Abstract
Changing gene expression patterns is the essential driver of developmental processes. Growth factors, micro-RNAs, long intergenic noncoding RNAs, and epigenetic marks, such as DNA methylation and histone modifications, all work by impacting gene expression. The key features of developing cells, including their ability to communicate with others, are defined primarily by their gene-expression profiles. It is therefore clear that a gene-expression atlas of the developing kidney can provide a useful tool for the developmental nephrology research community. Toward this end, the GenitoUrinary Development Molecular Anatomy Project (GUDMAP) consortium has worked to create an atlas of the changing gene-expression patterns that drive kidney development. In this article, the global gene-expression profiling strategies of GUDMAP are reviewed. The initial work used laser-capture microdissection to purify multiple compartments of the developing kidney, including cap mesenchyme, renal vesicle, S-shaped bodies, proximal tubules, and more, which were then gene-expression profiled using microarrays. Resolution of the atlas was then improved by using transgenic mice with specific cell types labeled with green fluorescent protein (GFP), allowing their purification and profiling. In addition, RNA-Seq replaced microarrays. Currently, the atlas is being pushed to the single-cell resolution using microfluidic approaches that allow high-throughput RNA-Seq analysis of hundreds of individual cells. Results can identify novel types of cells and define interesting heterogeneities present within cell populations.
Keywords: Kidney development, Cap mesenchyme, Renal vesicle, Podocyte, Mesangial cell, Gene expression atlas, RNA-Seq analysis of kidney
Introduction
Differential gene expression is the chief distinguishing mark of distinct cell types. Whereas all cells in the body are equipped with the same set of genes, they are employed in complex and unique combinations that differentiate one cell from another. The developmental biologist of today is working to understand how a human single-cell zygote, equipped withonly around 22,000 genes in its nucleus, is somehow able to construct a complete person. In this review, we focus on global gene-expression-profiling strategies designed to dissect the changing waves of gene expression that drive kidney development. Some of the early work is briefly revisited, a few current studies are examined, and possible future directions are discussed.
Early work
Sanjay Nigam pioneered the application of global gene-expression profiling to kidney development. Microarrays were used to provide—for the first time—broad views of genes expressed at different stages of rat kidney development [1]. This was then extended to the mouse system and to the ureteric bud (UB) and metanephric mesenchyme (MM) compartments that were separated by manual microdissection [2–5]. In addition, microarrays were used to great advantage to study gene-expression patterns in manipulated MM grown in organ culture [6].
The next stage in the gene-expression profile analysis of kidney development involved the use of more sophisticated techniques to isolate restricted compartments of the developing kidney. In particular, the generation of transgenic mice with green fluorescence protein (GFP) labeling specific cell types allowed their purification through fluorescence-activated cell sorting (FACS). This strategy was used to purify ureteric bud (UB) [3] and MM [7] cells. These studies provided some of the first unbiased and systematic analyses of genes expressed in early kidney development.
The Genitourinary Development Molecular Anatomy Project (GUDMAP) consortium then provided a more comprehensive study of the gene-expression patterns during kidney development [8, 9]. This project used in situ hybridizations to examine expression patterns of thousands of genes during kidney development. It also used microarrays coupled with FACS purification of GFP-labeled specific cell types and laser-capture microdissection (LCM) of distinct compartments to generate a global atlas of kidney development [10]. Fifteen different compartment/cell types were profiled, including MM, cap mesenchyme (CM), renal vesicle (RV), S-shaped bodies, glomerulus, proximal tubule, loop of Henle, UB, ureteric tips, cortical collecting ducts, medullary collecting ducts, and several distinct stromal compartments. Results described in intricate detail the changing waves of gene expression directing nephrogenesis. The data generated significant new insights into fundamental mechanisms, such as mesenchymal–epithelial transition, inductive signaling, branching morphogenesis, and nephron segmentation. All growth factors, receptors, and transcription factors expressed at the different stages and compartments of kidney development were defined. The GUDMAP data set provided a useful resource for the kidney-development research community.
Nevertheless, the original microarray-based atlas of kidney-development gene expression [10] was limited in several respects. First, micro-RNAs and other nonpolyadenylated and noncoding RNAs were not examined. This was partially resolved by moving from arrays to RNA-Seq. Second, limited numbers of developmental time points were examined. It is becoming increasingly clear, for example, that the CM of the E12.5 kidney is not the same as that at other time points. Third, many compartments isolated by LCM consisted of several distinct cell types. For example, the glomerulus has three main cell-type constituents: podocytes, mesangial cells, and endothelial cells. Array data based on LCM isolation of developing glomeruli therefore provide poor resolution. They may tell us that a gene is expressed in the glomerulus, but we still do not know in which of the three main cell types.
Higher resolution is better
The main disadvantage of global gene-expression strategies compared with in situ hybridization is the lack of spatial information. In situ hybridizations often generate beautiful images that can reveal exact expression boundaries and specific cells that express the gene of interest. Of course, the chief disadvantage of in situ hybridization is that it is largely a one-gene-at-a-time procedure. Enormous effort would be required to perform the tens of thousands of in situ hybridizations necessary to provide more comprehensive views.
An ideal solution would be to somehow combine the ease and sensitive and quantitative nature of global gene-expression profiling with the spatial definition provided by in situ hybridizations. The chief message of this review is that this can be achieved by increasing the resolution of the global gene-expression-profiling strategy. Instead of profiling total kidneys, we profile compartments; instead of profiling total compartments, we profile each cell type within the compartment. In some cases, analysis of ensemble averages of cell populations is inadequate. Interesting heterogeneities can exist within cell types that are best studied using single-cell gene-expression profiling approaches. In this manner, it is possible to produce a high-resolution atlas of gene-expression patterns that drive kidney development.
The podocyte
The podocyte offers an excellent example of how gene expression profiling resolution can be increased by moving from compartment-wide analysis to cell-type analysis. The podocyte is a remarkable-appearing cell, with multiple axon like projections from which extend the foot processes, which interdigitate. The podocytes have many functions, including glomerular basement membrane (GBM) synthesis and formation of slit diaphragms, which provide the final filtration barrier. In addition, podocytes function as pericytes, counteracting the distending forces of blood pressure on glomerular capillaries, and they are also thought to help clean the GBM filter, which is necessary in order to prevent clogging [11].Podocytes are derived from the CM and produce vascular endothelial growth factor (VEGF) in the S-shaped bodies, helping to recruit endothelial cells [12]. In addition, podocytes are of key importance in a number of kidney diseases in which the initial insult is often detected as podocyte effacement of foot processes and loss of slit diaphragms [13, 14].
One approach used for global profiling of podocytes is to use cells grown in culture. Conditionally immortalized podocyte mouse [15] and human [16] cell lines have been created, and microarrays have been used to define their changing gene-expression patterns as a function of exposure to high glucose levels [17] or mechanical stress [18]. Nevertheless, it is clear that cultured podocyte cells are not the same as those in vivo, and results need to be interpreted with caution [19].
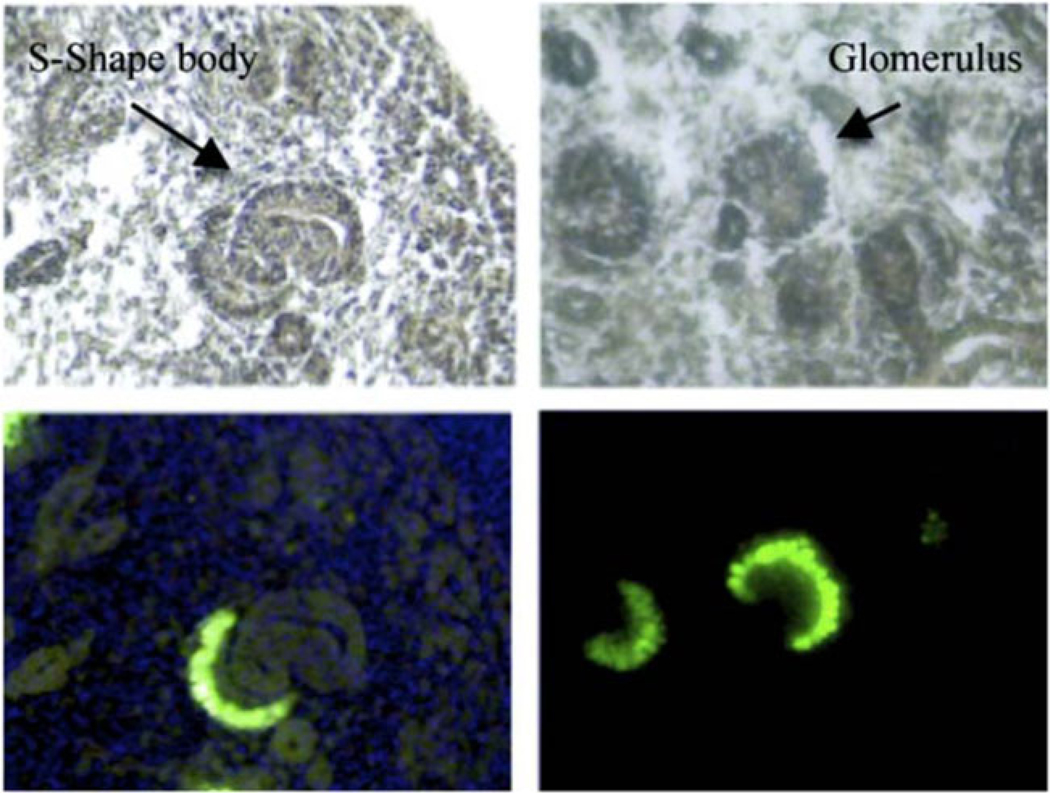
The MafB-GFP transgenic mouse specifically marks podocytes in the developing kidney [20]. Even the very early presumptive podocyte of the S-shaped body is GFP positive, as shown in Fig. 1. This made it possible to use a FACS/microarray strategy to define gene-expression profiles of podocytes from E13.5, E15.5, and adult kidneys. Embryonic kidneys are quite fragile, allowing rapid enzymatic dissociation to a single cell state, typically in under 5 min, followed by FACS, RNA purification, and microarray profiling. Rapid processing reduces the opportunity for artifact changes in gene expression that might occur during handling. For adult kidneys, however, glomeruli were first purified using a sieving procedure and then separated to single cells and used for FACS [20].
Fig. 1.
MafB-GFP mice show green fluorescent protein (GFP) expression restricted to podocytes. Top panels show cryostat sections with S-shaped body (left) and forming glomerulus (right) marked by arrows. Bottom panels show that GFP fluorescence is restricted to developing podocytes at E15.5. (Originally published in [20]
The results showed that the E13.5 podocyte is relatively undifferentiated, but >1,000 differentiation-related genes are elevated in expression in the adult podocyte [20]. Ontology analysis of these genes reveals the remarkable character of the podocyte. As might be expected, with the striking structure of the podocyte, many podocyte-enriched gene transcripts were associated with the cytoskeleton, including 65 cytoskeletal-binding proteins and 39 genes involved in the organization of the actin skeleton. The top biological processes to emerge included vesicle-mediated transport (72 genes), signaling regulation (99 genes), neurogenesis (74 genes), neuron-projection development (52 genes), chemotaxis (38 genes), phagocytosis (11 genes), and muscle (36 genes).Theseprocesses help define the remarkable multifunctional character of the podocyte. It was surprising to observe that podocytes exhibit robust expression of the transcription factor Foxd1 [20], a commonly accepted marker of the stromal compartment. This was confirmed by in situ hybridization. Podocytes are derived from CM, indicating that their Foxd1 expression does not reflect a stromal lineage. A total of 54 genes with highly elevated podocyte expression encoded transcription factors. Of interest, three of the most highly expressed were Foxc2, Wt1, and Pod1. It has been shown that Foxc2 and Wt1 expression, coupled with Notch signaling, is capable of driving podocyte development [21].
In summary, gene-expression profiling of developing and adult podocytes serves to delineate the complete in vivo gene-expression state of these remarkable cells and to better define their multifunctional character. Data reveals the complete set of transcription factors, growth factors, and receptors expressed by these cells. It also sets the baseline for future studies that investigate gene-expression changes that take place in podocytes as a function of disease.
Mesangial cells
Mesangial cells are another component of the glomerular triad. They construct the extracellular matrix framework of the glomerulus, have smooth-muscle-like properties that contribute to regulation of capillary flow, and have phagocytic-like properties that dispose of unwanted debris. They are also important players in kidney diseases, including diabetic nephropathy (type 2 diabetes is increasing in epidemic fashion and is now the most common cause of end-stage renal disease [22]).
Because of their importance, similar to podocytes, mesangial cells have been the subject of intense study, including global gene-expression profiling by microarrays. Most studies used mesangial cells grown in culture and focused on their gene-expression response to a variety of insults, including high glucose [23], static pressure [24], serum [25], kynurenine metabolites [26], endothelin [27], and platelet-derived growth factor (PDGF) [28]. These studies made many important discoveries, including the following: static pressure regulates connective-tissue growth-factor expression, endothelin increases collagen accumulation, and epidermal growth factor (EGF) regulates mesangial proliferation. Nevertheless, these studies were somewhat limited by their use of mesangial cells in culture, which can never fully recapitulate their in vivo counterparts. In addition, the sets of conditions used in cell culture cannot fully recreate those that mesangial cells are exposed to in vivo during kidney disease.
To better understand the transcriptional response of in vivo mesangial cells to diabetic nephropathy, the db/db mouse model was used. This mouse suffers disrupted leptin signaling and as a result becomes extremely obese and develops diabetic nephropathy. The Meis1-GFP transgenic mouse allows specific GFP labeling of both the stroma and mesangial cells in the kidney [10]. It was therefore possible to purify mesangial cells by first isolating glomeruli, using a sieving procedure, followed by cell dissociation and FACS [29].
Gene-expression profiles of wild type mesangial cells were first compared with those of podocytes, endothelial cells, total renal cortex, renal vesicles, and cap mesenchyme to better define their unique attributes [29]. The resulting list of genes was subjected to gene-ontology analysis to identify associated molecular functions and biological processes. Biological processes with P values of essentially zero included wound healing, coagulation, locomotion, regulation of body fluid levels and muscle contraction. Others with high statistical significance included ossification, cell adhesion, and extracellular matrix organization. Quite surprisingly, there was also the gene-expression signature of neural character, including cognition, learning axogenesis, and neuron development [29].
Another surprising finding was the fairly robust expression of Wt1 in mesangial cells. Wt1 is generally considered a podocyte marker in the adult kidney and is not thought to be expressed in mesangial cells. Nevertheless, array data showed Wt1 raw signal levels of 3,400 in podocytes and 650 in mesangial cells. This signal is extremely strong in podocytes, as expected, but still very significant—although about fivefold lower—in mesangial cells. This did not appear to be the result of contamination of the mesangial cells with podocytes, as other podocyte marker genes, such as Podxl, Nphs1, and Nphs2, showed very strong signal in podocytes and very low expression the mesangial cells, as expected. These results illustrate an important point that is confirmed repeatedly in global profiling analysis studies. In situ hybridizations and immunostains can suggest that a gene shows very restricted cell-type expression, whereas microarrays find lower, but significant, expression in other cell types. This is because microarrays are more sensitive and quantitative, and in situ/immunostain reactions are often optimized to give more on/off results: that is, the staining reaction is stopped after some cells show strong signals, thereby missing lower signals in other cells.
Mesangial cells expressed a number of growth factors, including very strong expression of pleiotrophin, with raw signals of around 6,000. Pleiotrophin is a powerful angiogenic factor, and one of its receptors, Ptprb, is expressed on glomerular endothelial cells, strongly suggesting paracrine function.
Mesangial cells from mice with db/db diabetic nephropathy showed elevated thrombospondin expression. This is particularly interesting because of the known role of transforming growth factor beta (TGF-β) in renal fibrosis. TGF-β is secreted as an inactive procytokine that must be activated to bind its receptors, and thrombospondin plays an important role in this process. Indeed, thrombospondin-mutant mice show an inflammatory phenotype that is similar to that of Tgf-β-mutant mice [30].
Another gene of particular interest upregulated in the db/db mesangial cells was Dcn, which encodes decorin [29]. Decorin is known to interact with TGF-β, and overexpression of decorin reduces fibrosis in many model systems [31]. Further, Dcn deficiency greatly increases the severity of diabetic nephropathy in mice with streptozotocin-induced diabetes [32]. This suggests that Dcn overexpression in db/db mesangial cells provides a protective effect.
CM and RV
Differentiation of CM to form RV is the first step in the conversion of the amorphous CM cloud into the intricate complexity of the nephron. The CM balance between self-renewal and differentiation is critically important in determining the final nephron count, which has important medical implications. In situ hybridizations are used to follow the changing gene-expression patterns as CM is induced to form RV [33], with the uninduced CM shown to express Six2 and Cited1, whereas induced CM expressed Six2 and Wnt4 but not Cited1. When the RV forms, it is already polarized in terms of gene expression, presaging the eventual proximal–distal segmentation of the nephron. The distal domain of the RV expresses Lhx1, Dll1, Dkk1, Jag1, Bmp2, and Brn1, whereas the proximal region expresses Tmem100 and Wt1 [33–36]. microarrays have also been used to globally define changes in gene expression as CM differentiates into RV, identifying >1,000 genes that change expression during this process [10].
RNA-Seq is superior to microarray gene-expression profiling in several ways [37]: It examines all transcripts, not just those on the array. It can also characterize alternative processing events, which is quite important, as >90 % of genes are now thought to give rise to alternate transcript forms. Finally, it offers a wider dynamic range and lower background than microarrays. In a recent study, RNA-Seq was used to characterize the CM to RV transition [38]. The Crym-GFP transgene primarily marks capping mesenchyme, similar to Six2-GFP [10]. This transgenic mouse can also be used for FACS enrichment of renal vesicle cells. In the mouse model, in the days immediately following birth, there is a burst of nephrogenesis that rapidly consumes the remaining CM [39]. At postnatal day four (P4), the CM is depleted, but there remains residual low-level Crym-GFP labeling of renal vesicles, likely through perdurance. It is therefore possible to isolate CM cells at P1 with a stringently gated FACS and to then isolate the RV from kidneys at P4, with FACS gating set for lower levels of GFP [38].
CM and RV RNA-Seq was carried out with two protocols, one looking only at polyadenylated RNA, and the other using a random primer reverse transcription reaction that looked at all transcripts. By comparing the resulting two sets of RNA-Seq data, it was possible to identify transcripts that are not polyadenylated. Of interest, most transcripts are not polyadenylated. For example, many enhancers are transcribed, giving rise to short (<2 kb) bidirectional transcripts that are not polyadenylated [40]. Therefore, RNA-Seq analysis, when carried out to examine nonpolyadenylated transcripts, can also generate an enhancer map.
Study results showed a surprising presence of opposite-stand transcripts in the 5′ regions of several genes of key importance in kidney development, including Sall1, Six2, Lhx1, and Emx2. In each case, there were both polyadenylated and nonpolyadenylated transcripts in the 5′ promoter regions. It is as if the polymerase could bind at the promoter region and move in the “normal” direction, giving rise to the standard coding transcripts for these genes, or in the opposite direction. We found there was generally coordinated regulation of transcripts in both directions. For example, for Six2, the opposite-strand transcript was strongly expressed in CM and only expressed at a very low level in RV. Also of interest, the polyadenylated opposite-strand transcripts were spliced, whereas the nonpolyadenylated transcripts were not. There was no evidence that any of these opposite-strand transcripts had coding functions.
Another interesting result was a wealth of unexpected variation in splicing patterns. This was particularly pronounced for Hox genes. The 39 Hox genes are found in four different clusters. They were among the first master regulators of development identified. In Drosophila, mutations in these genes can result in dramatic homeotic transformation of one body part into another. For example, the Antennapedia mutation causes imaginal discs that would normally create antennae to instead form legsthat now protrude from the head. In mammals, several Hox genes of the Hox10,11 groups play an important role in kidney development [41–43]. RNA-Seq results identified many novel Hox splicing patterns. For example, a predominant splice form for Hoxd11 in the CM actually used a novel 5′ exon, which then skipped directly to the last exon of the gene, giving a noncoding transcript. In the RV, however, the only splicing pattern detected was the normal canonical connection of the Hoxd11 first and second exons [38]. Indeed, for many Hox genes, novel splice patterns were found, and in many cases, there was intergenic splicing between exons of different genes. In addition, there was an abundance of antisense transcription, and many transcripts were derived from intergenic and intronic regions. The great complexities observed suggest that Hox clusters should actually be considered as supergenes, with many alternate transcription and processing possibilities, instead of collections of individual genes.
The future
There remains considerable room for improvement in the molecular atlas of kidney development. The adult kidney consists of many different cell categories, and perhaps we should start with a definition of each of their gene-expression states in order to determine the final cell-type products of the developmental process. First, we need to know where we are going before we can determine how to get there. Then, an ideal atlas would define the intermediate transition states, from progenitor to final differentiated cell type, to provide the full gene-expression pathway followed. Whereas this is a lofty goal, there are emerging technologies that suggest it will be possible to achieve. The continuing DNA sequencing revolution will keep driving down the cost of RNA-Seq analysis. The cost of DNA sequencing has dropped about 1-million-fold in the past decade, and there is no end in sight, as remarkable new technologies are being developed. This will facilitate the economic generation of the large numbers of RNA-Seq data sets required to define the many different lineage pathways of the developing kidney.
There is also an ongoing revolution in single-cell transcriptome analysis [44, 45]. This will provide a very powerful tool. For example, in looking at the cellular makeup of the adult kidney, we do not have the various transgenic-GFP tools required for FACS purification of each cell type present. However, if we could take the adult kidney, dissociate to single cells, and then RNA-Seq a large number of individual cells, we could generate gene-expression profiles for each cell type present. One of the great advantages of this strategy is that single cells that are microscopically validated are by definition pure. Other techniques, such as FACS and LCM, always result in some level of contamination.
There are considerable challenges in RNA-Seq analysis of single cells. Single cells often only have about five to ten total picograms of RNA, which equates to an average of only about ten transcripts per expressed gene per cell. It is technically very challenging to efficiently capture such a small number of transcripts. In addition, genes are expressed in a burst fashion, resulting in considerable “noise”, or variation, in transcript level over time. The end result is that the combination of biological and technical noise results in a variable and incomplete gene-expression profile for each cell examined. However, one advantage of RNA-Seq data is the ease of combining individual data sets. RNA-Seq reads for ten cells, for example, could be pooled to generate a very robust view of that particular cell type (Fig. 2). That is, by carrying out RNA-Seq on large numbers of cells, it is possible to assign individual cells to different categories, and then the RNA-Seq readings from that group can be pooled to generate a high-quality view of the gene-expression profile of that cell type.
Fig. 2.
Single-cell RNA-Seq analysis strategy for creating a gene-expression-profile atlas of organogenesis. The top shows an organ, which can be developing or adult. The cells are color coded, with cells that are more alike having similar colors. The cells are enzymatically dissociated, and single-cell RNA-Seq analysis is carried out. The resulting data is then analyzed, and bins, or categories are created. Whereas single-cell data are noisy, it is possible to pool RNA-Seq data sets from the cells of a category and produce a high-quality view of the gene-expression profile of each cell type. It is also possible to define interesting heterogeneities within types. Sets of marker genes for the different cell types are identified. Then, the three dimensional structure is re-created by performing combinations of in situ hybridizations and immunostains to define the locations of the different types of cells within the organ
In addition, there are situations during kidney development that show interesting cellular heterogeneity, which needs to be better defined. For example, as previously mentioned, the RV shows proximal–distal polarization, but our definition of this to date is restricted to in situ hybridization studies looking at one gene at a time. A more global look at the differing gene-expression programs of single cells within the early RV would be useful. Another example is the E11.5 MM, where different cells are destined to follow very distinct stromal, vascular, and nephrogenic lineages. A better definition of these early variations in gene expression that drive distinct developmental decisions would be highly interesting and valuable.
New microfluidic devices are being developed that allow high-throughput RNA-Seq analysis of single cells. The Fluidigm C1, for example, is capable of processing 96 cells at once, placing each in an individual chamber and then carrying out a series of biochemical reactions that can be harvested for RNA-Seq. Reactions are carried out in picoliter volumes, which saves reagent, and results in less nonspecific product. By creating large numbers of single-cell RNA-Seq data sets, by dividing the resulting profiles into pools, and then by combining them appropriately to generate robust readouts, it will be possible to create a single-cell resolution atlas of gene-expression patterns that force kidney development forward—from early progenitors to multiple differentiated cell types.
Acknowledgments
We thank all members of the Potter lab, including Mike Adam, Anna Raines, Bliss Magella, and Andrew Potter, for thoughtful discussions. This work was supported by 1RC4DK090891 from the National institutes of Health.
Abbreviations
- CM
Cap mesenchyme
- FACS
Fluorescent-activated cell sorting
- GFP
Green fluorescent protein
- GBM
Glomerular basement membrane
- GUDMAP
Genito Urinary Development Molecular Anatomy Project
- LCM
Laser-capture microdissection
- MM
Metanephric mesenchyme
- RV
Renal vesicle
- UB
Ureteric bud
References
- 1.Stuart RO, Bush KT, Nigam SK (2001) Changes in global gene expression patterns during development and maturation of the rat kidney. Proc Natl Acad Sci U S A 98:5649–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab K, Patterson LT, Aronow BJ, Luckas R, Liang HC, Potter SS (2003) A catalogue of gene expression in the developing kidney. Kidney Int 64:1588–1604 [DOI] [PubMed] [Google Scholar]
- 3.Challen G, Gardiner B, Caruana G, Kostoulias X, Martinez G, Crowe M, Taylor DF, Bertram J, Little M, Grimmond SM (2005) Temporal and spatial transcriptional programs in murine kidney development. Physiol Genom 23:159–171 [DOI] [PubMed] [Google Scholar]
- 4.Schwab K, Hartman HA, Liang HC, Aronow BJ, Patterson LT, Potter SS (2006) Comprehensive microarray analysis of Hoxa11/Hoxd11 mutant kidney development. Dev Biol 293:540–554 [DOI] [PubMed] [Google Scholar]
- 5.Stuart RO, Bush KT, Nigam SK (2003) Changes in gene expression patterns in the ureteric bud and metanephric mesenchyme in models of kidney development. Kidney Int 64:1997–2008 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, Yang J, Paragas N, Wallace VA, Dufort D, Pavlidis P, Jagla B, Kitajewski J, Barasch J (2007) beta-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development 134:3177–3190 [DOI] [PubMed] [Google Scholar]
- 7.Takasato M, Osafune K, Matsumoto Y, Kataoka Y, Yoshida N, Meguro H, Aburatani H, Asashima M, Nishinakamura R (2004) Identification of kidney mesenchymal genes by a combination of microarray analysis and Sall1-GFP knockin mice. Mech Dev 121: 547–557 [DOI] [PubMed] [Google Scholar]
- 8.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P, project G (2008) GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol 19:667–671 [DOI] [PubMed] [Google Scholar]
- 9.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Brunskill EW, Southard-Smith EM, Mendelsohn C, Baldock RA, Davies JA, Davidson D (2011) The GUDMAP database–an online resource for genitourinary research. Development 138: 2845–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT,Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS (2008) Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15: 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS (2008) Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costantini F, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J (2005) The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68:1562–1572 [DOI] [PubMed] [Google Scholar]
- 14.Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW (2007) Podocyte biology in diabetic nephropathy. Kidney Int Suppl (106):S36–S42 [DOI] [PubMed] [Google Scholar]
- 15.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R (1997) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258 [DOI] [PubMed] [Google Scholar]
- 16.Shankland SJ, Pippin JW, Reiser J, Mundel P (2007) Podocytes inculture: past, present, and future. Kidney Int 72:26–36 [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Yang S, Jung DS, Li JJ, Kim JJ, Kwak SJ, Kim DK, Moon SJ, Lee JE, Han DS, Kang SW (2008) Gene expression patterns in glucose-stimulated podocytes. Biochem Biophys Res Commun 370: 514–518 [DOI] [PubMed] [Google Scholar]
- 18.Endlich N, Sunohara M, Nietfeld W, Wolski EW, Schiwek D, Kranzlin B, Gretz N, Kriz W, Eickhoff H, Endlich K (2002) Analysis of differential gene expression in stretched podocytes: osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J 16:1850–1852 [DOI] [PubMed] [Google Scholar]
- 19.Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307 [DOI] [PubMed] [Google Scholar]
- 20.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS (2011) Defining the molecular character of the developing and adult kidney podocyte. PLoS One 6:e24640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JT, Zhang B, Cerqueira DM, Tran U, Wessely O (2010) Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development 137:1863–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins RC, Zimmet P (2010) Diabetic kidney disease: act now or paylater. Nephrology 15:20–22 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Uehara S, Ikeda T, Itadani H, Kotani H (2003) VitaminD3 up-regulated protein-1 regulates collagen expression in mesangial cells. Kidney Int 64:1632–1642 [DOI] [PubMed] [Google Scholar]
- 24.Hishikawa K, Oemar BS, Nakaki T (2001) Static pressure regulates connective tissue growth factor expression in human mesangial cells. J Biol Chem 276:16797–16803 [DOI] [PubMed] [Google Scholar]
- 25.Mishra R, Leahy P, Simonson MS (2002) Gene expression profiling reveals role for EGF-family ligands in mesangial cell proliferation. Am J Physiol Renal Physiol 283:F1151–F1159 [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura H, Sakai T, Kuwahara Y, Ito M, Tsuritani K, Hirasawa Y, Nagamatsu T (2009) Effects of kynurenine metabolites on mesangial cell proliferation and gene expression. Exp Mol Pathol 87:70–75 [DOI] [PubMed] [Google Scholar]
- 27.Simonson MS, Ismail-Beigi F (2011) Endothelin-1 increases collagen accumulation in renal mesangial cells by stimulating a chemokine and cytokine autocrine signaling loop. J Biol Chem 286:11003–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadlier DM, Ouyang X, McMahon B, Mu W, Ohashi R, Rodgers K, Murray D, Nakagawa T, Godson C, Doran P, Brady HR, Johnson RJ (2005) Microarray and bioinformatic detection of novel and established genes expressed in experimental anti-Thy1 nephritis. Kidney Int 68:2542–2561 [DOI] [PubMed] [Google Scholar]
- 29.Brunskill EW, Potter SS (2012) Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol 13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N (1998) Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93:1159–1170 [DOI] [PubMed] [Google Scholar]
- 31.Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S (2010) Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res 91:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams KJ, Qiu G, Usui HK, Dunn SR, McCue P, Bottinger E,Iozzo RV, Sharma K (2007) Decorin deficiency enhances progressive nephropathy in diabetic mice. Am J Path 171:1441–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugford JW, Yu J, Kobayashi A, McMahon AP (2009) Highresolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol 333:312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD,Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH (2009) Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332:273–286 [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR (2005) Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823 [DOI] [PubMed] [Google Scholar]
- 36.Nakai S, Sugitani Y, Sato H, Ito S, Miura Y, Ogawa M, Nishi M,Jishage K, Minowa O, Noda T (2003) Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development 130: 4751–4759 [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionarytool for transcriptomics. Nat Rev Genet 10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunskill EW, Potter SS (2012) RNA-Seq defines novel genes, RNA processing patterns and enhancer maps for the early stages of nephrogenesis: Hox supergenes. Dev Biol 368:4–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman HA, Lai HL, Patterson LT (2007) Cessation of renal morphogenesis in mice. Dev Biol 310:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME (2010) Widespread transcription at neuronal activity-regulated enhancers. Nature 465:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR (1995) Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375:791–795 [DOI] [PubMed] [Google Scholar]
- 42.Wellik DM, Hawkes PJ, Capecchi MR (2002) Hox11 paralogousgenes are essential for metanephric kidney induction. Genes Dev 16: 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yallowitz AR, Hrycaj SM, Short KM, Smyth IM, Wellik DM (2011) cell RNA-Seq reveals non-genetic gene expression heterogeneity. Hox10 genes function in kidney development in the differentiation Genome Biol 14:R31 and integration of the cortical stroma. PLoS One 6:e23410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasagawa Y, Nikaido I, Hayashi T, Danno H, Uno KD, Imai T, Ueda BJ, Potter SS, Gomez RA (2011) Genes that confer the identity of the HR (2013) Quartz-Seq: a highly reproducible and sensitive single-renin cell. J Am Soc Nephrol 22:2213–222522034642 [Google Scholar]
- 45.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA (2011) Genes that confer the identity of the renin cell. J Am Soc Nephrol 22:2213–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]