cereals will be exposed to higher average temperatures and more frequent extreme heat stress in the future (Lobell et al., 2011). Coupled with the requirements to increase yields by 60–70% over the next 50 years in order to maintain global food security, the effects of high temperature on development and the molecular mechanisms that underpin downstream responses should be investigated further to help generate thermotolerant germplasm (Jaggard et al., 2010).
To understand the effects of a warming climate, it is important to consider stages of development that are critically influenced by higher temperatures. The life cycle of cereals progresses through distinct growth phases: vegetative, reproductive (inflorescence/spike development), anthesis (flowering), grain set, and senescence. The transition between these growth phases is dependent on developmental programmes that are activated and modulated by environmental and endogenous stimuli (Huijser and Schmid, 2011). Temperature and photoperiod (the duration of light during the day) are critical environmental signals used as seasonal cues to coordinate developmental transitions (Fjellheim et al., 2014). During crop domestication, allelic variation for genes that regulate photoperiod and thermal-dependent initiation of flowering has broadened cultivation ranges, such that flowering and grain development occur under optimal conditions (Worland, 1996; Worland et al., 1998; Jung and Müller, 2009). Although cereals are widely adapted to grow in many areas of the world (Cockram et al., 2007), the late reproductive phases of development are particularly susceptible to temperature stress (Porter and Gawith, 1999; Wollenweber et al., 2003). The physiological response of cereals to high temperatures during late reproductive stages has been well studied, and our understanding of the molecular processes that underpin responses to warming temperatures is improving as we learn more about the genes that coordinate reproductive development. Further advances in our understanding of these genes will benefit from improved genetic knowledge and technical capabilities in wheat and barley research (Krasileva et al., 2017; Mascher et al., 2017; IWGSC, 2018; Ramírez-González et al., 2018; Watson et al., 2018).
Here, we discuss the effects of high temperature on the reproductive development of wheat and barley, including traits such as flowering time, inflorescence development, and grain filling. We consider exposure to high temperatures—average, acute, and night-time—as well as the response of other cereal crops to warmer conditions. We link historical phenology-based studies with more recent work investigating the genetic regulation of high-temperature responses, including the potential to learn from outcomes of research using model plants. In doing so, we set the stage for future research that aims to improve the thermal resilience of wheat and barley.
Temperature effects on reproductive development
Wheat and barley are temperate, facultative long-day plants that flower more rapidly under long-day photoperiods and are characterized by two growth types: winter and spring. Winter types show accelerated flowering after prolonged exposure to the cold temperatures of winter—a process known as vernalization—which coordinates floral development and delays the cold-sensitive reproductive growth phases until spring (Purvis, 1934; McKinney, 1940; Trevaskis, 2010). In contrast, spring types do not require a period of cold to flower (Trevaskis, 2010; Gol et al., 2017). Responses to these seasonal cues align flowering with the favourable conditions of spring, enabling the completion of fertilization and grain production before the onset of hot and dry conditions of summer (Fjellheim et al., 2014). The growth habit of the plant also influences its response to high temperatures (Craufurd and Wheeler, 2009; Dixon et al. 2019). For example, in field experiments using supplementary heating, spring wheat plants exposed to temperatures between 0 °C and 40 °C flowered significantly earlier with increasing temperature (White et al., 2011). In winter wheat, however, high-temperature treatments during or post-vernalization delay early and late stages of reproductive development, with the flowering of some cultivars occurring later at 25 °C than at 11 °C or 18 °C (Dixon et al., 2019).
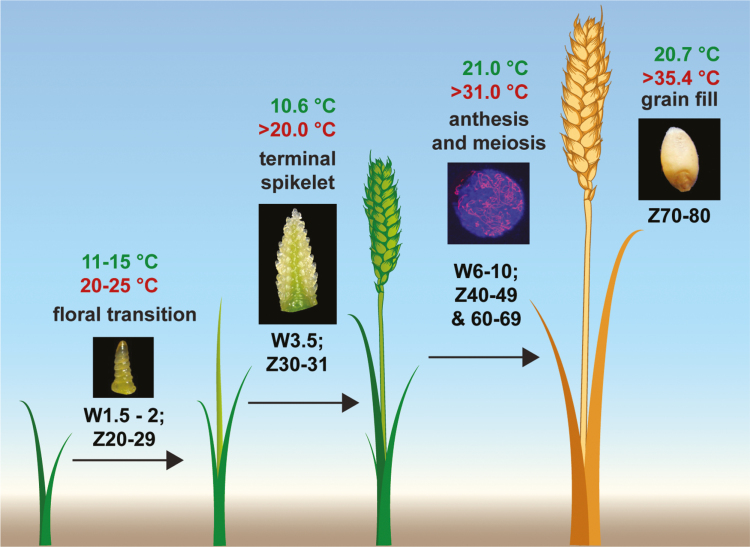
As expected for plants that have adapted to the progression of seasons from winter into spring, the ideal growth temperatures for early developmental stages are cooler than those of later stages. For example, the optimum temperatures for wheat at the terminal spikelet stage, anthesis, and grain filling, as determined from 65 published studies, are 10.6, 21.0, and 20.7 °C, respectively (Porter and Gawith, 1999; Fig. 1). As a consequence, the temperature at which thermal stress occurs is dependent on the developmental stage (Slafer and Rawson, 1995). During early wheat inflorescence development, when the number of spikelets is being determined (the terminal spikelet stage), an increase in temperature from 10 °C to 19 °C accelerates reproductive development, whereas temperatures >20 °C delay terminal spikelet initiation and reduce the number of spikelet primordia (Slafer and Rawson, 1995; Porter and Gawith, 1999). Similar results have been reported in spring and winter barley, where an increase in ambient temperature from 20 °C/16 °C (day/night) to 28 °C/24 °C and from 15 °C to 25 °C, respectively, delayed inflorescence development, and reduced floret number and grain per spike (florets are equivalent to spikelets in these cases, as the research used two-rowed cultivars) (Hemming et al., 2012; Ejaz and von Korff, 2017). The decrease in spikelet and floret number that accompanies delayed inflorescence development under high temperatures somewhat contradicts expectations, as slower development of the inflorescence is normally associated with the production of more spikelets (e.g. under short-day photoperiods). These results suggest that high temperatures inhibit both inflorescence and spikelet development during early reproductive stages of barley and wheat.
Fig. 1.
Optimum and maximum temperatures for the reproductive growth stages in wheat. Optimum temperatures are in green, and maximum temperatures (temperatures higher than which are damaging) are in red for key developmental stages of wheat and barley that are vulnerable to high temperatures. Insert images show a double ridge apex, terminal spikelet apex, prophase I meiotic cell with DAP1 (blue) and TaASY1 (red), and grain at maturity (from left to right). The respective Zadoks (Z; Zadoks et al., 1974) and Waddington (W; Waddington et al., 1983) scales for each growth stage are indicated.
The average optimum temperature for anthesis in wheat is 21.0 °C, and temperatures higher than 31 °C limit the success of pollination (Porter and Gawith, 1999; Fig. 1). Exposure of wheat and barley to high temperatures around anthesis results in a non-recoverable reduction of yield associated with floret infertility, due to adverse effects on ovary development and pollen viability, and a decreased rate of grain fill (Saini et al., 1983; Stone and Nicolas, 1996; Ferris et al., 1998; Wallwork et al., 1998; Sakata et al., 2000). For example, field-based analyses showed that short periods of high temperature (31.8 °C) immediately before anthesis significantly decreased grain mass, and Australian spring wheat varieties were sensitive to high temperatures (24 °C) within 3 d following anthesis, resulting in smaller grains at maturity (Chowdhury and Wardlaw, 1978; Stone and Nicolas, 1995c; Savin et al., 1999). However, at 8 d post-anthesis, high temperatures had little effect on grain mass or the number of deformed grains (Stone and Nicolas, 1995a, b). Short periods of moderately high temperatures are also detrimental to meiosis, with a treatment of 30 °C for 20–24 h preventing the progression of pollen mother cells through early meiotic stages, reducing pollen viability and grain number (Draeger and Moore, 2017). These outcomes highlight the importance of ongoing research investigating the effect of high temperatures on gamete development and fertilization, particularly because plants are more likely to encounter heat stress during late reproductive stages.
Grain development is another stage of the life cycle that is likely to experience thermal stress (Fig. 1). Exposure to high ambient temperatures during grain filling has an impact on yield by reducing grain number, weight, and quality (Stone and Nicolas, 1994; Wardlaw and Moncur, 1995; Gibson and Paulsen, 1999; Pradhan et al., 2012). The average optimum temperature for grain fill in wheat is 20.7 °C, and temperatures higher than 35.4 °C are damaging (Porter and Gawith, 1999; Shirdelmoghanloo et al., 2016). For example, plants grown in glasshouses and treated to temperatures of 40 °C during grain fill significantly reduced grain quality by decreasing protein accumulation (Stone and Nicolas, 1996). Regarding grain filling, high temperatures can increase assimilate supply; however, the duration of grain filling is reduced without a compensatory increase in the rate of grain fill, resulting in significantly lower yield (Tashiro and Wardlaw, 1989; Wardlaw and Moncur, 1995; Wardlaw et al., 1995; Lobell et al., 2012; Pradhan et al., 2012). The accelerated development of grains at high temperatures is consistent with transcriptome analyses, where treatments at 28, 37, and 42 °C advanced and compressed the expression of genes that regulate metabolic processes of grain development in wheat and barley, relative to control conditions (Altenbach and Kothari, 2004; Wan et al., 2008; Mangelsen et al., 2011).
Taken together, these analyses highlight the importance of investigating a broad range of traits and temperatures when considering the impact of a warming climate on wheat and barley reproduction. These detailed analyses of late reproductive development have set the stage for molecular experiments to now be performed, which aim to understand the genes that coordinate responses to warmer growth conditions.
Interactions between temperature and photoperiod
Photoperiod has an important role in determining the effects of high ambient temperature on reproductive development. For example, an ambient temperature of 30 °C delays spikelet initiation in barley in a photoperiod-dependent manner (Aspinall, 1969). In winter barley and fast-flowering barley lines (containing a mutation in EARLY FLOWERING3, ELF3), an increase in temperature from 15 °C to 25 °C resulted in rapid progression through reproductive development in long days but inhibited early developmental stages when plants were grown under short daylengths (Hemming et al., 2012). In long-day photoperiods, the increase in temperature from 15 °C to 25 °C coincided with the production of more spikes per plant. However, plants produced fewer primordia and florets per inflorescence, resulting in fewer florets per plant at 25 °C. In short days, the increase in temperature from 15 °C to 25 °C resulted in smaller shoot apices and fewer primordia (Hemming et al., 2012). In wheat, high temperatures caused a similar response under short daylengths, with early inflorescence development being delayed in isogenic lines containing photoperiod-insensitive or null alleles of Photoperiod-1 (Ppd-1) and a weak vernalization response (Rawson and Richards, 1993; Hemming et al., 2012). Under long days, high temperatures reduced the duration of time to double ridge and slowed the production of spikelet primordia, but plants flowered earlier at 25 °C than at 15 °C, suggesting that warmer conditions accelerate later developmental stages (Rawson and Richards, 1993). Similar results were found in a collection of spring and winter photoperiod-sensitive wheat lines containing different alleles of Ppd-1 and VERNALIZATION1 (VRN1), with warmer temperatures delaying reproductive development, particularly under short daylengths (Kiss et al., 2017). Together, these observations suggest that high temperatures inhibit reproductive development in non-inductive photoperiods, especially during early developmental stages, but accelerate reproduction under inductive daylengths. The interaction between temperature and photoperiod may indicate a conserved adaptation of temperate cereals to warmer conditions, which is dependent on progression through the season. For example, the interaction may help plants recognize that unseasonably warm temperatures during winter and early spring should be ignored as cues to flower, while warmer conditions during late spring and early summer signal that more hot and dry conditions are likely, promoting fertilization and grain fill before the onset of more extreme conditions.
Effects of chronic and acute high-temperature treatments on yield components
Climate projections suggest that extreme temperature events will become more intense and more frequent (Meehl et al., 2007; IPCC, 2013). In the field, stress events include short treatments of high temperatures (acute temperature stress) as well as sustained periods of warmer than average temperatures (chronic high-temperature stress). With both rising temperatures and frequency of acute high temperatures identified as significant threats to crop production (Challinor et al., 2005), it is important to consider the impact of both chronic and acute high temperatures for accurate prediction of crop performance under climate change scenarios (Barlow et al., 2015).
In wheat, the effects of acute and chronic warm temperatures are particularly significant during reproductive development, with treatments during early and late stages influencing various components of yield. For example, chronic exposure to warmer temperatures (2.6–5.4 °C above ambient) during early reproductive development (double ridge and terminal spikelet stages) of field-grown winter wheat decreased yield by reducing the number of grain-producing spikelets formed per inflorescence—the effect on reduced grain numbers was partially compensated by an increase in grain weight (Johnson and Kanemasu, 1983). Exposure to short or extended periods of high temperature (30 °C) later in development—including stages up to immediately prior to anthesis—dramatically reduced grain numbers and grain weight (Saini and Aspinall, 1982; Ugarte et al., 2007). Acute exposure to extremely high temperatures (40 °C) 2 weeks after anthesis and prolonged treatment of moderately high temperatures (21, 27, or 30 °C) 3 weeks after anthesis both led to a reduction in yield by decreasing grain weight (Stone et al., 1995). Four day treatments at 40 °C and prolonged exposure to 27 or 30 °C significantly reduced grain weight relative to control conditions; each 1 °C rise perturbed grain weight by 2.5% (Stone et al., 1995). In comparison, identical treatments during late developmental stages reduced grain weight without influencing grain number (Johnson and Kanemasu, 1983), and exposure to short periods of high temperature (30 °C for 3 d before anthesis) resulted in reduced grain set without a compensatory increase in grain weight (Saini and Aspinall, 1982). Taken together, these studies show that the effects of acute treatment of high temperatures and prolonged chronic stress under moderate heat merit equal investigation when studying the effects of warmer conditions on cereal productivity (Balla et al., 2019).
Heat acclimation has been identified as a possible strategy to mitigate yield losses of wheat caused by high-temperature stress (Stone and Nicolas, 1995b). One study investigated whether a sudden rise in temperature (20 °C to 40 °C) resulted in a more significant reduction in individual kernel mass than a gradual rise over the same temperature range (6 °C h–1), using wheat cultivars differing in heat tolerance. For the heat-sensitive genotype, the reduction of individual grain mass following sudden heat stress was higher than that resulting from gradual heat stress. On the other hand, for the heat-tolerant genotype, there was no significant difference in individual kernel mass of plants treated abruptly versus those warmed more gradually (Stone and Nicolas, 1995b). These results support the investigation of both chronic and acute heat stress on reproductive development, especially when considering cultivar-specific responses and the different ways plants are exposed to high temperatures in the mega-environments where wheat and barley are grown (Sonder, 2016).
High night-time temperatures
Climate models predict more substantial increases in night-time temperatures compared with daytime temperatures. In the past 100 years, global daily minimum (night-time) temperatures increased >2-fold compared with increases in daily maximum (daytime) temperatures (Easterling et al., 1997). Critical developmental stages are differentially sensitive to minimum and maximum temperatures, and historical yields of wheat are more strongly correlated with daily minimum temperatures compared with maximum temperatures (Lobell and Ortiz-Monasterio, 2007). Researchers and breeders should, therefore, consider the responses of wheat and barley to both increasing daytime and night-time temperatures in the pursuit of generating thermally resilient cultivars.
In wheat grown under controlled-environment conditions, exposure to high night-time temperature (HN) of ≥20 °C and constant daytime temperature (24 °C) decreased spikelet fertility, grains per spike, and grain size, compared with the control (14 °C night-time temperature; Prasad et al., 2008b). Furthermore, exposure to HN decreased the duration of grain filling (Prasad et al., 2008b), and each 1 °C increase in night temperatures reduced yield of winter wheat by 6%, and 4–7% in spring wheat and barley (García et al., 2015; Hein et al., 2019). Similar observations were made in the field using barley and wheat grown under ambient daytime temperatures and HN; HN reduced grain yields, which was associated with accelerated reproductive development and a shorter critical period for grain filling (García et al., 2015). The molecular processes that influence the response to HN are unknown; however, the effect of HN may be linked with decreases in the amount of photoassimilates available for plant growth and grain fill, caused by higher respiration at night (Garcia et al., 2016; Impa et al., 2019; Sadok and Jagadish, 2020). Similarly, it has been proposed that the HN treatment may depress photosynthesis-dependent processes, accelerating leaf senescence and reducing the effective period of light interception by the plant, which would reduce photoassimilate distribution to the grain (Shirdelmoghanloo et al., 2016; Lesjak and Calderini, 2017; Sadok and Jagadish, 2020). Dissection of the molecular responses that occur under warm night-time temperatures will therefore benefit from a multidisciplinary approach that includes physiology, developmental biology, and modelling.
Studies of winter wheat have investigated differences between exposure to HN, high daytime (HD), and both high daytime and night-time temperatures (HDN). One study suggested that the impact of HN and HD was similar on all reproductive traits; for example, both HN and HD during anthesis caused damage of comparable magnitude. However, more substantial decreases in seed set, grain number, and grain yield per inflorescence were observed at HDN, compared with either HD or HN (Narayanan et al., 2015). In contrast, a study under field conditions suggested that there was no significant difference in yield and biomass between plants exposed to HN versus HDN (Fang et al., 2015). The outcomes of these studies highlight the importance of considering high minimum as well as maximum temperatures in assessing the response of wheat and barley to a warming climate, particularly during late reproductive stages.
Responses of other cereals to high temperatures
In contrast to temperate cereals (wheat, barley, oat, and rye), tropical crops such as corn, rice, and sorghum are short-day plants that are often cultivated in warmer climates. Data describing responses of maize and rice to high temperatures, particularly during later developmental stages, may be used to improve thermal resilience of wheat and barley (Caddel and Weibel, 1972; Russell and Stuber, 1983; Izawa et al., 2000; Leff et al., 2004).
Studies comparing the effects of temperature on reproductive development between wheat and other cereals have shown that the optimum growth temperatures for corn, rice, and sorghum are higher than for wheat. For example, one study showed a significant difference between wheat (15/10 °C) and sorghum (27/22 °C) in optimum temperatures for kernel dry weight (Machado and Paulsen, 2001). Similar to wheat and barley, corn, rice, and sorghum are sensitive to high-temperature treatments—particularly during reproductive development, anthesis, and grain fill—resulting in decreased grain yield (Prasad et al., 2006, 2008a; Sánchez et al., 2014; Hatfield and Prueger, 2015). Nevertheless, corn, rice, and sorghum are more tolerant than wheat to high-temperature exposure. For example, although high-temperature conditions after anthesis in both wheat and rice result in smaller grains at maturity, grain size is much more stable at high temperatures in rice than in wheat (Chowdhury and Wardlaw, 1978; Tashiro and Wardlaw, 1989). Furthermore, a comparison of published studies suggests that around anthesis, wheat is sensitive to a lower maximum temperature (32 °C) than both maize and rice (37 °C; Sánchez et al., 2014). Conversely, maize has a shorter phase of anthesis (3–5 d) than wheat, rice, and sorghum (>1 week), which may reduce relative thermotolerance. A longer duration of anthesis reduces the likelihood of a single occurrence of an extreme event affecting all of the flowers (Hatfield and Prueger, 2015). Thus, the duration of anthesis must be considered in the context of projected climate models that predict increases in the incidences of acute high temperatures, and an extended period of anthesis could improve the fertility of wheat under warmer conditions (Lobell et al., 2011).
Taken together, these results suggest that there is potential to transfer knowledge about thermal response mechanisms from maize, rice, and sorghum into wheat and barley to improve their thermotolerance. This approach could be complemented by examining genetic variability for temperature responses among wild barley or wheat (e.g. Hordeum spontaneum and Aegilops species) that have adapted to warmer growth conditions, which could be utilized in breeding thermotolerant cultivated barley and wheat (Pradhan et al., 2012).
Molecular mechanisms influencing responses to temperature
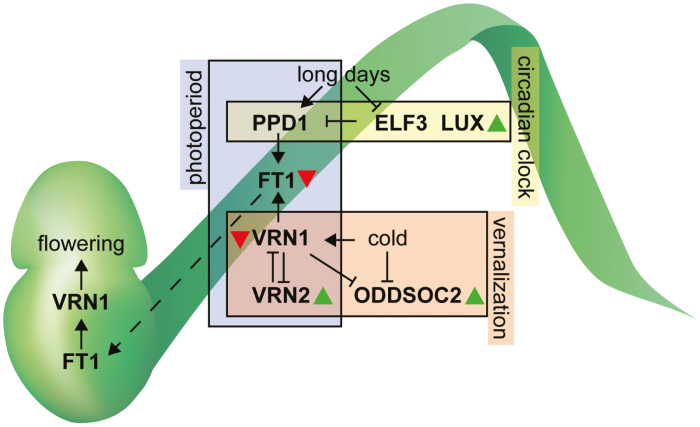
It is essential to understand the molecular mechanisms controlling developmental responses to temperature in order to select or generate varieties with improved tolerance to a warming climate. Many genes involved in the control of developmental responses to photoperiod and vernalization have been identified (Fig. 2; Fjellheim et al., 2014). Flowering in wheat and barley is promoted by long-day photoperiods, with transcriptional activation of FLOWERING LOCUS T1 (FT1) being a central driver of vegetative to floral transition (Yan et al., 2006; Hemming et al., 2008; Lv et al., 2014; Dixon et al., 2018). ELF3 suppresses flowering under non-inductive photoperiods by indirectly repressing FT1 expression and gibberellic acid (GA) production, which may occur via the modified activity of Ppd-1 (Faure et al., 2012; Boden et al., 2014; Gao et al., 2019, Preprint). Ppd-1 is a pseudo-response regulator that acts downstream of ELF3 to promote the expression of FT1; in wheat, photoperiod-insensitive alleles promote early flowering by misregulating diurnal expression of Ppd-1, by either modifying cis-regulatory regions or increasing gene copy number, while photoperiod responsiveness is modified in barley by missense alleles of Ppd-1 that delay flowering (Turner et al., 2005; Beales et al., 2007; Díaz et al., 2012; Shaw et al., 2013; Boden et al., 2015). FT1 protein is expressed in the leaf and translocated to the shoot apical meristem where it interacts with FLOWERING LOCUS D-LIKE (FDL) and 14-3-3 proteins to form the floral activating complex, which induces expression of meristem identity genes, such as the MADS-box transcription factor gene, VRN1 (Li and Dubcovsky, 2008; Li et al., 2015).
Fig. 2.
Thermally responsive components of the floral promoting pathway in wheat and barley. Schematic illustrating the interactions between FT1, VRN1, VRN2, ODDSOC2, PPD1, ELF3, and LUX, with their reported interactions with high growth temperatures. Boxes indicate genes that are photoperiod dependent, circadian clock regulated, and vernalization responsive. Green arrows indicate up-regulated genes and red arrows indicate down-regulated genes during exposure to high temperatures.
VRN1 and VERNALIZATION2 (VRN2) are key regulators of the vernalization pathway that activate and repress flowering, respectively. Winter and spring growth types are underpinned by natural variation in VRN1 and VRN2 (Danyluk et al., 2003; Trevaskis et al., 2003, 2006; Yan et al., 2003, 2004). During cold temperatures and short daylengths (winter growth types), VRN2 expression levels drop, reducing VRN2-mediated repression on VRN1, which induces expression of VRN1 and FT1 to promote flowering (Yan et al., 2006; Hemming et al., 2008; Li and Dubcovsky, 2008). Spring wheat and barley cultivars contain alleles of VRN1 that have insertions/deletions in the first intron or mutations in cis-regulatory regions, which activate VRN1 expression in the absence of cold treatment (Danyluk et al., 2003; Trevaskis et al., 2003; Yan et al., 2003). The deletion of VRN2 also promotes a spring growth habit (Yan et al., 2004; Dubcovsky et al., 2005; Trevaskis et al., 2006). Despite extensive characterization of the mechanisms underpinning low temperature- and photoperiod-dependent reproductive development, insight into the genetic basis of the high temperature responses is more limited.
In the model plant Arabidopsis thaliana, the accelerated flowering that occurs under warmer temperatures is underpinned by increased expression of FT—the orthologue of wheat and barley FT1 (Halliday et al., 2003; Balasubramanian et al., 2006). In contrast, analyses in barley have shown that high ambient temperatures repress FT1 expression, with the absolute transcript levels being dependent on the allelic variation for ELF3 or Ppd-1, or, alternatively, warmer conditions do not influence FT1 transcript levels (Hemming et al., 2012; Ejaz and von Korff, 2017). Similarly, in spring wheat, warmer ambient temperatures either reduce expression of FT1 or do not significantly affect FT1 transcript levels (Kiss et al., 2017; Dixon et al., 2018). Genetic experiments further support FT1-independent temperature responsiveness of flowering, with ft-b1 mutants flowering faster at 24 °C than at 20 °C, as does the wild type (Dixon et al., 2018; Finnegan et al., 2018). Moreover, high FT1 transcript levels in lines containing daylength-insensitive alleles of Ppd-1 do not induce rapid early reproductive development at high temperatures in short days (Hemming et al., 2012). In summary, these results suggest that genes or pathways other than the FT1-dependent regulation of flowering mediate developmental responses to high temperature in cereals.
In contrast to the photoperiod-dependent pathway, genes involved in vernalization interact strongly with temperature to control flowering time (Kiss et al., 2017; Dixon et al., 2019; Fig. 2). Delayed flowering of winter wheat following exposure to warm temperatures during and after cold treatment genetically maps to VRN1 (VRN-A1; Dixon et al., 2019). Growth at warmer temperatures leads to increased expression of VRN2 and a MADS-box transcription factor gene that represses flowering, known as ODDSOC2 (Kiss et al., 2017; Dixon et al., 2019; Fig. 2). The increased expression of VRN2 is associated with reduced levels of VRN1 and FT1 transcripts at warmer temperatures, relative to moderate and cool temperatures (Kiss et al., 2017; Dixon et al., 2019). Genetic analysis showed that allelic variation of VRN1 disrupts the balance of expression for activators (e.g. FT1) and repressors (e.g. VRN2 and ODDSOC2) of flowering in plants grown at 25 °C, relative to 11 °C, with reduced activity of FT1 delaying flowering and increasing spikelet number when the vernalization requirement has not been satisfied (Greenup et al., 2010; Dixon et al., 2019). Allelic variation for VRN1 also influences the expression of floral promoting factors in barley, with high-temperature treatments restricting FT1 expression more strongly in winter lines, relative to spring types, which was associated with stronger repression of VRN1 at high temperatures in the winter isogenic lines (Ejaz and von Korff, 2017). Similarly, warm growth temperatures inhibit reproductive development under short days but accelerate flowering under long days—the delay under short days coincides with the up-regulation of ODDSOC2 (Hemming et al., 2012). Taken together, the results highlight a key role for the vernalization pathway in coordinating the developmental response of wheat and barley to growth under warm temperatures.
High temperatures alter the expression of core circadian clock genes, including those for which allelic variation regulates flowering time (Ford et al., 2016; Ejaz and von Korff, 2017; Fig. 2). In barley, the high temperature-dependent increase in transcripts of circadian clock genes GIGANTEA (GI), LUX ARRHYTHMO (LUX), and PSEUDO RESPONSE REGULATOR (PRR) is dependent on ELF3, suggesting that ELF3 may be an essential regulator of photoperiod-dependent flowering during high temperatures (Ford et al., 2016). Under high ambient temperatures, a late-flowering mutant allele of Ppd-1 delayed floral development and reduced the number of florets and seeds per inflorescence in spring barley. In contrast, floral development occurred earlier, and seed numbers were maintained under high ambient temperatures in both wild-type Ppd-1 and elf3 mutants (Ejaz and von Korff, 2017). In wheat, allelic variation for ELF3 is likely to underpin an Earliness per se locus (Eps-D1), which interacts with temperature to regulate flowering time, spikelet number, and floret fertility (Lewis et al., 2008; Prieto et al., 2018; Ochagavía et al., 2019). Early-flowering alleles of Eps-D1 accelerate flowering, reduce spikelet number, and increase floret fertility, particularly when grown at cool temperatures (9–12 °C), while late alleles increase floret fertility at warmer temperatures (18 °C) (Lewis et al., 2008; Prieto et al., 2018; Ochagavía et al., 2019). The variable response of the Eps-D1 alleles at lower temperatures is potentially dependent on differences in ELF3 expression, which is significantly different between early and late alleles at 12 °C, but not 18 °C (Ochagavía et al., 2019). These observations suggest that high temperature affects inflorescence development, flowering time, and floret fertility in an ELF3- and Ppd-1-dependent manner. Given that genetic variation for Ppd-1 and ELF3 influences expression of these genes during the evening, it will be interesting to investigate the interaction between early flowering alleles of Ppd-1 and ELF3 with high night-time temperatures (Beales et al., 2007; Alvarez et al., 2016).
High temperatures also influence RNA synthesis and protein translation. For example, in the model temperate grass Brachypodium distachyon, H2A.Z-nucleosomes play a role in mediating the effects of increased temperature on gene transcription (Boden et al., 2013). H2A.Z-nucleosomes locate to transcription start sites that gate access of the transcriptional machinery into the gene body in a temperature-dependent manner (Raisner et al., 2005; Zhang et al., 2005; Kumar and Wigge, 2010). While H2A.Z levels do not fluctuate in chromatin of leaves as temperature increases, occupancy decreases with rising temperatures in chromatin of developing grain (Boden et al., 2013). The reduced grain weight caused by high temperatures was replicated by genetically perturbing H2A.Z occupancy in chromatin; these results demonstrate a potential mechanism for the reduced grain weight that occurs in wheat and barley under warmer temperatures (Boden et al., 2013).
Regarding translation, protein synthesis elongation factor Tu (EF-Tu) plays a central role in the elongation phase of protein production (Fu et al., 2008). In wheat, high temperatures increase the accumulation of chloroplast EF-Tu, and cultivars expressing greater EF-Tu under high-temperature stress display increased thermotolerance (Prasad et al., 2008b; Ristic et al., 2008). Following exposure to high-temperature stress, EF-Tu exhibits chaperone activity, and heterologous expression of EF-Tu decreases the thermal aggregation of leaf proteins and improves heat tolerance (Fu et al., 2008; Prasad et al., 2008b). High night-time temperatures increase the expression of EF-Tu in wheat, and EF-Tu has been implicated in circadian-regulated plant innate immunity in Arabidopsis (Prasad et al., 2008b; Bhardwaj et al., 2011; Wang et al., 2011). Taken together, these reports suggest that high temperature-dependent accumulation of EF-Tu is important for plant tolerance under thermal stress conditions, potentially in a photoperiod-dependent manner (Fu et al., 2008; Prasad et al., 2008b).
Future directions
Utilizing genetic diversity and gene editing techniques in cereals
The ability to map genetic traits in wheat and barley has improved dramatically in recent years following the assembly of reference genome sequences for several hexaploid wheat cultivars, tetraploid wheat, diploid progenitor species, and barley (Jia et al., 2013; Krasileva et al., 2017; Luo et al., 2017; Mascher et al., 2017; IWGSC, 2018; Ling et al., 2018). Together with the generation of sequenced mutant and mapping populations, we are now able to identify genes underpinning responses of wheat and barley to higher ambient temperatures (Krasileva et al., 2017; Boden and Østergaard, 2019; Adamski et al., 2020). For example, it is now possible to perform genetic screens using mutant populations to identify genes that confer improved thermal resilience—these screens could take advantage of our deep understanding of traits and developmental stages affected by both chronic and acute heat treatments. Luciferase-based thermal reporters and hyperspectral imaging could help detect genotypes from large populations with modified thermal responsiveness at different developmental stages—this approach has been used productively in Arabidopsis to identify genes that underpin developmental responses to high-temperature treatment (Kumar and Wigge, 2010). These screens could be complemented by an investigation of exotic germplasm grown under semi-arid versus elite conditions, to identify alleles used by landraces or wild progenitors to adapt to warmer conditions. This concept is supported by the D genome progenitor of wheat, Aegilops tauschii, which contains more copies of genes involved in abiotic stress and thermal regulation than other major cereals (Jia et al., 2013). Alternatively, small numbers of the crucial domestication alleles could be introduced into the backgrounds of temperature-resilient wild relatives of wheat and barley: a process termed ‘de novo domestication’ (Zsögön et al., 2018; Langridge and Waugh, 2019). Such analyses in barley and the diploid progenitors of wheat could help overcome the genetic redundancy that exists in tetraploid and hexaploid wheat, which masks the potential benefit of recessive alleles conferring improved temperature resilience. Alleles identified to improve thermal tolerance could be rapidly introduced into modern cultivars using gene editing such as CRISPR/Cas9 [clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9] to produce transgene-free genome-edited plants or through use of ‘speed breeding’ (Zhu et al., 2017; Watson et al., 2018).
Knowledge gained from Arabidopsis could improve our understanding of responses to high temperature in wheat and barley
While our ability to identify genes involved in temperature responses of cereals is improving, there is potential to use the knowledge gained in Arabidopsis to advance our understanding of the molecular pathways that respond to growth under warmer conditions. For example, research has identified multiple genes that contribute to the accelerated flowering of Arabidopsis under chronic warm ambient temperature treatments (e.g. 27 °C versus 17 °C), including PHYTOCHROME INTERACTING FACTOR4 (PIF4), FLOWERING LOCUS M (FLM), and SHORT VEGETATIVE PHASE (SVP) (Balasubramanian et al., 2006; Lee et al., 2007; Kumar et al., 2012). PIF4 expression increases as temperatures rise, and the encoded transcription factor binds to the promoter of FT to activate flowering (Kumar et al., 2012). Conversely, SVP interacts with an isoform of FLM (FLM-β) and MADS AFFECTING FLOWERING2 (MAF2) to suppress the expression of floral integrators, including FT, SUPPRESSOR OF CONSTANS (SOC1), TEMPRANILLO2 (TEM2), and Arabidopsis thaliana CENTRORADIALIS homologue (ATC) (Lee et al., 2007; Posé et al., 2013; Airoldi et al., 2015). High temperatures increase SVP protein degradation, reduce the production of FLM-β, and lower the abundance of the MAF2 isoform that can interact with SVP. Consequently, decreased levels of the SVP:FLM and SVP:MAF2 floral repressor complexes result in the promotion of flowering (Lee et al., 2013; Airoldi et al., 2015). Taken together, these studies highlight the potential that the phytochrome pathway or MADS-box transcription factors that control floral development may be involved in mechanisms regulating high-temperature responses in wheat and barley—indeed, ODDSOC2 is a MAF-like transcription factor that is more highly expressed under warmer temperatures to repress flowering (Hemming et al., 2012; Dixon et al., 2019). Whether these genes perform conserved roles in cereals remains to be seen; however, the identification of similar mechanisms that either activate or repress flowering would be useful in a breeding context for developing alleles that can fine-tune flowering responses under warmer ambient temperatures. These examples of molecular mechanisms controlling thermal responses in Arabidopsis have focused on the vegetative to floral transition—it would benefit wheat and barley research if future studies using models focused on late developmental stages in order to understand traits that are particularly vulnerable to thermal stress in cereals.
In Arabidopsis and Brachypodium, expression of heat shock protein 70 (HSP70) increases linearly with rising temperatures, identifying the HSP70 promoter as a potential ‘molecular thermometer’ (Kumar and Wigge, 2010; Boden et al., 2013). Lines containing a fusion of the HSP70 promoter to luciferase (HSP::LUC) have been used in forward genetic screens to identify mutations with altered responses to high temperature (Kumar and Wigge, 2010). Improved transformation capabilities of wheat and barley mean that such strategies could be used to identify genes involved in thermal responses in cereals, including those involved in acute and chronic high-temperature stress. An outcome of this approach in Arabidopsis includes the identification of H2A.Z as an important regulator of the temperature transcriptome in plants (Kumar and Wigge, 2010), suggesting that chromatin-based mechanisms may underpin thermal responses in temperate cereals. The results of this research have been transferred to grasses through work in Brachypodium (Boden et al., 2013); however, the potential for using this mechanism to improve thermal resilience in wheat and barley remains unexplored.
Modification of developmental and physical traits to enhance thermal resistance
As cereals are most likely to encounter temperature stress during their vulnerable late reproductive stages, modifying the development or thermal resilience of reproductive organs will be vital for maintaining wheat and barley yields under warmer temperatures. For example, rice is relatively tolerant of high temperatures, in part because the panicle architecture helps cool spikelets at the base of the inflorescence relative to those at the apex (Fu et al., 2016). Given that rice forms the majority of grain-producing spikelets at the base of the panicle, the overall yield penalty caused by higher temperatures is relatively low compared with wheat and barley, which produce most of their grain at the centre of the inflorescence. The spike architecture of a wheat and barley inflorescence could be modified to form a panicle-like structure with more spikelets at the base. This arrangement of spikelets may help reduce the adverse effects of warmer temperatures on grain development, which occurs following the emergence of the mature inflorescence. Several genes that regulate inflorescence architecture have been identified in wheat; for example, loss-of-function mutations in the FRIZZY PANICLE (FZP) gene are associated with an increase of lateral floral meristems, particularly at the base of the inflorescence (Dobrovolskaya et al., 2015; Poursarebani et al., 2015). Further investigation of genes that regulate spikelet arrangements could be used to engineer wheat and barley inflorescences with a higher proportion of florets/spikelets at the base, which may improve the fertility of florets in plants grown at higher temperatures.
Pollen is another reproductive tissue that is sensitive to high-temperature events (Hucl, 1996; Lawrie et al., 2006). Methods that extend the duration of pollination or increase pollen viability could be used to improve the resilience of wheat and barley to warm temperatures. For example, floral development in wheat consists of the primary and secondary openings of the flower, and fully or partially male-sterile lines have a prolonged second opening, which increases the opportunity for cross-pollination (Demotes-Mainard et al., 1996; Okada et al., 2018). Genotypes that produce florets with an extended period of second opening may help buffer the high temperature-dependent loss of viability so that pollen from a neighbouring floret can complete fertilization (Lukac et al., 2012). Alternatively, alleles that improve pollen viability could help enhance fertilization in plants grown at high temperatures. HSPs are involved in heat acclimation and acquired thermal tolerance of developing pollen (Rieu et al., 2017); however, several typical HSPs accumulate less in developing pollen than in vegetative tissue, which may result in increased protein unfolding (Müller and Rieu, 2016). The introduction of HSP alleles with increased expression in pollen may help improve resilience to high-temperature treatments.
The meiotic recombination gene, DMC1, has recently been identified as a candidate for high-temperature tolerance during wheat meiosis. Loss of DMC1 is associated with reduced synapsis and crossovers at 30 °C relative to 20 °C, indicating that DMC1 is required to maintain genome integrity under warmer growth conditions (Draeger et al., 2020). Targeting alleles that counterbalance the adverse effects of high temperatures on grain development could also be a novel strategy for maintaining yield in a warmer climate. Exposure to high temperatures during grain fill results in smaller grains at maturity (Chowdhury and Wardlaw, 1978; Stone and Nicolas, 1995c). Introducing alleles, such as the GW2 locus, that produce larger grains may partially mitigate the negative effect of high temperature on grain size (Simmonds et al., 2016).
Conclusions
The devastating consequences that warmer temperatures have on cereal yields provides a considerable driver for understanding the mechanisms controlling temperature perception in wheat and barley. The complicated relationship between acute versus constant high-temperature exposure, developmental stage, and time of day at which exposure occurs suggests that the investigation of temperature responses in these cereals will be a fruitful area for fundamental and applied research. Several genes influencing responses to temperature during development have been characterized; however, we have barely scratched the surface of understanding the molecular responses of wheat and barley to warming temperatures. Encouragingly, the emergence of diverse sequenced genomes and gene editing techniques provides hope for rapid improvement in thermal resilience of wheat and barley. This work will be supported by extensive understanding of the physiological response of wheat and barley to warmer temperatures, including the effects of heat on photosynthesis and respiration, and a strong understanding of the molecular mechanisms underpinning similar responses in model organisms.
Acknowledgements
We acknowledge the BBSRC (BB/P016855/1), the Royal Society (UF150081), and Waite Research Institute for funding that supported the authors’ research. We apologize to authors of papers which were not cited herein due to space constraints, and we acknowledge the reviewers for the valuable comments that improved the manuscript.
References
- Adamski NM, Borrill P, Brinton J, Harrington SA, Marchal C, Bentley AR, Bovill WD, Cattivelli L, Cockram J, Contreras-Moreira B. 2020. A roadmap for gene functional characterisation in crops with large genomes: lessons from polyploid wheat. eLife 9, e55646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi CA, McKay M, Davies B. 2015. MAF2 is regulated by temperature-dependent splicing and represses flowering at low temperatures in parallel with FLM. PLoS One 10, e0126516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach SB, Kothari KM. 2004. Transcript profiles of genes expressed in endosperm tissue are altered by high temperature during wheat grain development. Journal of Cereal Science 40, 115–126. [Google Scholar]
- Alvarez MA, Tranquilli G, Lewis S, Kippes N, Dubcovsky J. 2016. Genetic and physical mapping of the earliness per se locus Eps-A (m) 1 in Triticum monococcum identifies EARLY FLOWERING 3 (ELF3) as a candidate gene. Functional & Integrative Genomics 16, 365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall D. 1969. The effects of day length and light intensity on tile growth of barley. Australian Journal of Biological Sciences 22, 53–68. [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. 2006. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genetics 2, e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla K, Karsai I, Bónis P, Kiss T, Berki Z, Horváth Á, Mayer M, Bencze S, Veisz O. 2019. Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS One 14, e0222639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow KM, Christy BP, O’leary GJ, Riffkin PA, Nuttall JG. 2015. Simulating the impact of extreme heat and frost events on wheat crop production: a review. Field Crops Research 171, 109–119. [Google Scholar]
- Battisti DS, Naylor RL. 2009. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323, 240–244. [DOI] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. 2007. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theoretical and Applied Genetics 115, 721–733. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V, Meier S, Petersen LN, Ingle RA, Roden LC. 2011. Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS One 6, e26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden SA, Cavanagh C, Cullis BR, Ramm K, Greenwood J, Jean Finnegan E, Trevaskis B, Swain SM. 2015. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nature Plants 1, 14016. [DOI] [PubMed] [Google Scholar]
- Boden SA, Kavanová M, Finnegan EJ, Wigge PA. 2013. Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome Biology 14, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden SA, Østergaard L. 2019. How can developmental biology help feed a growing population? Development 146, dev172965. [DOI] [PubMed] [Google Scholar]
- Boden SA, Weiss D, Ross JJ, Davies NW, Trevaskis B, Chandler PM, Swain SM. 2014. EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. The Plant Cell 26, 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddel JL, Weibel DE. 1972. Photoperiodism in sorghum. Agronomy Journal 64, 473–476. [Google Scholar]
- Calderini DF, Abeledo LG, Savin R and Slafer GA. 1999. Final grain weight in wheat as affected by short periods of high temperature during pre- and post-anthesis under field conditions. Australian Journal of Plant Physiology 26, 453–458. [Google Scholar]
- Challinor AJ, Wheeler TR, Craufurd PQ, Slingo JM. 2005. Simulation of the impact of high temperature stress on annual crop yields. Agricultural and Forest Meteorology 135, 180–189. [Google Scholar]
- Chowdhury SI, Wardlaw IF. 1978. The effect of temperature on kernel development in cereals. Australian Journal of Agricultural Research 29, 205–223. [Google Scholar]
- Cockram J, Jones H, Leigh FJ, O’Sullivan D, Powell W, Laurie DA, Greenland AJ. 2007. Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. Journal of Experimental Botany 58, 1231–1244. [DOI] [PubMed] [Google Scholar]
- Craufurd PQ, Wheeler TR. 2009. Climate change and the flowering time of annual crops. Journal of Experimental Botany 60, 2529–2539. [DOI] [PubMed] [Google Scholar]
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F. 2003. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiology 132, 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demotes-Mainard S, Doussinault G, Meynard JM. 1996. Abnormalities in the male developmental programme of winter wheat induced by climatic stress at meiosis. Agronomie 16, 505–515. [Google Scholar]
- Díaz A, Zikhali M, Turner AS, Isaac P, Laurie DA. 2012. Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7, e33234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Farré A, Finnegan EJ, Orford S, Griffiths S, Boden SA. 2018. Developmental responses of bread wheat to changes in ambient temperature following deletion of a locus that includes FLOWERING LOCUS T1. Plant, Cell & Environment 41, 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Karsai I, Kiss T, Adamski NM, Liu Z, Ding Y, Allard V, Boden SA, Griffiths S. 2019. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development 146, dev172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaya O, Pont C, Sibout R, et al. 2015. FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiology 167, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger T, Martin AC, Alabdullah AK, Pendle A, Rey MD, Shaw P, Moore G. 2020. Dmc1 is a candidate for temperature tolerance during wheat meiosis. Theoretical and Applied Genetics 133, 809–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger T, Moore G. 2017. Short periods of high temperature during meiosis prevent normal meiotic progression and reduce grain number in hexaploid wheat (Triticum aestivum L.). Theoretical and Applied Genetics 130, 1785–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Chen C, Yan L. 2005. Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Molecular Breeding 15, 395–407. [Google Scholar]
- Easterling DR, Horton B, Jones PD, Peterson TC, Karl TR, Parker DE, Salinger MJ, Razuvayev V, Plummer N, Jamason P. 1997. Maximum and minimum temperature trends for the globe. Science 277, 364–367. [Google Scholar]
- Ejaz M, von Korff M. 2017. The genetic control of reproductive development under high ambient temperature. Plant Physiology 173, 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Cammarano D, Zhou G, Tan K, Ren S. 2015. Effects of increased day and night temperature with supplemental infrared heating on winter wheat growth in North China. European Journal of Agronomy 64, 67–77. [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA. 2012. Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proceedings of the National Academy of Sciences, USA 109, 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris R, Ellis RH, Wheeler TR, Hadley P. 1998. Effect of high temperature stress at anthesis on grain yield and biomass of field-grown crops of wheat. Annals of Botany 82, 631–639. [Google Scholar]
- Finnegan EJ, Ford B, Wallace X, Pettolino F, Griffin PT, Schmitz RJ, Zhang P, Barrero JM, Hayden MJ, Boden SA. 2018. Zebularine treatment is associated with deletion of FT-B1 leading to an increase in spikelet number in bread wheat. Plant, Cell & Environment 41, 1346–1360. [DOI] [PubMed] [Google Scholar]
- Fjellheim S, Boden S, Trevaskis B. 2014. The role of seasonal flowering responses in adaptation of grasses to temperate climates. Frontiers in Plant Science 5, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B, Deng W, Clausen J, Oliver S, Boden S, Hemming M, Trevaskis B. 2016. Barley (Hordeum vulgare) circadian clock genes can respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner. Journal of Experimental Botany 67, 5517–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Feng B, Zhang C, Yang Y, Yang X, Chen T, Zhao X, Zhang X, Jin Q, Tao L. 2016. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Frontiers in Plant Science 7, 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Momcilović I, Clemente TE, Nersesian N, Trick HN, Ristic Z. 2008. Heterologous expression of a plastid EF-Tu reduces protein thermal aggregation and enhances CO2 fixation in wheat (Triticum aestivum) following heat stress. Plant Molecular Biology 68, 277–288. [DOI] [PubMed] [Google Scholar]
- Gao M, Geng F, Klose C, et al. 2019. Phytochromes measure photoperiod in Brachypodium. bioRvix doi.10.1101/697169. [Preprint]. [Google Scholar]
- García GA, Dreccer MF, Miralles DJ, Serrago RA. 2015. High night temperatures during grain number determination reduce wheat and barley grain yield: a field study. Global Change Biology 21, 4153–4164. [DOI] [PubMed] [Google Scholar]
- Garcia G, Serrago R, Dreccer F, Miralles DJ. 2016. Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. Field Crops Research 195, 50–59. [Google Scholar]
- Gibson LR, Paulsen GM. 1999. Yield components of wheat grown under high temperature stress during reproductive growth. Crop Science 39, 1841–1846. [Google Scholar]
- Gol L, Tomé F, von Korff M. 2017. Floral transitions in wheat and barley: interactions between photoperiod, abiotic stresses, and nutrient status. Journal of Experimental Botany 68, 1399–1410. [DOI] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Talbot MJ, Dennis ES, Hemming MN, Trevaskis B. 2010. ODDSOC2 is a MADS box floral repressor that is down-regulated by vernalization in temperate cereals. Plant Physiology 153, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. 2003. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal 33, 875–885. [DOI] [PubMed] [Google Scholar]
- Hatfield JL, Prueger JH. 2015. Temperature extremes: effect on plant growth and development. Weather and Climate Extremes 10, 4–10. [Google Scholar]
- Hein NT, Wagner D, Bheemanahalli R, Šebela D, Bustamante C, Chiluwal A, Neilsen ML, Jagadish SVK. 2019. Integrating field-based heat tents and cyber-physical system technology to phenotype high night-time temperature impact on winter wheat. Plant Methods 15, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. 2008. Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiology 147, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Walford SA, Fieg S, Dennis ES, Trevaskis B. 2012. Identification of high-temperature-responsive genes in cereals. Plant Physiology 158, 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucl P. 1996. Out-crossing rates for 10 Canadian spring wheat cultivars. Canadian Journal of Plant Science 76, 423–427. [Google Scholar]
- Huijser P, Schmid M. 2011. The control of developmental phase transitions in plants. Development 138, 4117–4129. [DOI] [PubMed] [Google Scholar]
- Impa SM, Sunoj VSJ, Krassovskaya I, Bheemanahalli R, Obata T, Jagadish SVK. 2019. Carbon balance and source–sink metabolic changes in winter wheat exposed to high night-time temperature. Plant, Cell & Environment 42, 1233–1246. [DOI] [PubMed] [Google Scholar]
- IPCC 2013. Summary for policymakers. Climate change 2013: the physical science basis. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: and New York, USA: Cambridge University Press. [Google Scholar]
- IWGSC 2018. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 661, eaar7191. [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Tokutomi S, Okuno K, Shimamoto K. 2000. Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). The Plant Journal 22, 391–399. [DOI] [PubMed] [Google Scholar]
- Jaggard KW, Qi A, Ober ES. 2010. Possible changes to arable crop yields by 2050. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2835–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Zhao S, Kong X, et al. 2013. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496, 91–95. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Kanemasu ET. 1983. Yield and development of winter wheat at elevated temperatures 1. Agronomy Journal 75, 561–565. [Google Scholar]
- Jung C, Müller AE. 2009. Flowering time control and applications in plant breeding. Trends in Plant Science 14, 563–573. [DOI] [PubMed] [Google Scholar]
- Kiss T, Dixon LE, Soltész A, et al. 2017. Effects of ambient temperature in association with photoperiod on phenology and on the expressions of major plant developmental genes in wheat (Triticum aestivum L.). Plant, Cell & Environment 40, 1629–1642. [DOI] [PubMed] [Google Scholar]
- Krasileva KV, Vasquez-Gross HA, Howell T, et al. 2017. Uncovering hidden variation in polyploid wheat. Proceedings of the National Academy of Sciences, USA 114, E913–E921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA. 2012. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. 2010. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147. [DOI] [PubMed] [Google Scholar]
- Langridge P, Waugh R. 2019. Harnessing the potential of germplasm collections. Nature Genetics 51, 200–201. [DOI] [PubMed] [Google Scholar]
- Lawrie RG, Matus-Cádiz MA, Hucl P. 2006. Estimating out-crossing rates in spring wheat cultivars using the contact method. Crop Science 46, 247–249. [Google Scholar]
- Lee JH, Ryu HS, Chung KS, Posé D, Kim S, Schmid M, Ahn JH. 2013. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science 342, 628–632. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. 2007. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes & Development 21, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff B, Ramankutty N, Foley JA. 2004. Geographic distribution of major crops across the world. Global Biogeochemical Cycles 18, GB1009. [Google Scholar]
- Lesjak J, Calderini DF. 2017. Increased night temperature negatively affects grain yield, biomass and grain number in Chilean Quinoa. Frontiers in Plant Science 8, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S, Faricelli ME, Appendino ML, Valárik M, Dubcovsky J. 2008. The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat. Journal of Experimental Botany 59, 3595–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dubcovsky J. 2008. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. The Plant Journal 55, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lin H, Dubcovsky J. 2015. Factorial combinations of protein interactions generate a multiplicity of florigen activation complexes in wheat and barley. The Plant Journal 84, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HQ, Ma B, Shi X, et al. 2018. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 557, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Ortiz-Monasterio JI. 2007. Impacts of day versus night temperatures on spring wheat yields. Agronomy Journal 99, 469–477. [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. 2011. Climate trends and global crop production since 1980. Science 333, 616–620. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Sibley A, Ortiz-Monasterio JI. 2012. Extreme heat effects on wheat senescence in India. Nature Climate Change 2, 186. [Google Scholar]
- Lukac M, Gooding MJ, Griffiths S, Jones HE. 2012. Asynchronous flowering and within-plant flowering diversity in wheat and the implications for crop resilience to heat. Annals of Botany 109, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MC, Gu YQ, Puiu D, et al. 2017. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 551, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, Nitcher R, Han X, Wang S, Ni F, Li K, Pearce S, Wu J, Dubcovsky J, Fu D. 2014. Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS One 9, e94171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado S, Paulsen GM. 2001. Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant and Soil 233, 179–187. [Google Scholar]
- Mangelsen E, Kilian J, Harter K, Jansson C, Wanke D, Sundberg E. 2011. Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Molecular Plant 4, 97–115. [DOI] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, et al. 2017. A chromosome conformation capture ordered sequence of the barley genome. Nature 544, 427–433. [DOI] [PubMed] [Google Scholar]
- McKinney HH. 1940. Vernalization and the growth-phase concept. The Botanical Review 6, 25–47. [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye T, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A. 2007. Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, eds. IPCC, 2007: Climate Change 2007: the physical science basis. contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: and New York, USA: Cambridge University Press, 748–845. [Google Scholar]
- Müller F, Rieu I. 2016. Acclimation to high temperature during pollen development. Plant Reproduction 29, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Prasad PVV, Fritz AK, Boyle DL, Gill BS. 2015. Impact of high night-time and high daytime temperature stress on winter wheat. Journal of Agronomy and Crop Science 201, 206–218. [Google Scholar]
- Ochagavía H, Prieto P, Zikhali M, Griffiths S, Slafer GA. 2019. Earliness per se by temperature interaction on wheat development. Scientific Reports 9, 2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Jayasinghe JEARM, Nansamba M, Baes M, Warner P, Kouidri A, Correia D, Nguyen V, Whitford R, Baumann U. 2018. Unfertilized ovary pushes wheat flower open for cross-pollination. Journal of Experimental Botany 69, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JR, Gawith M. 1999. Temperatures and the growth and development of wheat: a review. European Journal of Agronomy 10, 23–36. [Google Scholar]
- Posé D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RG, Schmid M. 2013. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503, 414–417. [DOI] [PubMed] [Google Scholar]
- Poursarebani N, Seidensticker T, Koppolu R, et al. 2015. The genetic basis of composite spike form in barley and ‘miracle-wheat’. Genetics 201, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan GP, Prasad PVV, Fritz AK, Kirkham MB, Gill BS. 2012. High temperature tolerance in Aegilops species and its potential transfer to wheat. Crop Science 52, 292–304. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH Jr, Sheehy JE, Thomas JMG. 2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research 95, 398–411. [Google Scholar]
- Prasad PVV, Pisipati SR, Mutava RN, Tuinstra MR. 2008a Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Science 48, 1911–1917. [Google Scholar]
- Prasad PVV, Pisipati SR, Ristic Z, Bukovnik U, Fritz AK. 2008b Impact of nighttime temperature on physiology and growth of spring wheat. Crop Science 48, 2372–2380. [Google Scholar]
- Prieto P, Ochagavía H, Savin R, Griffiths S, Slafer GA. 2018. Physiological determinants of fertile floret survival in wheat as affected by earliness per se genes under field conditions. European Journal of Agronomy 99, 206–213. [Google Scholar]
- Purvis ON. 1934. An analysis of the influence of temperature during germination on the subsequent development of certain winter cereals and its relation to the effect of length of day. Annals of Botany 48, 919–957. [Google Scholar]
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD. 2005. Histone variant H2A.Z marks the 5' ends of both active and inactive genes in euchromatin. Cell 123, 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-González RH, Borrill P, Lang D, et al. 2018. The transcriptional landscape of polyploid wheat. Science 361, eaar6089. [DOI] [PubMed] [Google Scholar]
- Rawson HM, Richards RA. 1993. Effects of high temperature and photoperiod on floral development in wheat isolines differing in vernalisation and photoperiod genes. Field Crops Research 32, 181–192. [Google Scholar]
- Rieu I, Twell D, Firon N. 2017. Pollen development at high temperature: from acclimation to collapse. Plant Physiology 173, 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic Z, Bukovnik U, Momcilović I, Fu J, Vara Prasad PV. 2008. Heat-induced accumulation of chloroplast protein synthesis elongation factor, EF-Tu, in winter wheat. Journal of Plant Physiology 165, 192–202. [DOI] [PubMed] [Google Scholar]
- Russell WK, Stuber CW. 1983. Effects of photoperiod and temperatures on the duration of vegetative growth in maize 1. Crop science 23, 847–850. [Google Scholar]
- Sadok W, Jagadish SVK. 2020. The hidden costs of nighttime warming on yields. Trends in Plant Science 25, 644–651. [DOI] [PubMed] [Google Scholar]
- Saini HS, Aspinall D. 1982. Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Annals of Botany 49, 835–846. [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. 1983. Effect of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L.). Functional Plant Biology 10, 137–144. [Google Scholar]
- Sakata T, Takahashi H, Nishiyama I, Higashitani A. 2000. Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. Journal of Plant Research 113, 395–402. [Google Scholar]
- Sánchez B, Rasmussen A, Porter JR. 2014. Temperatures and the growth and development of maize and rice: a review. Global Change Biology 20, 408–417. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Turner AS, Herry L, Griffiths S, Laurie DA. 2013. Mutant alleles of Photoperiod-1 in wheat (Triticum aestivum L.) that confer a late flowering phenotype in long days. PLoS One 8, e79459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirdelmoghanloo H, Cozzolino D, Lohraseb I, Collins NC. 2016. Truncation of grain filling in wheat (Triticum aestivum) triggered by brief heat stress during early grain filling: association with senescence responses and reductions in stem reserves. Functional Plant Biology 43, 919–930. [DOI] [PubMed] [Google Scholar]
- Simmonds J, Scott P, Brinton J, Mestre TC, Bush M, Del Blanco A, Dubcovsky J, Uauy C. 2016. A splice acceptor site mutation in TaGW2-A1 increases thousand grain weight in tetraploid and hexaploid wheat through wider and longer grains. Theoretical and Applied Genetics 129, 1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slafer GA, Rawson HM. 1995. Base and optimum temperatures vary with genotype and stage of development in wheat. Plant, Cell & Environment 18, 671–679. [Google Scholar]
- Sonder K. 2016. Global map of wheat mega-environments. CIMMYT Research Data & Software Repository, version 4.
- Stone PJ, Nicolas ME. 1994. Wheat cultivars vary widely in their responses of grain yield and quality to short periods of post-anthesis heat stress. Functional Plant Biology 21, 887–900. [Google Scholar]
- Stone PJ, Nicolas ME. 1995a A survey of the effects of high temperature during grain filling on yield and quality of 75 wheat cultivars. Australian Journal of Agricultural Research 46, 475–492. [Google Scholar]
- Stone PJ, Nicolas ME. 1995b Comparison of sudden heat stress with gradual exposure to high temperature during grain filling in two wheat varieties differing in heat tolerance. I. Grain growth. Functional Plant Biology 22, 935–944. [Google Scholar]
- Stone PJ, Nicolas ME. 1995c Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Functional Plant Biology 22, 927–934. [Google Scholar]
- Stone PJ, Nicolas ME. 1996. Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. II. Fractional protein accumulation. Functional Plant Biology 23, 739–749. [Google Scholar]
- Stone PJ, Savin R, Wardlaw IF, Nicolas ME. 1995. The influence of recovery temperature on the effects of a brief heat shock on wheat. I. Grain growth. Functional Plant Biology 22, 945–954. [Google Scholar]
- Tashiro T, Wardlaw IF. 1989. A comparison of the effect of high temperature on grain development in wheat and rice. Annals of Botany 64, 59–65. [Google Scholar]
- Trevaskis B. 2010. The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Functional Plant Biology 37, 479–487. [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. 2003. MADS box genes control vernalization-induced flowering in cereals. Proceedings of the National Academy of Sciences, USA 100, 13099–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES. 2006. HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiology 140, 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. 2005. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034. [DOI] [PubMed] [Google Scholar]
- Ugarte C, Calderini DF, Slafer GA. 2007. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Research 100, 240–248. [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. 1983. A quantitative scale of spike initial and pistil development in wheat and barley. Annals of Botany 51, 119–130. [Google Scholar]
- Wallwork MAB, Jenner CF, Logue SJ, Sedgley M. 1998. Effect of high temperature during grain-filling on the structure of developing and malted barley grains. Annals of Botany 82, 587–599. [Google Scholar]
- Wan Y, Poole RL, Huttly AK, Toscano-Underwood C, Feeney K, Welham S, Gooding MJ, Mills C, Edwards KJ, Shewry PR. 2008. Transcriptome analysis of grain development in hexaploid wheat. BMC Genomics 9, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Barnaby JY, Tada Y, Li H, Tör M, Caldelari D, Lee DU, Fu XD, Dong X. 2011. Timing of plant immune responses by a central circadian regulator. Nature 470, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF, Dawson IA, Munibi P. 1989. The tolerance of wheat to high temperatures during reproductive growth. 2. Grain development. Australian Journal of Agricultural Research 40, 15–24. [Google Scholar]
- Wardlaw IF, Moncur L. 1995. The response of wheat to high temperature following anthesis. I. The rate and duration of kernel filling. Functional Plant Biology 22, 391–397. [Google Scholar]
- Wardlaw IF, Moncur L, Patrick JW. 1995. The response of wheat to high temperature following anthesis. II. Sucrose accumulation and metabolism by isolated kernels. Functional Plant Biology 22, 399–407. [Google Scholar]
- Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey M-D, Hatta MAM, Hinchliffe A, Steed A, Reynolds D. 2018. Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants 4, 23–29. [DOI] [PubMed] [Google Scholar]
- White JW, Kimball BA, Wall GW, Ottman MJ, Hunt LA. 2011. Responses of time of anthesis and maturity to sowing dates and infrared warming in spring wheat. Field Crops Research 124, 213–222. [Google Scholar]
- Wollenweber B, Porter JR, Schellberg J. 2003. Lack of interaction between extreme high-temperature events at vegetative and reproductive growth stages in wheat. Journal of Agronomy and Crop Science 189, 142–150. [Google Scholar]
- Worland AJ. 1996. The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 89, 49–57. [Google Scholar]
- Worland AJ, Borner A, Korzun V, Li WM, Petrovic S, Sayers EJ. 1998. The influence of photoperiod genes on the adaptability of European winter wheats. Euphytica 100, 385–394. [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences, USA 103, 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. 2004. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303, 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. 2003. Positional cloning of the wheat vernalization gene VRN1. Proceedings of the National Academy of Sciences, USA 100, 6263–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- Zaveri E, Lobell DB. 2019. The role of irrigation in changing wheat yields and heat sensitivity in India. Nature Communications 10, 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Roberts DN, Cairns BR. 2005. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123, 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Bortesi L, Baysal C, Twyman RM, Fischer R, Capell T, Schillberg S, Christou P. 2017. Characteristics of genome editing mutations in cereal crops. Trends in Plant Science 22, 38–52. [DOI] [PubMed] [Google Scholar]
- Zsögön A, Cermák T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Peres LEP. 2018. De novo domestication of wild tomato using genome editing. Nature Biotechnology 36, 1211–1216. [DOI] [PubMed] [Google Scholar]