Abstract
Human lifespans are exceptionally long compared with those of other primates. A key element in exploring the evolution of human longevity is understanding how modern humans grow older. Our current understanding of common age-related changes in human health and function stems mostly from studies in industrialized societies, where older adulthood is often associated with an increased incidence of chronic diseases. However, individuals who engage in different lifestyles across industrialized and non-industrialized contexts may display variance in age-related changes in health and function. Here, we explore aspects of physical function in a non-industrialized context using three objective measures of physical function. We assessed physical activity levels, walking endurance and muscle strength in two East African populations: Hadza hunter–gatherers in Tanzania and Pokot pastoralists in Kenya. Both Hadza and Pokot participants displayed significant age-related differences in most, but not all, functional measures. Our results suggest that some age-related differences in physical function seen in industrialized contexts could be consistently experienced by most humans, while other age-related differences may vary across populations. Studies of ageing should expand to include a broad range of populations so we can create a more comprehensive understanding of how senescence varies across different lifestyle contexts.
This article is part of the theme issue ‘Evolution of the primate ageing process’.
Keywords: ageing, physical function, non-industrial societies, physical activity, human longevity
1. Introduction
Human longevity sets us apart from other primate species [1,2]. While chimpanzees, humans' closest living relatives, rarely reach the age of 50 [3,4], humans in all populations frequently live into their 60s and 70s [5]. These differences in patterns of ageing may be ancient, as the hominin fossil record includes gradually increasing numbers of older individuals beginning around 2 million years ago with Homo erectus [6]. During this time, hominins likely began engaging in a hunting and gathering lifestyle characterized by higher-intensity activities [7–9], leading to several hypotheses linking this lifestyle with the evolution of old age (e.g. the grandmother hypothesis or the embodied capital hypothesis [10,11]). These hypotheses all have some support from a variety of datasets; however, a better understanding of how our hominin ancestors aged may help refine models for why lifespans increased during our evolutionary past.
Our current understanding of human ageing stems mostly from studies of industrialized societies, where age-related changes in health and function have become major public health concerns owing primarily to increasing numbers of older adults in the population [12,13]. In industrialized contexts, older adulthood is characterized by an increased incidence of one or more chronic diseases that limit functionality and increase mortality risk [13–16]. From an evolutionary perspective, such detrimental age-related changes in physical function would seem to be especially challenging to an older individual's ability to survive or contribute to their own or their kin's reproductive success. Indeed, in nonhuman primates, where support and care-taking from conspecifics is uncommon, there is limited evidence for age-related declines in physical function (although minor declines in body mass and walking speed have been documented [17–19]).
Large-scale epidemiological studies and meta-analyses reveal that several elements of lifestyle (e.g. diet, physical activity and social interactions) contribute to an individual's health status and chronic disease risk throughout life and may mitigate some of the age-related decline in physical function (e.g. [20–24]). People who live in less industrialized societies share many of these beneficial elements of lifestyle and many do not show the consistent age-related declines in measures of physical function that are commonly seen in industrialized contexts [25–27]. For example, in small-scale societies like the Tsimane—forager horticulturalists in Bolivia—or the Ache—hunter–gatherers of Paraguay—aspects of physical function like muscle strength and physical activity seem to decline more slowly when compared with more industrialized societies [25–27]. We have previously shown that in the Hadza hunter–gatherers of Tanzania and the Pokot pastoralists of Kenya, physical activity levels are higher than we see in more industrialized populations, including in older adults [28,29]. Exploring variation in how changes in health and function are expressed across modern human populations is key to understanding human senescence and consequently relevant for discussions of the evolution of human longevity.
Here, we use three objective measures of physical function to assess patterns of age-related differences in physical function in two modern small-scale societies in East Africa. We measured physical activity levels, walking endurance and grip strength in Hadza and Pokot participants. Both the Hadza and Pokot populations live in East African rift-valley environs and engage in non-industrial subsistence economies, but their lifestyles differ from each other in several important ways that could impact patterns of age-related differences in physical function. Key to this study is that the Pokot formally recognize an age-based division of labour, in which older men and women may delegate more strenuous tasks to younger individuals [30], while the Hadza do not have a formal shift in duties with age [31]. We therefore expected Hadza and Pokot participants to differ from each other and from industrialized populations in which measures of physical function are significantly associated with age [32–35].
2. Methods
(a). Participants: Hadza hunter–gatherers
The Hadza population lives near Lake Eyasi in northern Tanzania in small residential camps [31,36,37]. In Hadza culture, men and women engage in different kinds of foraging activities: adult men generally forage alone, carrying a bow and an axe, searching for wild honey or game [31,38,39]. Women typically forage in groups, carrying digging sticks, and their primary targets are tubers, fruit and seasonally, leafy greens [31,40]. The majority of the diet in the camps included in this study was derived from wild foods, including tubers, berries, Baobab seed pulp, honey, and meat from wild animals (e.g. bush pig, rock hyrax). Some Hadza men and women also smoke or chew tobacco [41]. In addition to walking, physical activity in Hadza adults includes climbing, digging, pounding Baobab seeds and carrying heavy loads including firewood and water. These general patterns also adequately summarize the activities of the men and women we observed in the camps studied here.
We recruited 128 Hadza participants (nmale = 78, nfemale = 50) from ages 4 to 84 years (age, mean ± s.e.m. = 34.3 ± 1.7 years) from two camps during the wet season and two camps during the dry season. Hadza participants completed a grip strength test and a subset of 32 Hadza participants (nmale = 18, nfemale = 14) also completed a walk test to assess endurance. A subset of 65 Hadza participants (nmale = 38, nfemale = 27) wore an accelerometer on the dominant wrist for at least one week. A subset of 14 Hadza participants (nmale = 7, nfemale = 7) then wore an accelerometer on the non-dominant wrist for at least a week, so we could determine if accelerometer placement impacts activity measures. Data collection took place in five two- to four-week intervals during July 2015; March, June and July 2018; October 2019. Approval was acquired from all appropriate agencies (Institutional Review Boards for the University of Arizona, Yale University, and the University of California Los Angeles; Tanzania Commission for Science and Technology) prior to data collection. All participants provided informed consent prior to and during data collection. Participant height and weight were also collected for analysis (table 1). Full participant data are provided in electronic supplementary material, table S1.
Table 1.
Descriptive statistics for Hadza and Pokot participants.
| group | sex | n | age mean [range] (years) | height (cm) | weight (kg) | BMI |
|---|---|---|---|---|---|---|
| Hadza | male | 78 | 36.1 [4–77] | 152.8 ± 1.7 | 45.4 ± 1.4 | 18.9 ± 0.3 |
| female | 50 | 31.4 [4–84] | 147.1 ± 2.3 | 42.1 ± 1.7 | 18.9 ± 0.4 | |
| Pokot | male | 22 | 36.4 [15–74] | 169.2 ± 1.4 | 54.0 ± 1.3 | 18.8 ± 0.3 |
| female | 23 | 37.9 [14–78] | 158.1 ± 1.3 | 47.3 ± 1.1 | 18.9 ± 0.4 |
Statistics displayed for height, weight and age are mean ± s.e.m.
(b). Participants: Pokot pastoralists
The Pokot population is one of several pastoralist groups who live in northern Kenya [42,43]. Owing to social, geographical and political changes over the past several decades, the Pokot have adopted a mixed economy of pastoralism, in which maintaining cattle, goats, camels and other livestock is supplemented by small-scale agriculture (‘kitchen gardening’) [43–45]. Like the Hadza, Pokot men and women engage in different daily activities: men maintain livestock herds and walk with animals to find grazing areas and water and protect herds from any threats. Women maintain homesteads, which entails building and maintaining herd and household structures, food preparation, childcare, and acquiring firewood and water [30,46]. Unlike the Hadza, the Pokot formally recognize an age-based division of labour, in which older men and women may delegate more strenuous tasks to younger individuals [30]. These patterns also adequately summarize the general activities of the men and women we observed in the goat-herding community studied here.
We recruited 45 Pokot participants (nmale = 23, nfemale = 24) from ages 14 to 78 years (age mean ± s.e.m. = 37.1 ± 2.9 years) from one community near Lake Baringo. Pokot participants wore an accelerometer on the non-dominant wrist for 1–4 days and completed both a walk test and grip strength test. Data collection took place over a one-week period in June 2016. Approval was acquired from appropriate agencies (Institutional Review Board of the University of Arizona, National Commission on Science and Technology and Innovation in Kenya) prior to data collection. All participants provided informed consent prior to and during study participation. Participant height and weight were also collected (table 1). Full participant data are provided in electronic supplementary material, table S1. Please note that the authors have previously published on one aspect of Pokot physical activity data (moderate-to-vigorous physical activity; see below) presented here [29].
(c). Physical activity levels: accelerometry
We used triaxial accelerometers (ActiGraph wGT3X-BT) to quantify physical activity in Hadza and Pokot participants. Differences in study protocols in Hadza and Pokot participants were related to logistics of fieldwork in these separate communities. Accelerometers were initialized through ActiGraph's ActiLife software (v. 6.13.13) and set to collect data continuously at 100 Hz. Raw accelerometry data were downloaded through ActiLife, then converted into raw files to process using the R [47] package GGIR, developed by van Hees et al. [48].
Using GGIR, we analysed raw accelerometry with methods described previously [33,49]. GGIR first identifies non-wear time, then calculates a three-dimensional vector magnitude (VM) using acceleration values from the three axes. Acceleration due to gravity (1g) is subtracted from the combined VM and reported as the Euclidian Norm Minus One (ENMO) [50]. GGIR reports time spent in moderate-to-vigorous physical activity (MVPA), calculated as time spent above an acceleration of 100 mg, a threshold for moderate activity used in industrialized populations [51]. Many epidemiological studies report time spent in MVPA and a growing number report ENMO, making both metrics useful for population comparisons [32,33].
After removing those participants who met our exclusion criteria (did not wear the accelerometer continuously or had less than one complete day of data (defined as 16 h of wear time)), accelerometry data from 53 Hadza participants (nmale = 29, nfemale = 24, age, mean ± s.e.m. = 42.0 ± 2.1 years) and 39 Pokot participants (nmale = 22, nfemale = 17, age, mean ± s.e.m. = 36.5 ± 3.0 years) were analysed in GGIR for ENMO and MVPA. Wear time among these 53 Hadza participants ranged from 6 to 9 days (mean ± s.e.m. = 8.1 ± 0.2 days). Wear time among these 39 Pokot participants ranged from 1 to 4 days (mean ± s.e.m. = 2.1 ± 0.1 days). Previous work has indicated that 1–2 days of accelerometer wear time is sufficient to capture estimates of habitual physical activity [52,53].
(d). Walking endurance: walk test performance
Walk tests that record distance travelled during a set time period are often used to assess walking endurance in industrialized populations [34,54]. In this study, we assessed walking endurance using a 2 min walk test developed for the NIH toolbox [55]. A flat 25-foot walkway was cleared and marked in each field site for the walk test. Participants were instructed to walk back and forth along the walkway as fast as they were able for 2 min while researchers tallied the number of laps travelled. At the end of 2 min, participants were instructed to immediately stop, and researchers measured total distance travelled.
(e). Physical strength: grip strength
Grip strength is frequently used to assess muscle strength in industrialized contexts [35,56]. A Camry digital hand dynamometer (South El Monte, CA) was used to measure grip strength. Participants used their dominant hand and were instructed to squeeze the dynamometer as hard as they were able for 3s per trial. Hand dominance was determined by identifying which hand a participant used to write or to throw a rock or stick. Participants completed three grip strength trials with at least 1 min of rest in between trials. The highest value a participant obtained across three grip strength trials was recorded as maximum grip strength (kg), following the protocol used in the U.S. National Health and Nutrition Examination Survey [56].
(f). Statistical analysis
All analyses were performed in R Studio (v1.2.1335) [47]. We stratified analyses by group owing to differences in study protocol across field sites. We used generalized additive models in the R package ‘mgcv' (v1.8-31) [57] to assess the effects of age and sex on physical activity levels (ENMO mg or time in MVPA), walk test performance (distance in metres) and grip strength (kilograms). Age was modelled as a spline function (order determined by model produced) to account for a potentially nonlinear effect of age on physical function measures. We tested for the effects of the interaction between age and sex on each measure. For measures where interactions were not significantly associated with the outcome, one age spline was fitted for both sexes. For measures where an interaction was significantly associated with the outcome, an age spline was fitted for males and females separately.
3. Results
(a). Physical activity
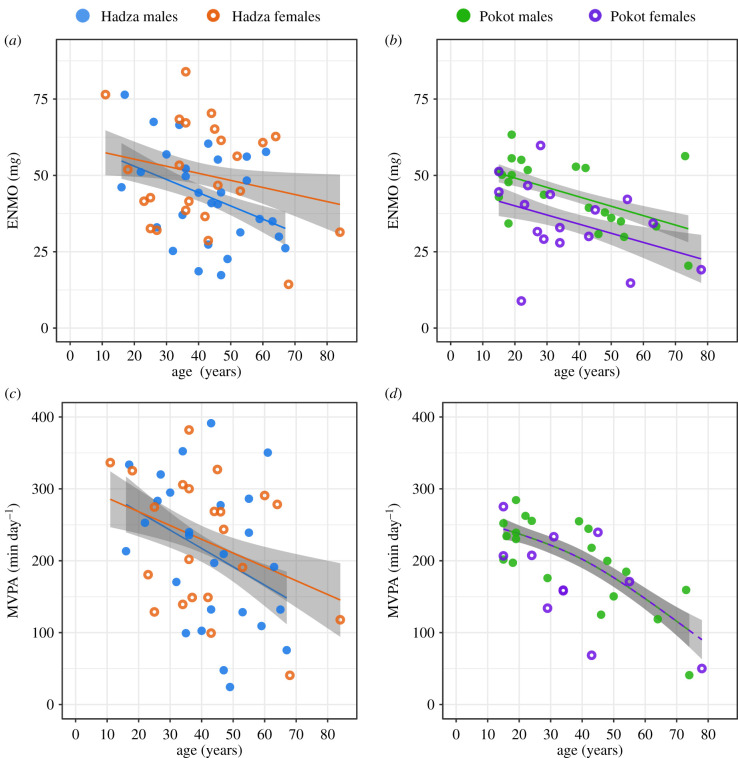
Accelerometer location (dominant versus non-dominant wrist) did not have a significant effect on physical activity levels in our Hadza sub-sample (see electronic supplementary material, tables S2 and S3). Average daily ENMO values for Hadza participants (figure 1a) were 43.23 ± 2.86 mg for males (n = 29) and 50.39 ± 3.52 mg for females (n = 24). Average daily ENMO for Pokot participants (figure 1b) was 44.10 ± 2.32 mg for males (n = 22) and 35.06 ± 3.18 mg for females (n = 17). Sex was not significantly associated with ENMO values for Hadza participants, but Pokot male participants displayed higher ENMO values than Pokot female participants (table 2). There was no interaction between age and sex on ENMO values for Hadza or Pokot participants (table 2). For both Hadza and Pokot participants, age was significantly negatively associated with ENMO values (table 2). Results did not change when BMI was included in the model (see electronic supplementary material, tables S8 and S12).
Figure 1.
Physical activity by age in Hadza and Pokot participants. Each data point represents one individual. Filled circles represent male participants and unfilled circles represent female participants. Lines fitting data represent a generalized additive model of the effect of age as a spline function on physical activity. Shaded region is the 95% confidence interval for the age spline. (a) ENMO for Hadza participants. (b) ENMO for Pokot participants. (c) MVPA for Hadza participants. (d) MVPA for Pokot participants.
Table 2.
Effect of sex and age on physical function measures. Results generated from generalized additive models (GAMs), stratified by group (no age × sex interaction) and by group and sex (age × sex interaction present). edf, estimated degrees of freedom; F, F-statistic; * indicates p-value is significant at the p = 0.05 level. See electronic supplementary material for full GAMs (electronic supplementary material, tables S4–S7), GAMs adjusted for body measurements (electronic supplementary material, tables S8–S15), summary statistics (electronic supplementary material, table S16) and generalized linear models (GLMs; for measures where edf = 1; electronic supplementary material, tables S17–S20).
| group | variable | n | sex × age interaction | effect of sex |
effect of s(age) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| est. | s.e. | t-value | p-value | sex | edf | F | p-value | ||||
| Hadza | ENMO | 53 (29M, 24F) | no; p = 0.49 | −6.74 | 4.32 | −1.56 | 0.13 | both | 1 | 5.11 | 0.03* |
| MVPA | 49 (27M, 22F) | no; p = 0.71 | −13.99 | 26.14 | −0.54 | 0.60 | both | 1 | 6.87 | 0.01* | |
| walk test | 32 (18M, 14F) | no; p = 0.34 | 14.54 | 8.88 | 1.64 | 0.11 | both | 1.15 | 1.27 | 0.33 | |
| gripstrength | 124 (75M, 49F) | yes; p = 0.02* | 4.90 | 1.11 | 4.40 | 2.51e−5* | male | 5.37 | 19.53 | <2e−16* | |
| female | 4.65 | 9.29 | 5.68e−8* | ||||||||
| Pokot | ENMO | 39 (22M, 17F) | no; p = 0.96 | 8.97 | 3.42 | 2.63 | 0.01* | both | 1 | 10.72 | 2.29e−3* |
| MVPAa | 31 (20M, 11F) | no; p = 0.72 | 27.95 | 16.91 | 1.65 | 0.11 | both | 1.02 | 28.52 | 6.69e−6* | |
| walk test | 45 (22M, 23F) | no; p = 0.86 | 15.06 | 7.54 | 2.00 | 0.05 | both | 1.98 | 3.91 | 0.03* | |
| grip strength | 45 (22M, 23F) | yes; p = 6.36e−4* | 13.11 | 2.05 | 6.40 | 1.46e−07* | male | 3.21 | 5.96 | 6.93e−4* | |
| female | 1 | 0.30 | 0.59 | ||||||||
aThe authors have previously published [29] on physical activity levels in this Pokot sample and used GLMs to assess the effect of age on MVPA.
Average daily time spent in MVPA for Hadza participants (figure 1c) was 210.67 ± 19.28 min per day for males (n = 27) and 227.12 ± 19.53 min per day for females (n = 22). Average daily time in MVPA for Pokot participants (figure 1d) was 201.39 ± 11.48 min per day for males (n = 20) and 173.03 ± 21.06 for females (n = 11). Sex was not significantly associated with time in MVPA for either Hadza or Pokot participants (table 2). There was no interaction between age and sex on time in MVPA for Hadza or Pokot participants (table 2). For both Hadza and Pokot participants, age was significantly negatively associated with time in MVPA (table 2). Results did not change when BMI was included in the model (see electronic supplementary material, tables S9 and S13).
(b). Walk test performance
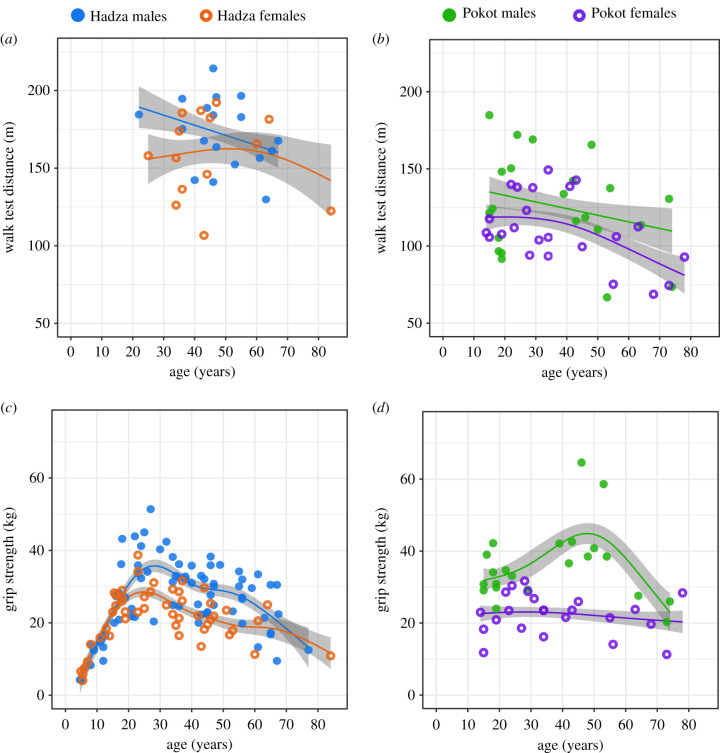
Hadza male participants (n = 18) travelled 172.17 ± 5.31 m and Hadza female participants (n = 14) travelled 158.63 ± 7.32 m during the 2 min walk test (figure 2a). Pokot male participants (n = 22) travelled 125.81 ± 6.71 m and Pokot female participants (n = 23) travelled 110.78 ± 4.73 m during the walk test (figure 2b). Sex was not significantly associated with walk test performance (distance travelled) for Hadza or Pokot participants (table 2). There was no interaction between age and sex on walk test performance for Hadza or Pokot participants (table 2). Age was not significantly associated with walk test performance for Hadza participants, but age was significantly associated with walk test performance for Pokot participants (table 2). Results did not change when BMI was included in the model (see electronic supplementary material, tables S10 and S14).
Figure 2.
Walk test performance and grip strength by age in Hadza and Pokot participants. Each data point represents one individual. Filled circles represent male participants and unfilled circles represent female participants. Lines fitting data represent a generalized additive model of the effect of age as a spline function on physical function tests. Shaded region is the 95% confidence interval for the estimated mean performance across age. (a) Walk test for Hadza participants. (b) Walk test for Pokot participants. (c) Grip strength for Hadza participants. (d) Grip strength for Pokot participants.
(c). Grip strength
Maximum grip strength among Hadza participants (figure 2c) averaged 26.7 ± 1.2 kg for males (n = 75) and 21.5 ± 1.1 kg for females (n = 49). Maximum grip strength among Pokot participants (figure 2d) averaged 36.1 ± 2.2 kg for males (n = 22) and 22.4 ± 1.2 kg for females (n = 23). Sex was significantly associated with grip strength for Hadza and Pokot participants, with male participants displaying a higher maximum grip strength compared with female participants (table 2). There was a significant interaction between age and sex on grip strength for both Hadza and Pokot participants (table 2). Age was significantly associated with maximum grip strength for Hadza male, Hadza female and Pokot male participants (table 2). Removal of the two Pokot male participants with large values did not alter the results (see electronic supplementary material, table S21 and figure S1). Age was not significantly associated with maximum grip strength for Pokot female participants (table 2). Results did not change when BMI was included in the model (see electronic supplementary material, tables S11 and S15).
4. Discussion
For Hadza and Pokot participants in this study, age was significantly inversely associated with most, though not all, measures of physical function. With the exception of walk test performance, Hadza and Pokot participants displayed patterns of age-related differences in functional measures that were similar to each other and to patterns seen in industrialized populations. While our results are based on small sample sizes, we suggest they provide preliminary evidence that (i) some age-related patterns of physical function may be more consistently expressed across populations and (ii) differences in age-based divisions of labour between Hadza and Pokot populations lead to modest variation in associations between age and one aspect of physical function: walking endurance. While speculative, we suggest that reductions in work-related activity in older aged pastoralists may play a role in their more consistent patterns of age-related shifts in physical function. Overall, however, it is clear that despite minor differences, both populations studied experience broadly similar age-related patterns of physical function.
(a). Comparison with industrialized populations
Hadza and Pokot participants in this study displayed patterns of age-related differences in physical function measures that were largely similar to patterns displayed by industrialized populations. In industrialized contexts, physical activity levels, walking endurance and grip strength decline with age. Similarly, we found significant associations between age and Pokot walk test performance and Hadza grip strength, in particular. We also found consistent patterns of inverse associations between age and physical activity levels across both Hadza and Pokot samples. However, Hadza and Pokot participants maintained objectively high physical activity levels even at older ages. For example, while the majority of adults in the US fail to meet the 150 min per week of MVPA recommended by health officials [58,59], activity levels for Hadza and Pokot participants across all ages exceeded these guidelines, often substantially. Hadza and Pokot participants over the age of 40 also generally displayed higher ENMO values than those reported from the UK BioBank (approx. 31 mg for adults ages 45–54), the largest epidemiological sample of adults over age 40 with accelerometer-derived ENMO reported to date [33]. Thus, both hunting and gathering and pastoralist lifestyles require high levels of physical activity across the lifespan, consistent with previous studies [28,60,61], marking a key area of difference with more industrialized societies. In industrialized populations, engaging in a physically active lifestyle, especially during older adulthood, is associated with decreased risk of disability and mortality [21,24]. Understanding how maintenance of high levels of physical activity at older ages in these foraging and pastoralist societies impacts overall health and function is a high priority for future work.
(b). Physical function and Hadza and Pokot lifestyles
Overall, Hadza and Pokot participants had similar age-related patterns of physical function. However, inter-population variation in both gender- and age-related patterns of measures could be an important reflection of differences in Hadza and Pokot subsistence strategies and divisions of labour. For example, Hadza men and women forage on a regular basis for the wild animal- and plant-based foods and continue to do so until advanced ages [31,40,62]. Although Hadza men generally travel greater distances than Hadza women [31], women forage for foods that involve high-intensity extraction and preparation activities, such as digging and pounding [31,40]. The lack of gender difference in Hadza activity levels in this study likely reflects the active requirements of foraging for both men and women, despite their gender-based division of labour. This result mirrors previous findings that show Hadza men and women engage in equally high levels of physical activity throughout adulthood [28].
The Pokot livelihood, by contrast, centres around livestock management [42,43]. Pokot men generally walk long distances with animals and build wells, and women maintain households (water and milk animals, prepare food, collect firewood and water) [30,46]. Intensity differences among the activities men and women engage in may help explain why male participants display higher ENMO values (average daily accelerometry intensity) than female participants, but not higher MVPA (time above the threshold for moderate activity). More detailed future analyses linking different behaviours with their intensities will help us better define the underlying causes of gender-based differences in activity levels in the Pokot people.
The two groups also differ in the association between age and walking endurance, which may be linked to the presence of an age-based division of labour among the Pokot, but not the Hadza. Hadza men and women continue foraging throughout life, without a formal shift in duties with age [31,62] and Hadza participants in this study do not show a significant age-related reduction in walking endurance. It is possible that continued engagement in foraging behaviours throughout adulthood could support the maintenance of walking endurance into older ages. By contrast, Pokot participants in this study show a more pronounced age effect, which could be linked to their cultural practice of delegating more strenuous tasks to younger Pokot individuals [30,46]. Future comparative research should explore this key difference between the Hadza and Pokot further in order to determine how cultural and lifestyle differences may influence walking endurance through life.
Finally, while Hadza participants display age-related patterns of grip strength more similar to those seen in industrialized contexts, Pokot male participants in this study display particularly high grip strength values in their 40s and 50s. It is possible that Pokot men engage in activities that maintain or build strength past young adulthood, and further work with a more robust sample size could help confirm and better explain this finding.
In several regards, our results echo findings from studies of other small-scale societies, where some measures of physical function differ across sampled ages and others do not (table 3). For example, most populations display similar age-related patterns in grip strength, while measures of endurance seem more variable across populations. Reduced strength could be a more common aspect of age-related change in physical function and therefore occur across different contexts, including foraging and pastoralism. Altogether, these findings suggest that age-related changes in some aspects of physical function (e.g. walking endurance) may depend more strongly on lifestyle than others.
Table 3.
Summary of age-related changes in measures of physical function across selected groups. Italics indicate findings from an industrialized population (UK). Bold type indicates findings different from those seen in industrialized populations.
| group | subsistence | measure | age-related decline? | reference |
|---|---|---|---|---|
| UK | industrialized | physical activity levels | yes | [33] |
| walking endurance | yes, after 60 | [34] | ||
| grip strength | yes | [35] | ||
| Hadza | hunter–gatherers | physical activity levels | noa | [28] |
| yes, but still high at older ages | this study | |||
| walking endurance | no | |||
| grip strength | yes | |||
| Pokot | pastoralists | physical activity levelsb | yes, but still high at older ages | |
| walking endurance | yes | |||
| grip strength | yes (M)/no (F) | |||
| Ache | hunter–gatherers | grip strength | yes | [27] |
| VO2maxc | yes (M)/no (F) | |||
| Shuar | forager horticulturalists | physical activity levels | no | [63] |
| Tsimane | forager horticulturalists | physical activity levels | yes (M)/no (F) | [64] |
| grip strength | yes | [25] | ||
| VO2max | yes, but slower rate of decline compared with a Canadian sample | [26] |
aRaichlen and colleagues [28] used heart rate monitors to measure average daily time spent in moderate-to-vigorous physical activity in Hadza hunter–gatherers and did not find evidence of age-related declines in activity levels.
bThe physical activity data presented in this paper have been previously published by the authors [29].
cVO2max (maximal oxygen uptake) is a measure of aerobic capacity and is often used as a proxy for endurance.
(c). Limitations and future directions
We believe our results provide preliminary evidence that age-related changes in some aspects of physical function may be more consistent across populations, while others may differ importantly across populations. However, this study had several limitations that could impact our findings. First, many important age-related changes seen in industrialized contexts begin to occur after the age of 60 [65] and in this study, we had small sample sizes in these older age groups. Future work that specifically aims to incorporate more older individuals could allow us to better assess how these groups experience ageing both across adulthood and into older adulthood. Furthermore, our study was cross-sectional, and longitudinal follow-up studies would allow us to evaluate changes in functional measures over time.
Second, we quantified physical activity levels using accelerometer-based measures. Accelerometers measure overall movement, but they do not measure heart rate, which may better capture the intensity of activities that are not associated with travel and movement [66]. Raichlen et al. [28] used heart rate monitors to measure physical activity levels in Hadza participants (n = 46) and found that time in MVPA (defined as heart rates greater than 55% of maximum heart rate) increased in older participants. It is possible that this difference between studies is associated with age-related changes in cardiovascular physiology, including decreased maximal heart rate with age [67]. In this case, at a given accelerometer intensity, older individuals may have a higher relative heart rate compared with younger individuals. These differences across studies suggest that more work is needed to develop ecologically relevant measures of physical activity in small-scale societies.
Third, we used walk test performance and grip strength to assess walking endurance and strength, respectively. These tests are used in clinical contexts to safely assess functional status, including an individual's ability to perform activities of daily life (ADLs) [16,68]. Since the Hadza and Pokot display subsistence strategies that differ both from one another and from industrialized populations, their ADLs could be fundamentally different from each other and from those of industrialized populations. More ecologically relevant assessments of health that account for population-specific ideas about ageing and functionality could be more appropriate for assessing function in different contexts.
Finally, Hadza and Pokot participants in this study displayed some group-based differences in the association between age and functional measures. Understanding how and why populations like these display different age-related patterns of functional measures is a key area of future work that will help us better evaluate how variation in cultural and lifestyle contexts impact age-related differences in behaviour and physiological status.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors would like to thank all Hadza and Pokot research participants for their involvement in the study and their incredible hospitality and generosity during our field seasons with them. We especially thank our Hadza and Pokot research assistants, without whom our field seasons would not have been successful: Mariamu Anyawire and Bunga Paolo (Hadza), Rachael Lorrus, Catherine Lokope and Willie Kibet Lotodo (Pokot). Thank you also to field team members Jacob Harris, Renée Hagen, Doreen Odera, Emma Bunkley and Cassidy Reeves for instrumental insight into data collection in the field. The authors declare no conflicts of interest.
Ethics
Approval for research with the Hadza foragers in Tanzania was acquired from all appropriate agencies (Institutional Review Boards for the University of Arizona, Yale University and the University of California Los Angeles; Tanzania Commission for Science and Technology) prior to data collection. Approval for research with the Pokot pastoralists in Kenya was acquired from all appropriate agencies (Institutional Review Board of the University of Arizona, National Commission on Science and Technology and Innovation in Kenya) prior to data collection. All Hadza and Pokot participants provided informed consent prior to and during study participation.
Data accessibility
Data are available as electronic supplementary material.
Authors' contributions
M.K.S., I.L.P. and D.A.R. designed the study. M.K.S., H.P., B.M.W., I.L.P., A.Z.P.M. and D.A.R. performed data collection. M.K.S., B.M.W. and D.A.R. analysed the data. M.K.S., H.P., G.E.A., B.M.W., A.Z.P.M., I.L.P. and D.A.R. discussed and interpreted the data. M.K.S. drafted the manuscript. M.K.S., G.E.A., B.M.W., I.L.P. and D.A.R. edited the manuscript for intellectual content. All authors gave final approval for publication and agree to be held accountable for the work performed herein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by: the National Science Foundation (REG Supplement 1637363 to BCS 1430790 (I.L.P.), BCS 1440867 (D.A.R.), BCS-0850815 (H.P.), BCS 1440867 (D.A.R.), BCS 1440841 (H.P.) and BCS 1440671 (B.M.W.)); the Max Planck Institute for Evolutionary Anthropology, Department of Human Behavior, Ecology, and Culture; the University of Arizona School of Anthropology William & Nancy Sullivan Scholarship Fund; and the Joseph & Mary Cacioppo Foundation.
References
- 1.Charnov EL. 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Charnov EL, Berrigan D. 1993. Why do female primates have such long lifespans and so few babies? or Life in the slow lane. Evol. Anthropol. 1, 191–194. ( 10.1002/evan.1360010604) [DOI] [Google Scholar]
- 3.Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. 2001. Mortality rates among wild chimpanzees. J. Hum. Evol. 40, 437–450. ( 10.1006/jhev.2001.0469) [DOI] [PubMed] [Google Scholar]
- 4.Wood BM, Watts DP, Mitani JC, Langergraber KE. 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol. 105, 41–56. ( 10.1016/j.jhevol.2017.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurven M, Kaplan H. 2007. Longevity among hunter-gatherers: a cross-cultural examination. Popul. Dev. Rev. 33, 321–365. ( 10.1111/j.1728-4457.2007.00171.x) [DOI] [Google Scholar]
- 6.Caspari R, Lee SH. 2004. Older age becomes common late in human evolution. Proc. Natl Acad. Sci. USA 101, 10 895–10 900. ( 10.1073/pnas.0402857101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bramble DM, Lieberman D. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352. ( 10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 8.Lieberman D. 2013. The story of the human body: evolution, health, and disease. New York, NY: Vintage Books. [PubMed] [Google Scholar]
- 9.Aiello LC, Wells JCK. 2002. Energetics and the evolution of the genus Homo. Ann. Rev. Anthropol. 31, 323–338. ( 10.1146/annurev.anthro.31.040402.085403) [DOI] [Google Scholar]
- 10.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 11.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 12.United Nations Department of Economic and Social Affairs. 2019. World Population Prospects 2019: Highlights, ST/ESA/SER.A/423 New York, NY: United Nations. [Google Scholar]
- 13.WHO. 2011. World report on disability: 2011 Geneva, Switzerland, World Health Organization.
- 14.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. 2000. Actual causes of death in the United States, 2000. JAMA 291, 1238–1245. ( 10.1001/jama.291.10.1238) [DOI] [PubMed] [Google Scholar]
- 15.Naghavi M, et al. 2017. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1151–1210. ( 10.1016/S0140-6736(17)32152-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubenstein LZ. 2006. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 35(Suppl. 2), ii37–ii41. ( 10.1093/ageing/afl084) [DOI] [PubMed] [Google Scholar]
- 17.Morbeck ME, Galloway A, Sumner DR. 2002. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees. Interdiscip. Top. Gerontol. 31, 48–62. ( 10.1159/000061458) [DOI] [Google Scholar]
- 18.Shively CA, et al. 2012. Aging and physical mobility in group-housed Old World monkeys. Age 34, 1123–1131. ( 10.1007/s11357-011-9350-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberts SC, Archie EA, Gesquiere LR, Altmann J, Vaupel JW, Christensen K. 2014. The male-female health-survival paradox: a comparative perspective on sex differences in aging and mortality. In Sociality, hierarchy, health: comparative biodemography: a collection of papers (eds Weinstein M, Lane MA), pp. 339–363. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 20.Blondell SJ, Hammersley-Mather R, Veerman JL. 2014. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14, 510 ( 10.1186/1471-2458-14-510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekelund U, et al. 2015. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 101, 613–621. ( 10.3945/ajcn.114.100065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Willett WC. 2002. Optimal diets for prevention of coronary heart disease. JAMA 288, 2569–2578. ( 10.1001/jama.288.20.2569) [DOI] [PubMed] [Google Scholar]
- 23.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. 2008. Reduced disability and mortality among aging runners. Arch. Intern. Med. 168, 1638–1646. ( 10.1001/archinte.168.15.1638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurven M, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, Hooper P, Kaplan H. 2017. The Tsimane Health and Life History Project: integrating anthropology and biomedicine. Evol. Anthropol. 26, 54–73. ( 10.1002/evan.21515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisor AC, Gurven M, Blackwell AD, Kaplan H, Yetish G. 2013. Patterns of senescence in human cardiovascular fitness: VO2max in subsistence and industrialized populations. Am. J. Hum. Biol. 25, 756–769. ( 10.1002/ajhb.22445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker R, Hill K. 2003. Modeling growth and senescence in physical performance among the Ache of eastern Paraguay. Am. J. Hum. Biol. 15, 196–208. ( 10.1002/ajhb.10135) [DOI] [PubMed] [Google Scholar]
- 28.Raichlen DA, et al. 2017. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 29, e22919 ( 10.1002/ajhb.22919) [DOI] [PubMed] [Google Scholar]
- 29.Sayre MK, Pike IL, Raichlen DA. 2019. High levels of objectively measured physical activity across adolescence and adulthood among the Pokot pastoralists of Kenya. Am. J. Hum. Biol. 31, e23205 ( 10.1002/ajhb.23205) [DOI] [PubMed] [Google Scholar]
- 30.Bollig M. 2000. Staging social structures: ritual and social organisation in an egalitarian society. The pastoral Pokot of northern Kenya. Ethnos 65, 341–365. ( 10.1080/00141840050198027) [DOI] [Google Scholar]
- 31.Marlowe F. 2010. The Hadza: hunter-gatherers of Tanzania. Berkeley, CA: University of California Press. [Google Scholar]
- 32.Troiano R, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. 2008. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exercise 40, 181–188. ( 10.1249/mss.0b013e31815a51b3) [DOI] [PubMed] [Google Scholar]
- 33.Doherty A, et al. 2017. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS ONE 12, e0169649 ( 10.1371/journal.pone.0169649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon RW, Wang YC, Gershon RC. 2015. Two-minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch. Phys. Med. Rehabil. 96, 472–477. ( 10.1016/j.apmr.2014.10.006) [DOI] [PubMed] [Google Scholar]
- 35.Dodds RM, et al. 2014. Grip strength across the life course: normative data from twelve British studies. PLoS One 9, e113637 ( 10.1371/journal.pone.0113637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blurton Jones N. 2016. Demography and evolutionary ecology of Hadza hunter-gatherers Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Blurton Jones NG, Smith LC, O'Connell JF, Hawkes K, Kamuzora CL. 1992. Demography of the Hadza, an increasing and high density population of savanna foragers. Am. J. Phys. Anthropol. 89, 159–181. ( 10.1002/ajpa.1330890204) [DOI] [PubMed] [Google Scholar]
- 38.Wood BM, Marlowe FW. 2013. Household and kin provisioning by Hadza men. Human Nat. 24, 80–317. ( 10.1007/s12110-013-9173-0) [DOI] [PubMed] [Google Scholar]
- 39.Marlowe FW. 2007. Hunting and fathering: the human sexual division of foraging labor. Cross-Cult. Res. 41, 170–195. ( 10.1177/1069397106297529) [DOI] [Google Scholar]
- 40.Hawkes K, O'Connell JF, Blurton Jones NG. 1989. Hardworking Hadza grandmothers. In Comparative socioecology (eds Standen V, Foley RA), pp. 341–366. London, UK: Basil Blackwell. [Google Scholar]
- 41.Crittenden AN, Sorrentino J, Moonie SA, Peterson M, Mabulla A, Ungar PS. 2017. Oral health in transition: the Hadza foragers of Tanzania. PLoS ONE 12, e0172197 ( 10.1371/journal.pone.0172197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollig M. 1992. East Pokot camel husbandry. Nomadic Peoples 31, 34–50. [Google Scholar]
- 43.Österle M. 2008. From cattle to goats: the transformation of East Pokot pastoralism in Kenya. Nomadic Peoples 12, 81–91. ( 10.3167/np.2008.120105) [DOI] [Google Scholar]
- 44.Greiner C, Alvarez M, Becker M. 2013. From cattle to corn: attributes of emerging farming systems of former pastoral nomads in East Pokot, Kenya. Soc. Nat. Resourc. 26, 1478–1490. ( 10.1080/08941920.2013.791901) [DOI] [Google Scholar]
- 45.Greiner C, Mwaka I. 2016. Agricultural change at the margins: adaptation and intensification in a Kenyan dryland. J. E. Afr. Stud. 10, 130–149. ( 10.1080/17531055.2015.1134488) [DOI] [Google Scholar]
- 46.Little MA. 1989. Human biology of African pastoralists. Am. J. Phys. Anthropol. 32, 215–247. ( 10.1002/ajpa.1330320510) [DOI] [Google Scholar]
- 47.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 48.van Hees V, Fang Z, Zhao J, Heywood J, Mirkes E, Sabia S, Migueles J. . 2020. GGIR: raw accelerometer data analysis. ( 10.5281/zenodo.1051064) [DOI]
- 49.van Hees VT, et al. 2014. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J. Appl. Physiol. 117, 738–744. ( 10.1152/japplphysiol.00421.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Hees VT, et al. 2013. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS ONE 8, e61691 ( 10.1371/journal.pone.0061691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildebrand M, van Hees VT, Hansen BH, Ekelund U. 2014. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exercise 46, 1816–1824. ( 10.1249/MSS.0000000000000289) [DOI] [PubMed] [Google Scholar]
- 52.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. 2008. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 167, 875–881. ( 10.1093/aje/kwm390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L. 2017. Measuring physical activity with hip accelerometry among U.S. older adults: how many days are enough? PLoS ONE 12, e0170082 ( 10.1371/journal.pone.0170082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rikli RE, Jones CJ. 1998. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J. Aging Phys. Act. 6, 363–375. ( 10.1123/japa.6.4.363) [DOI] [Google Scholar]
- 55.Connelly DM, Thomas BK, Cliffe SJ, Perry WM, Smith RE. 2009. Clinical utility of the 2-minute walk test for older adults living in long-term care. Physiother. Can. 61, 78–87. ( 10.3138/physio.61.2.78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perna FM, et al. 2016. Muscular grip strength estimates of the U.S. population from the National Health and Nutrition Examination Survey 2011–2012. J. Strength Cond Res. 30, 867–874. ( 10.1519/JSC.0000000000001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 73, 3–36. [Google Scholar]
- 58.Tucker JM, Welk GJ, Beyler NK. 2011. Physical activity in U.S. adults. Am. J. Prev. Med. 40, 454–461. ( 10.1016/j.amepre.2010.12.016) [DOI] [PubMed] [Google Scholar]
- 59.US DHHS. 2018. Physical activity guidelines for Americans, 2nd edn Washington: DC: U.S. Department of Health and Human Services. [Google Scholar]
- 60.Caldwell AE. 2016. Physical activity and energy expenditure in humans. In Human physical fitness and activity: an evolutionary and life history perspective (ed. Caldwell AE.), pp. 27–37. Cham, Switzerland: Springer. [Google Scholar]
- 61.Christensen DL, et al. 2012. Cardiorespiratory fitness and physical activity in Luo, Kamba, and Maasai of rural Kenya. Am. J. Hum. Biol. 24, 723–729. ( 10.1002/ajhb.22303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawkes K, O'Connell JF, Blurton Jones NG. 1997. Hadza women's time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Curr. Anthropol. 38, 551–577. ( 10.1086/204646) [DOI] [Google Scholar]
- 63.Madimenos FC, Snodgrass JJ, Blackwell AD, Liebert MA, Sugiyama LS. 2011. Physical activity in an indigenous Ecuadorian forager-horticulturalist population as measured using accelerometry. Am. J. Hum. Biol. 23, 488–497. ( 10.1002/ajhb.21163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurven M, Jaeggi AV, Kaplan H, Cummings D. 2013. Physical activity and modernization among Bolivian Amerindians. PLoS ONE 8, e55679 ( 10.1371/journal.pone.0055679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daley MJ, Spinks WL. 2000. Exercise, mobility and aging. Sports Med. 29, 1–12. ( 10.2165/00007256-200029010-00001) [DOI] [PubMed] [Google Scholar]
- 66.Schutz Y, Weinsier RL, Hunter GR. 2001. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obesity Res. 9, 368–379. ( 10.1038/oby.2001.48) [DOI] [PubMed] [Google Scholar]
- 67.Tanaka H, Monahan KD, Seals DR. 2001. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 37, 153–156. ( 10.1016/S0735-1097(00)01054-8) [DOI] [PubMed] [Google Scholar]
- 68.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. 2005. A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495. ( 10.1503/cmaj.050051) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as electronic supplementary material.